Abstract
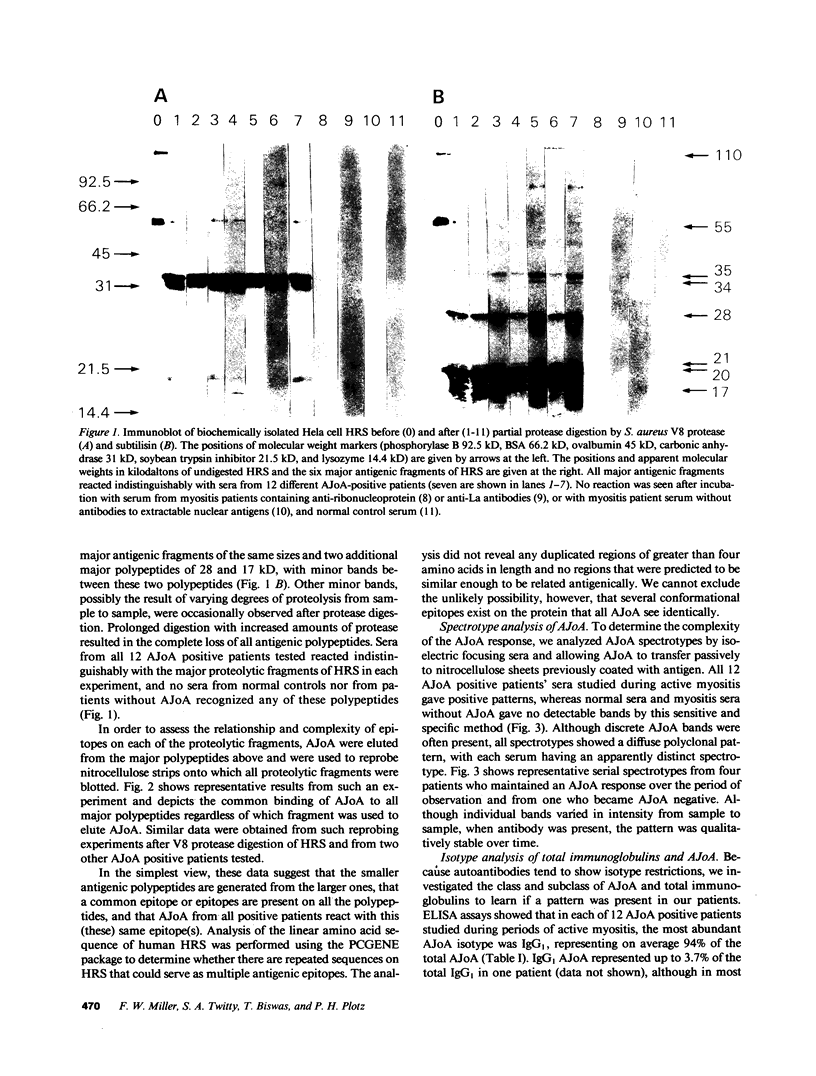
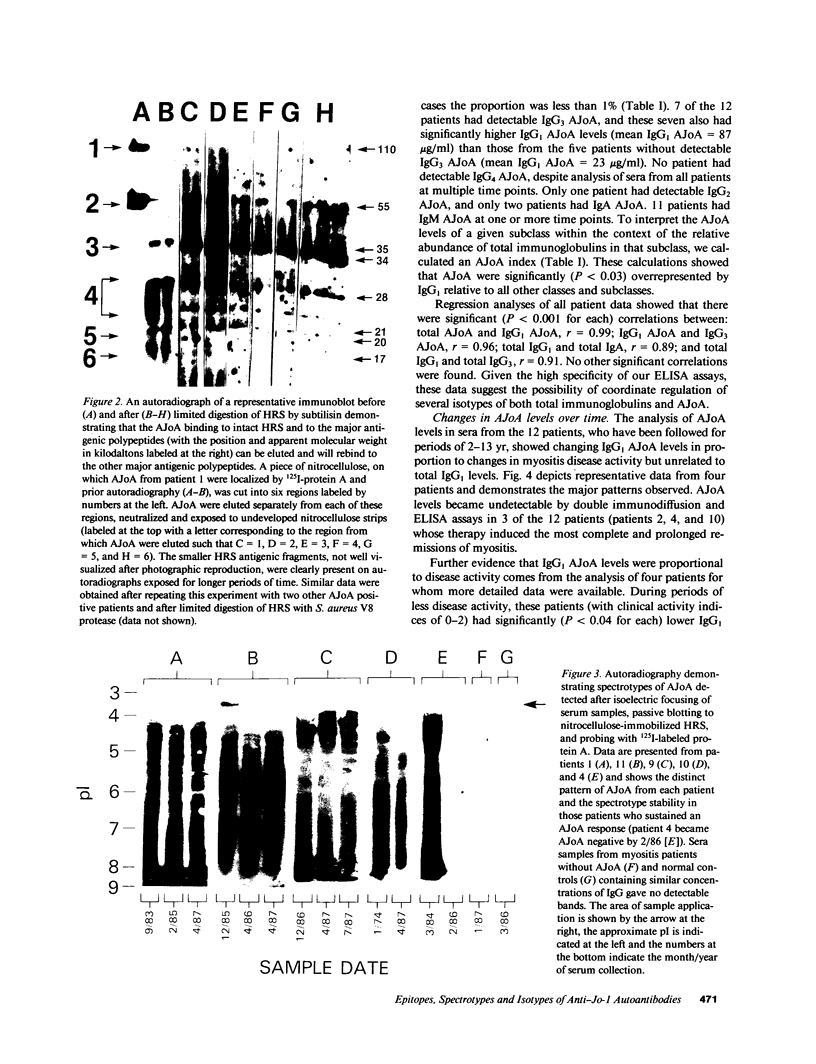
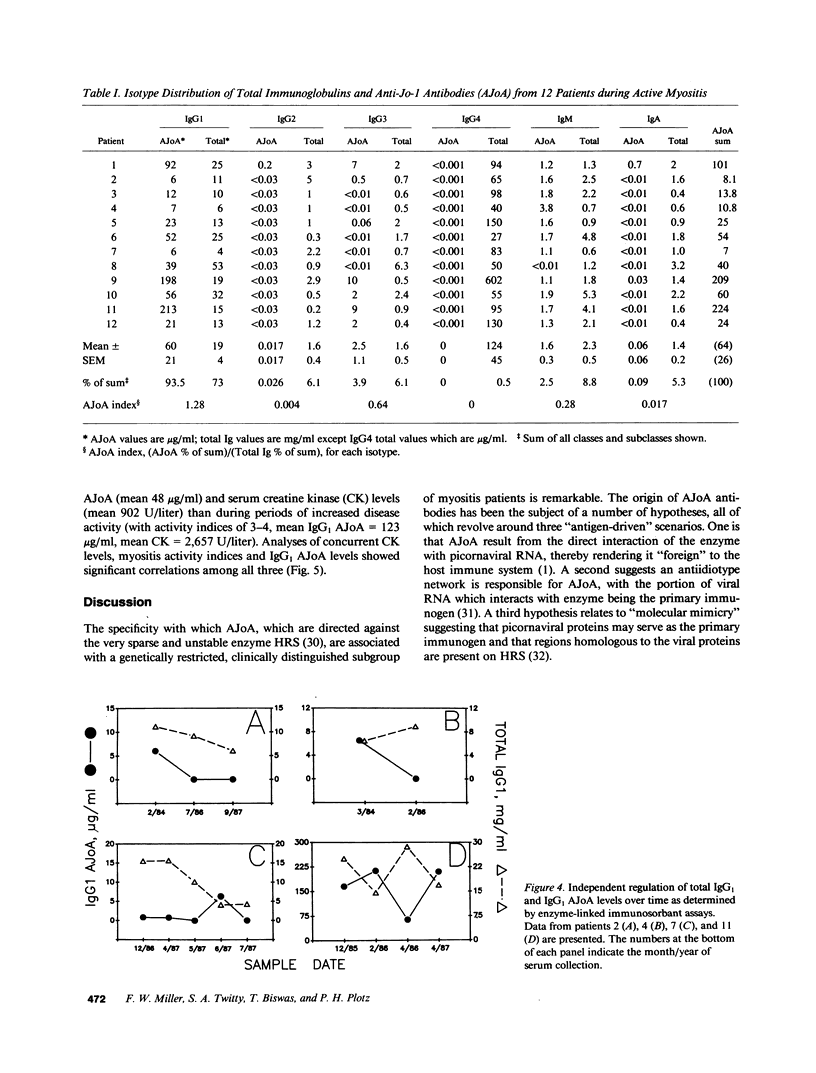
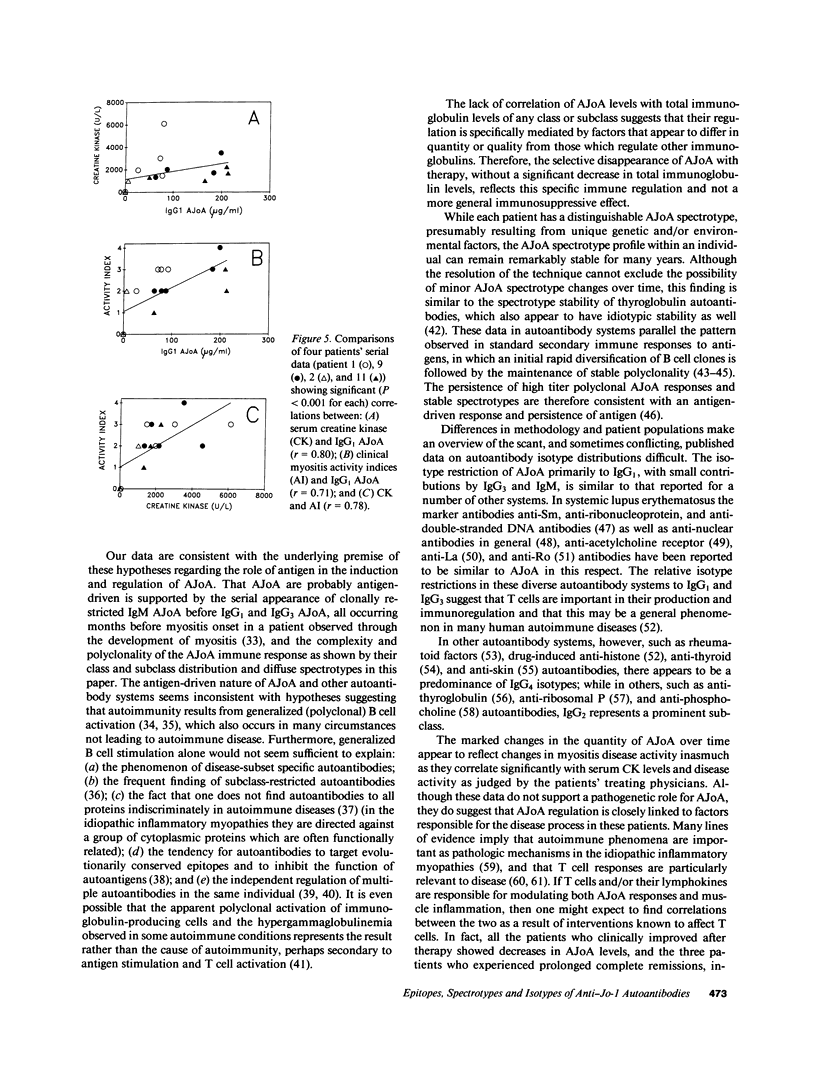
Anti-Jo-1 antibodies (AJoA), which bind to and inhibit the activity of histidyl-transfer RNA synthetase (HRS), are found in a genetically and clinically distinct subset of myositis patients. This specificity suggests that understanding the antigenic epitopes and immunoregulation governing the production of AJoA may result in clues to disease pathogenesis. Limited digestion of human HRS by V8 protease resulted in four major antigenic polypeptides of 35, 34, 21, and 20 kD; digestion with subtilisin gave four fragments of the same sizes and two additional major antigenic polypeptides of 28 and 17 kD. Sera from 12 AJoA positive patients reacted indistinguishably with these proteolytic fragments by Western blotting, and AJoA elution studies suggested a common epitope(s) on all six. Isoelectric focusing showed a different polyclonal pattern of AJoA in each patient, although serial analyses in individual patients revealed stable AJoA spectrotypes over years of observation. Enzyme-linked immunosorbent assays showed that the AJoA response was mainly restricted to the IgG1 heavy chain isotype. The levels of IgG1 AJoA varied in proportion to disease activity over time but were independent of total IgG1 levels, and three patients became AJoA negative as their myositis remitted after treatment. These findings suggest that AJoA are induced by an antigen-driven mechanism, bind to a common epitope or epitopes on HRS, and are modulated by an immune response closely linked to that which is responsible for myositis in these patients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Hirsch T. J., Bias W. B., Nishikai M., Reichlin M. The Jo-1 antibody system in myositis: relationships to clinical features and HLA. J Rheumatol. 1981 Nov-Dec;8(6):925–930. [PubMed] [Google Scholar]
