Removal of regulatory T cells precipitates tissue inflammation and a systemic autoimmune lympho- and myeloproliferative syndrome, even in germ-free mice.
Abstract
Suppression mediated by regulatory T cells (T reg cells) represents a unique, cell-extrinsic mechanism of in-trans negative regulation that restrains multiple types of immune cells. The loss of T reg cells leads to fatal, highly aggressive, and widespread immune-mediated lesions. This severe autoimmunity may be driven by commensal microbiota, the largest source of non-self ligands activating the innate and adaptive immune systems. Alternatively, T reg cells may primarily restrain T cells with a diverse self–major histocompatibility complex (MHC)–restricted T cell receptor repertoire independently of commensal microbiota. In this study, we demonstrate that in germ-free (GF) mice, ablation of the otherwise fully functional T reg cells resulted in a systemic autoimmune lympho- and myeloproliferative syndrome and tissue inflammation comparable with those in T reg cell–ablated conventional mice. Importantly, there were two exceptions: in GF mice deprived of T reg cells, the inflammation in the small intestine was delayed, whereas exocrine pancreatitis was markedly accelerated compared with T reg cell–ablated conventional mice. These findings suggest that the main function of T reg cells is restraint of self-MHC–restricted T cell responsiveness, which, regardless of the presence of commensal microbiota, poses a threat of autoimmunity.
The immune systems of multicellular organisms afford protection against a multitude of infectious agents while avoiding pathogenic responses to self. The activity of the immune cells and inflammation associated with the immune responses are controlled by numerous effector cell–intrinsic mechanisms and soluble factors. The emergence of the adaptive immune system based on antigen-specific receptors of unlimited diversity made the task of preventing pathogenic responses to self much more challenging. Regulatory T cells (T reg cells) expressing transcription factor Foxp3 (forkhead box protein 3) serve as key mediators of immunological tolerance and homeostasis in higher organisms. Loss of function mutations of the Foxp3 gene in mice and humans cause fatal, early-onset immune-mediated inflammation affecting multiple organs (Chatila et al., 2000; Bennett et al., 2001; Brunkow et al., 2001; Fontenot et al., 2003; Hori et al., 2003). Importantly, the loss of T reg cells in Foxp3− mice is responsible for all manifestations of the disease (Fontenot et al., 2003, 2005). Suppression mediated by T reg cells is vital not only during the neonatal period but throughout the lifespan of healthy animals. The latter notion is based on our previous studies of Foxp3DTR knockin mice, in which all Foxp3+ T reg cells express human diphtheria toxin (DT) receptor (DTR) and, therefore, can be efficiently eliminated upon treatment with DT (Kim et al., 2007; Lund et al., 2008). T reg cell ablation in healthy adult Foxp3DTR mice leads to fatal lympho- and myeloproliferative syndrome and widespread immune-mediated tissue lesions identical to those found in Foxp3− mice with the congenital T reg cell deficiency (Kim et al., 2007). Thus, suppression mediated by T reg cells represents a unique cell-extrinsic mechanism of negative regulation acting in-trans to restrain multiple types of immune effector cells.
Why is this mechanism of paramount importance? Why in its absence do cell-intrinsic mechanisms and soluble antiinflammatory factors elaborated by immune effector cells fail to maintain the delicate balance between immunity and tolerance? Depletion of CD4+ T effector cells along with T reg cells in adult Foxp3DTR mice alleviated expansion but not activation of DCs (Kim et al., 2007). These experiments suggested that self-reactive CD4+ T cells serve as potent amplifiers of activation of innate immune cells. The latter likely occurs upon direct recognition of products of microorganisms or metabolic changes resulting from their action and is a prerequisite for effective activation of CD4+ T cells and productive immune responses. Recent studies have shown that T reg cells control activity of innate immune cells, including DCs and NK cells (Kim et al., 2007; Wing et al., 2008; Feuerer et al., 2009; Liu et al., 2009). Under physiological conditions, commensal microbiota represent the largest source of ligands for toll-like receptors, which serve as the principal sensors of the innate immune system. Thus, it is reasonable to suggest that T reg cell–mediated suppression is indispensable because in its absence, unrestrained stimulation of the innate immune cells by commensal microbiota drives devastating autoimmune disease resulting from activation of self-reactive T cells, which have escaped thymic deletion, and of T cells specific for commensal microbiota. A corollary of this idea is that in contrast to conventional mice kept under specific pathogen-free (SPF) conditions, in germ-free (GF) mice, T reg cells are dispensable for or play a minor role in immune homeostasis. Alternatively, it is possible that cell-intrinsic mechanisms of tolerance are unable to restrain T cells with a diverse self-MHC–restricted TCR repertoire even in the absence of commensal microbiota. In other words, self-MHC–restricted T cell recognition either by itself or combined with the basal activity of innate sensors of infection and stress poses an imminent threat of autoimmunity, which cannot be successfully tackled by the aforementioned conventional mechanisms of tolerance without T reg cell–mediated suppression. According to this scenario, T reg cell ablation in GF mice is expected to lead to life-threatening immune-mediated lesions. To explore these possibilities, we examined a role for T reg cells in immune homeostasis in the absence and presence of commensal microbiota in GF and conventional Foxp3DTR mice.
