Killing of nonmalignant stroma requires cooperation between CD4+ and CD8+ T cells during the effector phase in the tumor microenvironment.
Abstract
Cancers frequently evade cytotoxic T lymphocyte–mediated destruction through loss or down-regulation of tumor antigens and antigen-presenting major histocompatibility complex molecules. Therefore, we have concentrated our efforts on immunological strategies that destroy nonmalignant stromal cells essential for the survival and growth of cancer cells. In this study, we developed a non–T cell receptor transgenic, immunocompetent tumor model to determine whether tumor-bearing hosts’ own immune systems could eliminate cancer cells through stromal targeting and what role CD4+ T cells play alongside CD8+ T cells in this process. We found that aggressive cancers could be eradicated by T cell targeting of tumor stroma. However, successful elimination required the cooperation of CD4+ and CD8+ T cells not only during the induction phase but also during the effector phase in the tumor microenvironment, implying a new role for CD4+ T cells that has not been previously described. Our study demonstrates the potential of stromal targeting as a cancer immunotherapy and suggests that successful anticancer strategies must facilitate cooperation between CD4+ and CD8+ T cells at the right times and the right places.
Activated CD8+ T cells can kill cancer cells directly by recognizing specific peptide–MHC complexes on the surface of the cancer cells. However, cancers can escape direct killing through down-regulation or loss of MHC or antigen targets, thus evading CTL-mediated destruction (Momburg et al., 1986; Smith et al., 1988; Andersson et al., 1991; Kaklamanis et al., 1992; Marincola et al., 2000; Vago et al., 2009). Cancer cells are embedded in tissue comprised of nonmalignant host cells and extracellular matrix, referred to as stroma. Furthermore, cancer cells are genetically diverse as a result of genomic instability and high mutation rate, and ultimately, therapy-resistant cancer variants cause relapse and death. In contrast, stromal cells are nonmalignant and are generally genetically stable; although chromosomal abnormalities occur (Moinfar et al., 2000; Wernert et al., 2001; Matsumoto et al., 2003; Allinen et al., 2004; Fukino et al., 2007; Patocs et al., 2007), they are rare and do not show the clonality characteristic of cancer cells (Qiu et al., 2008). Therefore, when stromal cells are targeted for destruction by chemo-, radiation, and/or immunotherapy, there is no escape of variant stromal cells. In addition, stromal cells have tumor-promoting and immunosuppressive effects, making them therapeutic targets of interest.
Previously, we showed that in certain experimental settings, T cells could eradicate or arrest growth of large established tumors, including cancer cell variants, by targeting stromal cells in the tumor: nonmalignant stromal cells pick up cancer antigens released by cancer cells, present antigenic epitopes on their surface MHC molecules, and become targets for T cells (Spiotto et al., 2004; Spiotto and Schreiber, 2005; Zhang et al., 2007). This stromal destruction then leads to bystander killing of cancer cells (Spiotto et al., 2004; Spiotto and Schreiber, 2005; Zhang et al., 2008). These studies used adoptively transferred, preactivated CD8+ T cells from TCR transgenic mice as effectors, and the tumor-bearing recipients were immunodeficient; therefore, the role of CD4+ T cells was not examined. CD4+ T cells are essential during the induction phase and for memory formation of CD8+ T cells (Keene and Forman, 1982; Hung et al., 1998; Hu et al., 2000; Gao et al., 2002; for reviews see Toes et al., 1999; Castellino and Germain, 2006). CD4+ T cells adoptively transferred into SCID, Rag-KO, or sublethally irradiated or lymphodepleted WT mice can up-regulate MHC class II expression on cancer cells for direct targeting (Muranski et al., 2008), amplify CD8+ T cells that directly target cancer cells (Greenberg et al., 1981), or eradicate MHC class II–negative cancer cells without CD8+ T cells (Greenberg et al., 1985; Frey, 1995; Monach et al., 1995; Mumberg et al., 1999; Perez-Diez et al., 2007) through IFN-γ effects on host stroma (Monach et al., 1995; Qin and Blankenstein, 2000; Egilmez et al., 2002; Broderick et al., 2005; Muranski et al., 2008), and CD4+ T cells have long been implicated in the activation of macrophages and other nonlymphoid tumoricidal effector cells (Greenberg, 1991; Hung et al., 1998).