- Askonas B. A., Williamson A. R., Wright B. E. Selection of a single antibody-forming cell clone and its propagation in syngeneic mice. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1398–1403. doi: 10.1073/pnas.67.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein R. M., Morgan S. H., Chapman J., Bunn C. C., Mathews M. B., Turner-Warwick M., Hughes G. R. Anti-Jo-1 antibody: a marker for myositis with interstitial lung disease. Br Med J (Clin Res Ed) 1984 Jul 21;289(6438):151–152. doi: 10.1136/bmj.289.6438.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bionda A., De Baets M. H., Tzartos S. J., Lindstrom J. M., Weigle W. O., Theophilopoulos A. N. Spectrotypic analysis of antibodies to acetylcholine receptors in experimental autoimmune myasthenia gravis. Clin Exp Immunol. 1984 Jul;57(1):41–50. [PMC free article] [PubMed] [Google Scholar]
- Biswas T., Miller F. W., Plotz P. H. Stimulation and partial stabilization of human histidyl-tRNA synthetase by hemoglobin. FEBS Lett. 1988 Feb 29;229(1):203–205. doi: 10.1016/0014-5793(88)80827-5. [DOI] [PubMed] [Google Scholar]
- Biswas T., Miller F. W., Takagaki Y., Plotz P. H. An enzyme-linked immunosorbent assay for the detection and quantitation of anti-Jo-1 antibody in human serum. J Immunol Methods. 1987 Apr 16;98(2):243–248. doi: 10.1016/0022-1759(87)90011-1. [DOI] [PubMed] [Google Scholar]
- Biswas T., Miller F. W., Twitty S. A., Plotz P. H. An efficient method for enrichment of histidyl-tRNA synthetase (Jo-1 antigen) from HeLa cells. J Immunol Methods. 1987 Apr 16;98(2):235–241. doi: 10.1016/0022-1759(87)90010-x. [DOI] [PubMed] [Google Scholar]
- Bohan A., Peter J. B. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975 Feb 13;292(7):344–347. doi: 10.1056/NEJM197502132920706. [DOI] [PubMed] [Google Scholar]
- Bonfa E., Chu J. L., Brot N., Elkon K. B. Lupus anti-ribosomal P peptide antibodies show limited heterogeneity and are predominantly of the IgG1 and IgG2 subclasses. Clin Immunol Immunopathol. 1987 Oct;45(1):129–138. doi: 10.1016/0090-1229(87)90119-x. [DOI] [PubMed] [Google Scholar]
