Abstract
Fission yeast genes identified in genetic screens are usually cloned by transformation of mutants with plasmid libraries. However, for some genes this can be difficult, and positional cloning approaches are required. The mutation swi5-39 reduces recombination frequency in homozygous crosses and has been used as a tool in mapping gene position (Schmidt, 1993). However, strain construction in swi5-39-based mapping is significantly more laborious than is desirable. Here we describe a set of strains designed to make swi5-based mapping more efficient and more powerful. The first improvement is the use of a swi5Δ strain marked with kanamycin (G418) resistance, which greatly facilitates identification of swi5 mutants. The second improvement, which follows directly from the first, is the introduction of a large number of auxotrophic markers into mapping strains, increasing the likelihood of finding close linkage between a marker and the mutation of interest. We combine these new mapping strains with a rec12Δ-based approach for initial mapping of a mutation to an individual chromosome. Together, the two methods allow an approximate determination of map position in only a small number of crosses. We used these to determine that mod22-1, a modifier of microtubule nucleation phenotypes, encodes a truncation allele of Swr1, a chromatin-remodelling factor involved in nucleosomal deposition of H2A.Z histone variant Pht1. Expression microarray analysis of mod22-1, swr1Δ and pht1Δ cells suggests that the modifier phenotype of mod22-1 mutants may be due to small changes in expression of one or more genes involved in tubulin function. Copyright © 2009 John Wiley & Sons, Ltd.
Keywords: Schizosaccharomyces pombe, gene mapping, γ-tubulin, Swr1, H2A.Z
Introduction
The most straightforward method of cloning fission yeast genes is to transform a mutant of interest with a plasmid library and identify transformants with restored wild-type phenotype. This is most often done at the colony level, normally by rescuing the lethality of temperature-sensitive mutants. Alternatively, other ‘macroscopic’ assays are possible, including colour change on phloxin B plates (Zimmerman et al., 2004) or after iodine staining (Ellermeier et al., 2004), or restoration of silencing of ade6+ on limiting-adenine plates (White, 2008). Rescue of phenotypes after plasmid library transformation can also be assayed microscopically (Chang et al., 1994; K.S., unpublished data), although to date this has generally been put to wider use in budding yeast (see e.g. Harkins et al., 2001).
In some cases, a plasmid-library transformation approach may not be successful. Reasons for this could include toxicity due to plasmid-based overexpression, impracticality in scoring complex (e.g. morphological) or poorly penetrant phenotypes on a large scale, or the absence of a potential rescuing clone from a plasmid library. In such cases, however, it is often possible to use classical genetics to map the position of a mutation. Information about map position can then be used in conjunction with additional methods, such as physical mapping and/or transformation of the mutant strain with cosmids covering the region of interest, in order to clone the gene of interest. Such an approach has been successful, for example, in the cloning of the cell-polarity regulator tea1+ (Mata and Nurse, 1997), the MAP kinase kinase kinase win1+ (Samejima et al., 1998) and the spindle pole body protein cdc11+, part of the septation initiation network (Krapp et al., 2001).
One important advance in determining map position was the introduction of the use of swi5-39 mutants in the first or second step of mapping (Schmidt, 1993). swi5 mutants were originally identified on the basis of their reduced mating-type switching in homothallic (h90) strains (Egel et al., 1984; Gutz and Schmidt, 1985). Subsequently it was shown that swi5 mutants have a reduced frequency of both intragenic recombination and intergenic recombination, throughout the genome, most likely due to defects in repair or belated repair of DNA double-stranded breaks (De Veaux et al., 1992; Schmidt, 1993; Schmidt et al., 1987, 1989; Young et al., 2004). In spite of this, viability of spores from swi5− meioses is comparable to wild-type (Schmidt, 1993; Young et al., 2004). Because genetic distance relative to physical distance is much smaller in swi5-39 mutants than in wild-type strains (approximately 5–15-fold, depending on the intergenic region), a genetic cross involving swi5-39 mutants can positively identify linkage between a mutant of interest and even a very distant marker gene. To this end, Schmidt (1993) created several swi5-39 mapping strains, each of which contained three or four markers on a single chromosome.
In spite of the reduced genetic distance in swi5-39 mutants and the concomitant benefits for mapping approximate gene position, current methods of swi5-based mapping are not ideal. Here we describe significant improvements to this method, using swi5Δ strains, and the construction and validation of a generic set of swi5Δ mapping strains with many additional auxotrophic markers. We also describe an alternative approach for the initial mapping of a mutation of interest to an individual chromosome, using recombination-deficient rec12Δ mutants. We used these methods to determine the map position of a novel mutation, mod22-1, which was originally identified as an enhancer of microtubule-nucleation defects when non-essential components of the γ-tubulin complex (γ-TuC) are deleted (Anders et al., 2006). In a further analysis, we found that mod22-1 is a nonsense allele of the fission yeast homologue of budding yeast chromatin-remodelling factor SWR1 (Kobor et al., 2004; Krogan et al., 2003; Mizuguchi et al., 2004), and that relatively small changes in gene expression are likely the cause of the mod22-1 mutant phenotype.
Materials and methods
Physical positions for various genes were derived from GeneDB at the Wellcome Trust Sanger Institute (http://www.genedb.org/genedb/pombe/), from the genome sequence current on 8 May 2008.
Yeast strains
Table 1 shows the final strains used for mapping and for our analysis of mod22/swr1. A large number of intermediate strains were also constructed (not shown). Apart from standard auxotrophic markers in our laboratory collection, we received marker strains from P. Fantes (University of Edinburgh, UK), G. Smith (Fred Hutchinson Cancer Research Center, Seattle, WA, USA), P. Nurse (Cancer Research UK, London, UK), and C. Hoffman (Boston College, Boston, MA, USA). We received swi5Δ (swi5-201::kanMX6) and rec12Δ (rec12-168::kanMX6) from G. Smith (Davis and Smith, 2003; Ellermeier et al., 2004). The swr1Δ, pht1Δ and swc2Δ deletion strains were obtained from Bioneer (http://pombe.bioneer.co.kr/).
Table 1.