Given the potential clinical application of stromal targeting in immunotherapy, especially in treating cancers that are prone to immune evasion, we thought it was important to test the efficacy of stromal targeting (recognition of cross-presented antigen on stromal cells by T cells) in a nontransgenic T cell model using immunocompetent mice. Therefore, the objective of the present study was twofold: (1) to use a physiologically relevant model to determine whether a normal host without prior immunization could eliminate cancer cells through stromal targeting and (2) to determine what role CD4+ T cells play alongside CD8+ T cells in killing cancer cells as bystanders in the tumor microenvironment. We analyzed immune responses of normal immunocompetent mice in which the T cells were host derived and activated by the cancer cells in the host. We used a highly aggressive cancer cell line derived from a spontaneous tumor, Ag104A, and engineered it to express defined CD4+ and CD8+ T cell–recognized epitopes. These epitopes cannot be presented by the cancer cells directly because Ag104A lacks the appropriate MHC molecules. Nevertheless, we found that normal hosts, without preimmunization, generated T cells that successfully eliminated tumors through stromal targeting. Surprisingly, CD4+ T cells were needed not only for optimal CD8+ T cell activation but also at the effector stage within the tumor microenvironment.
RESULTS
Cancer cells express tumor antigens that are cross-presented by host-derived cells to antigen-specific CD4+ or CD8+ T cells
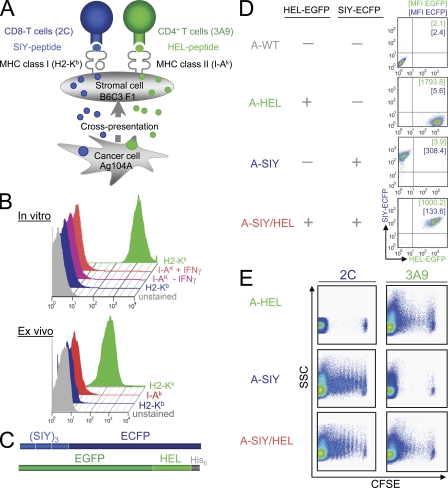
Ag104A is a highly aggressive cancer cell line that arose spontaneously in an aging mouse. Ag104A cancer cells injected subcutaneously produce tumors of ∼2 cm3 within 30 d in immunocompetent C57BL/6 × C3H/HeN F1 mice (B6C3; Wick et al., 1997; Yu et al., 2004). Ag104A cells were transduced to express the CD8+ T cell–recognized epitope SIYRYYGL (SIY) and/or the CD4+ T cell-recognized minimal hen egg lysozyme (HEL) epitope HEL(52–61) DYGILQINSR (Allen et al., 1987; Nelson et al., 1996; Carson et al., 1997). SIY is presented on H-2Kb and recognized by TCR transgenic 2C T cells (Sha et al., 1988), whereas HEL is presented on I-Ak and recognized by TCR transgenic 3A9 T cells (Fig. 1 A; Ho et al., 1994). Ag104A expresses H-2Kk but does not express H-2Kb or I-Ak and remains MHC class II negative even after 48 h of in vitro IFN-γ treatment (Fig. 1 B, top) or in vivo (Fig. 1 B, bottom). Thus, neither the SIY nor HEL antigen can be presented directly on the surface MHC of Ag104A cancer cells. However, CD11b-negative stromal cells from B6C3 mice (mostly fibroblasts) are H-2Kb positive, and CD11b-positive stromal cells (mostly monocytes and macrophages) express H-2Kb and I-Ak. Thus, T cells can only recognize the SIY or HEL antigens if they are cross-presented on the surface MHC of host stromal cells. Two antigen-encoding vectors were constructed: (1) a gene encoding the trimeric peptide (SIYRYYGL-AAY)3 fused to the enhanced CFP (ECFP)–encoding sequence and (2) a gene encoding the 25-mer peptide sequence [N44RNTDGSTDYGILQINSRWWCNDGR68] containing the known stimulatory HEL(52–61) epitope fused to the enhanced GFP (EGFP)–encoding sequence (Fig. 1 C). Using these two constructs, we established Ag104A tumor cell lines expressing the CD8+ T cell–recognized SIY antigen (A-SIY), the CD4+ T cell-recognized HEL antigen (A-HEL), or both the SIY and HEL antigens (A-SIY/HEL; Fig. 1 D).