RESULTS AND DISCUSSION
Comparable suppressor activity of T reg cells in GF and SPF mice
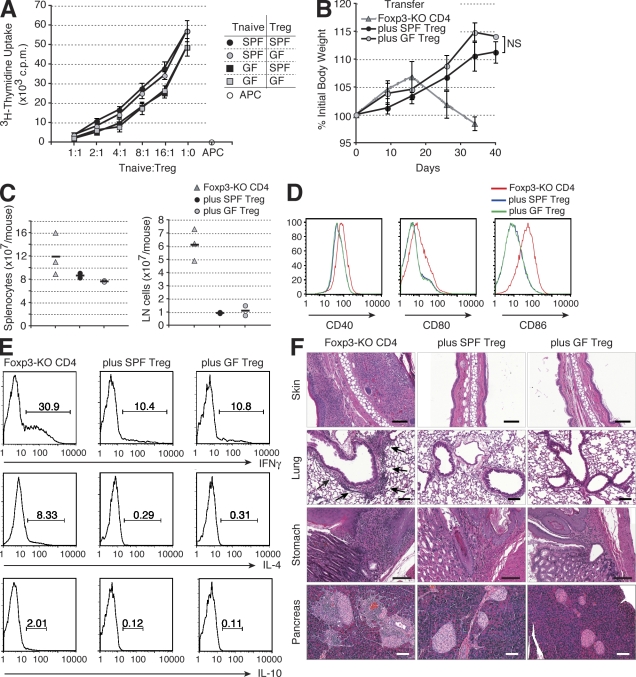
To explore a role for T reg cells in the absence of commensal microbiota, we generated GF Foxp3DTR mice. In agreement with previous studies (Min et al., 2007; Wen et al., 2008), T reg cells were present in normal numbers in GF Foxp3DTR and expressed unaltered amounts of Foxp3, CD25, CTLA-4 (cytotoxic T lymphocyte antigen 4), CD62L, CD44, CD69, and CD103 in comparison with SPF Foxp3DTR mice (unpublished data). Furthermore, purified T reg cells isolated from either GF or SPF Foxp3DTR mice potently suppressed in vitro proliferative responses of CD4+Foxp3− T cells (Fig. 1 A). To evaluate the in vivo suppressor activity, FACS-purified T reg cells from GF or SPF Foxp3DTR mice were adoptively transferred with effector CD4+ T cells isolated from Foxp3− mice into T cell–deficient (Tcrb−/−Tcrd−/−) recipients. As expected, within 5–6 wk after transfer, control animals that received the effector Foxp3− CD4+ T cells alone succumbed to fatal wasting disease accompanied by widespread tissue inflammation. In agreement with in vitro functional analyses (Fig. 1 A), T reg cells from SPF and GF Foxp3DTR mice prevented the body weight loss (Fig. 1 B), activation and expansion of CD4+ T cells, NK cells, DCs, and B cells (Fig. 1, C and D; and not depicted), CD4+ T cell cytokine production (Fig. 1 E), and inflammation with equal efficiency in all examined tissues, including those populated with commensal microbiota (skin, lungs, stomach, small and large intestine, and salivary glands; Fig. 1 F and not depicted). These results demonstrated that T reg cells developed normally in the absence of commensal microbiota and that the presence of microbes did not affect their ability to efficiently suppress immune-mediated inflammation.
Figure 1.