Fission yeast strains used in this study
| Strain | Genotype | Mapping strain number | Source |
|---|---|---|---|
| KS3733 | h+ura1-161 met5-1 ade3-58 lys2-97 arg3-124 his6-365 | Chr I A1 | This study |
| KS3731 | h−ura1-161 met5-1 ade3-58 lys2-97 arg3-124 his6-365 | Chr I A2 | This study |
| KS3728 | h+swi5Δ::kanMX6 ura1-161 met5-1 ade3-58 lys2-97 arg3-124 his6-365 | Chr I A3 | This study |
| KS3729 | h−swi5Δ::kanMX6 ura1-161 met5-1 ade3-58 lys2-97 arg3-124 his6-365 | Chr I A4 | This study |
| KS3393 | h+cyh1-7 leu2-120 ade4-31 | Chr I B1 | This study |
| KS3394 | h−cyh1-7 leu2-120 ade4-31 | Chr I B2 | This study |
| KS3421 | h+swi5Δ::kanMX6 cyh1-7 leu2-120 ade4-31 | Chr I B3 | This study |
| KS3419 | h−swi5Δ::kanMX6 cyh1-7 leu2-120 ade4-31 | Chr I B4 | This study |
| KS3402 | h+ade7-50 his3-D1 can1-1 leu1-32 | Chr II A1 | This study |
| KS3404 | h−ade7-50 his3-D1 can1-1 leu1-32 | Chr II A2 | This study |
| KS3478 | h+swi5Δ::kanMX6 ade7-50 his3-D1 can1-1 leu1-32 | Chr II A3 | This study |
| KS3480 | h−swi5Δ::kanMX6 ade7-50 his3-D1 can1-1 leu1-32 | Chr II A4 | This study |
| KS4251 | h+leu3-155 lys4-95 arg5-1 | Chr II B1 | This study |
| KS4253 | h−leu3-155 lys4-95 arg5-1 | Chr II B2 | This study |
| KS4278 | h+swi5Δ::kanMX6 leu3-155 lys4-95 arg5-1 | Chr II B3 | This study |
| KS4276 | h−swi5Δ::kanMX6 leu3-155 lys4-95 arg5-1 | Chr II B4 | This study |
| KS4853 | h+ura4-D18 ade6-M216 arg1-230 | Chr III A1 | This study |
| KS4851 | h−ura4-D18 ade6-M216 arg1-230 | Chr III A2 | This study |
| KS4855 | h+swi5Δ::kanMX6 ura4-D18 ade6-M216 arg1-230 | Chr III A3 | This study |
| KS4857 | h−swi5Δ::kanMX6 ura4-D18 ade6-M216 arg1-230 | Chr III A4 | This study |
| KS3358 | h+arg1-230 ade5-36 | Chr III B1 | This study |
| KS3356 | h−arg1-230 ade5-36 | Chr III B2 | This study |
| KS3452 | h+swi5Δ::kanMX6 arg1-230 ade5-36 | Chr III B3 | This study |
| KS3450 | h−swi5Δ::kanMX6 arg1-230 ade5-36 | Chr III B4 | This study |
| KS516 | h−ade6-210 leu1-32 ura4-D18 | Laboratory stock | |
| KS959 | h−gfh1Δ::kanMX6 ade6-M216 leu1-32 ura4-D18 | Laboratory stock | |
| KS963 | h−gfh1Δ::kanMX6 mod22-1 ade6-M216 leu1-32 ura4-D18 | Laboratory stock | |
| KS1470 | h−mod22-1 ade6-M210 leu1-32 ura4-D18 | Laboratory stock | |
| KS3250 | h−rec12Δ::kanMX6 lys1-37 leu1-32 ade6-M210 his7-366 | G. Smith | |
| KS3320 | h+swi5Δ::kanMX6 | G. Smith | |
| KS3322 | h−swi5Δ::kanMX6 | G. Smith | |
| KS3349 | h+gfh1Δ::hphMX6 mod22-1 | This study | |
| KS3484 | h+swi5Δ::kanMX6 gfh1Δ::hphMX6 mod22-1 | This study | |
| KS3488 | h−swi5Δ::kanMX6 gfh1Δ::hphMX6 cyh1-7 leu2-120 ade4-31 | This study | |
| KS3611 | h−gfh1Δ::hphMX6 cyh1-7 leu2-120 ade4-31 | This study | |
| KS4348 | h+swr1Δ::kanMX6 ade6-210 leu1-32 ura4-D18 | Bioneer | |
| KS4350 | h−gfh1Δ::hphMX6 swr1Δ::kanMX6 | This study | |
| KS4355 | h+pht1Δ::kanMX6 ade6-216 leu1-32 ura4-D18 | Bioneer | |
| KS4362 | h−swr1ΔC::kanMX6 ade6-210 leu1-32 ura4-D18 | This study | |
| KS4367 | h+gfh1Δ::hphMX6 pht1Δ::kanMX6 ade6-216 leu1-32 | This study | |
| KS4384 | h+gfh1Δ::hphMX6 swr1ΔC::kanMX6 ade6-210 leu1-32 ura4-D18 | This study | |
| KS4555 | h−gfh1Δ::hphMX6 mod22-1 leu1-32:p[leu1+:nmt1:swr1-Flag-Flag-His] | This study | |
| KS4645 | h+swc2Δ::kanMX ade6-216 leu1-32 ura4-D18 | Bioneer | |
| KS4666 | h+gfh1Δ::hphMX6 swc2Δ::kanMX leu1-32 ura4-D18 | This study |
Media and crosses
Standard fission yeast genetic techniques were used throughout (Moreno et al., 1991). Media were either yeast extract medium (YE5S) or Edinburgh minimal medium (EMM), or EMM plus glutamate (EMMG; EMM using 5 g/l Na glutamate as nitrogen source; also known as PMG). Supplements were used at 150–200 g/l. Canavanine (CAN) was added as hydrochloride from a filter-sterilized stock to autoclaved medium to a final concentration of 100 µg/ml. Cycloheximide (CHX), G418, nourseothricin and Hygromycin B were added to a final concentration of 100 µg/ml. Genetic crosses were carried out at 28 °C for 2–3 days on sporulation agar (SPA), including supplements as required. Tetrad analysis was done using a Singer micromanipulator on YE5S plates. Map distance was determined as described previously (Schmidt, 1993), using Perkins' (1949) formula and also a formula based on maximum-likelihood estimates (Munz et al., 1989).
Genomic DNA sequencing
Genomic DNA was amplified by yeast colony polymerase chain reaction (PCR), using a blend of Pwo and Taq polymerase. The resulting 2 kb PCR products were treated with exonuclease I and Antarctic phosphatase (NEB) and directly used in sequencing reactions. Lasergene (DNAStar) was used to assemble and analyse the sequences.
Truncation of Swr1
Truncation of Swr1 (swr1ΔC) was achieved by PCR-based one-step homologous recombination according to Bähler et al. (1998). The kanMX cassette was amplified by PCR from a plasmid, using a forward oligonucleotide corresponding to 80 bp upstream of the truncation site, but carrying a stop codon at its 3′-end, and a reverse oligonucleotide corresponding to 80 bp downstream of the original stop codon. The amplified fragment was transformed into a wild-type strain and stable integrants were selected. The truncation was confirmed by colony PCR.
Phenotype assay and microscopy
For morphology experiments, cell shape defects were determined by growing cells on YE5S plates for 2 days, replica-plating to fresh plates, and examining cell shape after 3 h at 32 °C (Snaith and Sawin, 2003). For imaging of cell shape by differential interference contrast (DIC) microscopy, cells were washed off the plates with deionized water and immediately fixed in 3% formaldehyde.
Phenotype rescue of gfh1Δ mod22-1 by swr1
The swr1 genomic clone (39/D03) from the ORFeome collection (Matsuyama et al., 2006) was cloned into pDUAL–FFH1c (Matsuyama et al., 2004) to create a leu1-integratable plasmid expressing C-terminally-tagged Swr1-2 × FLAG–6His under control of the nmt1 promoter. The plasmid was linearized with NotI and used for transformation of an Sz. pombe strain carrying the mod22-1 mutation in a gfh1Δ background. The phenotype of a clone stably expressing Swr1–2 × FLAG–6His was determined in the morphology assay described above (i.e. under repressing conditions for nmt1).
