Memory CD4+ T cells that produce both Th2 and Th17 cytokines are increased in the blood of patients with atopic asthma and in the lungs of asthmatic mice, where they contribute to inflammation.
Abstract
The inflammatory cytokine interleukin (IL)-17 is involved in the pathogenesis of allergic diseases. However, the identity and functions of IL-17–producing T cells during the pathogenesis of allergic diseases remain unclear. Here, we report a novel subset of TH2 memory/effector cells that coexpress the transcription factors GATA3 and RORγt and coproduce TH17 and TH2 cytokines. Classical TH2 memory/effector cells had the potential to produce IL-17 after stimulation with proinflammatory cytokines IL-1β, IL-6, and IL-21. The number of IL-17-TH2 cells was significantly increased in blood of patients with atopic asthma. In a mouse model of allergic lung diseases, IL-17–producing CD4+ TH2 cells were induced in the inflamed lung and persisted as the dominant IL-17–producing T cell population during the chronic stage of asthma. Treating cultured bronchial epithelial cells with IL-17 plus TH2 cytokines induced strong up-regulation of chemokine eotaxin-3, Il8, Mip1b, and Groa gene expression. Compared with classical TH17 and TH2 cells, antigen-specific IL-17–producing TH2 cells induced a profound influx of heterogeneous inflammatory leukocytes and exacerbated asthma. Our findings highlight the plasticity of TH2 memory cells and suggest that IL-17–producing TH2 cells may represent the key pathogenic TH2 cells promoting the exacerbation of allergic asthma.
Asthma is a common and heterogeneous inflammatory disorder of the airways (Anderson, 2008). Studies of patients and animal models suggest that TH2 memory cells that reside in the lung during disease remission contribute to the persistence and progression of asthma (Robinson et al., 1992; Epstein, 2006). In the allergic form of asthma, repetitive exposure to allergens activates allergen-specific resident TH2 memory cells to trigger production of chemokines and proinflammatory cytokines and recruitment of other inflammatory leukocytes (Cohn et al., 2004). In addition to allergens, environmental factors or infectious pathogens often trigger epithelial stress and altered innate immunity that induce different types of inflammation, thereby resulting in the heterogeneous forms of asthma (Simpson et al., 2006; Holgate, 2007).
Since the identification of IL-17 from activated T cell clones (Yao et al., 1995a), five additional family members have been discovered and designated as IL-17A–F (Li et al., 2000; Lee et al., 2001; Starnes et al., 2001). The discovery of the IL-17 cytokine family and the analysis of IL-23–mediated immune pathogenesis have led to the delineation of a new CD4+ T helper cell population termed TH17 (Yao et al., 1995b; Aarvak et al., 1999; Cua et al., 2003; Murphy et al., 2003; Harrington et al., 2005; Park et al., 2005a). The retinoic acid–related orphan receptor (RORγt) is the master transcription factor for the development of TH17 cell lineage, which can be characterized by their secretion of the proinflammatory cytokines IL-17, IL-17F, and IL-22 (Ivanov et al., 2006). Studies in vitro have observed that in the absence of IL-4 and IFN-γ, TGF-β, and IL-21 or IL-23 are important for the induction of RORγt expression, and that the proinflammatory cytokines IL-1β or IL-6 can trigger IL-17 cytokine production (Mangan et al., 2006; Veldhoen et al., 2006; Wilson et al., 2007; Manel et al., 2008; Volpe et al., 2008; Yang et al., 2008). During Th cell differentiation, transcription factors T-bet and GATA-3 are mutually inhibitory for TH2 and TH1 differentiation, respectively. Although T-bet is a negative regulator for TH17 differentiation, enforced expression of GATA-3 does not restrain the differentiation of IL-17–producing T cells, despite the loss of TH17-mediated pathology (van Hamburg et al., 2008). Additionally, an indispensible transcription factor for TH2 differentiation, IFN regulatory factor 4 (IRF4), is also required for TH17 cell development, suggesting that plasticity between the development and maintenance of TH2 and TH17 cells may exist (Brüstle et al., 2007).
The discovery of IL-17–producing T cells has added an additional layer of complexity to the regulation of allergic inflammation. In asthmatic patients, IL-17 expression is increased in the lungs, sputum, bronchoalveolar lavage (BAL) fluids, or sera, and the severity of airway hypersensitivity in patients correlates with IL-17 expression level (Molet et al., 2001; Chakir et al., 2003). IL-17 and IL-17F can provoke neutrophil infiltration in mouse models of asthma in an antigen-specific fashion (Hellings et al., 2003), probably by inducing lung structural cells to secrete proinflammatory cytokines and chemokines such as TNF, IL-1β, G-CSF, and IL-6 and CXCL1/Gro-α, CXCL2, and CXCL8/IL-8, respectively (Jovanovic et al., 1998; Laan et al., 1999; Ye et al., 2001; Jones and Chan, 2002). Importantly, IL-17R–deficient mice exhibit both reduced neutrophil and eosinophil recruitments (Ye et al., 2001), whereas IL-17A−/− mice exhibited reduced TH2 responses to antigen sensitization (Nakae et al., 2002). Although these studies demonstrate the importance of IL-17–producing cells in driving the exacerbation of allergic inflammation, the identity and characteristics of these cells during type-2 dominant immune response remain unclear. Herein, we demonstrate that a subset of TH2 cells in both mice and humans is capable of producing large amounts of the proinflammatory cytokines IL-17 and IL-22, in addition to classical TH2 cytokines. We suggest that IL-17–producing CD4+ TH2 cells may be a unique subset of lung resident TH2 memory/effector cells with additional inflammatory properties and contribute to the exacerbation of chronic allergic asthma.
RESULTS
A novel subset of human TH2 memory/effector cells produces IL-17
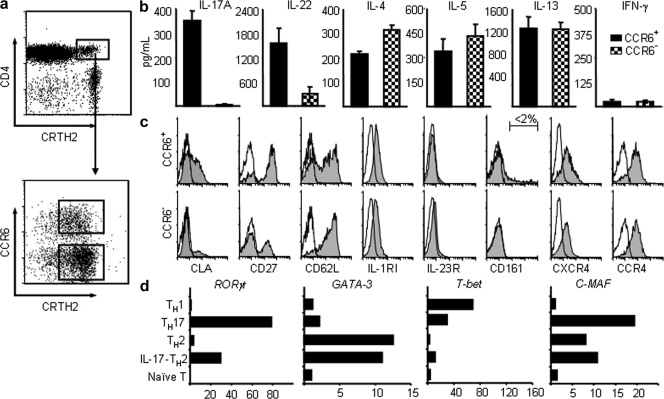
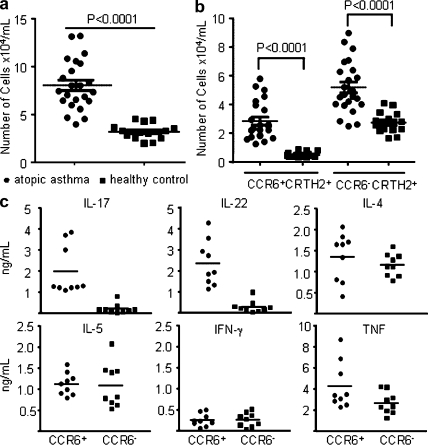
CCR6 was a useful marker for the identification of IL-17–producing cells in the human memory T cell pool (Acosta-Rodriguez et al., 2007). CRTH2 were reported to be the most reliable marker to identify human CD4+ TH2 memory cells (Cosmi et al., 2000; Wang et al., 2006). The characterization of human CRTH2+CD4+ TH2 cells led us to identify a distinctive subset of TH2 cells expressing a high level of CCR6 (Fig. 1 a). To investigate whether the CCR6+ subset of CRTH2+CD4+ TH2 cells also display features of TH17 cells, freshly purified CCR6+ and CCR6− subsets of CRTH2+CD4+ TH2 cells from the peripheral blood were stimulated with anti-CD3/CD28 mAbs for 24 h, and their secreted cytokines were examined using ELISA. Both subsets of CRTH2+CD4+ TH2 cells produced classical TH2 cytokines IL-4, IL-5, and IL-13, but not IFN-γ; notably, only the CCR6+ subset of TH2 cells could produce TH17 cytokines IL-17 and IL-22 (Fig. 1 b). Both subsets exhibit a memory T cell phenotype featuring the expression of CD45RO, CCR7, CD27, and CD62L, as well as CCR4 and CXCR4, the chemokine receptors expressed by TH2 cells (Fig. 1 c and not depicted). Notably, both TH2 cell subsets express low levels of surface IL-1RI, but do not express IL-23R; additionally, very few of them express CD161 (<2%), which is the marker of human TH17 precursors (Fig. 1 c; Acosta-Rodriguez et al., 2007; Annunziato et al., 2007; Cosmi et al., 2008). These findings reveal a novel subset of human TH2 cells that are capable of producing inflammatory IL-17 cytokine and expresses chemokine receptors for homing to the skin and other mucosal tissues.