- Bowles N. E., Dubowitz V., Sewry C. A., Archard L. C. Dermatomyositis, polymyositis, and Coxsackie-B-virus infection. Lancet. 1987 May 2;1(8540):1004–1007. doi: 10.1016/s0140-6736(87)92271-9. [DOI] [PubMed] [Google Scholar]
- Bunn C. C., Bernstein R. M., Mathews M. B. Autoantibodies against alanyl-tRNA synthetase and tRNAAla coexist and are associated with myositis. J Exp Med. 1986 May 1;163(5):1281–1291. doi: 10.1084/jem.163.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunn C. C., Mathews M. B. Autoreactive epitope defined as the anticodon region of alanine transfer RNA. Science. 1987 Nov 20;238(4830):1116–1119. doi: 10.1126/science.2446387. [DOI] [PubMed] [Google Scholar]
- Chou S. M., Gutmann L. Picornavirus-like crystals in subacute polymyositis. Neurology. 1970 Mar;20(3):205–213. doi: 10.1212/wnl.20.3.205. [DOI] [PubMed] [Google Scholar]
- Christensen M. L., Pachman L. M., Schneiderman R., Patel D. C., Friedman J. M. Prevalence of Coxsackie B virus antibodies in patients with juvenile dermatomyositis. Arthritis Rheum. 1986 Nov;29(11):1365–1370. doi: 10.1002/art.1780291109. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cohen P. L., Cheek R. L., Hadler J. A., Yount W. J., Eisenberg R. A. The subclass distribution of human IgG rheumatoid factor. J Immunol. 1987 Sep 1;139(5):1466–1471. [PubMed] [Google Scholar]
- Cronin M. E., Love L. A., Miller F. W., McClintock P. R., Plotz P. H. The natural history of encephalomyocarditis virus-induced myositis and myocarditis in mice. Viral persistence demonstrated by in situ hybridization. J Exp Med. 1988 Nov 1;168(5):1639–1648. doi: 10.1084/jem.168.5.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin M. E., Plotz P. H., Miller F. W. Abnormalities of the immune system in the idiopathic inflammatory myopathies. In Vivo. 1988 Jan-Feb;2(1):25–29. [PubMed] [Google Scholar]
- Davies T. F., Weber C. M., Wallack P., Platzer M. Restricted heterogeneity and T cell dependence of human thyroid autoantibody immunoglobulin G subclasses. J Clin Endocrinol Metab. 1986 May;62(5):945–949. doi: 10.1210/jcem-62-5-945. [DOI] [PubMed] [Google Scholar]