The suppressor activity of T reg cells from SPF and GF mice is comparable. (A) In vitro suppressor activity of sorted SPF and GF CD4+Foxp3+ T reg cells was assessed by [3H]thymidine incorporation in co-cultures with SPF and GF CD4+Foxp3−CD62LhighCD44low naive T cells (Tnaive) and irradiated T cell–depleted SPF splenocytes (APC) in the presence of anti-CD3. The data are representative of two independent experiments. (B) Analysis of the body weight of Tcrb−/−Tcrd−/− recipients of effector CD4+ T cells from Foxp3− mice alone (Foxp3-KO CD4) or together with T reg cells from SPF (plus SPF T reg) or GF (plus GF T reg) mice (n = 6 per group). (A and B) Error bars represent SD. (C) Spleen and LN cellularity of Tcrb−/−Tcrd−/− mice 35 d after the transfer of the indicated cell subsets. The horizontal bars indicate the mean values for each group. (D and E) Flow cytometric analyses of splenocytes of Tcrb−/−Tcrd−/− mice 35 d after the transfer of effector CD4+ T cells from Foxp3− mice with or without SPF or GF T reg cells. Two mice per group were analyzed in two independent experiments, and representative data are shown. The surface expression of the indicated co-stimulatory molecules on B cells (D) and the frequencies of the cytokine-producing cells among CD4+Foxp3− cells determined by flow cytometry after T cell stimulation with anti-CD3 and anti-CD28 (E) are shown. (F) Representative histopathology of the Tcrb−/−Tcrd−/− mice 35 d after the transfer of the indicated cell subsets. Inflamed area of the lung is marked by arrows. Bars, 100 µm.
Ablation of T reg cells in the absence of commensal microbiota leads to severe systemic inflammatory responses
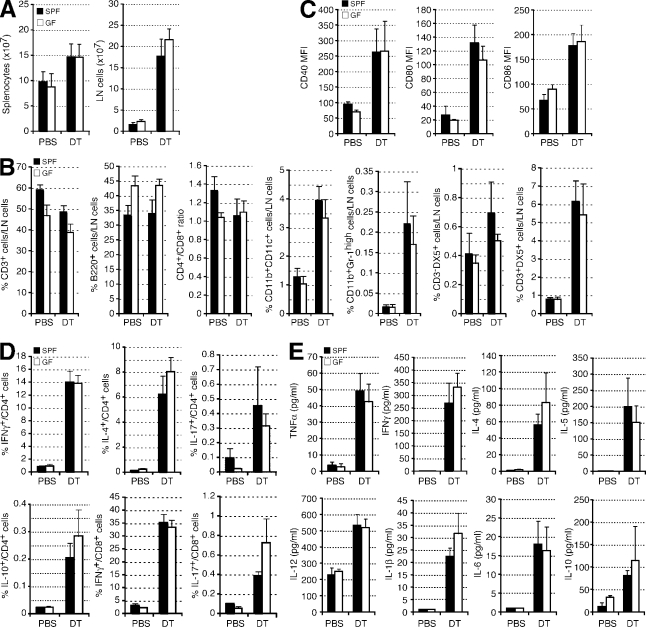
Next, we tested whether T reg cells are essential for immune homeostasis in the absence of commensal microbiota. To ablate T reg cells, SPF and GF Foxp3DTR mice were continuously treated with DT every third day. T reg cell depletion in both SPF and GF mice resulted in comparably severe autoimmune disease (Fig. 2 and Fig. S1). In the DT-treated mice, no difference in the extent of splenomegaly and lymphadenopathy (comparable cellularity of the spleen and LNs; Fig. 2 A) was observed. Likewise, the increases in the proportion and absolute numbers of myeloid cells and DCs, CD4+ and CD8+ T cells in the secondary lymphoid organs (Fig. 2 B), in the expression of CD40, CD80, and CD86 on DCs (Fig. 2 C), and in the cytokine production by CD4+ and CD8+ T cells (Fig. 2 D) were similar between the two groups. Serum cytokine levels and serum PGE2 (prostaglandin E2) levels were also similarly up-regulated in these mice, and the severity of the tissue inflammation was comparable in the majority of the organs analyzed (Fig. 2 E, Fig. S1, and not depicted). In addition to T cell expansion and cytokine production, ablation of T reg cells led to a comparable increase in all serum Ig isotypes in SPF and GF mice with one exception: the levels of IgA were markedly higher in SPF mice (Fig. S4). Overall, these results indicate that systemic immune-mediated inflammatory responses can commence upon T reg cell depletion without direct or indirect contributions from commensal microbiota, i.e., through induction of proinflammatory cytokines and co-stimulatory molecules. It is possible that exposure to microbial products via food or bedding could still contribute to the immune responses in T reg cell–ablated GF mice. Nevertheless, the essentially complete absence of Th17 cells in, and Th2 cytokine bias characteristic of, GF mice (Mazmanian et al., 2005; Ivanov et al., 2009; Fig. 3 D) suggests that this contribution is likely minor.