Microarray experiments
RNA isolation was performed as described (Lyne et al., 2003). Cy3 and Cy5 (GE Healthcare) incorporation was carried out using the Invitrogen Superscript direct cDNA labelling system, according to the manufacturer's instructions. Mutant samples were hybridized against wild-type samples on the same array. Two independent biological repeats were carried out, and dye swaps were applied for repeated experiments. Microarrays were scanned using an Axon GenePix 4000B scanner and analysed with GenePix 6.0 software. Quality control and data normalization was carried out as described (Lyne et al., 2003). Results were analysed with GeneSpring GX 7.3 (Agilent). Microarray data have been deposited in ArrayExpress (http://www.ebi.ac.uk/microarray-as/ae/) under Accession No. E-TABM-517. The processed microarray data are also available at: http://www.sanger.ac.uk/PostGenomics/S_pombe
Average fold-changes from two repeated experiments were used to generate lists of differentially expressed genes. The significance of gene list overlaps was calculated using a standard Fisher's exact test, and the p values were adjusted with a Bonferroni multiple testing correction. Each of the six overlap diagrams shown in Figure 3 generated three p values, from pairwise comparisons. For the three p values associated with Figure 3A, ‘> 1.25× down’, all p values were < 1 × 10−59. For Figure 3A, ‘> 1.5× down’, all p values were < 7 × 10−25, and for ‘> 2× down’, all were < 4 × 10−23. For the three p values associated with Figure 3B, ‘> 1.25× up’, all p values were < 1 × 10−06. For Figure 3B, ‘> 1.5× up’, all p values were < 0.0002. For Figure 3B, ‘> 2× up’, p values were either 0.03 or not significant.
Figure 3.

Venn diagrams showing numbers of genes changed in expression in mod22-1, swr1Δ and pht1Δ mutants, either unique to each mutant, common to pairs of mutants or common to all three mutants. (A) Genes with reduced expression in the mutants. (B) Genes with increased expression in the mutants
Results
The recessive mod22-1 mutation was originally identified in our laboratory as an enhancer of the microtubule and cell-shape defects seen upon deletion of any one or more of the non-essential γ-tubulin complex (γ-TuC) components Gfh1, Mod21 or Alp16 (Anders et al., 2006; see also Fujita et al., 2002; Venkatram et al., 2004). When grown to stationary phase and then refed with fresh medium, the double mutants mod22–1 gfh1Δ, mod22–1 mod21Δ and mod22–1 alp16Δ all grow with a curved cell shape, especially during the first cell cycle, as a consequence of a reduced frequency of cytoplasmic microtubule nucleation (Anders et al., 2006). By contrast, the single mutants gfh1Δ, mod21Δ and alp16Δ have a less pronounced reduction in microtubule nucleation, and thus tend to grow as straighter cells. The mod22-1 single mutant has nearly no observable morphological phenotype on its own, and this has suggested that mod22+ may be particularly important for γ-TuC-mediated microtubule nucleation when the large γ-TuC is not fully functional (Anders et al., 2006). We initially attempted to clone mod22+ by transformation of mod22–1 gfh1Δ double mutant cells with a plasmid library, followed by microscopy of individual replica-plated colonies to identify phenotype rescue. However, we recovered only gfh1+-containing plasmids (P.C.C. Lourenco and K.S., unpublished data). In a further set of experiments we transformed mod22-1 gfh1Δ mod21Δ cdc25-22 quadruple mutants, which might be rescued only by mod22+ and not by gfh1+ or mod21+ (the cdc25-22 mutation enhances the curved-cell phenotype at semi-permissive temperatures, facilitating rapid visual screening), but we did not identify any phenotype rescue in nearly 80 000 transformants. This suggested either that mod22+ may not be properly represented in our plasmid library, or that this method would not work for mod22+ in principle. Because of the time-intensive nature of microscopic screening, we undertook an alternative approach, involving positional gene mapping.
rec12Δ mapping to determine chromosomal location
A typical first step in positional mapping in fission yeast is to determine whether a gene of interest is located on chromosome I, II or III. Historically this has often been done by using the mutant mating-type allele mat2-B102 (also known as mei1-B102) to construct stable, non-sporulating diploids heterozygous for both the mutation of interest and different auxotrophic markers on each chromosome (Kohli et al., 1977). Haploidization is then induced using drugs such as fluoro-phenylalanine, thiabendazole or methyl benzimidazol-2yl carbamate, generating haploid strains without any prior meiotic crossing-over. Analysis of co-segregation of the mutation of interest with the auxotrophic markers can then reveal on which chromosome the mutation resides (Kohli et al., 1977). We took an alternative approach, using a rec12Δ::kanMX6 mutant with distinct markers on each chromosome (provided by Gerry Smith, Fred Hutchinson Cancer Research Center). rec12Δ mutants are viable but exhibit up to a 1000-fold reduction in recombination in homozygous crosses, reducing the total number of crossovers per meiosis per genome from 40–45 to practically nil (Davis and Smith, 2003; De Veaux et al., 1992), and the rec12Δ::kanMX6 allele can be easily identified in strain constructions by G418-resistance. rec12Δ-based mapping requires that both mating partners in a mapping cross must be rec12Δ; thus, depending on the complexity of the circumstances, one should be able to construct strains and map a mutation to an individual chromosome in two or three conventional crosses.
We constructed rec12Δ gfh1Δ mod22-1 ade6-210 leu1-32 lys1-37 mutants and crossed them to rec12Δ gfh1Δ mutants. The resulting progeny showed 96% co-segregation of mod22-1 with lys1-37, and random segregation of mod22-1 with ade6-210 and leu1-32 markers, indicating that mod22+ is located on chromosome I.
swi5Δ mapping strains for individual chromosomes
For swi5-based mapping, both mating partners must be swi5− mutants, and therefore mutations of interest must be crossed into swi5− backgrounds. However, identification of swi5-39 mutants is most easily done by iodine staining on sporulation plates, which is easy in homothallic (h90) strains but not possible in the heterothallic h− or h+ strains that are normally used in crosses. Thus, the presence of swi5-39 in a strain of interest can be determined only retrospectively, involving further crosses (alternatively, a UV-sensitivity assay can be employed; (Schmidt et al., 1989)). This can make the initial mapping strain construction laborious, especially if additional mutations or markers are to be incorporated, whether to uncover a mutant phenotype (as is necessary with mod22-1) or to increase the resolving power of linkage analysis. The swi5+ gene has been cloned independently by two groups and found to encode a very small (85-amino acid) non-essential protein (Akamatsu et al., 2003; Ellermeier et al., 2004). Interestingly, swi5Δ::kanMX6 strains have essentially the same phenotype as the original swi5-39 mutant. Because swi5Δ::kanMX6 strains can easily be identified by G418-resistance, we used swi5Δ strains rather than swi5-39 mutants to construct strains for mapping crosses.
We constructed two mapping strains for chromosome I, with six and three markers, respectively, in both swi5Δ and swi5+ backgrounds (Figure 1). We chose easily-scored auxotrophies (Egel, 2004; Kohli et al., 1977; Munz et al., 1989) and in some cases also optimized media conditions for easy screening (Table 2). A cross using our mapping strain for the short arm of chromosome I (chromosome IB) revealed close linkage between mod22 and ade4 by tetrad analysis in a swi5Δ × swi5Δ cross (Table 3). A further cross using the corresponding mapping strain in a swi5+× swi5+ context continued to show linkage between mod22 and ade4, with a genetic distance of approximately 8 cM (Table 4). Additional work on the identification and characterization of mod22+ is presented below.
