The intestinal parasite H. polygyrus secretes a TGF-β–like molecule that induces regulatory T cells, thus suppressing anti-parasitic effector T cell responses by the host.
Abstract
Foxp3-expressing regulatory T (T reg) cells have been implicated in parasite-driven inhibition of host immunity during chronic infection. We addressed whether parasites can directly induce T reg cells. Foxp3 expression was stimulated in naive Foxp3− T cells in mice infected with the intestinal helminth Heligmosomoides polygyrus. In vitro, parasite-secreted proteins (termed H. polygyrus excretory-secretory antigen [HES]) induced de novo Foxp3 expression in fluorescence-sorted Foxp3− splenocytes from Foxp3–green fluorescent protein reporter mice. HES-induced T reg cells suppressed both in vitro effector cell proliferation and in vivo allergic airway inflammation. HES ligated the transforming growth factor (TGF) β receptor and promoted Smad2/3 phosphorylation. Foxp3 induction by HES was lost in dominant-negative TGF-βRII cells and was abolished by the TGF-β signaling inhibitor SB431542. This inhibitor also reduced worm burdens in H. polygyrus–infected mice. HES induced IL-17 in the presence of IL-6 but did not promote Th1 or Th2 development under any conditions. Importantly, antibody to mammalian TGF-β did not recognize HES, whereas antisera that inhibited HES did not affect TGF-β. Foxp3 was also induced by secreted products of Teladorsagia circumcincta, a related nematode which is widespread in ruminant animals. We have therefore identified a novel pathway through which helminth parasites may stimulate T reg cells, which is likely to be a key part of the parasite’s immunological relationship with the host.
Regulatory T (T reg) cells are a subset of lymphocytes that play a key role in maintaining immune homeostasis by virtue of their ability to actively suppress the immune response (Sakaguchi et al., 2006; Zheng and Rudensky, 2007; Shevach, 2009). Natural T reg cells that emerge from the thymus with self-specificity limit activation and expansion of autoreactive T cells in the periphery, whereas inducible T (iT) reg cells derive from conventional T cells that are antigen stimulated in the presence of mediators such as TGF-β, IL-10, and retinoic acid (Hawrylowicz and O’Garra, 2005; Mucida et al., 2007; Curotto de Lafaille and Lafaille, 2009). In some systems, iT reg cells may initiate expression of the forkhead-winged-helix transcription factor Foxp3, which is constitutively expressed by natural T reg cells (Hori et al., 2003; Josefowicz and Rudensky, 2009). Deficiencies in the T reg cell subset are associated with poorly controlled allergic (Akdis et al., 2004; Hawrylowicz and O’Garra, 2005) or autoimmune (Ehrenstein et al., 2004) reactions, whereas more potent T reg cell activity may block protective immune responses against infection (Belkaid and Tarbell, 2009) or cancer (Zou, 2006).
When the immune system is challenged by an invading pathogen, Foxp3+ T reg cells play an essential role in controlling the voracity of the response. In general, they strike a balance that limits potentially harmful immune-mediated pathology to the host while still allowing sufficient immune pressure against the pathogen (Belkaid and Tarbell, 2009). However, when the Foxp3+ T reg cell component becomes dominant, the host fails to clear infection, in examples such as mycobacteria (Scott-Browne et al., 2007), leishmaniasis (Belkaid et al., 2002), and filariasis (Taylor et al., 2005). Skewing of the host response toward T reg cells may reflect either an intrinsic imbalance in host immune control or a targetted intervention by the pathogen to stimulate Foxp3+ T reg cell suppression. The observation that live, but not dead, filarial parasites stimulate murine Foxp3+ T reg cells in vivo (McSorley et al., 2008) supports the suggestion that the T reg cell response in helminth infection goes beyond a simple homeostatic balancing mechanism, but this has yet to be formally demonstrated.
It has been established that over the first 28 d of chronic infection with the gastrointestinal helminth parasite Heligmosomoides polygyrus, levels of Foxp3 expression within the CD4+ T cell population of the mesenteric LNs (MLNs) are significantly increased, and a higher degree of in vitro suppressive activity by purified CD4+CD25+ T reg cells is observed (Finney et al., 2007; Rausch et al., 2008). Moreover, the CD4+CD25+ population from infected mice, but not from uninfected mice, is able, upon adoptive transfer, to suppress allergic airway inflammation in sensitized recipient mice (Wilson et al., 2005). Although H. polygyrus infection is thus associated with amplification of regulatory cell activity, it is not known whether the parasite drives this population as part of its immune evasion strategy or if heightened regulation is a normal homeostatic mechanism of the immune system to minimize pathology.
We therefore set out to identify any mechanisms by which H. polygyrus was able to directly affect this arm of the immune response. In this paper, we demonstrate that some helminth parasites, including H. polygyrus and the related gastrointestinal nematode Teladorsagia circumcincta, secrete products capable of directly inducing Foxp3+ T reg cells. These results suggest a novel and effective ploy by parasites to exploit the immune system’s own self-regulatory signaling pathways.
RESULTS
H. polygyrus excretory-secretory antigen (HES) enhances the Foxp3+ T cell compartment in vitro
The helminth parasite H. polygyrus resides in the luminal zone of the upper gastrointestinal tract, and infection is associated with the expansion of functional T reg cells within the host (Wilson et al., 2005; Finney et al., 2007; Setiawan et al., 2007; Rausch et al., 2008). As many helminth parasites are known to release biologically active excretory-secretory (ES) antigens that directly modulate host immune function (Hewitson et al., 2009), we reasoned that ES products of H. polygyrus may have coevolved to target the Foxp3+ T reg cell compartment. Adult parasites were, therefore, maintained in vitro in serum-free tissue culture medium and their ES antigens collected and diafiltrated as HES.
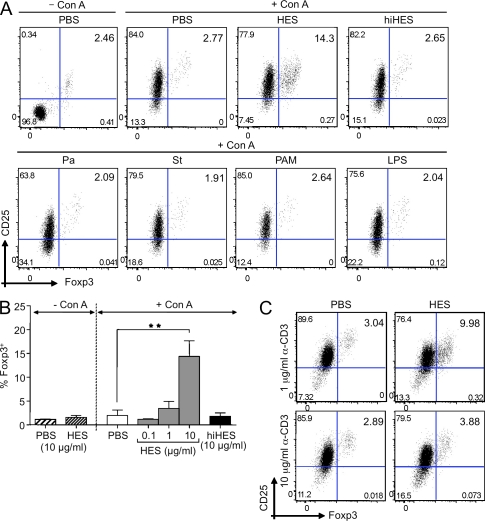
HES was tested for its ability to enhance expression of Foxp3 in naive splenic T cells cultured for 48 h in vitro. Because the conditions previously described for Foxp3 induction include polyclonal TCR ligation, we stimulated with Con A mitogen in some experimental groups, adding HES to cells 30 min before Con A addition to limit any possible direct binding of the lectin to HES glycans. Flow cytometric analysis revealed that in HES-treated cultures, the percentage of Foxp3+CD25+ cells within the CD4+ population increased more than fourfold over the 48-h period (Fig. 1, A and B). Cells treated with Con A alone showed strong up-regulation of CD25 (IL-2Rα), which is consistent with polyclonal activation, but no increase in Foxp3 expression. HES acted in a dose-dependent manner but did not up-regulate Foxp3 in the absence of Con A (Fig. 1 B). The ability of HES to enhance Foxp3 was abolished by heat treatment (Fig. 1, A and B), demonstrating the involvement of a heat-labile parasite component and showing that Foxp3 enhancement cannot be attributed to heat-stable contaminants such as LPS.
Figure 1.
HES increases the percentage of CD4+Foxp3+ T cells in mitogen-stimulated splenocyte cultures. (A) Representative plots of CD25 versus Foxp3 expression, gated on CD4+ T cells, from C57BL/6 splenocytes cultured in the presence of PBS alone, 2 µg/ml Con A, or combinations of Con A with pathogen products. Con A was added to cultures 30 min after pathogen products, and flow cytometry was performed 48 h later. Top row, PBS alone, Con A, Con A plus 10 µg/ml HES, and Con A plus 10 µg/ml of heat-inactivated (hi) HES. Heat inactivation was performed for 30 min at 100°C. Bottom row, Con A plus 10 µg/ml Propionibacterium acnes extract (Pa), 10 µg/ml Salmonella typhimurium extract (St), 1 µg/ml Pam-3-CSK4, or 1 µg/ml LPS. (B) Percentage of Foxp3+ cells within the CD4+ T cell population of splenocytes exposed to the indicated stimuli. Data represent mean ± SD from three replicate cultures with cells from individual C57BL/6 mice. Results of Student’s t test: **, P < 0.005. (C) Foxp3 induction in splenocytes, from naive mice, exposed to PBS or HES in combination with low (1 µg/ml) or high (10 µg/ml) doses of α-CD3 antibody. Data are representative of at least three experiments performed using different batches of HES.