Figure 2.
T reg cell–depleted GF mice developed severe inflammatory disease. SPF and GF Foxp3DTR mice were treated with either PBS or DT and analyzed at day 8 after the start of the treatment. (A) The numbers of cells in the spleen and LNs in the indicated mice (n = 7–16 per group). (B and C) Flow cytometric analyses of the cellular composition of LNs in the indicated mice (n = 7–16 per group). (B) Percentage of cells expressing the indicated surface markers. (C) Mean fluorescence intensity (MFI) of the indicated co-stimulatory molecules on CD11c+ MHC class IIhigh DCs. (D) Splenocytes isolated from the indicated mice were stimulated in vitro with anti-CD3 and anti-CD28, and production of the indicated cytokine was measured by flow cytometry. Frequencies of the cytokine-producing cells among CD4+ or CD8+ T cells were determined (n = 3–9 per group). (E) The serum cytokine levels (n = 5–8 per group) were measured by Luminex multiplex bead cytokine assay. (A–E) Error bars represent SD.
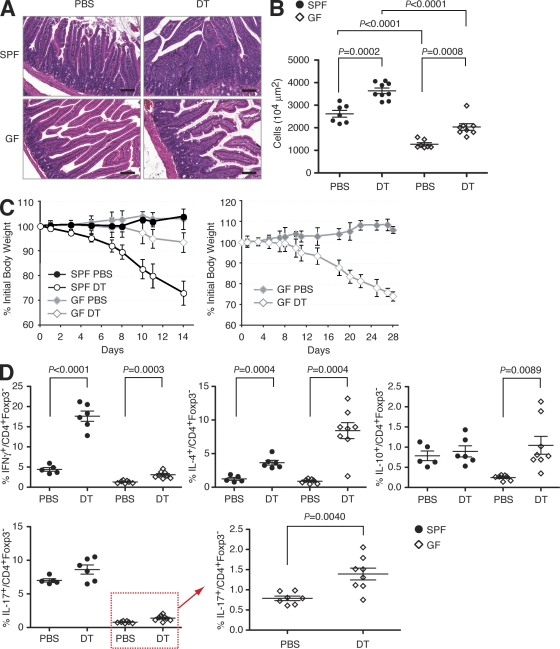
Figure 3.
Inflammation in the small intestine of T reg cell–depleted SPF mice was more severe than in GF mice. (A) Representative histopathology of the small intestine of SPF and GF Foxp3DTR mice 8 d after the start of the treatment with PBS or DT. Bars, 100 µm. (B) The cell numbers in the lamina propria of the small intestine in the indicated mice were determined by morphometry. (C) Analysis of the body weight of SPF and GF Foxp3DTR mice treated with DT or PBS (control). The left panel shows changes in body weights between days 0 and 14 (n = 13 for each group). The right panel shows changes in body weights between days 0 and 28 (n = 4 for each group). Error bars represent SD. (D) Leukocytes isolated from the small intestinal lamina propria of the indicated mice were stimulated in vitro with anti-CD3 and anti-CD28 in the presence of monensin, and intracellular staining for the indicated cytokines was performed. The frequencies of cytokine-producing cells among CD4+Foxp3− cells were determined by flow cytometric analysis. Absolute numbers of the cells are shown in Fig. S2. Data are representative of two independent experiments. (B and D) The horizontal lines indicate the mean ± SEM.