Figure 1.

Diagram showing the positions of markers for the six different mapping-strain chromosomes, drawn to scale with the genome sequence. Black circles, centromeres; grey-dashed regions, ribosomal DNA repeats at either end of chromosome III, which can vary in length (Pasero and Marilley, 1993)
Table 2.
Mapping strain markers
| Chromosome | Screening medium | Physical position on chromosomea (bp) | Physical distance to next marker genea (bp) | Genetic distancea (cM) |
|---|---|---|---|---|
| I | ||||
| ura1-161 | EMMG − U | 739 300 | 403 815 | 50 |
| met5-1b | EMMG − M | 1 143 115 | 186 147 | 52 |
| ade3-58 | EMMG − A | 1 329 262 | 346 468 | 76 |
| lys2-97 | EMMG − Kc | 1 675 730 | 598 282 | 48 |
| arg3-124 | EMMG − R | 2 274 012 | 558 784 | 122 |
| his6-365 | EMMG − H | 2 832 796 | 937 204d | 250 |
| cyh1-7 | YE5S + CHX | 3 770 000d | 670 096d | 118 |
| leu2-120 | EMMG − L | 4 440 096 | 924 264 | 170 |
| ade4-31 | EMMG − A | 5 364 360 | N/A | N/A |
| II | ||||
| ade7-50 | EMMG − A | 1 157 118 | 331 093 | 64 |
| his3-D1 | EMMG − H | 1 488 211 | 401 953f | 92 |
| can1-1 | EMM + CANe | 1 890 164f | 84 324f | 32 |
| leu1-32 | EMMG − L | 1 974 488 | 714 104 | 100 |
| leu3-155 | EMMG − L | 2 688 592 | 817 459 | 140 |
| lys4-95 | EMMG − Kc | 3 506 051 | 588 597 | 188 |
| arg5-1 | EMMG − R | 4 094 648 | N/A | N/A |
| III | ||||
| ura4-D18 | EMMG − U | 115 781 | 1 200 556 | 260 |
| ade6-M210 | EMMG − A | 1 316 337 | 296 987 | 100 |
| arg1-230 | EMMG − R | 1 613 324 | 799 041 | 144 |
| ade5-36 | EMMG − A | 2 412 365 | N/A | N/A |
Physical positions are from GeneDB at the Wellcome Trust Sanger Institute (http://www.genedb.org/genedb/pombe/) on 8 May 2008. Genetic distances were estimated using the map of Munz et al. (1989).
met5 = met9.
Use sodium glutamate as nitrogen source. Ammonium chloride must not be used, as in our hands Ura- Ade- Arg- Lys+ strains and Ura−Met−His−Lys+ strains failed to grow on ammonium chloride-based medium lacking lysine. There reasons for this are not yet clear.
Exact position of cyh1 is unknown and therefore estimated from position of centromere, which is very closely linked.
Use ammonium chloride as nitrogen source. Sodium glutamate can be used as well, but ammonium chloride gives better results.
Exact position of can1 is unknown and therefore estimated from position of spo14, which is very closely linked.
N/A, not applicable.
Table 3.
Mapping of test genes by tetrad analysis
|
swi5+× swi5+ |
swi5Δ × swi5Δ |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mapping strain | Test gene A | Marker B | Distance A–B (kb) | PD | NPD | T | N | dpa (cM) | dla (cM) | PD | NPD | T | N | dpa (cM) | dla (cM) |
| Chr I A | mod21 | ura1 | 521 | 14 | 1 | 29 | 44 | 40 | 61 | 24 | 0 | 0 | 24 | 0 | 0 |
| mod21 | met5 | 925 | 13 | 4 | 27 | 44 | 58 | 80 | 22 | 0 | 2 | 24 | 4.2 | 4.4 | |
| mod21 | ade3 | 1111 | 11 | 11 | 22 | 44 | ND | ND | 20 | 1 | 3 | 24 | 19 | 12 | |
| mod21 | lys2 | 1457 | 11 | 7 | 26 | 44 | ND | ND | 19 | 1 | 4 | 24 | 21 | 14 | |
| mod21 | arg3 | 2055 | 11 | 6 | 25 | 44 | ND | ND | 19 | 1 | 4 | 24 | 21 | 14 | |
| mod2 | his6 | 2614 | 8 | 3 | 23 | 44 | ND | ND | 18 | 1 | 5 | 24 | 23 | 17 | |
| Chr I B | mod22 | cyh1 | 1507b,c | 18 | 2 | 27 | 47 | 42 | 54 | ||||||
| mod22 | leu2 | 838b | 30 | 0 | 17 | 47 | 18 | 22 | |||||||
| mod22 | ade4 | 86b | 45 | 1 | 1 | 47 | 7.5 | 3.3 | |||||||
| Chr II A | alp6 | ade7 | 756 | 8 | 9 | 44 | 61 | ND | ND | 16 | 0 | 12 | 28 | 21 | 28 |
| alp6 | his3 | 1087 | 10 | 11 | 40 | 61 | ND | ND | 11 | 1 | 16 | 28 | 39 | 51 | |
| alp6 | can1 | 1489c | 11 | 14 | 36 | 61 | ND | ND | 9 | 3 | 16 | 28 | ND | ND | |
| alp6 | leu1 | 1574 | 10 | 13 | 38 | 61 | ND | ND | 11 | 4 | 13 | 28 | ND | ND | |
| Chr III A | alp16 | ura4 | 317 | 12 | 8 | 43 | 63 | ND | ND | 43 | 0 | 11 | 54 | 10.2 | 11.4 |
| alp16 | ade6 | 884 | 12 | 9 | 42 | 63 | ND | ND | 41 | 1 | 12 | 54 | 17 | 23 | |
| alp16 | arg1 | 1181 | 17 | 4 | 42 | 63 | 52 | 79 | 33 | 3 | 18 | 54 | 33 | 29 | |
| Chr III B | cdc11 | arg1 | 436 | 13 | 4 | 39 | 56 | 56 | 91 | 35 | 0 | 11 | 46 | 12 | 14 |
| cdc11 | ade5 | 363 | 13 | 8 | 35 | 56 | ND | ND | 43 | 0 | 3 | 46 | 3.3 | 3.4 | |
Map distance dp is based on Perkins' formula, and map distance dl is derived from maximum likelihood estimate (see Materials and methods), as distances based on Perkins' formula are likely to be underestimates when NPD > 5% of total tetrads. ND, not determined, because data do not suggest linkage.
Distance determined retrospectively, after identification of mod22 as swr1.
Estimated distance; see Table 2.
PD, parental ditype; NPD, non-parental ditype; T, tetratype. Our criterion for linkage in tetrad analysis is PD > NPD, with statistical significance p < 0.05 by χ2 test. A more stringent criterion would be p < 0.01 (Kohli et al., 1977).
Table 4.