As this was the first demonstration of a pathogen-associated ligand that can interact with host cells to induce Foxp3, we ascertained whether this was a more general property of pathogen-derived products. We also tested preparations of the gram-negative bacteria Salmonella typhimurium, as well as the gram-positive bacteria Propionibacterium acnes, both of which were devoid of T reg cell–expanding activity (Fig. 1 A). Similarly, the TLR-2 and -4 ligands Pam-3-CSK4 and LPS had no effect (Fig. 1 A).
Addition of exogenous TGF-β to activated T cell cultures is known to induce Foxp3 expression in vitro (Chen et al., 2003; Peng et al., 2004). In this setting, stimulation of Foxp3 is maximal at concentrations of anti-CD3 below those used for effector T cell activation (Kim and Rudensky, 2006). We therefore tested the ability of HES to increase the percentage of Foxp3+ cells using 1 and 10 µg/ml of plate-bound anti-CD3. In these experiments, although both doses stimulated similar expression of CD25, the lower dose was found to be significantly more effective in the induction of Foxp3 (Fig. 1 C).
HES induces Foxp3+ T reg cells in vitro
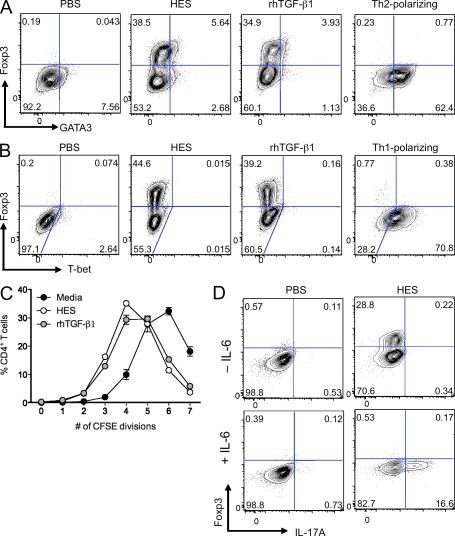
The expansion in Foxp3-expressing CD4+ T cell numbers in response to HES may represent de novo Foxp3 induction, proliferation of natural Foxp3+CD4+ T cells, or selective death of Foxp3−CD4+ T cells. To distinguish between these possibilities, we sorted naive enhanced GFP (eGFP)−CD4+ T cells from Foxp3-eGFP transgenic mice and co-cultured them with splenic DC and anti-CD3 in the presence of PBS, HES, or recombinant human (rh) TGF-β1 as a Foxp3-inducing control. Foxp3 expression was analyzed 3 d later by intracellular staining (Fig. 2, A and B). After this time period, a similar proportion of cells expressed Foxp3 in the HES and rhTGF-β1–treated wells, demonstrating that HES, like TGF-β, is able to induce de novo expression of Foxp3 in activated CD4+ T cells.
Figure 2.
HES shows TGF-β–like activity and directly induces Foxp3 expression in CD4+ T cells. (A) Foxp3 and GATA3 expression in FACS-sorted naive Foxp3-eGFP–negative CD4+ T cells cultured for 3 d with splenic DC in the presence of α-CD3 alone and in combination with HES, rhTGF-β1, or Th2-polarizing cytokines. (B) Foxp3 and T-Bet expression in FACS-sorted naive Foxp3-eGFP–negative CD4+ T cells, cultured as in A with HES, rhTGF-β1, or Th1-polarizing cytokines. (C) Proliferation measured by CFSE labeling of FACS-sorted naive Foxp3-eGFP–negative CD4+ T cells after 3 d of culture with splenic DC in the presence of α-CD3 alone and in combination with HES or rhTGF-β1. Error bars represent SEM. (D) Intracellular IL-17A and Foxp3 expression in FACS-sorted naive Foxp3-eGFP–negative CD4+ T cells cultured for 4 d with splenic DC in the presence of α-CD3, with or without IL-6 and in combination with HES or PBS control. Data are representative of at least three similar experiments performed using different batches of HES.
Many ES products have been described to induce Th2 or Th1 polarization of T cells (Hewitson et al., 2009). HES specifically induced Foxp3 expression in T cells, and there was no evidence of expression of the canonical Th2 transcription factor GATA3 (Fig. 2 A) or Th1 transcription factor T-bet (Fig. 2 B) when compared with cytokine-polarized controls.
Moreover, in addition to its Foxp3-inducing ability, HES demonstrated additional TGF-β–like activities. HES suppressed proliferation of CFSE-labeled naive eGFP−CD4+ T cells to a similar extent as rhTGF-β1 (Fig. 2 C). In the presence of IL-6, HES was also able to drive IL-17A production from naive CD4+ T cells (Fig. 2 D).
HES contains a TGF-β–like activity
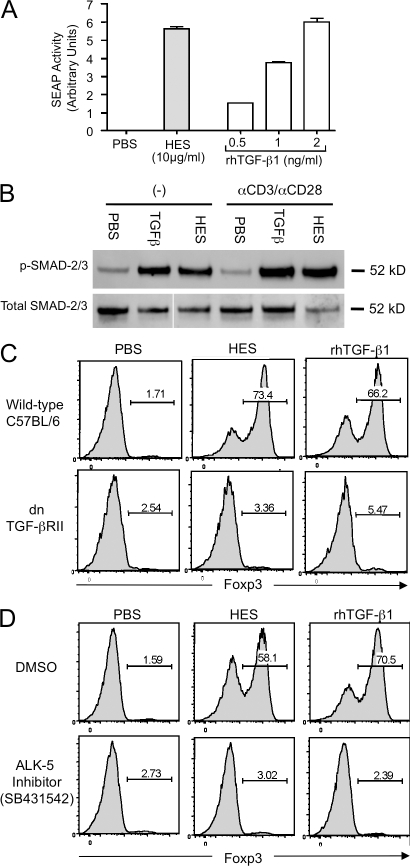
The similarities observed between the effects of HES and TGF-β indicated clearly that HES may contain a functional mimic of TGF-β. Using a cell line derived from TGF-β1−/− fibroblasts transfected with a TGF-β–responsive alkaline phosphatase reporter construct (Tesseur et al., 2006), we assessed whether HES was able to drive TGF-β signaling. It was found that 10 µg/ml HES activated these reporter cells, with a similar effect to that of 1–5 ng/ml rhTGF-β1 (Fig. 3 A). Previously, this cell line has been shown to be specifically responsive to TGF-βs and not other TGF-β superfamily members (Tesseur et al., 2006), suggesting that a true TGF-β mimic is present in HES. To confirm that HES activates the canonical signaling pathway downstream of the TGF-β heterodimeric receptor (TGF-βR), we also probed HES and TGF-β–treated T cells for phosphorylation of the SMAD-2/3 signaling molecules (Fig. 3 B). These studies showed that in the presence of either ligand, significant levels of phospho-SMADs were detectable, and that SMAD phosphorylation indicative of canonical TGF-βR ligation (Attisano and Wrana, 2002) occurred without the requirement for additional TCR stimulation.
Figure 3.
HES activates TGF-β–responsive cells, induces SMAD-2/3 phosphorylation, and induces Foxp3 expression through the TGF-β signaling pathway. (A) HES activates the TGF-β–responsive reporter cell line MFB-F11, inducing expression of alkaline phosphatase activity. Error bars represent SEM of triplicate wells of MFB-F11 cells stimulated with each treatment. (B) Phosphorylation of SMAD-2/3 signaling molecules, activated by TGF-β ligation, was measured in C57BL/6 CD4+ cells stimulated in the absence (−) or presence of plate-bound anti-CD3/anti-CD28, together with PBS, 5 ng/ml rhTGF-β1, or 10 μg/ml HES. Cell lysates were probed by Western blotting with phospho-SMAD-2/3–specific antibody (top) and then stripped and reprobed with antibody to SMAD-2/3 peptide backbone. The figure represents a single determination, with internal replication (with and without anti-CD3/CD28). (C) CD4+ cells were purified from WT C57BL/6 or dominant-negative TGF-βRII mice and stimulated with plate-bound anti-CD3/anti-CD28 in the presence of IL-2, with either 10 µg/ml HES or 5 ng/ml rhTGF-β1. After 72 h, Foxp3 expression was analyzed by flow cytometry. (D) WT CD4+ cells were cultured as in C but were treated with 5 µM of the ALK-5/TGF-βRI inhibitor SB431542 (or DMSO as a control) alongside HES or rhTGF-β1. After 72 h, Foxp3 expression was analyzed by flow cytometry. Results shown in A, C, and D, are representative of at least three similar experiments performed with different batches of HES.