The small intestine and pancreas are differentially affected by inflammation in SPF and GF mice
Although the severity of the tissue inflammation in T reg cell–deprived SPF and GF mice was comparable in the majority of the organs analyzed, in the small intestine, inflammatory lesions were much more pronounced in SPF compared with GF mice. As shown in Fig. 3 (A and B), the extent of myeloid and lymphoid cell infiltration in the lamina propria of the small intestine in SPF mice was markedly higher than that in GF mice, and T reg cell–deprived SPF mice lost weight more quickly than GF mice, likely reflecting the severity of intestinal inflammation (Fig. 3 C). The extent of cell infiltration in the lamina propria of the large intestine was also higher in SPF mice than in GF mice, albeit the difference was less significant than in the small intestine. In contrast to similarly augmented levels of proinflammatory cytokines in the serum, production of cytokines by T cells, and the levels of activation of DCs in the secondary lymphoid organs (Fig. 2), the profiles of cytokine production by lamina propria lymphocytes and the levels of activation of lamina propria DCs were different between SPF and GF mice. The surface expression of co-stimulatory molecules (CD40, CD80, and CD86) on lamina propria DCs was higher in control SPF mice than in control GF mice. Upon T reg cell depletion, these molecules were up-regulated more significantly in SPF than GF mice (Fig. S3). Whereas significant numbers of T cells in the small intestine of control SPF mice produced IFN-γ or IL-17 at the basal condition, very few T cells were producing these proinflammatory cytokines in GF mice in the presence of T reg cells (Fig. 3 D and Fig. S2). Lack of IL-17 production in GF mice was in agreement with the studies showing an essential role of specific gut microbes in the induction of Th17 cells (Ivanov et al., 2008, 2009; Gaboriau-Routhiau et al., 2009). T reg cell depletion in SPF mice resulted in a considerable increase in IFN-γ–producing T cells but only a moderate rise in IL-4–producing T cells, whereas GF mice showed the opposite trend. The latter observation was consistent with the previously reported bias toward Th2 responses in GF mice (Mazmanian et al., 2005). Unexpectedly, T reg cell ablation also resulted in a significant boost in Th17 cells not only in SPF, but also in a proportionally similar increase in GF mice (Fig. 3 D). This result suggested that T reg cells restrain Th17 cell differentiation both in the presence and absence of commensal microbiota and that the relief of T reg cell–mediated suppression leads to Th17 responses in the absence of microbiota. However, even after the depletion of T reg cells, the numbers of Th17 cells in the small intestinal lamina propria in GF mice did not reach those in SPF mice, which is consistent with the recently recognized role for segmented filamentous bacteria (Ivanov et al., 2008, 2009; Gaboriau-Routhiau et al., 2009) and potentially other yet to be defined bacterial species in Th17 induction and expansion. Commensal microbiota not only provide signals to innate immune cells but also are arguably the largest source of non-self antigens. The difference in non-self antigen spectrum and load and in T cell repertoire might also underlie the difference in the local immune response in the intestine of SPF and GF mice.
In addition to cytokine production, we measured the circulating antibodies specific for intestinal and food antigens in SPF and GF mice. T reg cell depletion resulted in increased levels of autoantibodies reactive with the protein extracts from small and large intestine as well as antibodies reactive with food antigens in both SPF and GF mice (unpublished data), suggesting that the immune responses against autoantigens as well as the food antigens in the intestine are also controlled by T reg cells regardless of the presence of commensal microbiota.
It is noteworthy that the lung and skin, two other environmental interfaces harboring commensal microbiota, were similarly affected by severe inflammation induced upon T reg cell depletion in SPF and GF mice (Fig. S1). This might be because of the difference in the bacterial load or composition of microbiota in these organs. An additional, but arguably less likely, explanation for the observed differences is potential variation in the activity of innate immune sensors triggered by endogenous ligands in the lung and skin versus the gastrointestinal tract.