Figure 1.
Cancer cells express tumor antigens that are cross-presented and activate TCR transgenic antigen-specific CD4+ or CD8+ T cells. (A) Model of indirectly presented CD4+ or CD8+ T cell–recognized tumor antigens. Ag104A cancer cells, which lack the necessary MHC molecules to directly present the CD8+ T cell–recognized SIY peptide and the CD4+ T cell–recognized HEL peptide, release these antigens into the surrounding stroma where they are picked up by macrophages, dendritic cells, and other stromal cells and are presented on their surface MHC. Antigen-specific T cells recognize the cross-presented antigens on stromal cells. (B, top) In vitro cultured Ag104A cancer cells were cultured where indicated for 48 h with IFN-γ and stained with PE-conjugated antibodies specific for the indicated MHC molecule (right of histogram). (bottom) Ag104A cancer cells isolated from established tumors in vivo (identified by positive surface staining for an Ag104A tumor-specific antigen recognized by the monoclonal antibody 237mAb) were stained with the indicated antibodies. Histograms were gated on 237mAb-positive cells. Similar results were obtained in two additional experiments. (C) Schematic diagram of the CD4+ and CD8+ T cell–recognized antigens. The CD8+ T cell–recognized epitope SIYRYYGLAAY (3× repeat) was fused to ECFP. The CD4+ T cell–recognized epitope HEL (25 mer) was fused to EGFP and flanked by a 6xHis tag. (D) Fluorescence intensity of Ag104A tumor cell lines transduced to express SIY-ECFP (A-SIY), HEL-EGFP (A-HEL), or both (A-SIY/HEL). Numbers indicate the mean fluorescence intensity (MFI). Untransduced Ag104A cells (A-WT) are included for comparison. (E) Indicated cancer cell suspensions were injected subcutaneously into the lower back of B6C3 mice. 24 h later, naive CFSE-labeled 2C and HEL transgenic T cells were adoptively transferred intravenously. 5 d later, draining lymph nodes were reisolated, and CFSE dilution was analyzed. CFSE plots were generated by gating on CD4+ (3A9)- or CD8+ (2C)-positive cells. Similar results were obtained in two additional experiments. SSC, side scatter.
To determine whether the SIY and HEL antigens were released from transduced Ag104A cells and cross-presented by B6C3-derived host cells, we used proliferation of naive TCR transgenic CD8+ and CD4+ T cells in vivo as a readout of antigen uptake and presentation by host cells. B6C3 mice were inoculated with A-HEL, A-SIY, or A-SIY/HEL cancer cells. 24 h later, CFSE-labeled naive CD8+ 2C T cells or CD4+ 3A9 T cells were adoptively transferred into the inoculated mice, and 5 d later, draining lymph nodes were reisolated to analyze proliferation of adoptively transferred T cells (Fig. 1 E). CFSE-labeled 2C T cells proliferated in lymph nodes draining inocula of A-SIY or A-SIY/HEL cancer cells but not in lymph nodes draining inocula of A-HEL cancer cells, whereas 3A9 T cells proliferated in lymph nodes draining inocula of A-HEL or A-SIY/HEL cancer cells but not in those draining inocula of A-SIY cancer cells. Thus, in B6C3 mice, antigen-presenting host cells cross-presented SIY and HEL antigens released from the transduced Ag104A cancer cells, inducing proliferation of antigen-specific T cells.