We then tested T cells from mice expressing a dominant-negative form of the TGF-βRII (Gorelik and Flavell, 2000), which failed to expand Foxp3 expression in response to either HES or TGF-β (Fig. 3 C). This result also confirmed that HES acts directly on the T cell, as this receptor construct is restricted to the T cell lineage, and signaling in bystander populations (such as dendritic cells) is not compromised. Cells were also treated with the specific TGF-β type I receptor (TGF-βRI)/ALK-5 inhibitor SB431542 (Inman et al., 2002) for 1 h before addition of HES and anti-CD3. After treatment with the inhibitor, neither HES nor rhTGF-β1 were able to induce Foxp3 expression (Fig. 3 D), formally demonstrating that Foxp3 induction by HES is dependent on signaling through the TGF-β pathway.
HES and mammalian TGF-β are immunologically distinct
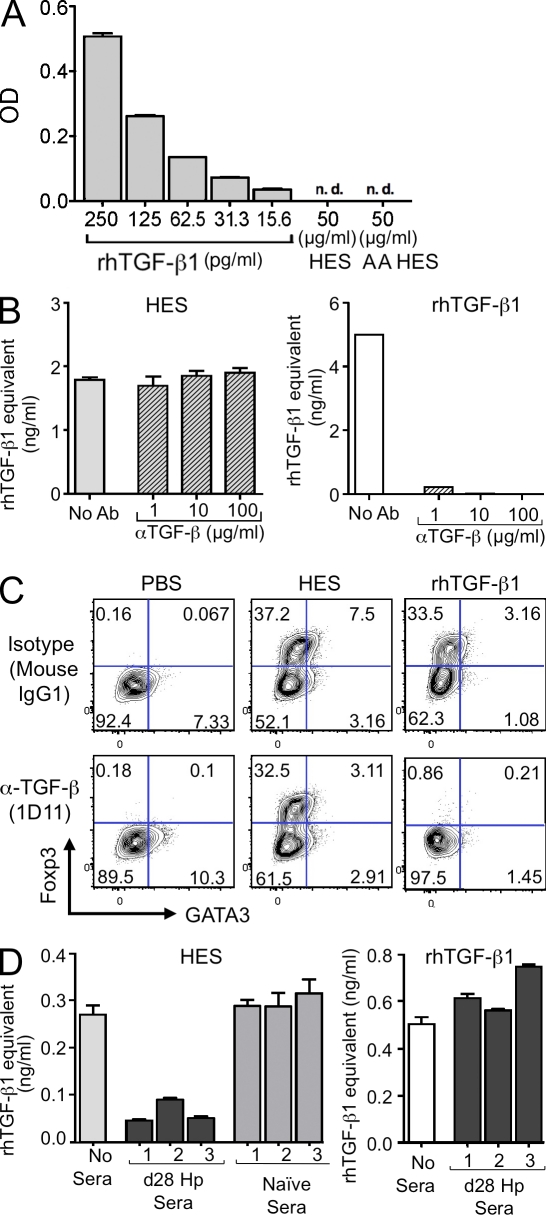
We evaluated the possibility that, in vivo, parasites might sequester host TGF-β and release it under in vitro conditions into the HES. We first tested whether HES contained a substance that would be reactive in the standard ELISA for mammalian TGF-β. As shown in Fig. 4 A, this assay detected as little as 15 pg/ml rhTGF-β1 but was negative when incubated with 50 µg/ml HES, whether or not the HES was subjected to an acid activation step that would release TGF-β from its latent form.
Figure 4.
HES and mammalian TGF-β are not recognized or cross-inhibited by specific antibodies to each other. (A) ELISA assay sensitive to 15 pg/ml rhTGF-β1 fails to react with native HES or acid-activated (AA) HES. (B) HES activation of MFB-F11 cells, calibrated by a standard curve of rhTGF-β, is not diminished even when preincubated with 100 µg/ml of the pan–TGF-β antibody 1D11 (left). In contrast, rhTGF-β1 activity is dramatically inhibited by as little as 1 µg/ml 1D11 (right). Data are representative of three similar experiments performed using different batches of HES. (C) Induction of Foxp3 expression in CD4+ T cells by rhTGF-β1, but not by HES, is ablated by preincubation with 30 µg/ml 1D11. Data are representative of three similar experiments performed using different batches of HES. (D) Day-28 sera from H. polygyrus (Hp)–infected mice, but not naive mice, blocks the ability of HES to activate MFB-F11 cells (left). In contrast, rhTGF-β1 activity was not affected by infection sera (right). HES or rhTGF-β1 were preincubated for 60 min with the indicated antibody before testing on MFB-F11 cells. Data are representative of three similar experiments. Error bars in A, C, and D represent SEM of triplicate wells of MFB-F11 cells stimulated with each treatment.
We then tested the activity of HES in the presence of pan-vertebrate anti–TGF-β blocking antibody. This antibody, 1D11, has previously been shown to interact with mammalian TGF-β1, 2, and 3 as well as amphibian TGF-β5. We preincubated HES or rhTGF-β1 for 1 h with increasing concentrations of 1D11. Samples were then used to activate the MFB-F11 cell line (Fig. 4 B). Even at 100 µg/ml 1D11, the ability of HES to stimulate the TGF-β–reporter cell line could not be inhibited. In contrast, the activity of 5 ng/ml rhTGF-β1 was dramatically inhibited with as little as 1 µg/ml 1D11. The resistance of HES to 1D11 neutralization was also observed at the level of Foxp3 induction, under conditions which completely blocked the effects of mammalian TGF-β (Fig. 4 C), and the ability of HES to directly inhibit CD4+ effector cell proliferation was likewise insensitive to 1D11 (Fig. S1 A). Similarly, blocking host TGF-β activity did not reveal any underlying property of HES to polarize expression toward GATA3 (Fig. S1 B) or T-bet (Fig. S1 C). Additionally, the inability of 1D11 to affect Foxp3 induction in vitro excludes the possibility that HES functions by stimulating an accessory cell population to release TGF-β.
As HES is able to reduce effector cell proliferation, the possibility that it differentially affects the survival of effector and regulatory subsets was investigated. In fact, HES exerts a modest protection against apoptosis of effector T cells, which closely replicates the effect of host TGF-β1 (Fig. S2 A). Interestingly, HES also prevents Foxp3+ T reg cells from undergoing apoptosis in vitro (Fig. S2 B). Collectively, these data argue that HES does not produce expansion of the T reg cell compartment by inducing a higher rate of cell death among the effector population.
If the parasite TGF-β–like ligand is dissimilar to mammalian TGF-β, there is a possibility that it will stimulate an antibody response in the host. We tested sera from chronically infected mice and found that they were able to neutralize TGF-β signaling by HES (Fig. 4 D). Naive mouse sera did not interfere with HES signaling. Notably, the same antisera produced no effect on the action of mammalian TGF-β in this system, confirming that host and parasite ligands are immunologically distinct entities.
Blocking TGF-β signaling, but not host TGF-β alone, boosts immunity to infection
To test the role of host TGF-β in host immunity, we compared the effects of neutralizing monoclonal antibody with activity against all host isoforms, with the outcome of treatment with the ALK 5 inhibitor shown previously (Fig. 3 D), to block the induction of Foxp3 by HES and host TGF-β alike. Using a protocol in which chronically infected (>28 d) mice were treated, monoclonal antibody administration did not alter the final worm burden (Fig. S3), whereas recipients of ALK5 inhibitor showed a significant reduction in parasite load (Fig. 5 A). Interestingly, at the time of assay, treated mice showed a significant increase in IL-4–secreting Th2-like effector cells (unpublished data). This represents the first description of an intervention at the level of immune system signaling that produces a marked reduction in worm load.
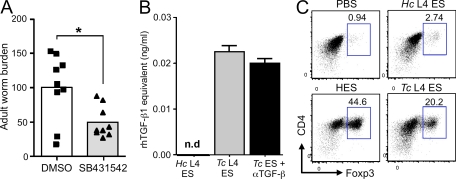
Figure 5.