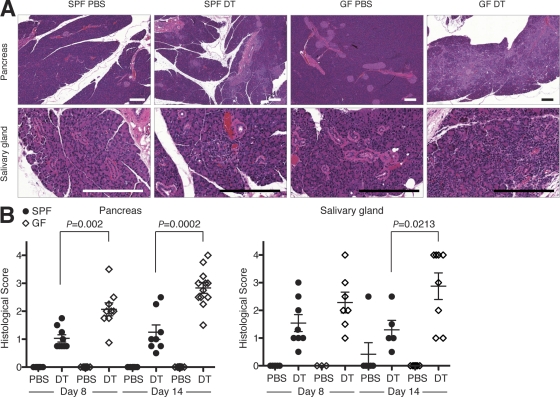
Unlike attenuated inflammatory response in the intestine, the inflammatory responses in the salivary glands and pancreata were more severe in T reg cell–depleted GF mice (Fig. 4). The inflammation in DT-treated SPF Foxp3DTR mice was mostly confined to the exocrine pancreas with a lack of or with only minor insulitis. These features were unchanged in GF mice, too, but the degree of the exocrine pancreatitis was more severe in GF mice. The inflammation in the salivary gland was also more pronounced in GF mice, although the observed difference was relatively minor. In accordance with histological observations, pancreas-resident DCs of T reg cell–deprived GF mice showed a more activated phenotype than those in SPF mice (Fig. S3). We also measured the levels of antibodies specific for several candidate pancreatic autoantigens previously implicated in autoimmune exocrine pancreatitis (Okazaki et al., 2001; Meagher et al., 2008; Ise et al., 2010). We found that at day 14, the levels of antibodies against PDIA2 (protein disulfide isomerase family A, member 2) were higher in GF than in SPF mice, whereas the levels of antibodies against α-amylase, carboxypeptidase B, and insulin were comparable (Fig. S4 B). PDIA2 is expressed predominantly in pancreatic acinar cells, and the immune response against PDIA2 is reported to cause exocrine pancreatitis (Niki et al., 2006; Ise et al., 2010). The increased amounts of anti-PDIA2 antibodies are consistent with heightened exocrine pancreatitis observed in T reg cell–depleted GF mice. These observations suggested that some yet to be identified components of the commensal microbiota protected these organs from autoimmunity in a T reg cell–independent manner. The mechanism of the observed protection also remains to be elucidated. Recently, we observed microorganism-dependent protection from autoimmunity in a spontaneous autoimmune diabetes model in nonobese diabetic mice (Wen et al., 2008), although the role of T reg cells in such protection remains to be elucidated.
Figure 4.
Commensal microbiota contributed to protection from autoimmunity in the pancreas and the salivary gland. (A) Representative histopathology of the pancreas and the salivary gland of SPF and GF Foxp3DTR mice 14 d after the start of the treatment with DT or PBS (control). Bars, 200 µm. (B) Histological scores of the pancreas and salivary gland of the indicated mice. The horizontal lines indicate the mean ± SEM.
Together, our experiments suggest that the systemic and local inflammatory responses to self and possibly environmental antigens need to be continuously restrained by T reg cells regardless of the presence or absence of commensal microbiota. Therefore, the commensal microbiota are not essential for induction of pathogenic immune responses against self in the absence of T reg cell–mediated suppression. Our observations also indicate that the commensal microbiota minimally influence the severity of inflammation induced in the majority of the organs upon relief of T reg cell–mediated suppression. Thus, it seems that the interactions between the adaptive immune system and commensal microbiota do not underlie the absolute reliance of immune homeostasis on the suppressive function of T reg cells. Our results argue that self-MHC–restricted T cell recognition, likely in combination with basal activity of innate immune sensors, makes T reg cell–mediated negative regulation of adaptive and innate immunity indispensable for immunological restraint.
MATERIALS AND METHODS
Mice and DT treatment.
Foxp3− and Foxp3DTR mice were described previously (Fontenot et al., 2003; Kim et al., 2007). Foxp3DTR mice were rederived and kept GF at Taconic and housed in the GF animal facility at the University of Chicago during the experiment. Tcrb−/−Tcrd−/− mice were purchased from The Jackson Laboratory. All mice other than GF mice were maintained in the SPF animal facility at the Memorial Sloan-Kettering Cancer Center. All experiments were approved by and performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (“CD4 T cell Biology and Antigen Presentation” # 08-10-023), the Research Animal Resource Center of Memorial Sloan-Kettering Cancer Center and The University of Chicago, and the National Institutes of Health “Guide for the Care and Use of Laboratory Animals.” DT was purchased from Sigma-Aldrich and dissolved in PBS. The endotoxin levels of DT and PBS used were <0.01 EU/ml, as determined by a chromogenic endotoxin assay kit (GenScript). Mice were intraperitoneally injected with 40 µg DT per kg of body weight three times a week.
Histology.
Tissues were removed from mice, and hematoxylin and eosin–stained sections were made for histological analysis. The severity of the organ inflammation was determined by scoring the degree of the inflammatory cellular infiltration into tissues (0 = none; 1 = mild; 2 = moderate; 3 = moderate and diffuse or severe but focal; 4 = severe and diffuse). The scores of two to three sections were averaged to give a final score.
Measurements of serum cytokine, PGE2, and Ig levels.