Figure 1.
Phenotypic and functional characteristics of CRTH2+CD4+ TH2 cell subsets. (a) Enriched human CRTH2hiCD4+ T cells could be divided into two subsets based on their surface expression of CCR6. Purified resting CCR6+ and CCR6− of CRTH2+CD4+ TH2 cells were stimulated with anti-CD3/CD28 mAbs for the measurements of cytokines in the culture supernatants by ELISA (b) or analyzed for the expression of other surface markers (c). Filled histograms represent the staining of indicated cell subset with markers shown below the histogram; open histograms represent the isotype. (d) cDNA templates made from the indicated T cell subsets were prepared as described in the Materials and methods. Expression levels of indicated genes that are involved in T cell differentiation were measured by real-time PCR as described in methods. Fold differences in gene expression level between cell types marked in the left panel are indicated in horizontal axis. The results in a–d are from separate experiments. Data represent the mean (±SD) of five experiments (b). Data are from one of three independent experiments (c and d). IL-17-TH2, IL-17–producing TH2 cells.
Because RORγt expression is essential for the generation of the classical IL-17–producing CD4+ T cells (Ivanov et al., 2006), we analyzed the expression of RORγt transcript in the following cell populations: CCR6+CRTH2+ IL-17–producing TH2 cells, classical CCR6−CRTH2+ TH2 cells, CCR6+CRTH2− TH17 cells (Acosta-Rodriguez et al., 2007), in vitro-generated TH1 cells, and CD4+CD45RO− naive T cells. Both TH17 and IL-17–producing TH2 cells were found to express a significant level of RORγt transcript; in contrast, classical TH2, TH1, or naive T cells did not (Fig. 1 d). Although both IL-17–producing TH2 and classical TH2 cells expressed the master transcription factor for TH2 development, GATA3, these TH2 cell subsets did not express T-bet, the transcription factor for TH1 development (Fig. 1 d). These data demonstrate that CCR6+CRTH2+ IL-17–producing TH2 cells can concurrently express RORγt and GATA3, which are the master transcription factors for TH17 cells and TH2 cells, respectively.
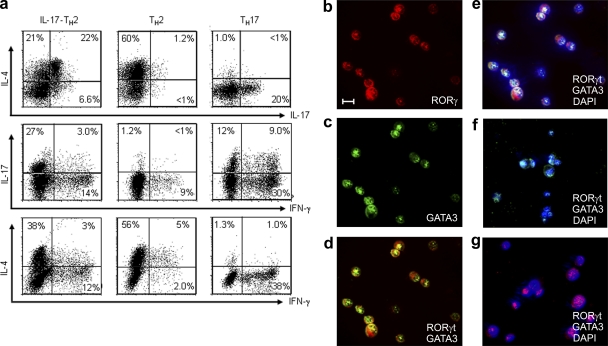
To further test whether IL-17 and IL-4 are concomitantly produced at the single-cell level, IL-17–producing TH2 cells, classical TH2 cells, and TH17 cells were isolated and maintained with homeostatic cytokines (IL-7 and IL-15) for 3 d before intracellular cytokine analyses. We found that a significant fraction of IL-17–producing TH2 cells produced cytokines IL-4 and IL-17 concurrently, whereas classical TH2 cells produced mostly IL-4, but little IL-17 (Fig. 2 a). Conversely, the classical TH17 cells produced mostly IL-17, but little IL-4 (Fig. 2 a). To examine whether GATA3 and RORγt can be coexpressed at single-cell level, the three aforementioned T helper cell subsets were activated by anti-CD3 mAb before immunofluorescence analyses for expression of GATA3 and RORγt. Notably, IL-17–producing TH2 cells were found to coexpress GATA3 and RORγt in the nucleus (Fig. 2, b–e). Corresponding to their cytokine production pattern, classical TH2 cells expressed only GATA3 and not RORγt (Fig. 2 f), whereas classical TH17 cells expressed only RORγt and not GATA3 in their nucleus (Fig. 2 g). These results demonstrate that IL-17–producing TH2 cells express both GATA3 and RORγt transcription factors.
Figure 2.
The novel human CCR6+CRTH2+CD4+ TH2 cell subset displays features of both TH2 and TH17 cell lineages. Sorted CCR6+ (b-e) and CCR6− (f) subsets of CRTH2+CD4+ TH2 and CCR6+CRTH2−CD4+ TH17 (g) cells were cultured in the homeostatic cytokines IL-7/IL-15 in the presence of anti–IL-4 and anti–IFN-γ mAbs for 3 d. Expanded cells were collected and restimulated with PMA plus ionomycin for analysis of intracellular cytokine production (a) or activated with anti-CD3 before two-color immunofluorescent staining with anti–human RORγt (red) or GATA-3 (green) mAb (b-g). Cell nuclei were identified with DAPI (blue). Numbers within the quadrants indicate the percentage of cultured cells that stained positive for each respective cytokine. Bars, 5 µm. Data are from one of three independent experiments (a–g). IL-17-TH2, IL-17–producing TH2 cells.
Proinflammatory cytokines induce classical TH2 memory cells to produce IL-17
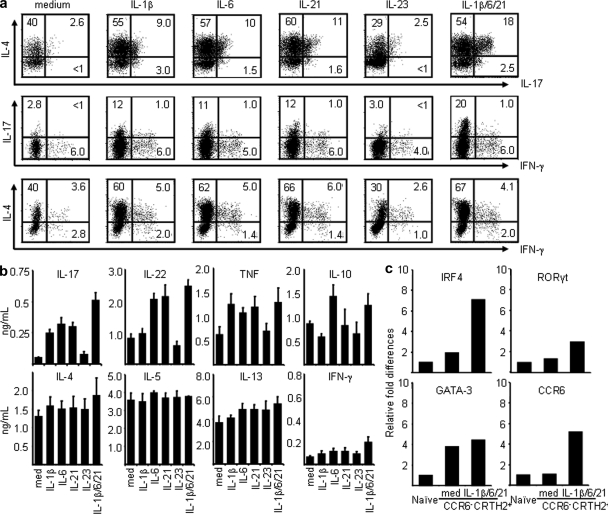
Several studies demonstrated that TH1-polarizing signals could reprogram committed TH2 memory/effector cells to produce IFN-γ, suggesting the existence of plasticity within committed TH2 cells (Brugnolo et al., 2003; Filì et al., 2006; Hegazy et al., 2010). To test whether classical TH2 cells have the potential to produce IL-17 cytokine under TH17-polarizing signals, purified classical CCR6−CRTH2+ TH2 cells were cultured with homeostatic cytokines (IL-7 and IL-15) plus anti–IL-4 and anti–IFN-γ mAbs in the absence or presence of select TH17-polarizing cytokines for 6 d. As shown in Fig. 3 (a and b), we found that the TH17 polarizing cytokine IL-1β, IL-6, or IL-21, but not IL-23, is capable of inducing the classical TH2 cells to produce significant amounts of IL-17 as determined by ELISA and intracellular cytokine analyses. Notably, the treatment combination of IL-1β, IL-6, and IL-21 together is most effective at inducing the classical TH2 cells to produce IL-17 and IL-22 cytokines (Fig. 3, a and b), possibly via up-regulation of the expression of TH17-associated transcription factors IRF4 and RORγt, as well as CCR6 transcripts (Fig. 3 c). Collectively, these results suggest that committed TH2 memory/effector cells possess the plasticity to become IL-17–producing cells after stimulation with proinflammatory cytokines.
Figure 3.
Proinflammatory stimuli induce classical CD4+ TH2 cells to produce IL-17 cytokine. Sorted CCR6−CRTH2+CD4+ TH2 cells were maintained with homeostatic cytokines IL-7/IL-15 plus anti–IL-4 and anti–IFN-γ mAbs in the presence or absence of the indicated proinflammatory cytokines for 6 d. Expanded cells were restimulated with PMA plus ionomycin for the analysis of intracellular cytokine production (a) or anti-CD3/CD28 for measurement of cytokines in the culture supernatants by ELISA (b) or were used as cDNA templates for the indicated gene expression analysis by real-time PCR (c). Fold differences in gene expression level between cell/treatment groups marked in the horizontal axis are indicated in the left panel. Data represent the mean (±SD) of five experiments (b). Data are from one of three independent experiments (a and c).