- Delves P. J., Roitt I. M. Long term spectrotypic and idiotypic stability of thyroglobulin autoantibodies in patients with Hashimoto's thyroiditis. Clin Exp Immunol. 1988 Mar;71(3):459–463. [PMC free article] [PubMed] [Google Scholar]
- Dziarski R. Preferential induction of autoantibody secretion in polyclonal activation by peptidoglycan and lipopolysaccharide. I. In vitro studies. J Immunol. 1982 Mar;128(3):1018–1025. [PubMed] [Google Scholar]
- Eisenberg R. A., Dyer K., Craven S. Y., Fuller C. R., Yount W. J. Subclass restriction and polyclonality of the systemic lupus erythematosus marker antibody anti-Sm. J Clin Invest. 1985 Apr;75(4):1270–1277. doi: 10.1172/JCI111826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A. G., Arahata K. Mononuclear cells in myopathies: quantitation of functionally distinct subsets, recognition of antigen-specific cell-mediated cytotoxicity in some diseases, and implications for the pathogenesis of the different inflammatory myopathies. Hum Pathol. 1986 Jul;17(7):704–721. doi: 10.1016/s0046-8177(86)80180-0. [DOI] [PubMed] [Google Scholar]
- Gharavi A. E., Chu J. L., Elkon K. B. Autoantibodies to intracellular proteins in human systemic lupus erythematosus are not due to random polyclonal B cell activation. Arthritis Rheum. 1988 Nov;31(11):1337–1345. doi: 10.1002/art.1780311101. [DOI] [PubMed] [Google Scholar]
- Gray D., Skarvall H. B-cell memory is short-lived in the absence of antigen. Nature. 1988 Nov 3;336(6194):70–73. doi: 10.1038/336070a0. [DOI] [PubMed] [Google Scholar]
- Jefferis R., Reimer C. B., Skvaril F., de Lange G., Ling N. R., Lowe J., Walker M. R., Phillips D. J., Aloisio C. H., Wells T. W. Evaluation of monoclonal antibodies having specificity for human IgG sub-classes: results of an IUIS/WHO collaborative study. Immunol Lett. 1985;10(3-4):223–252. doi: 10.1016/0165-2478(85)90082-3. [DOI] [PubMed] [Google Scholar]