Serum cytokine levels were determined by Luminex multiplex assays (Invitrogen). For the determination of serum PGE2 levels, indomethacin was included in the tubes to collect blood samples to minimize de novo production of PGE2. PGE2 levels were determined using the PGE2 EIA kit (Cayman Chemical). Serum IgM, IgG1, IgG2a, IgG2b, IgG2c, IgG3, and IgA levels were determined by ELISA using the SBA Clonotyping system (SouthernBiotech). IgE ELISA was performed using biotinylated anti-IgE antibody (BD) and streptavidin-conjugated horseradish peroxidase. Antibodies reactive with the pancreatic proteins were detected by ELISA using horseradish peroxidase–conjugated anti–mouse Ig (H + L) antibody. Recombinant mouse PDIA2, bovine carboxypeptidase B, porcine α-amylase, and bovine insulin were gifts from K. Murphy (Washington University in St. Louis, St. Louis, MO).
Flow cytometry and cell sorting.
Single-cell suspensions were prepared from the spleen, LNs (axillary, inguinal, submandibular, and mesenteric LNs), intestine, and pancreas. The cells from the small intestine lamina propria and pancreas were isolated by collagenase digestion followed by Percoll centrifugation. Cells were stained with fluorescently tagged antibodies purchased from eBioscience, BD, or R&D Systems and analyzed using an LSR II flow cytometer (BD). Flow cytometry data were analyzed using FlowJo software (Tree Star, Inc.). For in vitro suppression assays and in vivo transfer experiments, CD4+Foxp3 (GFP)+ T reg cells and CD4+Foxp3− cells were sorted using a FACSAria II (BD). For intracellular cytokine staining, cells were stimulated for 5 h with 5 µg/ml anti-CD3 and 5 µg/ml anti-CD28 antibodies in the presence of brefeldin A or monensin, harvested, and stained with an intracellular cytokine assay kit (BD).
In vitro suppression assays and cell transfers.
4 × 104 CD4+Foxp3−CD62Lhigh naive T cells FACS purified from SPF Foxp3DTR mice were cultured with graded numbers of CD4+Foxp3+ T reg cells FACS purified from SPF or GF Foxp3DTR mice in the presence of 105 irradiated T cell–depleted splenocytes and 1 µg/ml anti-CD3 antibody in a 96-well round-bottom plate for 80 h. Cell proliferation was assessed by [3H]thymidine incorporation during the final 8 h of culture. For adoptive T cell transfers, 2 × 106 CD4+ T cells from SPF Foxp3− mice were intravenously injected into T cell–deficient Tcrb−/−Tcrd−/− mice maintained under SPF conditions together with or without 4 × 105 of SPF or GF CD4+Foxp3+ T reg cells. The recipient mice were analyzed for immune cell activation and tissue inflammation 35 d after the transfer.
Statistical analysis.
For statistical analysis, we used the Student’s t test. A p-value of <0.05 was considered to be statistically significant.
Online supplemental material.
Fig. S1 shows the histopathology of organs of SPF and GF Foxp3DTR mice treated either with PBS or DT. Fig. S2 shows the cell numbers of the cytokine-producing cells in the small intestinal lamina propria of SPF and GF Foxp3DTR mice treated with PBS or DT. Fig. S3 shows the levels of activation markers on the DCs in the small intestine and the pancreas of SPF and GF Foxp3DTR mice treated with PBS or DT. Fig. S4 shows the serum antibody levels in SPF and GF Foxp3DTR mice treated with PBS or DT and the levels of serum antibodies, which react with pancreatic proteins. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20101235/DC1.
Acknowledgments
Recombinant mouse PDIA2, bovine carboxypeptidase B, porcine α-amylase, and bovine insulin were gifts from Dr. Kenneth Murphy.
This work is supported by the Japan Society for the Promotion of Science (T. Chinen), the National Institutes of Health (A.Y. Rudensky and A.V. Chervonsky), the Juvenile Diabetes Research Foundation (A.V. Chervonsky), and the Howard Hughes Medical Institute (A.Y. Rudensky).
The authors disclose no financial conflict of interest.