IL-17 and TH2 cytokines synergistically induce chemokine production
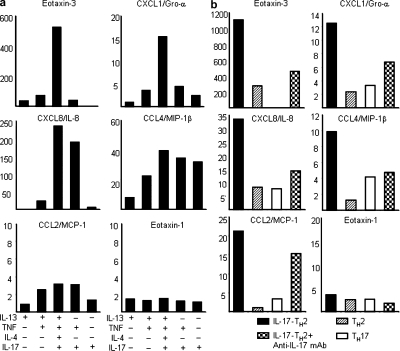
Severe asthma is often associated with elevated IL-17 expression and intense infiltration of neutrophils and eosinophils in the airway of atopic patients (Kolls et al., 2003). To address whether the combination of IL-17 and TH2 cytokines can synergistically induce chemokine production, which would enhance the recruitment of inflammatory cells, we treated normal human bronchial epithelial cells or bronchial epithelial cell lines (BEAS-2) with various combinations of IL-17 and TH2 cytokines. Compared with the treatments of IL-13, TNF, IL-17, IL-13 with TNF, or IL-17 with TNF, the combination of IL-17, TH2 cytokines, and TNF induced the greatest increase in gene expression of eotaxin-3 (>500-fold), IL-8 (>200-fold), MIP-1β (>40-fold), and Gro-α (>15-fold), but not MCP-1 or eotaxin-1, in the normal human bronchial epithelial cells and bronchial epithelial cell lines (BEAS-2; Fig. 4 a and not depicted). In a parallel experiment, we examined the effect of supernatants collected from activated IL-17–producing CD4+CCR6+CRTH2+ TH2, classical CD4+CCR6−CRTH2+ TH2, or CD4+CCR6+CRTH2− TH17 cells on the induction of chemokine gene expression in normal human bronchial epithelial cells. Compared with the supernatants collected from activated classical TH2 or TH17 cells, we found that the cytokine milieu secreted by IL-17–producing TH2 cells was the most effective in inducing the up-regulation of eotaxin-3 (>1,000-fold), IL-8 (>30-fold), Gro-α (>12-fold), MCP-1 (>20-fold), and MIP-1β (∼10-fold), but not eotaxin-1 gene expression in normal bronchial epithelial cells (Fig. 4 b). Notably, neutralizing anti–IL-17 mAbs can block the up-regulation of select chemokine genes induced by treatment with supernatant from IL-17–producing TH2 cells (Fig. 4 b). These results suggest that the IL-17 and TH2 cytokines produced concurrently by IL-17–producing TH2 cells can selectively enhance the expression of pro-allergic chemokine genes in lung epithelial cells, particularly eotaxin-3.
Figure 4.
Combinations of IL-17 and TH2 cytokines induce profound up-regulation of chemokine gene expression. cDNA templates made from cultured normal human bronchial epithelial cells treated with or without the indicated cytokines (a) or supernatants collected from indicated T helper subsets activated by anti-CD3/CD28 mAbs for 24 h in the presence or absence of anti–IL-17 neutralizing antibodies (b). Expression levels of indicated chemokine genes were measured by real-time PCR as described in the Materials and methods. Fold differences in gene expression level between treatment groups marked in the horizontal axis are indicated in the left panel. Data are from one of three independent experiments (a and b). IL-17-TH2, IL-17–producing TH2 cells.
Increased frequency of IL-17–producing TH2 cells in patients with atopic asthma
Previous studies have showed that CRTH2+CD4+ TH2 cells circulate in the peripheral blood of all healthy subjects tested, ranging from 2–4% of total CD4+ T cells, and that the frequency of these cells is elevated in patients with atopic dermatitis (Cosmi et al., 2000). To examine whether an increased frequency of circulating IL-17–producing TH2 cells is associated with patients with atopic asthma, 39 subjects were recruited for the study (23 subjects with atopic asthma and 16 healthy control subjects). Subject characteristics are summarized in Table I. The number of total CD4+CRTH2+ TH2 memory/effector cells and of CCR6+ and CCR6− of CD4+CRTH2+ TH2 cell subset cells from the peripheral blood of subjects were analyzed and compared by flow cytometry. Consistent with a previous study (Cosmi et al., 2000), the number of circulating total CD4+CRTH2+ TH2 memory/effector cells in subjects with atopic asthma was significantly higher than that of healthy controls (8.05 ± 2.58 × 104 [n = 23] vs. 3.22 ± 0.76 × 104 [n = 16], mean ± SD; Fig. 5 a). Notably, the number of circulating CCR6+CD4+CRTH2+ IL-17–producing TH2 cells in subjects with atopic asthma is 5.8-fold higher than that found in the healthy donors (2.84 ± 1.33 vs. 0.49 ± 0.19 × 104, mean ± SD), whereas the number of classical CCR6−CD4+CRTH2+ TH2 cells is 1.9-fold higher than that in the healthy donors (5.19 ± 2.73 vs. 2.73 ± 0.71 × 104, mean ± SD; Fig. 5 b). Purified CCR6+CD4+CRTH2+ TH2 cells obtained from asthma patients (n = 9) produced large amounts of inflammatory cytokines, IL-17, IL-22, and TNF, together with classical TH2 cytokines; in contrast, isolated CCR6−CD4+CRTH2+ TH2 cells produced only TH2 cytokines and a lesser amount of TNF (Fig. 5 c). These results suggest that patients with atopic asthma may have increased frequency of inflammatory IL-17–producing TH2 cells in their blood.
Table I.
Characteristics of the study population
| Charactristics | Atopic asthma subjects | Healthy control subjects |
| n = 23 | n = 16 | |
| Age, yr | 50 (27–65) | 34 (24–46) |
| Sex (male/female) | 9/14 | 10/7 |
| Onset of asthma (childhood/adulthood) | 8/17 | – |
| FEV1, percentage predicted | 68 (31–98) | 107 (102–121) |
| Percentage of PEF variability | 20 (12–69) | ND |
| Skin test positive | 23 (100%) | ND |
Data are presented as median with interquartile ranges. FEV1, forced expiratory volume in the first second; PEF, peak expiratory flow; ND, not done.
Figure 5.
An increased number of circulating IL-17–producing TH2 cells is associated with patients with allergic asthma. Total CD4+ T cells from peripheral blood of healthy donors (n = 16) or subjects with allergic asthma (n = 23) were enriched and counted by hemacytometer. Cell numbers of total CRTH2+CD4+ TH2 cells (a) and of CCR6+ and CCR6− subsets of CRTH2+CD4+ TH2 cells (b) were obtained based on their the percentage within enriched CD4+ T cells by multicolor immunofluorescence analyses. (c) Sorted CCR6+ or CCR6− subsets of CRTH2+CD4+ TH2 cells from subjects with atopic asthma (n = 9) were restimulated with anti-CD3/CD28 mAbs for 24 h before measurement of the secreted cytokines in the supernatants by ELISA.
IL-17–producing TH2 cells are induced in inflamed lung in vivo
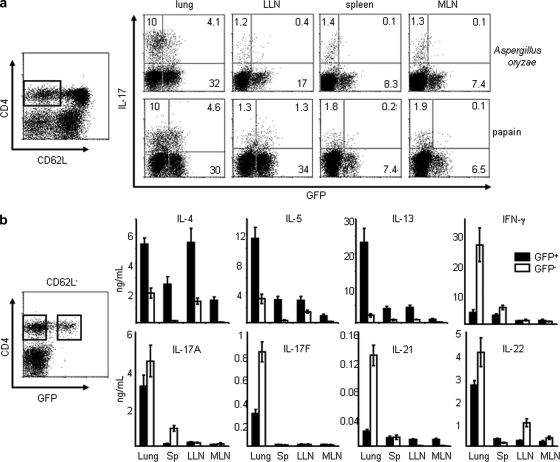
To further establish the function of IL-17–producing TH2 cells in the pathogenesis of asthma in vivo, we used a previously described mouse model of allergic lung diseases by challenging IL-4-eGFP knock-in (4GET) mice six times intranasally with Aspergillus oryzae or papain (Henderson et al., 1996). 1 d after the last challenge, CD4+CD62L−CD44hi memory/effector T cells isolated from lung or other lymphoid tissues were stimulated with PMA and ionomycin for intracellular cytokine analyses. As shown in Fig. 6 a, two populations of the infiltrating CD4+ cells in the lung of mice challenged with either allergen Aspergillus oryzae or papain expressed either IL-4 (GFP+; ∼36%) or IL-17 (∼10%), indicative of the conventional TH2 and TH17 cells, respectively. Notably, a third population (∼4%) in inflamed lung produced both IL-4 (GFP+) and IL-17 cytokines (Fig. 6 a). Very few IL-4/IL-17–double-producing T cells were detected in the lung draining lymph nodes, spleen, or intestine mesenteric lymph nodes, although a significant number of conventional IL-4+ (GFP+)/IL-17– TH2 or TH17 cells could be observed in these lymphoid organs (Fig. 6 a). ELISA analyses further showed that GFP+CD4+ TH2 cells isolated from inflamed lung concomitantly produced the classical TH2 cytokines IL-4, IL-5, and IL-13 and the inflammatory TH17 cytokines IL-17A, IL-17F, and IL-22 after restimulation with anti-CD3/CD28 mAbs for 24 h (Fig. 6 b). In contrast, GFP+CD4+ TH2 T cells isolated from spleen, lung draining lymph nodes, or intestine mesenteric lymph nodes could produce moderate levels of TH2 cytokines, but very low levels of IL-17A, IL-17F, and IL-22 (Fig. 6 b). In addition, GFP−CD4+ non-TH2 cells isolated from inflamed lung, but not other lymphoid organs, produced large amounts of IL-17A, IL-17F, IL-22, IL-21, and IFN-γ, but very little of IL-4, IL-5, and IL-13, indicative of the classical TH17 or TH1 cell subsets (Fig. 6 b). These data suggest that the IL-17/IL-4–double- producing TH2 cells were induced selectively in inflamed lung, but not in the draining lymph nodes or other lymphoid tissues in this mouse model of asthma.