- Jones C. C., Hamilton R. G., Jordon R. E. Subclass distribution of human IgG autoantibodies in pemphigus. J Clin Immunol. 1988 Jan;8(1):43–49. doi: 10.1007/BF00915155. [DOI] [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987 Jun 1;165(6):1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knisley K. A., Rodkey L. S. Affinity immunoblotting. High resolution isoelectric focusing analysis of antibody clonotype distribution. J Immunol Methods. 1986 Dec 4;95(1):79–87. doi: 10.1016/0022-1759(86)90320-0. [DOI] [PubMed] [Google Scholar]
- Lieu T. S., Reimer C. B., Sontheimer R. D. Immunoglobulin class and subclass profile of the Ro/SS-A autoantibody response. J Invest Dermatol. 1988 Feb;90(2):158–164. doi: 10.1111/1523-1747.ep12462142. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M. Myositis autoantibody inhibits histidyl-tRNA synthetase: a model for autoimmunity. Nature. 1983 Jul 14;304(5922):177–179. doi: 10.1038/304177a0. [DOI] [PubMed] [Google Scholar]
- Mathews M. B., Reichlin M., Hughes G. R., Bernstein R. M. Anti-threonyl-tRNA synthetase, a second myositis-related autoantibody. J Exp Med. 1984 Aug 1;160(2):420–434. doi: 10.1084/jem.160.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan S. M., Feldt-Rasmussen U., Young E. T., Middleton S. L., Dlichert-Toft M., Siersboek-Nielsen K., Date J., Carr D., Clark F., Rees Smith B. IgG subclass distribution of thyroid autoantibodies: a 'fingerprint' of an individual's response to thyroglobulin and thyroid microsomal antigen. Clin Endocrinol (Oxf) 1987 Mar;26(3):335–346. doi: 10.1111/j.1365-2265.1987.tb00791.x. [DOI] [PubMed] [Google Scholar]
- Miller F. W., Love L. A., Biswas T., McClintock P. R., Notkins A. L., Plotz P. H. Viral and host genetic factors influence encephalomyocarditis virus-induced polymyositis in adult mice. Arthritis Rheum. 1987 May;30(5):549–556. doi: 10.1002/art.1780300509. [DOI] [PubMed] [Google Scholar]
- Papadea C., Check I. J., Reimer C. B. Monoclonal antibody-based solid-phase immunoenzymometric assays for quantifying human immunoglobulin G and its subclasses in serum. Clin Chem. 1985 Dec;31(12):1940–1945. [PubMed] [Google Scholar]