Mapping of test genes by random spore analysis
| Mapping strain | swi5 background | Test gene (A) | Marker (B) | Physical distance (A to B; kb) | Recombinants (%) | n | χ2 | p |
|---|---|---|---|---|---|---|---|---|
| Chr I B | swi5+ | mod22 | cyh1 | 1507a, b | 49 | 120 | 0.033 | 0.8551 |
| mod22 | leu2 | 838a | 54 | 120 | 0.833 | 0.3613 | ||
| mod22 | ade4 | 86a | 8 | 120 | 83.333 | < 0.0001 | ||
| Chr II B | swi5+ | tea2 | leu3 | 1205 | 47 | 280 | 0.914 | 0.3390 |
| tea2 | lys4 | 387 | 44 | 280 | 3.657 | 0.0558 | ||
| tea2 | arg5 | 201 | 25 | 280 | 68.014 | < 0.0001 | ||
| Chr II B | swi5Δ | tea2 | leu3 | 1205 | 13 | 560 | 306 | < 0.0001 |
| tea2 | lys4 | 387 | 7 | 560 | 414 | < 0.0001 | ||
| tea2 | arg5 | 201 | 4 | 560 | 475 | < 0.0001 |
Distance determined retrospectively, after identification of mod22 as swr1.
Estimated distance; see Table 2.
We validated the swi5Δ mapping strain for the long arm of chromosome I (chromosome I A) by tetrad analysis of a cross with a swi5Δ mod21Δ strain, in which a non-essential component of the fission yeast γ-tubulin complex is deleted (Anders et al., 2006) (Table 3). In this swi5Δ × swi5Δ cross, mod21 showed very close linkage to ura1, as well as linkage to other markers, as expected. By contrast, in a comparable swi5+× swi5+ cross, only weak linkage of mod21 to ura1 was observed, with essentially no linkage to other markers.
The potential general usefulness of this method led us to develop similar mapping strains for chromosomes II and III, in both swi5Δ and swi5+ backgrounds (Figure 1, Table 2). We constructed two strains for chromosome II with seven markers in total, and two strains for chromosome III with four markers in total (see Materials and methods). We validated these strains in a similar manner to the chromosome I strains, by ‘mapping’ the position of known genes involved in cytoskeletal function (Browning et al., 2000; Fujita et al., 2002; Krapp et al., 2001; Tomlin et al., 2002; Vardy and Toda, 2000). All of the mapping strains behaved as desired (Tables 3, 4), revealing linkage between the test gene and at least one auxotrophic marker in all swi5Δ × swi5Δ crosses, but only rarely in swi5+× swi5+ crosses.
Identification of mod22 as swr1
As described above, with both swi5Δ and swi5+ strains we found close linkage of mod22 to ade4 on chromosome I, and additional crosses indicated that mod22 was approximately 4–8 cM away from ade4, towards the centromere.
Attempts to rescue the curved-cell phenotype of mod22-1 gfh1Δ mod21Δ cdc25-22 mutants with cosmids from this region were not successful (data not shown). We therefore directly sequenced a large region of genomic DNA from mod22-1 mutants, as well as from wild-type control cells. In a 92 kb interval from ORFs SPAC4D7.02c to SPAPJ760.02c, our experimentally determined wild-type sequence showed a 100% match to the published genome sequence (Wood et al., 2002), and we found only a single nucleotide change in mod22-1 mutants relative to wild-type cells. This was in the uncharacterized ORF SPAC11E3.01c (also known as SPAC2H10.03c), which encodes a 1288 amino acid protein that is most closely related to the budding yeast SWI2/SNF2 family chromatin-remodelling factor Swr1p (43% identity; Kobor et al., 2004; Krogan et al., 2003; Mizuguchi et al., 2004). We will designate the fission yeast gene as swr1+ and its protein product as Swr1. The identified mutation is a C to T transition at nucleotide 2209, converting a CGA (Arg) codon to a TGA (stop) codon and truncating the predicted Swr1 protein at about 60% of its predicted length (Figure 2A).
Figure 2.
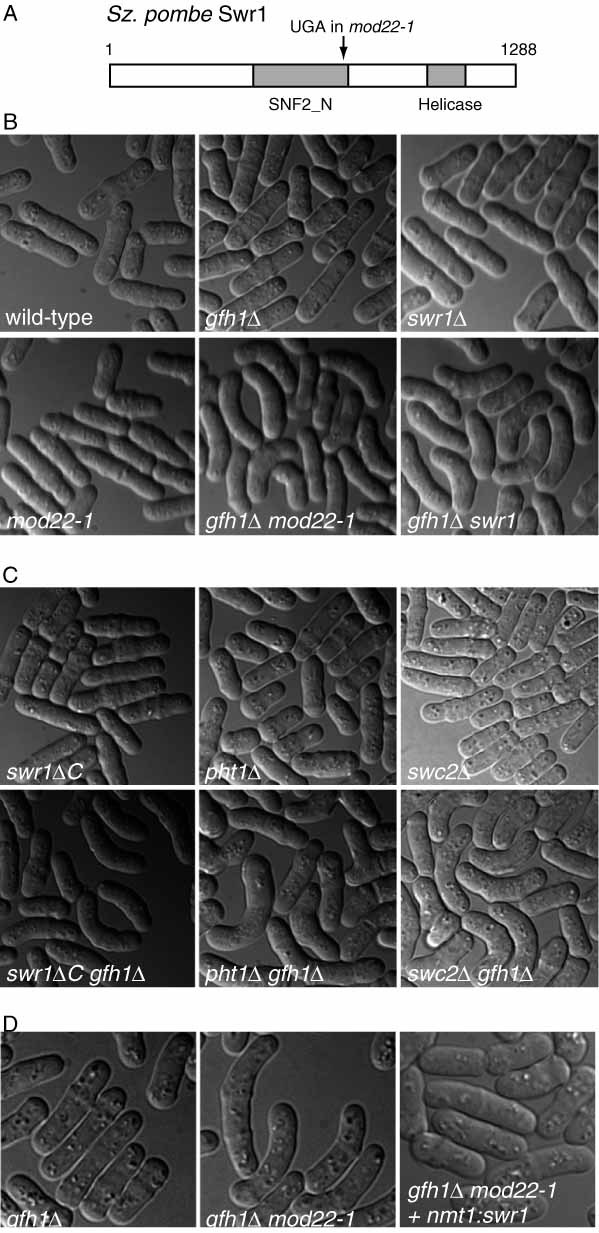
Allelism of mod22 and swr1, a gene encoding a chromatin-remodelling factor. (A) Schematic of fission yeast Swr1 protein, showing position of the nonsense mutation in mod22-1. Shaded areas indicate two regions of high identity (>75%) to budding yeast Swr1p. (B) Cell-shape phenotypes in the indicated mutants, showing synthetic effect of combining either mod22-1 or swr1Δ with gfh1Δ. (C) Cell-shape phenotypes in the indicated mutants, showing that swr1ΔC (a de novo-created truncation-equivalent of mod22-1), pht1Δ (deletion of fission yeast H2A.Z) and swc2Δ (deletion of a component of the SWR1–SWR-C complex) all have similar phenotypes to mod22-1 and swr1Δ. D. Rescue of the cell-shape phenotype of gfh1Δ mod22-1 double mutant by plasmid-based expression of wild-type Swr1
To confirm that mod22-1 is allelic to swr1+, we created a gfh1Δ swr1Δ double mutant and found that it displayed a curved-cell phenotype, while neither single mutant did (Figure 2B). In addition, using PCR-based gene targeting we created a truncation mutant, swr1ΔC, to mimic the nonsense mutation present in mod22-1. This, too, led to curved cells in, and only in, gfh1Δ backgrounds (Figure 2C). Finally, we transformed the mod22-1 gfh1Δ mutant with an integrating plasmid containing swr1+ (with nmt1 promoter, repressed) and were able to partially rescue the curved-cell phenotype of the mutant (Figure 2D). These three lines of evidence confirm that mod22-1 is a nonsense allele of swr1.