Blocking of TGF-β signaling reduces worm burden, and ES from a related nematode parasite has TGF-β–like and Foxp3-inducing activity. (A) WT C57BL/6 mice were infected with 200 H. polygyrus L3 cells by gavage. On days 35, 37, and 39, mice were intraperitoneally injected with 200 µl of a 1:1 DMSO/PBS solution containing 2 mg/ml SB431542 or 200 µl of DMSO/PBS alone. Mice were sacrificed on day 40 of infection and adult worms in the lumen of the gut counted. Data are combined from two identical experiments, each with four to five mice per group. Data points show individual mice and bars represent mean values. Results of Student’s t test: *, P < 0.05. (B) ES from 10 µg/ml T. circumcincta fourth-stage larvae (TcL4ES) is able to activate the MFB-F11 TGF-β reporter assay, and this activity is unaffected by high levels (100 µg/ml) of α–TGF-β (1D11) antibody. The equivalent ES from 10 µg/ml H. contortus (HcL4ES) had no detectable TGF-β activity. Error bars represent SEM. (C) 30 µg/ml TcL4ES induces significant Foxp3 expression after 72 h in purified CD4+ cultures stimulated with plate-bound anti-CD3/anti-CD28 and exogenous IL-2. In contrast, 30 µg/ml HcL4ES has no significant effect. Results presented in B and C are representative of three similar experiments.
TGF-β–like activity is present in other helminth ES
To examine whether release of TGF-β ligands is a general feature of all helminth parasites, we tested ES from two prominent gastrointestinal nematodes that cause chronic infection in the ruminant animals Haemonchus contortus and Teladorsagia circumcincta. We found that although no detectable TGF-β stimulation was present within H. contortus ES, the secretions of T. circumcincta fourth-stage larvae (L4) both ligated TGF-β receptor in reporter cell lines in a manner not inhibitable by 1D11 monoclonal anti–TGF-β antibody (Fig. 5 B) and induced Foxp3 in mammalian T cells (Fig. 5 C). Hence, H. polygyrus is not unique in its release of a TGF-β activity. In addition, we found that NES, the secreted antigens from Nippostrongylus brasiliensis, a gastrointestinal nematode which is quickly expelled from the gut (Holland et al., 2000), does not induce Foxp3 expression under the same conditions used for HES (unpublished data).
HES-induced T reg cells are functionally suppressive in vitro and in vivo
To ascertain whether HES-induced Foxp3+ T cells are indeed functional T reg cells, we first assessed the competence of HES iT reg cells in suppressing responder T cell proliferation. Foxp3− CD4+ naive T cells from Foxp3-eGFP mice were sorted and cultured in an APC-free system with plate-bound anti-CD3/anti-CD28 in the presence of HES or rhTGF-β1. After 7 d, cells that were typically >80% Foxp3+ were harvested and resorted, based on GFP expression, to isolate iT reg cell populations. Freshly sorted naive CD4+ responder cells were CFSE labeled and co-cultured with increasing ratios of both HES- and rhTGF-β1–induced iT reg cells. HES- and rhTGF-β1–induced iT reg cells inhibited anti-CD3–stimulated responder T cell proliferation to a similar extent (Fig. 6 A). HES-treated T cells, therefore, not only express the transcription factor Foxp3 but are able to act in the same manner as conventionally induced T reg cells.
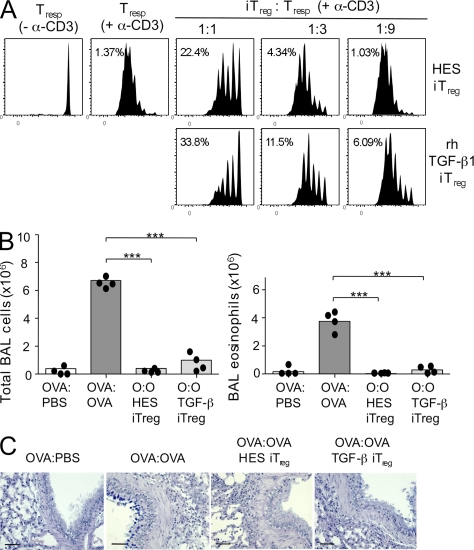
Figure 6.
HES-induced Foxp3+ T reg cell are functionally suppressive in vitro and in vivo in a Th2 environment. (A) The response of CD4+Foxp3− CFSE-labeled index responder T cells stimulated with α-CD3 for 4 d was measured by flow cytometry with and without additional unlabeled suppressor cells induced in vitro. Left, CFSE-labeled CD4+Foxp3− index responder T cells alone. Right, CFSE-labeled responder cells mixed with graded numbers of induced Foxp3+ T cells generated in vitro by co-culture of FACS-sorted CD4+Foxp3− T cells with HES or rhTGF-β1. Data are representative of three similar experiments performed using different batches of HES. (B) C57BL/6 mice were sensitized to OVA by two intraperitoneal (i.p.) injections with 10 µg OVA adsorbed to Alum 10 d apart. On days 16 and 19, 106 HES or rhTGF-β1 iT reg cells (generated as in A) were transfered intravenously in 200 µl PBS. Control mice were injected with 200 µl PBS alone. 1 d after each iT reg cell transfer, mice were given soluble OVA by direct tracheal inoculation. Lavage cells were recovered 24 h after the final airway challenge, and total cellularity (left) and eosinophilia (right) were enumerated. Data points represent results from individual mice and are representative of two identical experiments., and bars represent mean values. (C) Histological sectioning of lung tissues from allergen-sensitized mice receiving HES or rhTGF-β1 iT reg cells, as described in B. Bars, 100 µm.
Previously, work from our laboratory has demonstrated that CD4+CD25+ T reg cells isolated from the MLN of H. polygyrus–infected animals are able to suppress immune responses in a murine model of Th2-associated allergic disease (Wilson et al., 2005). We therefore next tested the ability of HES- and rhTGF-β1–induced iT reg cells to mediate suppression in this in vivo model. Mice were sensitized by intraperitoneal injection on days 0 and 10 with OVA adsorbed to alum, followed by intratracheal challenge with OVA on days 17 and 20. Subsequently, on day 21 bronchoalveolar lavage fluid was taken to measure inflammatory cell recruitment and lungs removed and fixed for histology. Mice that had received either HES- or rhTGF-β1–induced iT reg cells intravenously 1 d before each OVA challenge had reduced airway cellular infiltrates (Fig. 6 B), with differential counting of cells demonstrating a dramatic reduction in eosinophils (Fig. 6 B). Moreover, Alcian blue–periodic acid schiff revealed that accumulation of goblet cells in the connecting airways was inhibited in the iT reg cell–treated groups (Fig. 6 C). This demonstrates that HES-induced iT reg cells, as has been reported for rhTGF-β1–induced iT reg cells (Chen et al., 2003), are functionally able to suppress development of Th2-mediated pathology.
In vivo Foxp3+ T reg cell conversion is enhanced during H. polygyrus infection
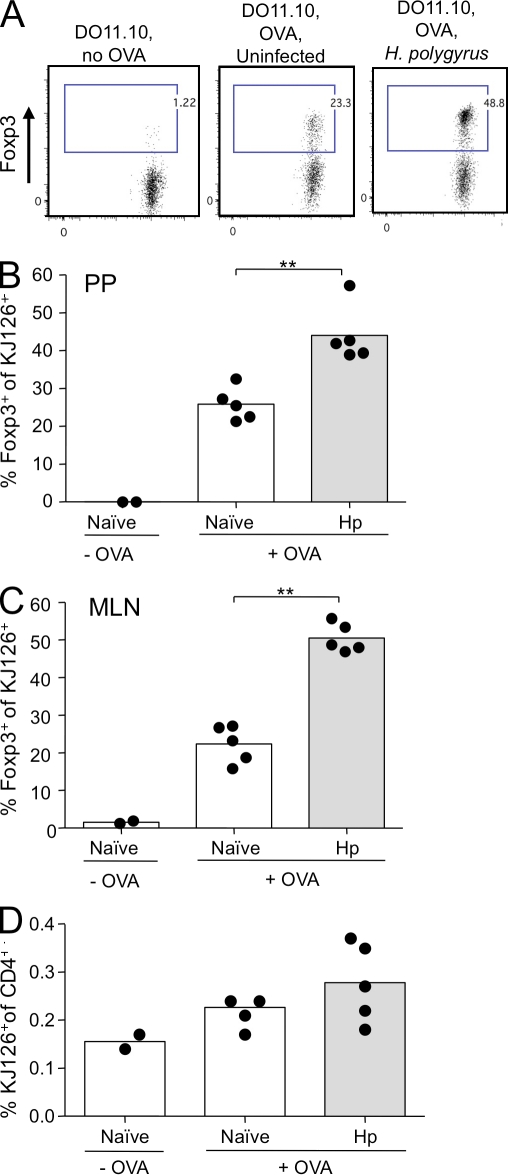
To assess whether H. polygyrus infection in vivo, like HES in vitro, can promote de novo Foxp3+ T reg cell conversion, we used a previously described model of oral antigen exposure (Thorstenson and Khoruts, 2001; Zhang et al., 2001). Naive eGFP− T cells, isolated from DO11.10 × Foxp3-eGFP mice, were transfered into BALB/c recipients that had been infected the previous day with H. polygyrus or uninfected controls. Recipient mice subsequently received OVA protein dissolved in their drinking water for five consecutive days. After this period, KJ126 antibody was used to detect the expanded TCR transgenic cells in the gut-associated lymphoid tissue (GALT), including the MLN and Peyer’s patches (PPs).