Coexpression of SIY and HEL antigen is required for the bystander elimination of cancer cells
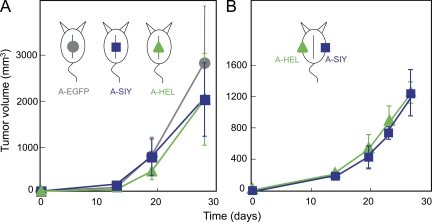
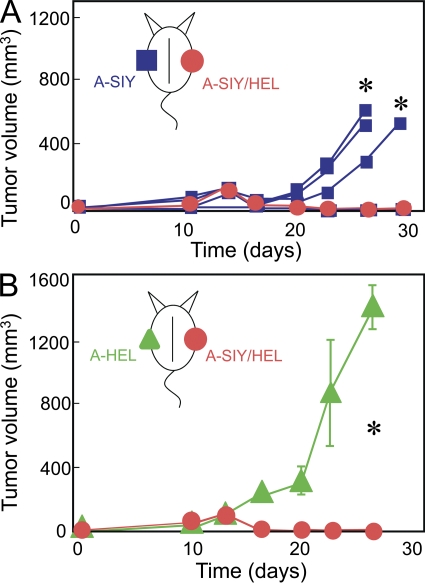
Once we had established that SIY and HEL antigen could be released from Ag104A and presented on host cells, we investigated whether endogenous, nontransgenic T cells could target antigens on tumor stromal cells and cause bystander destruction of cancer cells. Naive B6C3 mice were injected with A-SIY, A-HEL, or Ag104A cells overexpressing only EGFP as control (A-EGFP). We used a high injection dose, 5 × 105 Ag104A cells, a dose which is 50-fold higher than the 100% lethal dose of 104 Ag104A parental cancer cells. There were no differences in tumor outgrowth between A-EGFP, A-SIY, or A-HEL (Fig. 2 A), and all tumors grew progressively, killing the host within weeks. Thus, high level expression of either CD4+ or CD8+ T cell–recognized tumor antigens alone is not sufficient to allow endogenous CD4+ or CD8+ T cells to target tumor stroma and prevent tumor outgrowth. We also injected A-SIY and A-HEL into the same mouse on opposite flanks (Fig. 2 B). Again, both tumors grew progressively with similar growth kinetics.
Figure 2.
High expression of either SIY or HEL tumor antigen is insufficient to prevent tumor outgrowth. (A) 5 × 105 A-SIY (n = 4), A-HEL (n = 4), or A-EGFP (n = 4) cells were injected subcutaneously into B6C3 mice. Tumor volume was monitored. Data are representative of at least five independent experiments. (B) Mice were inoculated with A-SIY cells on one flank and A-HEL cells on the contralateral flank (n = 4). Tumor volume was monitored. Data are representative of two independent experiments. (A and B) Error bars show SEM.
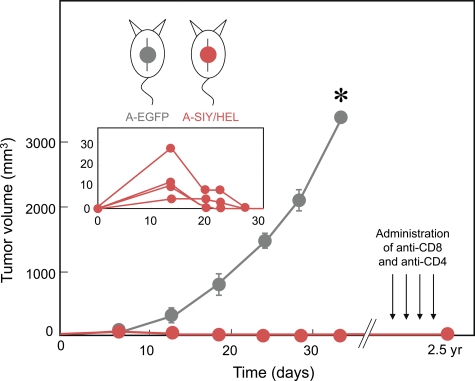
CD4+ T cells have been shown to be important for efficient CD8+ T cells responses through promotion of CD8+ T cell proliferation and clonal expansion, induction of effector function, and generation of long-lived memory. Because the presence of tumor-derived CD4+ or CD8+ T cell–recognized antigen in the same mouse but on opposite flanks had no impact on outgrowth, we next asked whether simultaneous expression of CD4+ and CD8+ T cell–recognized tumor antigens on the same cancer cell could delay or prevent tumor growth. B6C3 mice were challenged with 5 × 105 A-SIY/HEL cancer cells. Of note, A-SIY/HEL cancer cells express only half the amount of the SIY and HEL antigen in comparison with single antigen–expressing A-SIY or A-HEL (Fig. 1 D). Although small tumors initially developed within 2–3 wk to a volume of 20–40 mm3, they then regressed completely, and the mice remained tumor-free for >2 yr (Fig. 3).