A large-scale localization screen has shown the fission yeast Swr1 is a nuclear protein (Matsuyama et al., 2006). In budding yeast, Swr1p functions primarily as part of a multi-protein SWR1–SWR-C complex that regulates gene expression by facilitating exchange of variant histone H2A.Z Htz1p into chromatin (Kobor et al., 2004; Krogan et al., 2003; Mizuguchi et al., 2004). This suggested that fission yeast Swr1 may exert its normal function via a similar mechanism, involving Pht1, the fission yeast homologue of Htz1p (Carr et al., 1994). We found that pht1Δ gfh1Δ double mutant cells also displayed a curved-cell phenotype, as well as a low frequency of branched cells (Figure 2C). The simplest interpretation of these results is that the phenotypic effects of mod22-1 and swr1 mutations are mediated via Pht1 and, more generally, that these effects may be indirect consequences of altered gene expression in the mutants. Consistent with this, when we combined gfh1Δ with a deletion of swc2, a different component of the SWR1–SWR-C complex (Kobor et al., 2004; Krogan et al., 2003; Mizuguchi et al., 2004), we also obtained a curved-cell phenotype (Figure 2C).
Previous analysis has suggested that mod22+ is particularly important for microtubule nucleation when the non-essential or ‘non-core’ components of the fission yeast γ-tubulin complex are deleted, leaving behind only the essential core γ-tubulin small complex (γ-TuSC), which contains γ-tubulin (Gtb1) as well as Alp4 and Alp6, the fission yeast homologues of human GCP2 and GCP3 (Anders et al., 2006). To determine whether we could identify one or more critical target genes whose altered expression might be responsible for the mod22-1/swr1Δ curved-cell phenotype, we performed a transcription microarray analysis of mod22-1, swr1Δ and pht1Δ mutants. Transcriptome analysis in budding yeast has revealed small but statistically significant changes in the expression of a large number of genes in swr1Δ and htz1Δ mutants, with considerable overlap between the two mutants, although very few genes show more than even a two-fold change in expression in both mutants (Kobor et al., 2004; Krogan et al., 2003; Mizuguchi et al., 2004). This may be a general feature for mutants affecting chromatin, as similarly small global effects in gene expression were observed in fission yeast mutants affecting gene silencing and RNAi pathways (Hansen et al., 2005). Because of these previous observations, we took a non-conservative approach to analysing our microarray data, in order to include as many potential candidate target genes as possible (see Materials and methods).
We identified 121 genes (out of approximately 5000 in fission yeast) whose average expression was reduced more than 1.25-fold in all three mutants relative to wild-type cells (Figure 3A; see also Supporting information, Table S3, and Materials and methods for statistical significance). Smaller numbers of genes showed increased expression in the mutants (Figure 3B; see also Supporting information, Table S3). Only 13 genes were reduced in expression more than two-fold, and none of these has any known functional link to the microtubule cytoskeleton (Table S3). A possible exception is the gene encoding the CENP-C centromere protein Cnp3 (SPBC1861.01c; SPBC56F2.13), but this is involved in intranuclear mitotic spindle function rather than cytoplasmic microtubule function (Holland et al., 2005).
Interestingly, among the genes reduced more than 1.25-fold in all mutants was the gene encoding the γ-TuSC subunit Alp6, which was reduced in expression an average of 33%, 22% and 38% in mod22-1, swr1Δ and pht1Δ mutants, respectively. In addition, the gene for ADP-ribosylation factor-like protein Alp41, which is important for the function of tubulin-folding co-factor proteins (Bhamidipati et al., 2000; Radcliffe et al., 2000), was reduced in expression an average of 50%, 42% and 39%, respectively, in the same mutants. A gene encoding a putative tubulin–tyrosine ligase-like protein (SPAC12B10.04) was also reduced in expression an average of 42%, 37% and 58%, respectively, in mod22-1, swr1Δ and pht1Δ mutants, but the relevance of this remains unclear, as to date there is no evidence for actual tubulin–tyrosine ligase activity in yeast (Badin-Larcon et al., 2004). No other significant changes in gene expression for other γ-TuC proteins, or for tubulin itself, were observed. Overall, these results suggest that the morphological phenotype of mod22-1, swr1Δ and pht1Δ mutants may be due to small changes in expression of one or more genes, including alp6 and alp41, possibly in conjunction with other genes indirectly affecting microtubule nucleation and/or dynamics.
Discussion
Here we have described general methods and swi5Δ strains that should considerably improve the efficiency of mapping gene position in fission yeast. Using these methods, we were able to show that the enhancement of microtubule nucleation defects by mod22-1 mutants is due to a premature stop codon in the chromatin-remodelling factor Swr1. Our evidence further suggests that the role of mod22/swr1 in microtubule nucleation is likely an indirect effect of changes in gene expression. This is not surprising, given the global importance of SWI2/SNF2 family proteins in regulating chromatin structure in general (Kobor et al., 2004; Krogan et al., 2003; Mizuguchi et al., 2004).
It is much easier to introduce mutations into swi5Δ strains than into swi5-39 strains, and our new strains provide up to nine markers per chromosome, giving higher resolution in the earliest steps of mapping compared to the original strains introduced by Schmidt (1993). With the rec12Δ and swi5Δ mapping strains, a novel mutation can be mapped to an individual chromosome and further mapped within a given chromosome in a relatively small number of crosses, including both strain construction and the actual mapping crosses. The large number of markers in our mapping strains should make it possible to infer not only the approximate position of a mutation of interest in relation to known markers, but also gene order, as is normally done in ‘three-factor’ crosses, although we did not exploit this directly in the present work. We expect that these strains will provide advantages over previous methods whenever positional mapping is required.
For our mapping strains, we constructed two strains per chromosome. To obtain the most information from the smallest number of crosses, one would like to have as many markers as possible in a single cross, as this not only improves the likelihood of finding linkage but also allows a more accurate determination of approximate map position. However, there are practical reasons against trying to introduce too many markers into a single strain, and against making a single mapping strain for a given chromosome. First, the frequency of obtaining a desired multiple-mutant strain in heterozygous crosses is halved with each additional marker introduced. Second, most chromosomes contain several different auxotrophic markers for the same nutrient (e.g. ade2, ade3 and ade4 on chromosome I), and in some cases use of more than one of these may be beneficial. This is easily achieved by putting two markers in different mapping strains. Third, it is likely that too many auxotrophies or specific combinations of auxotrophies in a single strain may compromise growth (Kohli et al., 1977). Indeed, during strain construction we found some unexpected incompatibilities, such as the failure of his2 leu3 lys4 arg5 quadruple mutants to grow on media individually lacking either methionine, uracil or adenine (data not shown), which forced a change in our strategy for construction of one of the mapping strains for chromosome II.