Figure 6.
The induction of IL-17–producing TH2 cells occurred in the inflamed lung after allergen exposure. 4GET mice (n = 4) were challenged with indicated allergens plus OVA intranasally every other day for a total of six times before sacrifice. Sorted total (a) or GFP+ and GFP− subsets (b) of CD4+CD62L−CD44hi memory/effector T cells from lung or indicated lymphoid tissues were restimulated with PMA plus ionomycin for the analysis of intracellular cytokine production (a) or with anti-CD3/CD28 mAbs for 24 h before measurement of cytokines in the supernatants by ELISA (b). Data are from one of three independent experiments (a and b). Data represented as the mean (±SD); four mice per group. LLN, lung draining LNs; MLN, intestine mesenteric LNs.
IL-17–producing TH2 cells are increased in inflamed lung during chronic phase
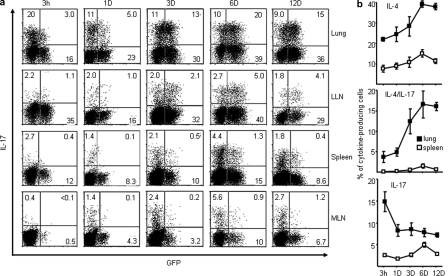
Allergen-specific TH2 memory cells that reside in lung during disease remission are the principle cell type responsible for the exacerbation of allergic asthma (Epstein, 2006). To characterize lung-resident memory T cells after remission, we analyzed intracellular cytokine production by CD4+CD62L− memory/effector T cells in the lung and other lymphoid tissues from mice that rested for different periods of time after the last allergen challenge. 3 h after the last challenge, the majority of lung CD4+CD62L− memory/effector T cells were found to be the conventional IL-17+IL-4−(GFP−) TH17 cells (20%) or the classical IL-17–IL-4+(GFP+) TH2 cells (16%); only 3% of lung memory/effector CD4+ T cells were IL-17–producing TH2 cells (IL-17+GFP+; Fig. 7 a). Notably, the frequency of conventional TH17 cells that reside in the lung declined rapidly to ∼10% 1 d after the last challenge. Conversely, the frequency of the IL-17–producing TH2 cells accumulated to ∼20% of total lung resident CD4+CD62L− memory/effector T cells in mice that rested for 6 d, and this increase was maintained for at least 12 d after the last challenge (Fig. 7, a and b). In addition, IL-17–producing TH2 cells could be found in the lung draining lymph nodes (>5%) and spleen (>1.3%), but not in the mesenteric lymph nodes 6 d after the last challenge, suggesting that some of IL-17–producing TH2 cells migrate out of the inflammatory sites into the periphery (Fig. 7, a and b). These results suggest that upon exposure to allergens, the influx of TH17 cells into inflammatory lung can occur rapidly within 3 h, but that the IL-17–producing TH2 cells persist in lung and may be the dominant IL-17–producing T cells during the chronic phase of inflammation.
Figure 7.
Inflammatory IL-17–producing TH2 cells persist in the inflamed lung during the chronic phase of allergic asthma. 4GET mice (n = 4) were challenged with Aspergillus Orazae plus OVA intranasally every other day for a total of six times (a and b). After the last challenge, mice rested for the indicated time frame (a and b; horizontal axis) before sacrifice. Purified CD4+CD62L−CD44hi memory/effector T cells from lung or other lymphoid tissues were restimulated with PMA plus ionomycin for the analysis of intracellular cytokine production (a). The percentage of indicated cytokine producing cells within the total CD4+ memory/effector T cell pool were numerated as shown on the left axis (b). Data are representative of two independent experiments. Data represented as the mean (±SD); four mice per group.
IL-17–producing TH2 cells exacerbate allergic inflammation
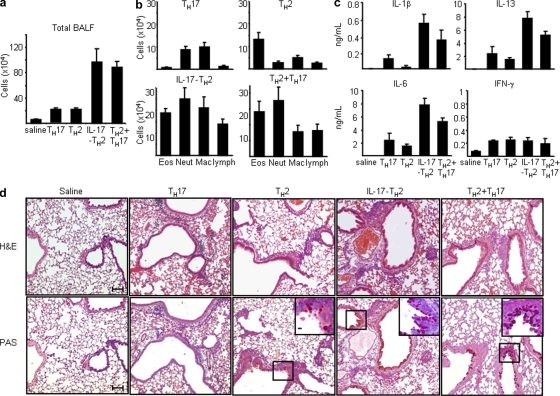
Because the expression level of IL-17 is associated with the severity of asthma in patients (Molet et al., 2001; Chakir et al., 2003), we assessed the relative roles of the classical TH17, classical TH2, and IL-17–producing TH2 cells in the pathogenesis of allergic asthma. To generate OVA-specific IL-17–producing TH2 or classical TH2 cells, splenic CD4+ TH2 cells were first isolated from OVA/alum adjuvant–sensitized 4GET mice carrying OVA-specific DO11.10 TCR transgene (DO11.10X4GET). OVA-specific IL-17–producing TH2, or classical TH2 cells, were then further induced by co-culturing splenic CD4+ TH2 cells with APCs pulsed with OVA peptides in the presence of IL-1β, IL-6, IL-23, and anti–IFN-γ or IL-4 and anti-IFN-γ, respectively. OVA-specific TH17 cells were generated by co-culturing splenic CD4+ T cells from naive DO11.10X4GET mice with APC pulsed with OVA peptides in TH17-polarizing conditions. BALB/c mice were transferred intravenously with a single OVA-specific, IL-17–producing TH2, classical TH2, or TH17 cell subset, with both the classical TH2 and TH17 cell subsets, or with saline only; mice were then challenged with OVA intranasally once a day for 2 d. As shown in Fig. 8 a, bronchoalveolar lavage fluid (BALF) from mice transferred with OVA-specific, IL-17–producing TH2 cells or both the classical TH2 and TH17 cell subsets contained about threefold more infiltrating cells than those from mice transferred with only OVA-specific classical TH2 or TH17 cells or saline alone. Moreover, we found that mice transferred with OVA-specific IL-17–producing TH2 cells or both the classical TH2 and TH17 cell subsets exhibited markedly enhanced recruitments of eosinophils, neutrophils, macrophage, and lymphocytes into the airway, whereas mice transferred with only OVA-specific classical TH2 or TH17 cells showed a moderate influx of eosinophils or neutrophils and macrophages into the airway, respectively, after intranasal OVA challenges (Fig. 8 b). Interestingly, a significant increase of inflammatory cytokines, IL-1β and IL-6, and a moderate increase of IL-5 and IL-13, but not IFN-γ production, were detected in the BALF of mice transferred with OVA-specific, IL-17–producing TH2 cells or both the classical TH2 and TH17 cell subsets compared with those in BALF of mice transferred with OVA-specific classical TH2 or TH17 cells or saline only (Fig. 8 c). Histological analyses of lungs from mice transferred with OVA-specific, IL-17–producing TH2 cells or both the classical TH2 and TH17 cell subsets exhibited markedly enhanced peribronchial inflammation with infiltrated eosinophils and neutrophils, more prominent mucin production, and goblet cell hyperplasia compared with lungs from mice transferred with conventional TH2 or TH17 cells or saline only (Fig. 8 d). These results suggest that during the allergen recall response, antigen-specific IL-17–producing TH2 cells may have additional inflammatory properties, similar to those of the combined TH2 and TH17 cells, which promote the infiltration of heterogeneous leukocytes and exacerbate the immunopathology of allergic asthma.
Figure 8.
Antigen-specific inflammatory IL-17–producing TH2 cells promote the exacerbation of allergic asthma. Five groups of BALB/c mice were intranasally challenged once a day for 2 d with OVA 24 h after being adoptive transferred with 0.9% saline as a control or OVA-specific IL-17–producing TH2, classical TH2, classical TH17, or classical TH2 and TH17 cells generated in vitro, as described in Materials and methods. BALF of individual mice of each group were collected for the measurement of total cell counts (a) and differential cell counts (b), indicating that the total numbers of individual inflammatory cells in each group or (c) concentrations of indicated cytokines by ELISA. (d) Histological analysis of representative lung bronchovascular bundles stained with hematoxylin and eosin (H&E; top) or stained with periodic acid Schiff (PAS; bottom). The insets at the corner depict higher magnification images of the airway epithelium stained with PAS, showing that much more abundant mucus-producing cells (pink cytoplasm) are lining the airway epithelium of mice receiving indicated T helper cell subsets. Data are representative of three independent experiments. Data represented as the mean (±SD); four mice per group. Bars: (capped) 100 µm; (uncapped) 10 µm.