Figure 3.
Cancer cells coexpressing CD8+ and CD4+ antigens are rejected. Mice were injected with either 5 × 105 A-EGFP control (n = 3) or 5 × 105 A-SIY/HEL (n = 5) cancer cells coexpressing both the SIY and HEL antigens. Tumor volume was monitored. The inset shows small tumors (40 mm3) that initially developed but then regressed completely. Error bars show SEM. A-SIY/HEL recipient mice remained tumor free for >2 yr. These mice were then treated four times with the CD4-depleting antibody GK1.5 and three times with the CD8-depleting antibody YTS196 over a 3-mo interval. Even after administration of antibodies, all mice remained tumor free. Data are representative of at least four independent experiments (*, P < 0.0001).
Because it was possible that stromal targeting might not completely eradicate cancer cells but instead arrest their proliferation, resulting in dormancy or an equilibrium state (Zhang et al., 2008), we treated these tumor-free mice with four doses of a CD4+ T cell–depleting antibody and three doses of a CD8+ T cell–depleting antibody. Although both effector and memory CD8+ T cells have been shown to be effectively depleted by antibody treatment (Zhang et al., 2008), memory CD4+ T cell depletion is inefficient compared with naive CD4+ T cell depletion (Chace et al., 1994). However, given that HEL-specific CD4+ T cells do not cause tumor regression in the absence of CD8+ T cells in our model, effective CD8+ T cell depletion should lead to outgrowth of any remaining A-SIY/HEL cancer cells. Only 1 of the 20 mice developed a tumor near but not at the site of previous tumor inoculation. Subsequent analysis of this tumor revealed that it was not caused by outgrowth of the previous A-SIY/HEL inoculum but was in fact a new spontaneous tumor: the reisolated cancer cells were (a) positive for H-2Kb and Db in addition to H-2Kk MHC class I molecules, indicating that the tumor arose from B6C3 hybrid cells, (b) negative for the Ag104A-specific 237mAb antigen, an antigen which has never been observed to be lost by Ag104A (Schietinger et al., 2006), and (c) negative for both the SIY and HEL antigens expressed by the initial inoculum. These results demonstrated that highly aggressive cancer cells could be completely eliminated by CD4+ and CD8+ T cells even though only host stromal cells were directly targeted. However, synergy of CD4+ and CD8+ T cells was required.