- Pearce D. C., Yount W. J., Eisenberg R. A. Subclass restriction of anti-SS-B (La) autoantibodies. Clin Immunol Immunopathol. 1986 Jan;38(1):111–119. doi: 10.1016/0090-1229(86)90128-5. [DOI] [PubMed] [Google Scholar]
- Pisetsky D. S., McCarty G. A., Peters D. V. Mechanisms of autoantibody production in autoimmune MRL mice. J Exp Med. 1980 Nov 1;152(5):1302–1310. doi: 10.1084/jem.152.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotz P. H. Autoantibodies are anti-idiotype antibodies to antiviral antibodies. Lancet. 1983 Oct 8;2(8354):824–826. doi: 10.1016/s0140-6736(83)90740-7. [DOI] [PubMed] [Google Scholar]
- Rubin R. L., Tang F. L., Chan E. K., Pollard K. M., Tsay G., Tan E. M. IgG subclasses of autoantibodies in systemic lupus erythematosus, Sjogren's syndrome, and drug-induced autoimmunity. J Immunol. 1986 Oct 15;137(8):2528–2534. [PubMed] [Google Scholar]
- Rødgaard A., Nielsen F. C., Djurup R., Somnier F., Gammeltoft S. Acetylcholine receptor antibody in myasthenia gravis: predominance of IgG subclasses 1 and 3. Clin Exp Immunol. 1987 Jan;67(1):82–88. [PMC free article] [PubMed] [Google Scholar]
- Schur P. H. IgG subclasses--a review. Ann Allergy. 1987 Feb;58(2):89-96, 99. [PubMed] [Google Scholar]
- Scott M. G., Briles D. E., Shackelford P. G., Smith D. S., Nahm M. H. Human antibodies to phosphocholine. IgG anti-PC antibodies express restricted numbers of V and C regions. J Immunol. 1987 May 15;138(10):3325–3331. [PubMed] [Google Scholar]
- Siekevitz M., Kocks C., Rajewsky K., Dildrop R. Analysis of somatic mutation and class switching in naive and memory B cells generating adoptive primary and secondary responses. Cell. 1987 Mar 13;48(5):757–770. doi: 10.1016/0092-8674(87)90073-0. [DOI] [PubMed] [Google Scholar]