Consistent with other studies, although systemic dissemination of transfered cells was evident (not depicted), converted Foxp3+ T reg cells were only present in the TGF-β–rich environment of the GALT in both groups (Fig. 7). Strikingly, the proportion of OVA-specific Foxp3+ DO11.10 T cells was increased over twofold in the MLN (Fig. 7, A and B) and PP (Fig. 7 C) of H. polygyrus–infected animals, reaching levels of 50% of all transgenic cells that had been Foxp3-negative 7 d earlier. Survival of donor transgenic cells was similar in all groups of mice (Fig. 7 D), indicating that increased proportions of Foxp3-expressing cells in infected mice does not reflect differential survival. These data demonstrate for the first time that conversion to T reg cells specific to an oral antigen can not only be maintained while a productive Th2-polarized immune response is being initiated (Mohrs et al., 2005) but that it can be expanded.
Figure 7.
H. polygyrus infection amplifies de novo Foxp3 expression among OVA-specific DO11.10 T cells. Male BALB/c mice were seeded on day 1 of infection with 106 eGFP-negative CD4+ T cells from (Foxp3-eGFP.BALB/c × DO11.10) F1 male mice and given 1.5% OVA in drinking water from day 2. MLN and PP were harvested at day 7. (A) Representative flow cytometry plots of MLN DO11.10 cells identified with clonotypic antibody KJ126 in control mice, those receiving cells alone, and those given both cells and oral OVA in the absence or presence of infection. (B) Percentage of KJ126+CD4+ T cells expressing Foxp3 in the MLN. **, P < 0.01. (C) Percentage of KJ126+CD4+ T cells expressing Foxp3 in the PP. **, P < 0.01. (D) Percentage of CD4+ T cells expressing KJ126 in the MLNs of cell recipients given cells alone, or oral OVA with or without infection. Results shown are representative of two similar experiments, with control groups of two mice and OVA-treated groups of five mice in each case. Data points represent individual mice and bars represent mean values.
DISCUSSION
Our experiments have established several new central tenets of immunomodulation in helminth parasite infections. First, the induction of T reg cells observed in vivo can be elicited in vitro by worm-secreted products; second, helminth molecules can promote a regulatory phenotype in naive peripheral T cells; and third, switching noncognate T cells into the regulatory state provides a mechanistic explanation for the hygiene hypothesis, in which infections may protect against allergies and autoimmunity. We show in this paper that the excretory-secretory products of H. polygyrus can indeed drive expression of the signature transcription factor Foxp3, in a manner analogous to TGF-β, when added to naive non–T reg cells, and that they do so directly without the requirement for an antigen-presenting accessory population.
Arguably, helminth parasites may be expected to be particularly adept at manipulating host regulatory pathways (Maizels et al., 2004; Elliott et al., 2007). They are complex eukaryotic organisms that can often infect an immunocompetent human host for several decades. Data from both human and animal studies support a role for T reg cells in the generation of a suppressive environment favorable to parasite persistence. T cell clones from patients with the human filarial helminth Onchocerca volvulus produce antigen-specific IL-10 and TGF-β, cytokines characteristic of a T reg cell phenotype (Satoguina et al., 2002), whereas a broader profiling of polyclonal responses in lymphatic filariasis shows raised Foxp3 expression alongside reduced effector cytokines (Babu et al., 2006). In corroboration with this, in the mouse model of filarial infection Litomosoides sigmodontis, parasite killing occurs if antibodies to T reg cell surface marker proteins are administered (Taylor et al., 2005, 2007). Although other helminth products, such as Schistosome egg antigen, can promote T reg cell activity indirectly through eliciting host TGF-β production (Zaccone et al., 2009), ours is the first study to report a pathogen directly mimicking the host ligand for the purposes of immune down-regulation.
Immunity to H. polygyrus in the mouse is known to be highly Th2 dependent and mediated through successive phases by alternatively activated macrophages in the mucosal wall (Anthony et al., 2006), followed by goblet cell mediators, such as RELM-β, acting in the luminal environment (Herbert et al., 2009). In addition, antibodies can play a significant role, for example, in limiting worm fecundity in vivo (McCoy et al., 2008). The protective Th2 response can only develop if competing Th1 and Th17 responses are sufficiently dampened at an early stage; thus, we have recently observed that IL-23p19–deficient mice have enhanced resistance to H. polygyrus infection (unpublished data), suggesting that the extent to which parasite-derived TGF-β is able to induce a Th17 response (Fig. 2 D) may reflect a secondary strategy to block protective Th2 immunity. Similarly, mice expressing the dominant-negative TGF-βRII, which we show in this paper prevents the regulatory action of HES, exhibit extraordinarily high Th1 responses and are unable to expel H. polygyrus (Ince et al., 2009). Competition between Th subsets is particularly evident in the early phases of infection when the parasite has entered the intestinal wall, which may explain why inhibition of TGF-β–mediated signaling (unpublished data) or depletion of Foxp3+ T reg cells (Rausch et al., 2009) in the first 14 d of infection does not alter the outcome in terms of worm burden.
The immune system’s self-regulatory mechanisms offer an essential safeguard against autoimmunity; however, pathogens may have evolved to exploit such pathways and forestall the development of protective immunity to infection (Maizels et al., 2004; Scott-Browne et al., 2007; Belkaid and Tarbell, 2009). In chronic infections, the development of a stronger regulatory environment does not necessarily denote induction by the pathogen, as immune responsiveness often needs to be restrained to minimize tissue pathology (Baumgart et al., 2006; Layland et al., 2007; D’Elia et al., 2009; Maizels et al., 2009). However, by demonstrating that the expansion of T reg cell activity observed during infection in vivo can be reproduced by parasite products in vitro, one must conclude that the increased T reg cell activity goes beyond an intrinsic homeostatic dynamic of the immune system.
A major theme of infection research has been the bystander effect of microbes and parasites on the immune response to autoantigens and allergens (Wilson and Maizels, 2004). Previously, studies have demonstrated suppression of allergy by T reg cells in helminth-infected mice (Wilson et al., 2005; Dittrich et al., 2008) but have not established if the cells in question are parasite antigen specific. Our data demonstrate that not only are OVA-specific T reg cells stimulated during H. polygyrus infection in the presence of antigen but that a polyclonal Foxp3+ population generated in vitro is able to suppress airway allergy. Hence, although certain helminth antigens selectively drive T reg cells (Zaccone et al., 2009), the majority of T reg cells activated in vivo by infection are likely to be noncognate for parasite antigen.
It is unlikely that the Foxp3+ T reg cell subset is uniquely responsible for parasite-mediated immune regulation and, indeed, we have recently established that in C57BL/6 mice infected with H. polygyrus an IL-10–independent regulatory B cell population is also generated (Wilson et al., 2010). Hence, depletion of the Foxp3+ T cell subset at one particular time may not necessarily reverse the regulatory environment (Rausch et al., 2009). More broadly, across a range of helminth parasite systems there are examples of CD8+ T reg cells (Metwali et al., 2006), as well as IL-10–dependent B cells (Mangan et al., 2004) and suppressive macrophages (Jenkins and Allen, 2010), each contributing to a proregulatory environment. Thus, although immunoregulation is a shared strategy for diverse helminth species, the composition of the regulatory cell population is not necessarily the same in each case. It is interesting, however, that TGF-β–mediated signaling is common to many of these regulatory pathways.