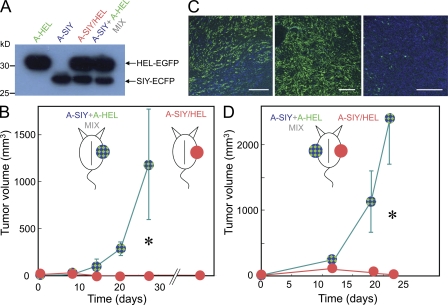
Given that the presence of tumor-derived CD4+ and CD8+ antigens in the same mouse (injection of A-SIY and A-HEL on opposite flanks; Fig. 2 B) had no effect on tumor outgrowth, whereas the A-SIY/HEL cancer cells were eradicated (Fig. 3), we reasoned that copresentation of CD4+ and CD8+ T cell–recognized antigens in the tumor-draining lymph nodes was necessary for optimal tumor elimination. To further elucidate the spatial and temporal mechanics of CD4+/CD8+ T cell stromal targeting, we injected B6C3 mice with either A-SIY/HEL cancer cells (5 × 105) or with a mixture of A-SIY (2.5 × 105) and A-HEL (2.5 × 105) cancer cells (A-SIY + A-HEL mix). Immunoblotting demonstrated that A-SIY/HEL and the A-SIY + A-HEL mix contained the same amount of antigen (Fig. 4 A). Thus, both inocula contained the same number of Ag104A cells and the same quantity of tumor antigens. The only difference was that in A-SIY/HEL, both antigens were expressed by each Ag104A cancer cell, whereas in the A-SIY + A-HEL mix, each antigen was expressed by half of the cells in the mixed cell inoculum. The same total amount of CD4+ and CD8+ T cell–recognized antigen should be cross-presented in the draining lymph nodes. Unexpectedly, the two inocula behaved very differently in vivo (Fig. 4 B). Although mice challenged with A-SIY/HEL remained tumor free for >2 yr, A-SIY + A-HEL mix tumors grew out progressively (Fig. 4 B) with growth rates similar to controls (A-EGFP, A-SIY, or A-HEL; Fig. 2 A). Microscopic analysis of A-SIY + A-HEL mix tumors at 2–3 wk showed that the growing tumors were not homogenous mixtures of A-SIY and A-HEL cancer cells, but rather had a mosaic appearance with large areas of either A-SIY–positive blue or A-HEL–positive green tumor (Fig. 4 C). The A-HEL areas of the tumor had very few A-SIY cells and vice versa. This finding that mixed cancer cells grew as mosaic tumors was demonstrated previously and is explained by the fact that after cancer cells are inoculated as cell suspensions, many of the inoculated cancer cells die after injection (Schreiber et al., 2006), and subsequently, individual cells expand clonally, creating small pockets of each cancer cell clone. In spite of the mosaicism, overall, the tumor had a 50:50 mix of A-SIY and A-HEL cancer cells; therefore, the amount of SIY and HEL antigen in draining lymph nodes was the same as that in draining lymph nodes from A-SIY/HEL tumors.
Figure 4.
CD4+ and CD8+ T cell-recognized antigens must be expressed in the same cancer cell during the effector phase. (A) Lysates from 105 cell equivalents of A-SIY/HEL, A-SIY, and A-HEL cancer cells were analyzed by immunoblotting with anti-EGFP. A mixture of 5 × 104 A-SIY and 5 × 104 A-HEL cells was also analyzed. (B) 2.5 × 105 A-SIY were mixed with 2.5 × 105 A-HEL and injected subcutaneously into B6C3 mice (n = 4). 5 × 105 A-SIY/HEL cancer cells were injected subcutaneously into other B6C3 mice (n = 6). Tumor volume was monitored. Error bars show SEM. Data are representative of five independent experiments (*, P = 0.0052). (C) Confocal fluorescence microscopy images of frozen tumor tissue sections of early (<2 wk old) A-SIY+HEL-mix tumors. Green indicates HEL-EGFP; blue indicates SIY-ECFP. Each panel shows a different region of a tumor. Bars, 200 µm. (D) 2.5 × 105 A-SIY and 2.5 × 105 A-HEL cells were mixed and injected subcutaneously into one flank, and 5 × 105 A-SIY/HEL cancer cells were injected into the opposite flank of B6C3 mice (n = 5). Tumor volume was monitored. Error bars show SEM (*, P = 0.0023).