DISCUSSION
Severe asthma is a heterogeneous disorder with distinct types of inflammatory processes. Although the discovery of IL-17–producing T cells has shed light on the understanding of the underlying mechanisms that contribute to the heterogeneity and severity of asthma, the identity of IL-17–producing T cells in allergic diseases, including asthma remain elusive. In this study, we identified a distinct population of human IL-17–producing TH2 cells characterized by (a) the capability of concomitantly producing the classical TH2 cytokines IL-4, IL-5, and IL-13, and the inflammatory TH17 cytokines IL-17 and IL-22; (b) dual expression of the TH17-transcription factor RORγt and the TH2-transcription factor GATA3; and (c) surface expression of CD45RO, the chemokine receptors CCR4 and CXCR4, and CLA, suggesting a memory phenotype and readiness to migrate into inflammatory mucosa sites. Notably, the number of circulating IL-17–producing TH2 cells is significantly increased in patients with atopic asthma. Moreover, we demonstrated that the mouse counterpart of IL-17–producing CD4+ TH2 cells are selectively induced in the inflamed lung and are the dominant IL-17–producing T cells during the chronic phase of asthma in vivo. Mice transferred with antigen-specific, IL-17–producing TH2 cells exhibited profound immunopathological allergic inflammation after recall response. These data suggest that the IL-17–producing CD4+ TH2 cells may represent the inflammatory TH2 cells that promote the pathophysiology of asthma.
The TH17 cell lineage possesses a unique genetic program and is described as an additional branch of the T helper cell subsets (Harrington et al., 2005; Park et al., 2005b; Ivanov et al., 2006). Studies in vitro demonstrated that the absence of TH2 and TH1 cytokines is the prerequisite for TH17 cell differentiation from naive T cells (Veldhoen et al., 2006) and that the TGF-β, IL-21, or IL-23, as well as the proinflammatory cytokines IL-1β or IL-6, are important for the induction of IL-17 cytokine production (Mangan et al., 2006; Veldhoen et al., 2006; Wilson et al., 2007; Manel et al., 2008; Volpe et al., 2008; Yang et al., 2008). However, recent findings suggest that some cytokines, such as IL-1β (Tillie-Leblond et al., 1999; Nakae et al., 2003; Chung et al., 2009) and IL-21 (Fröhlich et al., 2007; Nurieva et al., 2007; Leonard et al., 2008; Yang et al., 2008), and the transcription factor IRF4 (Rengarajan et al., 2002; Brüstle et al., 2007; Honma et al., 2008) are important for the development of both TH2 and TH17 immune responses, suggesting that the plasticity between the development and maintenance of TH2 and TH17 cells may exist. Our finding of the novel subset of CD4+ TH2 memory/effector cells capable of producing IL-17 supports this hypothesis. Notably, we showed that the proinflammatory cytokine IL-1β, IL-6, and IL-21 could directly induce the up-regulation of IRF4 and RORγt gene expression and the production of IL-17 in classical TH2 memory/effector cells in vitro. At the early phase of allergic inflammation in an animal model of allergic lung diseases, IL-17–producing TH2 cells could primarily be found in the inflamed lung along with other T helper subsets, including classical TH2 and TH17 cells, and some TH1 cells. Notably, these resident IL-17–producing TH2 cells persist in inflamed lung as the dominant IL-17–producing T cells at the chronic stage of airway allergic inflammation. Collectively, these observations suggest that substantial plasticity exists within CD4+ TH2 memory/effector cells and that this plasticity may be controlled by local inflammatory cues. In theory, naive CD4+ T cells or TH17 cells may also have the potential to become IL-4/IL-17–dual-producing cells. It is possible that some naive T cells may not have to undergo the TH2 or TH17 differentiation pathway and may become IL-4/IL-17 double producers through subsequent regulation from unique microenvironments in vivo. Recent studies have reported that TH17 cells have the plasticity to become other cell lineages (Lee et al., 2009; Zhu and Paul, 2010). To test whether TH2-polarizing signals could induce TH17 cells to become IL-4/IL-17 double producers, in our pilot studies, we found that TH2-polarizing stimuli (thymic stroma lymphopoietin–activated DCs or IL-4) could induce the freshly isolated human CCR6+CRTH2− TH17 cells to produce IL-4, but shut down their IL-17 production in vitro (unpublished data). However, during the revision of this study, findings from the characterizations of human CD4 T cell clones point to the possibility that TH17 cells may have the potential to become IL-4/IL-17 dual-producing cells (Cosmi et al., 2010). Understanding the cellular origin and the underlying mechanisms that drive the induction of IL-4/IL-17 double producers during allergic inflammation is the basis for further investigations.
The severity of asthma is correlated with the level of IL-17 cytokine found in the lung, sputum, BALF, or serum of patients (Molet et al., 2001; Chakir et al., 2003). One of the major functions of the cytokine IL-17 during asthmatic reactions is to orchestrate the sustained neutrophilic mobilization (Kolls et al., 2003; Lindén et al., 2005). However, the mixed eosinophilic, neutrophilic, and granulocytic infiltrations with greatly increased total cell number are often observed in the sputum of patients in some subtypes of severe allergic asthma (Simpson et al., 2006), and the underlying cellular and molecular mechanisms remain unknown. One of the mechanisms involved in the IL-13–mediated pathophysiological features of asthma is the induction of chemokine production by airway structural cells (Zimmermann et al., 2003). We found that the combination of inflammatory IL-17 and TH2 cytokines IL-4 and IL-13 or the use of a cytokine milieu produced by IL-17–producing TH2 cells have profound synergistic effects on the induction of various chemokine genes in primary lung bronchial epithelial cells, such as MIP-1β, MCP-1, Gro-α, IL-8, and eotaxin-3, which is particularly effected. The effects of IL-17–producing TH2 cells on promoting the recruitments of inflammatory leukocytes were further substantiated in this animal model of asthma in vivo. Transfer of antigen-specific, IL-17–producing TH2 cells triggered much stronger influx of heterogeneous leukocytes, including neutrophils, eosinophils, macrophage, and lymphocytes, which resulted in profound goblet hyperplasia as well as elevated mucin production after antigen sensitization. In contrast, mice transferred with conventional TH2 or TH17 cells exhibited fewer airway infiltrations of eosinophils or neutrophils, respectively, and limited pathophysiological features. The finding that the frequency of circulated IL-17–producing TH2 cells is significantly elevated in atopic asthma patients further highlights the potential role of this novel cell subset in the exacerbation of allergic diseases. Future analyses on the frequency and characteristics of the inflammatory IL-17–producing TH2 cells in patients with different subtypes of asthma may facilitate the understanding of the heterogeneity and severity of allergic asthma.
Patients with severe allergic asthma during remission often have elevated nitric oxide breath levels that are indicative of their persistent lung inflammation and are possibly mediated by resident allergen-specific TH2 memory cells (Yurovsky et al., 1998; Bates and Silkoff, 2003). The identification of the TH17 cell lineage and its confounding roles in the pathogenesis of allergic inflammation have has further unveiled the complexity of atopy (Nakae et al., 2002; Schnyder-Candrian et al., 2006) and raises new questions on how TH17 cells and TH2 cells cooperate to mediate the severity and heterogeneity of allergic asthma. The temporal recruitments and interplay between these two T helper subsets have been suggested as the cause of the heterogeneity in the pathology of severe asthma (Larché et al., 2003; Schmidt-Weber et al., 2007). In an animal model of asthma, we showed that the influx of TH17 cells could occur within the first 3 h after the last challenge; however, the majority of IL-17–producing T cells persisted in the lung from day 3 after the last challenge and were found to express low-to-high levels of GFP (IL-4) that were indicative of their TH2 characteristics. Our findings suggest that the rapid influx of TH17 cells may be part of the inflammatory processes triggered by the injured epithelial cells or altered innate immunity induced by environmental stimuli or invaded pathogens at the acute phase of allergic asthma. Antigen-specific classical TH2 or IL-17–producing TH2 cells that respond to allergen sensitization may reside in the lung and contribute to the persistence and progression of chronic allergic asthma. Designing curative therapy for chronic allergic diseases in a phase-specific manner may require not only the understanding of the factors that regulate the balance for the development of various T helper subsets, but also their temporal sequences and potential interactions in the induction of immunopathology of allergic asthma.
MATERIALS AND METHODS
Cell culture and isolation of human TH2 and TH17 memory cell subsets.