We are now actively seeking to identify the molecular principle of TGF-β–like activity within HES. In this regard, it should be noted that 10 µg/ml of unfractionated HES exerts a similar biological effect to 0.5–5 ng/ml rhTGF-β1 (Fig. 3 A). Hence, a biochemical approach may be problematic given the limiting quantities of parasite-derived material that is available. In the absence of an available genome sequence for this organism, we are undertaking a cDNA-based transcriptomic survey to identify candidate gene products that will be tested for their ability to drive T reg cell differentiation in vitro. Among these are members of the broader TGF-β superfamily that are present in H. polygyrus and other nematodes (McSorley et al., 2010).
In conclusion, we have shown that helminth parasites have evolved a mechanism to directly expand Foxp3+ T reg cells. It can be envisaged that by inducing T reg cells the parasite can limit the impact of host effector responses, thereby preventing its expulsion. Although we have shown that a polyclonal population of naive T cells can be stimulated by parasite products to develop regulatory properties, the dependence of this process on TCR ligation makes it probable that, in situ, T cells with parasite-specific receptors are most likely to be subject to conversion to the regulatory phenotype. We are currently addressing whether infection engenders pathogen-specific T reg cells and, if so, which parasite antigens are the targets of such cells.
MATERIALS AND METHODS
Mice.
C57BL/6 and BALB/c mice were bred in house. Foxp3-eGFP transgenic mice (Fontenot et al., 2005) were backcrossed to C57BL/6 for 6–10 generations. DO11.10 mice were bred in house and crossed with a line of Foxp3-eGFP mice which had been backcrossed to BALB/c for 10 generations. Mice carrying the dominant-negative TGF-βRII construct were as previously described (Gorelik and Flavell, 2000). Mice were infected with 200 infective larvae (L3) of H. polygyrus, and antibodies collected 28 d later. Protocols were approved by the University of Edinburgh Ethical Review Committee, and animal studies were performed under UK Home Office Licences.
H. polygyrus, HES, and other pathogen products.
Parasites were maintained as previously described (Wilson et al., 2005). To collect HES, adult parasites taken 14 d after infection were maintained for 21 d in serum-free tissue culture medium, which was subsequently diafiltrated and concentrated over a 10,000-mol-wt Amicon membrane (Millipore) as described elsewhere (Harcus et al., 2009). Parasites remained viable throughout, and no major differences were seen by SDS-PAGE analysis of proteins released. H. contortus and T. circumcincta ES were collected from fourth-stage larvae under similar conditions for 10 d. P. acnes and S. typhimurium extracts were gifts from A. MacDonald (Institute of Immunology and Infection Research, Edinburgh, Scotland). LPS and Pam-3-CSK4 were purchased from Sigma-Aldrich and InvivoGen, respectively.
TGF-β and SMAD assays.
MFB-F11 cells (Tesseur et al., 2006) were adhered for 4 h to 96-well flat-bottomed plates at 4 × 104 cells/well in DME GlutaMAX, 10% FCS, 100 U/ml penicillin, and 100 µg/ml streptomycin. Cells were washed once with PBS, and then 50 µl of test samples was applied. Doubling dilutions of rhTGF-β1 (R&D Systems) starting at 4 ng/ml were used as a standard. After 24 h, samples were measured on a LumiStar luminometer (BMG Labtech) using the Great Escape SEAP (Takara Bio Inc.) kit as per the manufacturer’s instructions.
Cell transfer and oral antigen administration.
Uninfected or day-1 H. polygyrus–infected male BALB/c mice were given 106 FACS-purified CD4+eGFP− splenocytes from male Foxp3-eGFPxDO11.10 F1 mice intravenously. Starting on the next day (day 2), mice were given drinking water containing 1.5% OVA (Sigma-Aldrich) until MLN and PP were harvested on day 7.
Flow cytometry.
An LSR-II (BD) was used for flow cytometry with anti–CD4-PerCP or -PB (BD or eBioscience; 1:200); anti–CD25-PE (Invitrogen; 1:50); anti–Foxp3-APC or -FITC (eBioscience; 1:50); anti–GATA3-AF647 (BD; 1:25); anti–Tbet-PE (eBioscience; 1:150); anti–IL-17A-PerCP/Cy5.5 (eBioscience); and anti–CD44-APC (eBioscience; 1:300). Isotype controls were rat IgG2a-APC (eBioscience; 1:50), rat IgG2a-FITC (eBioscience), and rat IgG1-PE (BioLegend; 1:50).
In vitro splenocyte cultures.
Naive splenocytes were cultured in 96-well round-bottomed plates at 5 × 105 cells/well in RPMI1640, 10% FCS, 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, 50 µM 2-mercaptoethanol. Parasite products were added for 30 min, followed by addition of 2 µg/ml concanavalin A. After 48 h, cells were removed and stained for flow cytometric analysis.
In vitro T cell cultures.
CD4+ cells were first MACS purified with an LS column and then stained for CD4-PerCP and sorted for eGFP− population on a FACSAria (BD). 5 × 105 CD4+Foxp3− cells were then cultured in 24-well plates in complete RPMI 1640. Cells were stimulated with 2 µg/ml of plate-bound anti-CD3 (clone 145-2C11), 2 µg/ml of anti-CD28 (clone 37.51), 20 µg/ml of recombinant murine IL-2 (R&D Systems), and either 2 ng/ml rhTGF-β1 or 10 µg/ml HES. The ALK5 inhibitor SB-431542 (Tocris Bioscience; Inman et al., 2002) was dissolved at 10 mM in DMSO and used at a final concentration of 5 µM.
DC purification.
To isolate splenic DC, spleens were sliced into fragments and digested in 50 µg/ml Liberase TL (Roche) and 150 µg/ml DNase I (Sigma-Aldrich) and then treated with EDTA in Ca2+-free medium to disaggregate cells. After digestion, cells were passed through a 70-µM cell strainer, resuspended in 1.077 g/cm3 iso-osmotic NycoPrep medium (Accurate Chemical & Scientific Corp.), overlayed with RPMI 1640, and centrifuged at 1,650 g. The low-density fraction was then collected and washed thoroughly, and CD11c+ DCs were sorted from this fraction by positive selection using an autoMACS (Miltenyi Biotech) before using in co-culture with CD4+ T cells.
DC/T cell co-culture.
FACSAria-sorted CD4+CD44− Foxp3-eGFP–negative T cells were cultured in 96-well round-bottomed plates at 5 × 105 cells/well, with 5 × 104 purified splenic DCs/well, in complete RPMI 1640. Cells were stimulated with 1 µg/ml of soluble α-CD3 in the presence of 10 µg/ml HES or 2 ng/ml rhTGF-β1. 50 ng/ml rmIL-6 was added to some cultures to drive Th17 polarization. Additionally, CD4+ T cells were polarized under Th1 (10 ng/ml rmIL-12 + 10 µg/ml of anti-IL-4) and Th2 (10 ng/ml rmIL-4 + 10 µg/ml of anti-IL-12) conditions for control cultures. After 4 d, cells were removed and stained for flow cytometric analysis.
Suppression assay.
After culture, Foxp3-eGFP–positive and –negative populations were FACS sorted. 5 × 104 cells of either population were cultured with the same number of FACS-sorted CD4+Foxp3-GFP− effector cells with 105 irradiated APCs and 1 µg/ml of anti-CD3. Responder cells were stained with 1 ml of 20 µM CMTMR (Invitrogen) at 37°C for 30 min, followed by washing and a further 30-min incubation. Cells were then washed and cultured. After 90 h, cells were stained with CD4-PerCP for analysis on an LSR II. For CFSE labeling, sorted CD4+ T cells were labeled at 106 cells/100 µl in PBS containing CFSE at a concentration of 2 µM for 10 min at 37°C. Cells were then washed repeatedly in complete RPMI 1640 before addition to DC co-cultures or suppression assays.
In vivo treatment of infected mice.
Monoclonal anti–TGF-β1 antibody was grown in house using the 1D11 cell line and purified from cell supernatants on a Protein G column. Control mouse IgG1 was purchased from Sigma-Aldrich. ALK5 inhibitor SB431542 (Sigma-Aldrich) was dissolved in DMSO vehicle.
Allergen-induced airway inflammation.