- Stevens T. L., Bossie A., Sanders V. M., Fernandez-Botran R., Coffman R. L., Mosmann T. R., Vitetta E. S. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature. 1988 Jul 21;334(6179):255–258. doi: 10.1038/334255a0. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Antinuclear antibodies: diagnostic markers and clues to the basis of systemic autoimmunity. Pediatr Infect Dis J. 1988 May;7(5 Suppl):S3–S9. [PubMed] [Google Scholar]
- Targoff I. N., Reichlin M. Measurement of antibody to Jo-1 by ELISA and comparison to enzyme inhibitory activity. J Immunol. 1987 May 1;138(9):2874–2882. [PubMed] [Google Scholar]
- Tojo T., Friou G. J., Spiegelberg H. L. Immunoglobulin G subclass of human antinuclear antibodies. Clin Exp Immunol. 1970 Jan;6(1):145–151. [PMC free article] [PubMed] [Google Scholar]
- Travers R. L., Hughes G. R., Cambridge G., Sewell J. R. Coxsackie B neutralisation titres in polymyositis/dermatomyositis. Lancet. 1977 Jun 11;1(8024):1268–1268. doi: 10.1016/s0140-6736(77)92487-4. [DOI] [PubMed] [Google Scholar]
- Tsui F. W., Siminovitch L. Isolation, structure and expression of mammalian genes for histidyl-tRNA synthetase. Nucleic Acids Res. 1987 Apr 24;15(8):3349–3367. doi: 10.1093/nar/15.8.3349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E. J., Jeffrey P. D. Sequence homology between encephalomyocarditis virus protein VPI and histidyl-tRNA synthetase supports a hypothesis of molecular mimicry in polymyositis. Med Hypotheses. 1988 Jan;25(1):21–25. doi: 10.1016/0306-9877(88)90041-2. [DOI] [PubMed] [Google Scholar]
- Ytterberg S. R., Mahowald M. L., Messner R. P. Coxsackievirus B 1-induced polymyositis. Lack of disease expression in nu/nu mice. J Clin Invest. 1987 Aug;80(2):499–506. doi: 10.1172/JCI113098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Borg E. J., Horst G., Hummel E., Jaarsma D., Limburg P. C., Kallenberg C. G. Sequential development of antibodies to specific Sm polypeptides in a patient with systemic lupus erythematosus: evidence for independent regulation of anti-double-stranded DNA and anti-Sm antibody production. Arthritis Rheum. 1988 Dec;31(12):1563–1567. doi: 10.1002/art.1780311215. [DOI] [PubMed] [Google Scholar]