Footnotes
Abbreviations used:
- DT
- diphtheria toxin
- DTR
- DT receptor
- GF
- germ free
- SPF
- specific pathogen free
References
- Bennett C.L., Christie J., Ramsdell F., Brunkow M.E., Ferguson P.J., Whitesell L., Kelly T.E., Saulsbury F.T., Chance P.F., Ochs H.D. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27:20–21 10.1038/83713 [DOI] [PubMed] [Google Scholar]
- Brunkow M.E., Jeffery E.W., Hjerrild K.A., Paeper B., Clark L.B., Yasayko S.A., Wilkinson J.E., Galas D., Ziegler S.F., Ramsdell F. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27:68–73 10.1038/83784 [DOI] [PubMed] [Google Scholar]
- Chatila T.A., Blaeser F., Ho N., Lederman H.M., Voulgaropoulos C., Helms C., Bowcock A.M. 2000. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J. Clin. Invest. 106:R75–R81 10.1172/JCI11679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerer M., Shen Y., Littman D.R., Benoist C., Mathis D. 2009. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 31:654–664 10.1016/j.immuni.2009.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J.D., Gavin M.A., Rudensky A.Y. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4:330–336 10.1038/ni904 [DOI] [PubMed] [Google Scholar]
- Fontenot J.D., Rasmussen J.P., Williams L.M., Dooley J.L., Farr A.G., Rudensky A.Y. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 22:329–341 10.1016/j.immuni.2005.01.016 [DOI] [PubMed] [Google Scholar]
- Gaboriau-Routhiau V., Rakotobe S., Lécuyer E., Mulder I., Lan A., Bridonneau C., Rochet V., Pisi A., De Paepe M., Brandi G., et al. 2009. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 31:677–689 10.1016/j.immuni.2009.08.020 [DOI] [PubMed] [Google Scholar]
- Hori S., Nomura T., Sakaguchi S. 2003. Control of regulatory T cell development by the transcription factor Foxp3. Science. 299:1057–1061 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- Ise W., Kohyama M., Nutsch K.M., Lee H.M., Suri A., Unanue E.R., Murphy T.L., Murphy K.M. 2010. CTLA-4 suppresses the pathogenicity of self antigen-specific T cells by cell-intrinsic and cell-extrinsic mechanisms. Nat. Immunol. 11:129–135 10.1038/ni.1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., Frutos Rde.L., Manel N., Yoshinaga K., Rifkin D.B., Sartor R.B., Finlay B.B., Littman D.R. 2008. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 4:337–349 10.1016/j.chom.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I.I., Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V., et al. 2009. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 139:485–498 10.1016/j.cell.2009.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.M., Rasmussen J.P., Rudensky A.Y. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8:191–197 10.1038/ni1428 [DOI] [PubMed] [Google Scholar]
- Liu K., Victora G.D., Schwickert T.A., Guermonprez P., Meredith M.M., Yao K., Chu F.F., Randolph G.J., Rudensky A.Y., Nussenzweig M. 2009. In vivo analysis of dendritic cell development and homeostasis. Science. 324:392–397 10.1126/science.1170540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J.M., Hsing L., Pham T.T., Rudensky A.Y. 2008. Coordination of early protective immunity to viral infection by regulatory T cells. Science. 320:1220–1224 10.1126/science.1155209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian S.K., Liu C.H., Tzianabos A.O., Kasper D.L. 2005. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 122:107–118 10.1016/j.cell.2005.05.007 [DOI] [PubMed] [Google Scholar]
- Meagher C., Tang Q., Fife B.T., Bour-Jordan H., Wu J., Pardoux C., Bi M., Melli K., Bluestone J.A. 2008. Spontaneous development of a pancreatic exocrine disease in CD28-deficient NOD mice. J. Immunol. 180:7793–7803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B., Thornton A., Caucheteux S.M., Younes S.A., Oh K., Hu-Li J., Paul W.E. 2007. Gut flora antigens are not important in the maintenance of regulatory T cell heterogeneity and homeostasis. Eur. J. Immunol. 37:1916–1923 10.1002/eji.200737236 [DOI] [PubMed] [Google Scholar]
- Niki S., Oshikawa K., Mouri Y., Hirota F., Matsushima A., Yano M., Han H., Bando Y., Izumi K., Matsumoto M., et al. 2006. Alteration of intra-pancreatic target-organ specificity by abrogation of Aire in NOD mice. J. Clin. Invest. 116:1292–1301 10.1172/JCI26971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki K., Uchida K., Chiba T. 2001. Recent concept of autoimmune-related pancreatitis. J. Gastroenterol. 36:293–302 10.1007/s005350170094 [DOI] [PubMed] [Google Scholar]
- Wen L., Ley R.E., Volchkov P.Y., Stranges P.B., Avanesyan L., Stonebraker A.C., Hu C., Wong F.S., Szot G.L., Bluestone J.A., et al. 2008. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 455:1109–1113 10.1038/nature07336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Miyara M., Fehervari Z., Nomura T., Sakaguchi S. 2008. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 322:271–275 10.1126/science.1160062 [DOI] [PubMed] [Google Scholar]