This study was approved by the institutional review board for human research at The University of Texas M.D. Anderson Cancer Center (Houston, Texas). Human CD4+ TH2 memory/effector T cells were enriched by the depletion of other lineage cells using microbeads and then sorted as CD4+CRTH2+CCR6+Lineage− or CD4+CRTH2+CCR6−Lineage− cells. TH17 cells were sorted as CD4+CRTH2−CCR6+ Lineage− cells with purity >99%, as previously described (Wang et al., 2006). In some experiments, purified CD4+CRTH2+CCR6− TH2 cells were cultured with 20 ng/ml IL-7 and 10 ng/ml IL-15 plus 2 µg/ml anti–IL-4 and 1 µg/ml anti–IFN-γ mAbs (R&D Systems) in the presence or absence of 10 ng/ml IL-1β, 25 ng/ml IL-6, 10 ng/ml IL-21, or 25 ng/ml IL-23 (R&D Systems), or in the combination of these cytokines for the induction of IL-17 production. Human primary normal bronchial epithelial cells (Lonza) were maintained in bronchial epithelial growth medium (Lonza), and the bronchial epithelial cell line (BEAS-2) was cultured following the instructions of American Type Culture Collection.
Analysis of cytokine production.
Freshly isolated or expanded human TH2 cell subsets or TH17 cells were stimulated with immobilized anti-CD3 (OKT3; 5 µg/ml) and soluble anti-CD28 (L293.1; 1 µg/ml) for 24 h. Collected supernatants were assessed by ELISA for IL-4, IL-5, IL-13, IL-17, IL-22, IFN-γ, IL-10, and TNF production (R&D Systems). In mouse experiments, sorted GFP+ or GFP− CD4+CD62L− memory/effector T cells, isolated from 4GET mice challenged intranasally with the allergen Aspergillus oryzae 6 times were stimulated with immobilized 5 µg/ml anti-CD3 and soluble 1 µg/ml anti-CD28 for 24 h. Collected supernatants were assessed by ELISA for IL-4, IL-5, IL-13, IL-17A, IL-17F, IFN-γ, IL-10, TNF (R&D Systems), and IL-22 production (Antigenic American). Intracellular cytokine analyses were performed using PE-IL-4, FITC-IFN-γ (BD), and APC-IL-17 (eBioscience) in human studies and PE-IL-17 (eBioscience) and APC-IFN-γ (BD) in mouse experiments.
RNA isolation and real-time quantitative PCR.
Total RNA samples from sorted or cultured cells were isolated by RNeasy kit (QIAGEN). The cDNA templates were synthesized using SuperScript II (Life Technologies). Oligonucleotide primers were selected using Primer Express 2.0 (Applied Biosystems). Real-time quantitative PCR was performed with the ABI Prism 7900 (Applied Biosystems) detection system. For the analysis of GATA3, c-maf, T-bet, NFATc1, IL-4, and IL-5 gene expression, real-time PCR probes were purchased directly from the manufacturer (Applied Biosystems). For analyses of chemokine gene expression, transcripts were amplified using an annealing temperature of 60°C and the following primers: CXCL1/Gro-α; 5′-AGGGAATTCACCCCAAGAACAT-3′ and 5′-GATGCAGGATTGAGGCAAGCT-3′; CXCL-8, 5′-CTCTTGGCAGCCTTCCTGATT-3′ and 5′-TATGCACTGACATCTAAGTTCTTTAGCA-3′; Eotaxin-3, 5′-CTGGGTGCGAAGCTATGAAT-3′ and 5′-TCTTGCCTCTTTTGGTAGTGAA-3′; and CCL-3/MIP-α, 5′-CACCTCCCGGCAGATTCC-3′ and 5′-GCCGGCTTCGCTTGGTTA-3′. For each sample, mRNA abundance was normalized to the amount of 18S rRNA or GAPDH and expressed as arbitrary units, as described previously (Wang et al., 2006).
Immunohistology.
For immunofluorescent staining, cultured human T helper subsets were activated by anti-CD3 for 6 h before being cytospun onto the slides. The tested slides were fixed and incubated with goat anti-GATA3 (Santa Cruz Biotechnology, Inc.) and mouse anti–human RORγt mAb (provided by D. Littman, Skirball Institute of Biomolecular Medicine, New York University School of Medicine, New York, NY) at room temperature for 1 h, followed by Alexa Fluor 488-F(ab’)2 fragment rabbit anti–goat IgG antibody (Invitrogen). After washing and blocking, cells were counterstained with biotinylated horse anti–mouse antibodies and visualized by Alexa Fluor 549–conjugated streptavidin (Invitrogen).
Induction and analysis of a mouse model of allergic lung diseases.
We used a previously well-established animal model of allergen-induced airway disease (Henderson et al., 1996). In brief, 4GET or DO10.11X4GET mice were anesthetized with isoflurane and subjected to intranasal inhalation of 50 µl of OVA only, Aspergillus oryzae with OVA, or Papain with OVA every other day for a total of 6 challenges. Potential endotoxin contamination was removed from OVA by endotoxin-removing gel (Thermo Fisher Scientific). After the last challenge, mice were sacrificed at various time points and CD4+CD62L− memory/effector T cells were sorted from the lung, spleen, lung draining lymph nodes, and mesenteric lymph nodes. Purified T cells were analyzed for cytokine production by intracellular cytokine staining or ELISA as described above.
Generation of OVA-specific TH subsets.
For the generation of OVA-specific TH17 cells, CD4+ T cells were obtained from splenocytes of naive DO11.10 × 4GET mice, enriched by magnetic anti-CD4 microbeads, and then cultured with irradiated CD4− spleen cells pulsed with OVA 323–339 peptide in the presence of 5 ng/ml TGF-β (Peprotech), 20 ng/ml IL-6 (Peprotech), 10 µg/ml IL-23 (R&D systems), 20 µg/ml anti–IL-4 mAb (BD), and 20 µg/ml anti–IFN-γ mAb (BD). For the generation of OVA-specific TH2 subsets, DO11.10X4GET mice were first immunized intraperitoneally with 100 µg of OVA in 2 mg of aluminum hydroxide (Thermo Fisher Scientific). OVA-specific IL-17–producing TH2 cells or conventional TH2 cells were then generated from enriched CD4+ T cells cultured with irradiated CD4− spleen cells pulsed with OVA323-339 peptide in the presence of 20 ng/ml IL-1β (R&D systems), 20 ng/ml IL-6, and 10 µg/ml IL-23 plus 20 µg/ml anti–IFN-γ mAbs or 10 ng/ml IL-4 (Peprotech) and 20 µg/ml anti-IFN-γ mAb, respectively.
Adoptive transfer experiments for antigen-induced airway inflammation.
The IL-17–producing TH2 cells, conventional TH2 cells, or TH17 cells were transferred intravenously into BALB/c mice (2 × 106 cells/mouse). Control mice received saline intravenously. 1 d after transfer, mice were intranasally challenged with OVA (50 µg/ml) every day for a total of two times. Mice were sacrificed 24 h after the last challenge. Total cell numbers or numbers of eosinophils, neutrophils, macrophage, and lymphocytes in the BALF were numerated, and the levels of IL-1β, IL-6, IL-5, IL-13, and IFN-γ in the BALF were evaluated by ELISA. For the histological analyses, individual lung were fixed in 10% buffer formalin. Hematoxylin and eosin, Giemsa, and periodic-acid Shiff staining were performed by Histology Consultation Services, Inc.
Subjects and study design.
Study participants were recruited from patients diagnosed in the Bernstein Allergy Group, and the Clinical Research Center in the Division of Allergy and Immunology at the University of Cincinnati. The study was approved by the University of Cincinnati Institutional Review Board. Patients taking any oral topical skin medication were excluded from the study. Exclusion criteria include: (a) having had an acute viral infection within at least 1 mo before the study; (b) being a smoker or ex-smoker who has had ≥10 pack per year smoking history; (c) having any unstable chronic disease (other than asthma), or (d) being pregnant. Inclusion criteria were as follows: (a) age 18–65 yr; (b) history of allergic asthma lasting for 1 yr or longer based on previous diagnosis in our clinics; (c) positive skin prick tests (wheal diameter >5 mm) to one or more of the following allergens: timothy grass pollen, ragweed, cockroach, mold, house dust mite, or cat dander in the presence of positive histamine and negative vehicle control; (d) receiving inhaled corticosteroids with or without other medications for asthma, including β2-agonists, leukotriene modifying agents or sustained release theophylline for at least 2 mo. All asthmatic subjects must have a ≥12% improvement in forced expiratory volume in the first second of exhalation (FEV1) or a fall of >20% or more in response to a provocative methacholine dose ≤10mg/ml, confirming airway hyperresponsiveness. All subjects underwent a thorough history (including an asthma control test), physical examination, weight to determine body mass index, and allergy skin prick testing. Atopy is defined by one or more positive skin prick tests to at least one common inhalant allergen.
Statistical analysis.
Data were analyzed using GraphPad Prism 5 software. Data are presented as mean value ± SD and analyzed using Student’s t test (n = 2 groups). P values of <0.05 were considered significant.