In constructing general-use swi5Δ mapping strains, we used easily-scored markers, almost all of which are auxotrophies, in order for the strains to have a wide application. We avoided using temperature-sensitive alleles, in case the mutation of interest to be mapped was also temperature-sensitive, and we avoided including alleles marked with antibiotic resistance (e.g. nourseothricin or hygromycin; swi5Δ is already marked with G418 resistance) because some synthetic phenotypes may require the introduction of such markers for phenotype detection, as with our gfh1Δ mod22-1 double mutant. However, in some cases, specific additional markers could be included in the mapping strains to provide better resolution, e.g. near the end of the short arm of chromosome II and in chromosome III, which contains a disproportionately small number of known auxotrophies. Further rational modification of the swi5Δ mapping strains should be straightforward.
Is it better to use random spore or tetrad analysis with the swi5Δ mapping strains? Depending on sample size, tetrad analysis can confidently identify linkage at 50 cM and beyond (with less accuracy in determining actual genetic distance), so in principle it will have an advantage over random spore analysis (Kohli et al., 1977). In essentially all of our swi5Δ × swi5Δ test crosses, linkage to a marker would most likely have been identified by random spore analysis, based on marker-to-marker distances (see Supporting information, Tables S1 and S2). However, we note that the fold-reduction in recombination in swi5−× swi5− crosses varies across the genome (Schmidt, 1993; also our own data). Therefore, if a mutation of interest were present in a large marker-free interval (e.g. between ade7 and the telomere on chromosome II; see Figure 1) and recombination were not maximally reduced in this region, it is likely that tetrad analysis would be beneficial.
It is worth pointing out how swi5Δ mapping could be used in the context of recent technical advances and genomic resources in fission yeast and beyond. Subsequent to swi5Δ mapping, further fine-mapping of a mutation of interest is required before other methods can be used for gene identification (e.g. transforming mutants with phage clones, cosmid clones or ORFeome clones; Matsuyama et al., 2006). For this next step, the swi5+ mapping strains could be used, or swi5Δ strains crossed to the mutant of interest in a swi5+ background, but in most cases one is unlikely to find linkage to markers. Further fine-mapping could take advantage of non-essential gene deletions from the fission yeast gene deletion set (http://pombe.bioneer.co.kr/). As each deletion is marked with G418 resistance, it would be straightforward to cross a mutation of interest with an evenly-spaced set of deletions.
With the increasing availability of massively parallel sequencing technologies (e.g. Illumina or 454 sequencing), one can identify mutations by sequencing an entire mutant genome at a reasonable price. However, a mutagenized genome from a mutant hunt is likely to contain several mutations, most of which are not of interest. In this case, swi5Δ mapping would allow one to focus attention on the region where the mutation of interest is known to reside. In the future, it is also possible that single-nucleotide polymorphisms (SNPs) among different fission yeast strains (e.g. natural geographical isolates vs. laboratory strains; Patch and Aves, 2007) could be used to help map gene position, in conjunction with massively parallel sequencing. Here again swi5Δ mapping could be of value, by providing a genomic region of interest for high-resolution SNP-based mapping, and thus reducing the amount of unnecessary screening.
Acknowledgments
We thank P. Fantes, G. Smith, P. Nurse and C. Hoffman for providing strains and many helpful suggestions and advice concerning their use; M. Keogh for useful discussions and sharing of unpublished data; P. Lourenco for early work on mod22-1; and H. Ohkura for advice on genomic sequencing. K.E.S. is a Wellcome Trust Senior Research Fellow in Basic Biomedical Sciences. This work was supported by the Wellcome Trust (to K.E.S.) and Cancer Research UK (to J.B.).
Supporting Information
Supporting information may be found in the online version of this article.
Table S1. Distance between markers, from tetrad analysis
Table S2. Distance between markers, from random spore analysis
Table S3. Lists of up- and downregulated genes common to mod22-1, swr1Δ and pht1Δ mutants
References
- Akamatsu Y, Dziadkowiec D, Ikeguchi M, et al. Two different Swi5-containing protein complexes are involved in mating-type switching and recombination repair in fission yeast. Proc Natl Acad Sci USA. 2003;100:15770–15775. doi: 10.1073/pnas.2632890100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders A, Lourenco PC, Sawin KE. Noncore components of the fission yeast γ-tubulin complex. Mol Biol Cell. 2006;17:5075–5093. doi: 10.1091/mbc.E05-11-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badin-Larcon AC, Boscheron C, Soleilhac JM, et al. Suppression of nuclear oscillations in Saccharomyces cerevisiae expressing Glu tubulin. Proc Natl Acad Sci USA. 2004;101:5577–5582. doi: 10.1073/pnas.0307917101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bähler J, Wu JQ, Longtine MS, et al. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998;14:943–951. doi: 10.1002/(SICI)1097-0061(199807)14:10<943::AID-YEA292>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Bhamidipati A, Lewis SA, Cowan NJ. ADP ribosylation factor-like protein 2 (Arl2) regulates the interaction of tubulin-folding cofactor D with native tubulin. J Cell Biol. 2000;149:1087–1096. doi: 10.1083/jcb.149.5.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning H, Hayles J, Mata J, et al. Tea2p is a kinesin-like protein required to generate polarized growth in fission yeast. J Cell Biol. 2000;151:15–28. doi: 10.1083/jcb.151.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AM, Dorrington SM, Hindley J, et al. Analysis of a histone H2A variant from fission yeast: evidence for a role in chromosome stability. Mol Gen Genet. 1994;245:628–635. doi: 10.1007/BF00282226. [DOI] [PubMed] [Google Scholar]
- Chang EC, Barr M, Wang Y, et al. Cooperative interaction of Sz. pombe proteins required for mating and morphogenesis. Cell. 1994;79:131–141. doi: 10.1016/0092-8674(94)90406-5. [DOI] [PubMed] [Google Scholar]
- Davis L, Smith GR. Nonrandom homolog segregation at meiosis I in Schizosaccharomyces pombe mutants lacking recombination. Genetics. 2003;163:857–874. doi: 10.1093/genetics/163.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veaux LC, Hoagland NA, Smith GR. Seventeen complementation groups of mutations decreasing meiotic recombination in Schizosaccharomyces pombe. Genetics. 1992;130:251–262. doi: 10.1093/genetics/130.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egel R. Fission yeast in general genetics. In: Egel R, editor. The Molecular Biology of Schizosaccharomyces pombe. Berlin: Springer; 2004. pp. 1–12. [Google Scholar]