C57BL/6 mice were sensitized using 10 µg OVA emulsified in alum (Imject Alum; Thermo Fisher Scientific) and then, 10 d later, boosted with the same antigen. On days 16 and 19, 106 FACS-sorted HES- or rhTGF-β1–induced T reg cells were transferred intravenously by tail vein injection into sensitized mice. In concert with these cell transfers, mice were challenged by the intratracheal route with 10 µg OVA in PBS on days 17 and 20. Mice were killed 24 h after this final challenge and airways assessed for inflammation by cannulation of the trachea and lavage of airspaces with 0.5 ml PBS, followed by an additional 1-ml wash. Collected fluids were spun at 1,200 g and pellets resuspended for cellular analysis. Cytospins were prepared by spinning ∼5 × 105 cells onto poly-L-lysine–coated slides and staining with Diff Quick (Boehringer Ingelheim). Cell counts were performed on at least 200 cells at 100× magnification. Histopathology was performed on formalin-fixed lungs that were embedded in paraffin, sectioned, and stained with Alcian blue–periodic acid schiff to visualize mucus-containing goblet cells.
Online supplemental material.
Fig. S1 shows that HES and TGF-β do not affect proliferation or induce GATA3 or T-bet expression in naive CD4+ T cells. Fig. S2 shows that both HES and TGF-β extend the survival of both T reg and effector CD4+ T cells. Fig. S3 shows that in vivo administration of monoclonal antibody against mammalian TGF-β to H. polygyrus–infected mice does not significantly change worm burden. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20101074/DC1.
Acknowledgments
We thank Andrew MacDonald for insightful support and Judith Allen for extensive discussion and critical comments on the manuscript. We are also extremely grateful to Yvonne Gibson for careful husbandry and to Andrew Sanderson and Martin Waterfall for flow cytometry support.
J.R. Grainger thanks the Wellcome Trust for studentship support through the 4-year PhD Program, H.J. McSorley, K.J. Filbey, and C.A.M. Finney thank the Medical Research Council for studentship support, E.J.D. Greenwood thanks the Wellcome Trust for an undergraduate summer studentship, and K.A. Smith, J.P. Hewitson, Y. Harcus, and R.M. Maizels thank the Wellcome Trust for Programme Grant support. A.Y. Rudensky is a Howard Hughes Medical Institute Investigator and is supported by a National Institutes of Health grant.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- eGFP
- enhanced GFP
- HES
- Heligmosomoides polygyrus excretory-secretory antigen
- MLN
- mesenteric LN
- PP
- Peyer’s patch
References
- Akdis M., Verhagen J., Taylor A., Karamloo F., Karagiannidis C., Crameri R., Thunberg S., Deniz G., Valenta R., Fiebig H., et al. 2004. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J. Exp. Med. 199:1567–1575 10.1084/jem.20032058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony R.M., Urban J.F., Jr., Alem F., Hamed H.A., Rozo C.T., Boucher J.L., Van Rooijen N., Gause W.C. 2006. Memory T(H)2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat. Med. 12:955–960 10.1038/nm1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attisano L., Wrana J.L. 2002. Signal transduction by the TGF-β superfamily. Science. 296:1646–1647 10.1126/science.1071809 [DOI] [PubMed] [Google Scholar]
- Babu S., Blauvelt C.P., Kumaraswami V., Nutman T.B. 2006. Regulatory networks induced by live parasites impair both Th1 and Th2 pathways in patent lymphatic filariasis: implications for parasite persistence. J. Immunol. 176:3248–3256 [DOI] [PubMed] [Google Scholar]
- Baumgart M., Tompkins F., Leng J., Hesse M. 2006. Naturally occurring CD4+Foxp3+ regulatory T cells are an essential, IL-10-independent part of the immunoregulatory network in Schistosoma mansoni egg-induced inflammation. J. Immunol. 176:5374–5387 [DOI] [PubMed] [Google Scholar]
- Belkaid Y., Tarbell K. 2009. Regulatory T cells in the control of host-microorganism interactions (*). Annu. Rev. Immunol. 27:551–589 10.1146/annurev.immunol.021908.132723 [DOI] [PubMed] [Google Scholar]
- Belkaid Y., Piccirillo C.A., Mendez S., Shevach E.M., Sacks D.L. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 420:502–507 10.1038/nature01152 [DOI] [PubMed] [Google Scholar]
- Chen W., Jin W., Hardegen N., Lei K.J., Li L., Marinos N., McGrady G., Wahl S.M. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 198:1875–1886 10.1084/jem.20030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curotto de Lafaille M.A., Lafaille J.J. 2009. Natural and adaptive Foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 30:626–635 [DOI] [PubMed] [Google Scholar]
- D’Elia R., Behnke J.M., Bradley J.E., Else K.J. 2009. Regulatory T cells: a role in the control of helminth-driven intestinal pathology and worm survival. J. Immunol. 182:2340–2348 10.4049/jimmunol.0802767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich A.M., Erbacher A., Specht S., Diesner F., Krokowski M., Avagyan A., Stock P., Ahrens B., Hoffmann W.H., Hoerauf A., Hamelmann E. 2008. Helminth infection with Litomosoides sigmodontis induces regulatory T cells and inhibits allergic sensitization, airway inflammation, and hyperreactivity in a murine asthma model. J. Immunol. 180:1792–1799 [DOI] [PubMed] [Google Scholar]
- Ehrenstein M.R., Evans J.G., Singh A., Moore S., Warnes G., Isenberg D.A., Mauri C. 2004. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti–TNF-α therapy. J. Exp. Med. 200:277–285 10.1084/jem.20040165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott D.E., Summers R.W., Weinstock J.V. 2007. Helminths as governors of immune-mediated inflammation. Int. J. Parasitol. 37:457–464 10.1016/j.ijpara.2006.12.009 [DOI] [PubMed] [Google Scholar]
- Finney C.A.M., Taylor M.D., Wilson M.S., Maizels R.M. 2007. Expansion and activation of CD4(+)CD25(+) regulatory T cells in Heligmosomoides polygyrus infection. Eur. J. Immunol. 37:1874–1886 10.1002/eji.200636751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J.D., Rasmussen J.P., Williams L.M., Dooley J.L., Farr A.G., Rudensky A.Y. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 22:329–341 10.1016/j.immuni.2005.01.016 [DOI] [PubMed] [Google Scholar]
- Gorelik L., Flavell R.A. 2000. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 12:171–181 10.1016/S1074-7613(00)80170-3 [DOI] [PubMed] [Google Scholar]
- Harcus Y., Nicoll G., Murray J., Filbey K., Gomez-Escobar N., Maizels R.M. 2009. C-type lectins from the nematode parasites Heligmosomoides polygyrus and Nippostrongylus brasiliensis. Parasitol. Int. 58:461–470 10.1016/j.parint.2009.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrylowicz C.M., O’Garra A. 2005. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat. Rev. Immunol. 5:271–283 10.1038/nri1589 [DOI] [PubMed] [Google Scholar]
- Herbert D.R., Yang J.-Q., Hogan S.P., Groschwitz K., Khodoun M.V., Munitz A., Orekov T., Perkins C., Wang Q., Brombacher F., et al. 2009. Intestinal epithelial cell secretion of RELM-β protects against gastrointestinal worm infection. J. Exp. Med. 206:2947–2957 10.1084/jem.20091268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitson J.P., Grainger J.R., Maizels R.M. 2009. Helminth immunoregulation: the role of parasite secreted proteins in modulating host immunity. Mol. Biochem. Parasitol. 167:1–11 10.1016/j.molbiopara.2009.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland M.J., Harcus Y.M., Riches P.L., Maizels R.M. 2000. Proteins secreted by the parasitic nematode Nippostrongylus brasiliensis act as adjuvants for Th2 responses. Eur. J. Immunol. 30:1977–1987 [DOI] [PubMed] [Google Scholar]
- Hori S., Nomura T., Sakaguchi S. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- Ince M.N., Elliott D.E., Setiawan T., Metwali A., Blum A., Chen H.L., Urban J.F., Flavell R.A., Weinstock J.V. 2009. Role of T cell TGF-β signaling in intestinal cytokine responses and helminthic immune modulation. Eur. J. Immunol. 39:1870–1878 10.1002/eji.200838956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman G.J., Nicolás F.J., Callahan J.F., Harling J.D., Gaster L.M., Reith A.D., Laping N.J., Hill C.S. 2002. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 62:65–74 10.1124/mol.62.1.65 [DOI] [PubMed] [Google Scholar]