Acknowledgments
We thank Dr. Marc E. Rothenberg for critically reading the manuscript and discussions; Dr. Dan Littman for providing anti–human RORγt mAb; Dr. David Corry for suggestions; Karen Ramirez and Zhiwei He for cell sorting assistance; and Shawna Hottinger for editorial assistance.
This work was supported by the National Institute of Allergy and Inflammatory Disease grants to Y.-J. Liu (R01 AI061645-01 and U19 AI071130-01) and Y.-H. Wang (R01 AI090129-01), the Dana Foundation (Y.-J. Liu), the Sandler Foundation (Y.-J. Liu), and American Lung Association and American Academy of Asthma, Allergy, and Immunology Foundation (Y.-H. Wang).
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- BAL
- bronchoalveolar lavage
- BALF
- BAL fluid
- IRF4
- IFN regulatory factor 4
- RORγt
- retinoic acid–related orphan receptor
References
- Aarvak T., Chabaud M., Miossec P., Natvig J.B. 1999. IL-17 is produced by some proinflammatory Th1/Th0 cells but not by Th2 cells. J. Immunol. 162:1246–1251 [PubMed] [Google Scholar]
- Acosta-Rodriguez E.V., Rivino L., Geginat J., Jarrossay D., Gattorno M., Lanzavecchia A., Sallusto F., Napolitani G. 2007. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8:639–646 10.1038/ni1467 [DOI] [PubMed] [Google Scholar]
- Anderson G.P. 2008. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 372:1107–1119 10.1016/S0140-6736(08)61452-X [DOI] [PubMed] [Google Scholar]
- Annunziato F., Cosmi L., Santarlasci V., Maggi L., Liotta F., Mazzinghi B., Parente E., Filì L., Ferri S., Frosali F., et al. 2007. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 204:1849–1861 10.1084/jem.20070663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates C.A., Silkoff P.E. 2003. Exhaled nitric oxide in asthma: from bench to bedside. J. Allergy Clin. Immunol. 111:256–262 [DOI] [PubMed] [Google Scholar]
- Brugnolo F., Sampognaro S., Liotta F., Cosmi L., Annunziato F., Manuelli C., Campi P., Maggi E., Romagnani S., Parronchi P. 2003. The novel synthetic immune response modifier R-848 (Resiquimod) shifts human allergen-specific CD4+ TH2 lymphocytes into IFN-gamma-producing cells. J. Allergy Clin. Immunol. 111:380–388 10.1067/mai.2003.102 [DOI] [PubMed] [Google Scholar]
- Brüstle A., Heink S., Huber M., Rosenplänter C., Stadelmann C., Yu P., Arpaia E., Mak T.W., Kamradt T., Lohoff M. 2007. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat. Immunol. 8:958–966 10.1038/ni1500 [DOI] [PubMed] [Google Scholar]
- Chakir J., Shannon J., Molet S., Fukakusa M., Elias J., Laviolette M., Boulet L.P., Hamid Q. 2003. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J. Allergy Clin. Immunol. 111:1293–1298 10.1067/mai.2003.1557 [DOI] [PubMed] [Google Scholar]
- Chung Y., Chang S.H., Martinez G.J., Yang X.O., Nurieva R., Kang H.S., Ma L., Watowich S.S., Jetten A.M., Tian Q., Dong C. 2009. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 30:576–587 10.1016/j.immuni.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn L., Elias J.A., Chupp G.L. 2004. Asthma: mechanisms of disease persistence and progression. Annu. Rev. Immunol. 22:789–815 10.1146/annurev.immunol.22.012703.104716 [DOI] [PubMed] [Google Scholar]
- Cosmi L., Annunziato F., Galli M.I.G., Maggi R.M.E., Nagata K., Romagnani S., Nagata K., Romagnani S. 2000. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur. J. Immunol. 30:2972–2979 [DOI] [PubMed] [Google Scholar]
- Cosmi L., De Palma R., Santarlasci V., Maggi L., Capone M., Frosali F., Rodolico G., Querci V., Abbate G., Angeli R., et al. 2008. Human interleukin 17–producing cells originate from a CD161+CD4+ T cell precursor. J. Exp. Med. 205:1903–1916 10.1084/jem.20080397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosmi L., Maggi L., Santarlasci V., Capone M., Cardilicchia E., Frosali F., Querci V., Angeli R., Matucci A., Fambrini M., et al. 2010. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J. Allergy Clin. Immunol. 125:222–230 [DOI] [PubMed] [Google Scholar]
- Cua D.J., Sherlock J., Chen Y., Murphy C.A., Joyce B., Seymour B., Lucian L., To W., Kwan S., Churakova T., et al. 2003. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 421:744–748 10.1038/nature01355 [DOI] [PubMed] [Google Scholar]
- Epstein M.M. 2006. Targeting memory Th2 cells for the treatment of allergic asthma. Pharmacol. Ther. 109:107–136 10.1016/j.pharmthera.2005.06.006 [DOI] [PubMed] [Google Scholar]
- Filì L., Ferri S., Guarna F., Sampognaro S., Manuelli C., Liotta F., Cosmi L., Matucci A., Vultaggio A., Annunziato F., et al. 2006. Redirection of allergen-specific TH2 responses by a modified adenine through Toll-like receptor 7 interaction and IL-12/IFN release. J. Allergy Clin. Immunol. 118:511–517 10.1016/j.jaci.2006.05.027 [DOI] [PubMed] [Google Scholar]
- Fröhlich A., Marsland B.J., Sonderegger I., Kurrer M., Hodge M.R., Harris N.L., Kopf M. 2007. IL-21 receptor signaling is integral to the development of Th2 effector responses in vivo. Blood. 109:2023–2031 10.1182/blood-2006-05-021600 [DOI] [PubMed] [Google Scholar]
- Harrington L.E., Hatton R.D., Mangan P.R., Turner H., Murphy T.L., Murphy K.M., Weaver C.T. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123–1132 10.1038/ni1254 [DOI] [PubMed] [Google Scholar]
- Hegazy A.N., Peine M., Helmstetter C., Panse I., Fröhlich A., Bergthaler A., Flatz L., Pinschewer D.D., Radbruch A., Löhning M. 2010. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity. 32:116–128 10.1016/j.immuni.2009.12.004 [DOI] [PubMed] [Google Scholar]
- Hellings P.W., Kasran A., Liu Z., Vandekerckhove P., Wuyts A., Overbergh L., Mathieu C., Ceuppens J.L. 2003. Interleukin-17 orchestrates the granulocyte influx into airways after allergen inhalation in a mouse model of allergic asthma. Am. J. Respir. Cell Mol. Biol. 28:42–50 10.1165/rcmb.4832 [DOI] [PubMed] [Google Scholar]
- Henderson W.R., Jr., Lewis D.B., Albert R.K., Zhang Y., Lamm W.J., Chiang G.K., Jones F., Eriksen P., Tien Y.T., Jonas M., Chi E.Y. 1996. The importance of leukotrienes in airway inflammation in a mouse model of asthma. J. Exp. Med. 184:1483–1494 10.1084/jem.184.4.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgate S.T. 2007. The epithelium takes centre stage in asthma and atopic dermatitis. Trends Immunol. 28:248–251 10.1016/j.it.2007.04.007 [DOI] [PubMed] [Google Scholar]
- Honma K., Kimura D., Tominaga N., Miyakoda M., Matsuyama T., Yui K. 2008. Interferon regulatory factor 4 differentially regulates the production of Th2 cytokines in naive vs. effector/memory CD4+ T cells. Proc. Natl. Acad. Sci. USA. 105:15890–15895 10.1073/pnas.0803171105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133 10.1016/j.cell.2006.07.035 [DOI] [PubMed] [Google Scholar]
- Jones C.E., Chan K. 2002. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 26:748–753 [DOI] [PubMed] [Google Scholar]
- Jovanovic D.V., Di Battista J.A., Martel-Pelletier J., Jolicoeur F.C., He Y., Zhang M., Mineau F., Pelletier J.P. 1998. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J. Immunol. 160:3513–3521 [PubMed] [Google Scholar]
- Kolls J.K., Kanaly S.T., Ramsay A.J. 2003. Interleukin-17: an emerging role in lung inflammation. Am. J. Respir. Cell Mol. Biol. 28:9–11 10.1165/rcmb.2002-0255PS [DOI] [PubMed] [Google Scholar]
- Laan M., Cui Z.H., Hoshino H., Lötvall J., Sjöstrand M., Gruenert D.C., Skoogh B.E., Lindén A. 1999. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 162:2347–2352 [PubMed] [Google Scholar]
- Larché M., Robinson D.S., Kay A.B. 2003. The role of T lymphocytes in the pathogenesis of asthma. J. Allergy Clin. Immunol. 111:450–463, quiz :464 10.1067/mai.2003.169 [DOI] [PubMed] [Google Scholar]
- Lee J., Ho W.H., Maruoka M., Corpuz R.T., Baldwin D.T., Foster J.S., Goddard A.D., Yansura D.G., Vandlen R.L., Wood W.I., Gurney A.L. 2001. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J. Biol. Chem. 276:1660–1664 10.1074/jbc.M008289200 [DOI] [PubMed] [Google Scholar]