- Egel R, Beach DH, Klar AJ. Genes required for initiation and resolution steps of mating-type switching in fission yeast. Proc Natl Acad Sci USA. 1984;81:3481–3485. doi: 10.1073/pnas.81.11.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellermeier C, Schmidt H, Smith GR. Swi5 acts in meiotic DNA joint molecule formation in Schizosaccharomyces pombe. Genetics. 2004;168:1891–1898. doi: 10.1534/genetics.104.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita A, Vardy L, Garcia MA, Toda T. A fourth component of the fission yeast γ-tubulin complex, Alp16, is required for cytoplasmic microtubule integrity and becomes indispensable when γ-tubulin function is compromised. Mol Biol Cell. 2002;13:2360–2373. doi: 10.1091/mbc.02-01-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutz H, Schmidt H. Switching genes in Schizosaccharomyces pombe. Curr Genet. 1985;9:325–331. doi: 10.1007/BF00421601. [DOI] [PubMed] [Google Scholar]
- Hansen KR, Burns G, Mata J, et al. Global effects on gene expression in fission yeast by silencing and RNA interference machineries. Mol Cell Biol. 2005;25:590–601. doi: 10.1128/MCB.25.2.590-601.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkins HA, Page N, Schenkman LR, et al. Bud8p and Bud9p, proteins that may mark the sites for bipolar budding in yeast. Mol Biol Cell. 2001;12:2497–2518. doi: 10.1091/mbc.12.8.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland S, Ioannou D, Haines S, Brown WR. Comparison of Dam tagging and chromatin immunoprecipitation as tools for the identification of the binding sites for Sz. pombe CENP-C. Chromosome Res. 2005;13:73–83. doi: 10.1007/s10577-005-7062-z. [DOI] [PubMed] [Google Scholar]
- Kobor MS, Venkatasubrahmanyam S, Meneghini MD, et al. A protein complex containing the conserved Swi2/Snf2-related ATPase Swr1p deposits histone variant H2A.Z into euchromatin. PLoS Biol. 2004;2:E131. doi: 10.1371/journal.pbio.0020131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli J, Hottinger H, Munz P, et al. Genetic mapping in Schizosaccharomyces pombe by mitotic and meiotic analysis and induced haploidization. Genetics. 1977;87:471–489. doi: 10.1093/genetics/87.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krapp A, Schmidt S, Cano E, Simanis V. Sz. pombe cdc11p, together with sid4p, provides an anchor for septation initiation network proteins on the spindle pole body. Curr Biol. 2001;11:1559–1568. doi: 10.1016/s0960-9822(01)00478-x. [DOI] [PubMed] [Google Scholar]
- Krogan NJ, Keogh MC, Datta N, et al. A Snf2 family ATPase complex required for recruitment of the histone H2A variant Htz1. Mol Cell. 2003;12:1565–1576. doi: 10.1016/s1097-2765(03)00497-0. [DOI] [PubMed] [Google Scholar]
- Lyne R, Burns G, Mata J, et al. Whole-genome microarrays of fission yeast: characteristics, accuracy, reproducibility, and processing of array data. BMC Genom. 2003;4:27. doi: 10.1186/1471-2164-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata J, Nurse P. tea1 and the microtubular cytoskeleton are important for generating global spatial order within the fission yeast cell. Cell. 1997;89:939–949. doi: 10.1016/s0092-8674(00)80279-2. [DOI] [PubMed] [Google Scholar]
- Matsuyama A, Arai R, Yashiroda Y, et al. ORFeome cloning and global analysis of protein localization in the fission yeast Schizosaccharomyces pombe. Nat Biotechnol. 2006;24:841–847. doi: 10.1038/nbt1222. [DOI] [PubMed] [Google Scholar]
- Matsuyama A, Shirai A, Yashiroda Y, et al. pDUAL, a multipurpose, multicopy vector capable of chromosomal integration in fission yeast. Yeast. 2004;21:1289–1305. doi: 10.1002/yea.1181. [DOI] [PubMed] [Google Scholar]
- Mizuguchi G, Shen X, Landry J, et al. ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science. 2004;303:343–348. doi: 10.1126/science.1090701. [DOI] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular analysis of the fission yeast Schizosaccharomyces pombe. Meth Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Munz P, Wolf K, Kohli J, Leupold U. Genetics overview. In: Nasim A, Young P, Johnson BF, editors. Molecular Biology of the Fission Yeast. New York: Academic Press; 1989. pp. 1–30. [Google Scholar]
- Pasero P, Marilley M. Size variation of rDNA clusters in the yeasts Saccharomyces cerevisiae and Schizosaccharomyces pombe. Mol Gen Genet. 1993;236:448–452. doi: 10.1007/BF00277147. [DOI] [PubMed] [Google Scholar]
- Patch AM, Aves SJ. Fingerprinting fission yeast: polymorphic markers for molecular genetic analysis of Schizosaccharomyces pombe strains. Microbiology. 2007;153:887–897. doi: 10.1099/mic.0.2006/001669-0. [DOI] [PubMed] [Google Scholar]
- Perkins DD. Biochemical mutants in the smut fungus Ustilago maydis. Genetics. 1949;34:607–626. doi: 10.1093/genetics/34.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliffe PA, Vardy L, Toda T. A conserved small GTP-binding protein Alp41 is essential for the cofactor-dependent biogenesis of microtubules in fission yeast. FEBS Lett. 2000;468:84–88. doi: 10.1016/s0014-5793(00)01202-3. [DOI] [PubMed] [Google Scholar]
- Samejima I, Mackie S, Warbrick E, et al. The fission yeast mitotic regulator win1+ encodes an MAP kinase kinase kinase that phosphorylates and activates Wis1 MAP kinase kinase in response to high osmolarity. Mol Biol Cell. 1998;9:2325–2335. doi: 10.1091/mbc.9.8.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt H. Effective long range mapping in Schizosaccharomyces pombe with the help of swi5. Curr Genet. 1993;24:271–273. doi: 10.1007/BF00351803. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Kapitza P, Gutz H. Switching genes in Schizosaccharomces pombe: their influence on cell viability and recombination. Curr Genet. 1987;11:303–308. [Google Scholar]
- Schmidt H, Kapitza-Fecke P, Stephen ER, Gutz H. Some of the swi genes of Schizosaccharomyces pombe also have a function in the repair of radiation damage. Curr Genet. 1989;16:89–94. doi: 10.1007/BF00393400. [DOI] [PubMed] [Google Scholar]
- Snaith HA, Sawin KE. Fission yeast mod5p regulates polarized growth through anchoring of tea1p at cell tips. Nature. 2003;423:647–651. doi: 10.1038/nature01672. [DOI] [PubMed] [Google Scholar]
- Tomlin GC, Morrell JL, Gould KL. The spindle pole body protein Cdc11p links Sid4p to the fission yeast septation initiation network. Mol Biol Cell. 2002;13:1203–1214. doi: 10.1091/mbc.01-09-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy L, Toda T. The fission yeast γ-tubulin complex is required in G1 phase and is a component of the spindle assembly checkpoint. EMBO J. 2000;19:6098–6111. doi: 10.1093/emboj/19.22.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatram S, Tasto JJ, Feoktistova A, et al. Identification and characterization of two novel proteins affecting fission yeast γ-tubulin complex function. Mol Biol Cell. 2004;15:2287–2301. doi: 10.1091/mbc.E03-10-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. 2008. Charcterization of factors affecting RNAi-directed heterochromatin assembly in fission yeast. PhD thesis, University of Edinburgh.
- Wood V, Gwilliam R, Rajandream MA, et al. The genome sequence of Schizosaccharomyces pombe. Nature. 2002;415:871–880. doi: 10.1038/nature724. [DOI] [PubMed] [Google Scholar]
- Young JA, Hyppa RW, Smith GR. Conserved and nonconserved proteins for meiotic DNA breakage and repair in yeasts. Genetics. 2004;167:593–605. doi: 10.1534/genetics.103.023762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman S, Tran PT, Daga RR, et al. Rsp1p, a J domain protein required for disassembly and assembly of microtubule organizing centers during the fission yeast cell cycle. Dev Cell. 2004;6:497–509. doi: 10.1016/s1534-5807(04)00096-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