- Jenkins S.J., Allen J.E. 2010. Similarity and diversity in macrophage activation by nematodes, trematodes, and cestodes. J. Biomed. Biotechnol. 2010:262609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefowicz S.Z., Rudensky A. 2009. Control of regulatory T cell lineage commitment and maintenance. Immunity. 30:616–625 10.1016/j.immuni.2009.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.M., Rudensky A. 2006. The role of the transcription factor Foxp3 in the development of regulatory T cells. Immunol. Rev. 212:86–98 10.1111/j.0105-2896.2006.00426.x [DOI] [PubMed] [Google Scholar]
- Layland L.E., Rad R., Wagner H., da Costa C.U. 2007. Immunopathology in schistosomiasis is controlled by antigen-specific regulatory T cells primed in the presence of TLR2. Eur. J. Immunol. 37:2174–2184 10.1002/eji.200737063 [DOI] [PubMed] [Google Scholar]
- Maizels R.M., Balic A., Gomez-Escobar N., Nair M., Taylor M.D., Allen J.E. 2004. Helminth parasites—masters of regulation. Immunol. Rev. 201:89–116 10.1111/j.0105-2896.2004.00191.x [DOI] [PubMed] [Google Scholar]
- Maizels R.M., Pearce E.J., Artis D., Yazdanbakhsh M., Wynn T.A. 2009. Regulation of pathogenesis and immunity in helminth infections. J. Exp. Med. 206:2059–2066 10.1084/jem.20091903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan N.E., Fallon R.E., Smith P., van Rooijen N., McKenzie A.N., Fallon P.G. 2004. Helminth infection protects mice from anaphylaxis via IL-10-producing B cells. J. Immunol. 173:6346–6356 [DOI] [PubMed] [Google Scholar]
- McCoy K.D., Stoel M., Stettler R., Merky P., Fink K., Senn B.M., Schaer C., Massacand J., Odermatt B., Oettgen H.C., et al. 2008. Polyclonal and specific antibodies mediate protective immunity against enteric helminth infection. Cell Host Microbe. 4:362–373 10.1016/j.chom.2008.08.014 [DOI] [PubMed] [Google Scholar]
- McSorley H.J., Harcus Y.M., Murray J., Taylor M.D., Maizels R.M. 2008. Expansion of Foxp3+ regulatory T cells in mice infected with the filarial parasite Brugia malayi. J. Immunol. 181:6456–6466 [DOI] [PubMed] [Google Scholar]
- McSorley H.J., Grainger J.R., Harcus Y.M., Murray J., Nisbet A.J., Knox D.P., Maizels R.M. 2010. daf-7-related TGF-β homologues from Trichostrongyloid nematodes show contrasting life-cycle expression patterns. Parasitology. 137:159–171 10.1017/S0031182009990321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metwali A., Setiawan T., Blum A.M., Urban J., Elliott D.E., Hang L., Weinstock J.V. 2006. Induction of CD8+ regulatory T cells in the intestine by Heligmosomoides polygyrus infection. Am. J. Physiol. Gastrointest. Liver Physiol. 291:G253–G259 10.1152/ajpgi.00409.2005 [DOI] [PubMed] [Google Scholar]
- Mohrs K., Harris D.P., Lund F.E., Mohrs M. 2005. Systemic dissemination and persistence of Th2 and type 2 cells in response to infection with a strictly enteric nematode parasite. J. Immunol. 175:5306–5313 [DOI] [PubMed] [Google Scholar]
- Mucida D., Park Y., Kim G., Turovskaya O., Scott I., Kronenberg M., Cheroutre H. 2007. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 317:256–260 10.1126/science.1145697 [DOI] [PubMed] [Google Scholar]
- Peng Y., Laouar Y., Li M.O., Green E.A., Flavell R.A. 2004. TGF-β regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc. Natl. Acad. Sci. USA. 101:4572–4577 10.1073/pnas.0400810101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch S., Huehn J., Kirchhoff D., Rzepecka J., Schnoeller C., Pillai S., Loddenkemper C., Scheffold A., Hamann A., Lucius R., Hartmann S. 2008. Functional analysis of effector and regulatory T cells in a parasitic nematode infection. Infect. Immun. 76:1908–1919 10.1128/IAI.01233-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch S., Huehn J., Loddenkemper C., Hepworth M.R., Klotz C., Sparwasser T., Hamann A., Lucius R., Hartmann S. 2009. Establishment of nematode infection despite increased Th2 responses and immunopathology after selective depletion of Foxp3+ cells. Eur. J. Immunol. 39:3066–3077 10.1002/eji.200939644 [DOI] [PubMed] [Google Scholar]
- Sakaguchi S., Ono M., Setoguchi R., Yagi H., Hori S., Fehervari Z., Shimizu J., Takahashi T., Nomura T. 2006. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 212:8–27 10.1111/j.0105-2896.2006.00427.x [DOI] [PubMed] [Google Scholar]
- Satoguina J., Mempel M., Larbi J., Badusche M., Löliger C., Adjei O., Gachelin G., Fleischer B., Hoerauf A. 2002. Antigen-specific T regulatory-1 cells are associated with immunosuppression in a chronic helminth infection (onchocerciasis). Microbes Infect. 4:1291–1300 10.1016/S1286-4579(02)00014-X [DOI] [PubMed] [Google Scholar]
- Scott-Browne J.P., Shafiani S., Tucker-Heard G., Ishida-Tsubota K., Fontenot J.D., Rudensky A.Y., Bevan M.J., Urdahl K.B. 2007. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J. Exp. Med. 204:2159–2169 10.1084/jem.20062105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan T., Metwali A., Blum A.M., Ince M.N., Urban J.F., Jr., Elliott D.E., Weinstock J.V. 2007. Heligmosomoides polygyrus promotes regulatory T-cell cytokine production in the murine normal distal intestine. Infect. Immun. 75:4655–4663 10.1128/IAI.00358-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach E.M. 2009. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 30:636–645 10.1016/j.immuni.2009.04.010 [DOI] [PubMed] [Google Scholar]
- Taylor M.D., LeGoff L., Harris A., Malone E., Allen J.E., Maizels R.M. 2005. Removal of regulatory T cell activity reverses hyporesponsiveness and leads to filarial parasite clearance in vivo. J. Immunol. 174:4924–4933 [DOI] [PubMed] [Google Scholar]
- Taylor M.D., Harris A., Babayan S.A., Bain O., Culshaw A., Allen J.E., Maizels R.M. 2007. CTLA-4 and CD4+ CD25+ regulatory T cells inhibit protective immunity to filarial parasites in vivo. J. Immunol. 179:4626–4634 [DOI] [PubMed] [Google Scholar]
- Tesseur I., Zou K., Berber E., Zhang H., Wyss-Coray T. 2006. Highly sensitive and specific bioassay for measuring bioactive TGF-β. BMC Cell Biol. 7:15 10.1186/1471-2121-7-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorstenson K.M., Khoruts A. 2001. Generation of anergic and potentially immunoregulatory CD25+CD4 T cells in vivo after induction of peripheral tolerance with intravenous or oral antigen. J. Immunol. 167:188–195 [DOI] [PubMed] [Google Scholar]
- Wilson M.S., Maizels R.M. 2004. Regulation of allergy and autoimmunity in helminth infection. Clin. Rev. Allergy Immunol. 26:35–50 10.1385/CRIAI:26:1:35 [DOI] [PubMed] [Google Scholar]
- Wilson M.S., Taylor M.D., Balic A., Finney C.A.M., Lamb J.R., Maizels R.M. 2005. Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J. Exp. Med. 202:1199–1212 10.1084/jem.20042572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.S., Taylor M.D., O’Gorman M.T., Balic A., Barr T.A., Filbey K., Anderton S.M., Maizels R.M. 2010. Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur. J. Immunol. 40:1682–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccone P., Burton O., Miller N., Jones F.M., Dunne D.W., Cooke A. 2009. Schistosoma mansoni egg antigens induce Treg that participate in diabetes prevention in NOD mice. Eur. J. Immunol. 39:1098–1107 10.1002/eji.200838871 [DOI] [PubMed] [Google Scholar]
- Zhang X., Izikson L., Liu L., Weiner H.L. 2001. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J. Immunol. 167:4245–4253 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Rudensky A.Y. 2007. Foxp3 in control of the regulatory T cell lineage. Nat. Immunol. 8:457–462 10.1038/ni1455 [DOI] [PubMed] [Google Scholar]
- Zou W. 2006. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 6:295–307 10.1038/nri1806 [DOI] [PubMed] [Google Scholar]