- Lee Y.K., Turner H., Maynard C.L., Oliver J.R., Chen D., Elson C.O., Weaver C.T. 2009. Late developmental plasticity in the T helper 17 lineage. Immunity. 30:92–107 10.1016/j.immuni.2008.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard W.J., Zeng R., Spolski R. 2008. Interleukin 21: a cytokine/cytokine receptor system that has come of age. J. Leukoc. Biol. 84:348–356 10.1189/jlb.0308149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Chen J., Huang A., Stinson J., Heldens S., Foster J., Dowd P., Gurney A.L., Wood W.I. 2000. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc. Natl. Acad. Sci. USA. 97:773–778 10.1073/pnas.97.2.773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindén A., Laan M., Anderson G.P. 2005. Neutrophils, interleukin-17A and lung disease. Eur. Respir. J. 25:159–172 10.1183/09031936.04.00032904 [DOI] [PubMed] [Google Scholar]
- Manel N., Unutmaz D., Littman D.R. 2008. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 9:641–649 10.1038/ni.1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan P.R., Harrington L.E., O’Quinn D.B., Helms W.S., Bullard D.C., Elson C.O., Hatton R.D., Wahl S.M., Schoeb T.R., Weaver C.T. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 441:231–234 10.1038/nature04754 [DOI] [PubMed] [Google Scholar]
- Molet S., Hamid Q., Davoine F., Nutku E., Taha R., Pagé N., Olivenstein R., Elias J., Chakir J. 2001. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 108:430–438 10.1067/mai.2001.117929 [DOI] [PubMed] [Google Scholar]
- Murphy C.A., Langrish C.L., Chen Y., Blumenschein W., McClanahan T., Kastelein R.A., Sedgwick J.D., Cua D.J. 2003. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198:1951–1957 10.1084/jem.20030896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae S., Komiyama Y., Nambu A., Sudo K., Iwase M., Homma I., Sekikawa K., Asano M., Iwakura Y. 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 17:375–387 10.1016/S1074-7613(02)00391-6 [DOI] [PubMed] [Google Scholar]
- Nakae S., Komiyama Y., Yokoyama H., Nambu A., Umeda M., Iwase M., Homma I., Sudo K., Horai R., Asano M., Iwakura Y. 2003. IL-1 is required for allergen-specific Th2 cell activation and the development of airway hypersensitivity response. Int. Immunol. 15:483–490 10.1093/intimm/dxg054 [DOI] [PubMed] [Google Scholar]
- Nurieva R., Yang X.O., Martinez G., Zhang Y., Panopoulos A.D., Ma L., Schluns K., Tian Q., Watowich S.S., Jetten A.M., Dong C. 2007. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 448:480–483 10.1038/nature05969 [DOI] [PubMed] [Google Scholar]
- Park H., Li Z., Yang X.O., Chang S.H., Nurieva R., Wang Y.H., Wang Y., Hood L., Zhu Z., Tian Q., Dong C. 2005a. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133–1141 10.1038/ni1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Li Z., Yang X.O., Chang S.H., Nurieva R., Wang Y.H., Wang Y., Hood L., Zhu Z., Tian Q., Dong C. 2005b. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133–1141 10.1038/ni1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengarajan J., Mowen K.A., McBride K.D., Smith E.D., Singh H., Glimcher L.H. 2002. Interferon regulatory factor 4 (IRF4) interacts with NFATc2 to modulate interleukin 4 gene expression. J. Exp. Med. 195:1003–1012 10.1084/jem.20011128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.S., Hamid Q., Ying S., Tsicopoulos A., Barkans J., Bentley A.M., Corrigan C., Durham S.R., Kay A.B. 1992. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N. Engl. J. Med. 326:298–304 10.1056/NEJM199201303260504 [DOI] [PubMed] [Google Scholar]
- Schmidt-Weber C.B., Akdis M., Akdis C.A. 2007. TH17 cells in the big picture of immunology. J. Allergy Clin. Immunol. 120:247–254 10.1016/j.jaci.2007.06.039 [DOI] [PubMed] [Google Scholar]
- Schnyder-Candrian S., Togbe D., Couillin I., Mercier I., Brombacher F., Quesniaux V., Fossiez F., Ryffel B., Schnyder B. 2006. Interleukin-17 is a negative regulator of established allergic asthma. J. Exp. Med. 203:2715–2725 10.1084/jem.20061401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J.L., Scott R., Boyle M.J., Gibson P.G. 2006. Inflammatory subtypes in asthma: assessment and identification using induced sputum. Respirology. 11:54–61 10.1111/j.1440-1843.2006.00784.x [DOI] [PubMed] [Google Scholar]
- Starnes T., Robertson M.J., Sledge G., Kelich S., Nakshatri H., Broxmeyer H.E., Hromas R. 2001. Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J. Immunol. 167:4137–4140 [DOI] [PubMed] [Google Scholar]
- Tillie-Leblond I., Pugin J., Marquette C.H., Lamblin C., Saulnier F., Brichet A., Wallaert B., Tonnel A.B., Gosset P. 1999. Balance between proinflammatory cytokines and their inhibitors in bronchial lavage from patients with status asthmaticus. Am. J. Respir. Crit. Care Med. 159:487–494 [DOI] [PubMed] [Google Scholar]
- van Hamburg J.P., de Bruijn M.J., Ribeiro de Almeida C., van Zwam M., van Meurs M., de Haas E., Boon L., Samsom J.N., Hendriks R.W. 2008. Enforced expression of GATA3 allows differentiation of IL-17-producing cells, but constrains Th17-mediated pathology. Eur. J. Immunol. 38:2573–2586 10.1002/eji.200737840 [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Hocking R.J., Atkins C.J., Locksley R.M., Stockinger B. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24:179–189 10.1016/j.immuni.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Volpe E., Servant N., Zollinger R., Bogiatzi S.I., Hupé P., Barillot E., Soumelis V. 2008. A critical function for transforming growth factor-beta, interleukin 23 and proinflammatory cytokines in driving and modulating human T(H)-17 responses. Nat. Immunol. 9:650–657 10.1038/ni.1613 [DOI] [PubMed] [Google Scholar]
- Wang Y.H., Ito T., Wang Y.H., Homey B., Watanabe N., Martin R., Barnes C.J., McIntyre B.W., Gilliet M., Kumar R., et al. 2006. Maintenance and polarization of human TH2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 24:827–838 10.1016/j.immuni.2006.03.019 [DOI] [PubMed] [Google Scholar]
- Wilson N.J., Boniface K., Chan J.R., McKenzie B.S., Blumenschein W.M., Mattson J.D., Basham B., Smith K., Chen T., Morel F., et al. 2007. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 8:950–957 10.1038/ni1497 [DOI] [PubMed] [Google Scholar]
- Yang L., Anderson D.E., Baecher-Allan C., Hastings W.D., Bettelli E., Oukka M., Kuchroo V.K., Hafler D.A. 2008. IL-21 and TGF-beta are required for differentiation of human T(H)17 cells. Nature. 454:350–352 10.1038/nature07021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z., Fanslow W.C., Seldin M.F., Rousseau A.M., Painter S.L., Comeau M.R., Cohen J.I., Spriggs M.K. 1995a. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 3:811–821 10.1016/1074-7613(95)90070-5 [DOI] [PubMed] [Google Scholar]
- Yao Z., Painter S.L., Fanslow W.C., Ulrich D., Macduff B.M., Spriggs M.K., Armitage R.J. 1995b. Human IL-17: a novel cytokine derived from T cells. J. Immunol. 155:5483–5486 [PubMed] [Google Scholar]
- Ye P., Rodriguez F.H., Kanaly S., Stocking K.L., Schurr J., Schwarzenberger P., Oliver P., Huang W., Zhang P., Zhang J., et al. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194:519–527 10.1084/jem.194.4.519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurovsky V.V., Weersink E.J., Meltzer S.S., Moore W.C., Postma D.S., Bleecker E.R., White B. 1998. T-cell repertoire in the blood and lungs of atopic asthmatics before and after ragweed challenge. Am. J. Respir. Cell Mol. Biol. 18:370–383 [DOI] [PubMed] [Google Scholar]
- Zhu J., Paul W.E. 2010. Heterogeneity and plasticity of T helper cells. Cell Res. 20:4–12 10.1038/cr.2009.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann N., Hershey G.K., Foster P.S., Rothenberg M.E. 2003. Chemokines in asthma: cooperative interaction between chemokines and IL-13. J. Allergy Clin. Immunol. 111:227–242 [DOI] [PubMed] [Google Scholar]