Cooperation of CD4+ and CD8+ T cells is required during the effector phase in the local tumor microenvironment
Because similar amounts of antigen entered the draining lymph node whether coming from A-SIY + A-HEL mix or A-SIY/HEL, both inocula should have induced similar activation/proliferation of HEL-specific CD4+ T cells and SIY-specific CD8+ T cells. However, Mitchison and O’Malley (1987) showed that CD4+ and CD8+ T cell epitope linkage enhances cytolytic responses in vivo when antigens are present in low concentration. At low antigen concentrations, APCs may pick up one or the other antigen but not both, whereas at high concentrations, most APCs will pick up both antigens, even when they are not linked (or derived from the same cell). Therefore, the antitumor response to A-SIY/HEL could be explained by enhanced activation and proliferation of CD4+ and CD8+ T cells during the induction phase because in A-SIY/HEL, both antigens were present in the same cell. To clarify the role of CD4+ T cells during the induction stage versus the effector stage, we injected A-SIY/HEL cancer cells into one flank and the mixture of A-SIY and A-HEL cancer cells into the opposite flank of the same mouse (Fig. 4 D). If the requirement for CD4+ T cell help was solely during induction, CD4+ and CD8+ T cells optimally induced by the A-SIY/HEL inoculum should circulate, enter the A-SIY + A-HEL mix cancer cells, and prevent outgrowth by the mixed tumor. After 21 d, A-SIY/HEL tumors again were eliminated or very small; however, A-SIY + A-HEL mix tumors grew progressively on the contralateral flank. This result suggests that for bystander elimination of cancer cells, synergy of CD4+ and CD8+ T cells is also required during the effector phase in the local tumor stroma.
A-SIY + A-HEL mix tumors were reisolated from moribund mice, readapted to culture, and analyzed for the presence of Ag104A expressing SIY or HEL (Table I). We found that >98% of the cancer cells were HEL positive with almost complete elimination of SIY-positive cancer cells. The same selection against SIY-positive cancer cells occurred in mice that had not received A-SIY/HEL in the opposite flank, suggesting that A-SIY + A-HEL mix cancer cells also induced effective systemic anti-SIY immune responses (Table I). Thus, there was no evidence that A-SIY/HEL cancer cells were more effective in inducing anti-SIY–specific CD8+ T cell responses than A-SIY + A-HEL mix tumors. Because the aforementioned experiments demonstrated a selection against SIY-expressing cancer cells, we tested whether the systemic response induced by A-SIY/HEL was sufficient to eliminate A-SIY tumors. When mice were injected on one flank with A-SIY/HEL cancer cells and A-SIY on the opposite side, 5/5 mice rejected A-SIY/HEL tumors; however, 3/5 mice developed progressive contralateral A-SIY tumors (Fig. 5 A and Table I). We reisolated and analyzed the A-SIY tumors for SIY expression, and all of the tumors retained the SIY antigen (Table I); thus, the anti-SIY immune response induced by A-SIY/HEL inocula was not sufficient to prevent A-SIY tumor outgrowth in mice. Similarly, when mice were injected on one flank with A-SIY/HEL cancer cells and A-HEL on the opposite site, A-SIY/HEL tumors were rejected, whereas all of the contralateral A-HEL tumors grew progressively (Fig. 5 B), retaining the HEL antigen. The A-SIY tumors that did grow out (3/5) grew at a slower rate than the A-HEL tumors (Fig. 5, A and B), thus the A-SIY/HEL inoculum induced a systemic anti-SIY response. However, without the presence of the HEL antigen in the cancer cells, stromal targeting and tumor elimination was insufficient. Collectively, these results demonstrate that for optimal stromal targeting, cooperation of CD4+ T cells and CD8+ T cells is not only required during the induction phase but also during the effector phase in the local tumor microenvironment.
Table I.
Expression of SIY and HEL antigens on reisolated tumors
| Inoculation on side A | Inoculation on side B | Animal number | Tumor on side B | |||
| Days of growth | Volume | Antigen expression (% of tumor cells positive) | ||||
| SIY only | HEL only | |||||
| mm3 | % | % | ||||
| Experiment I | ||||||
| A-SIY/HEL | A-SIY + A-HEL | 1 | 21 | 2,600 | <1 | 93 |
| A-SIY/HEL | A-SIY + A-HEL | 2 | 21 | 1,950 | <1 | 96 |
| A-SIY/HEL | A-SIY + A-HEL | 3 | 21 | 4,900 | <1 | 89 |
| A-SIY/HEL | A-SIY + A-HEL | 4 | 21 | 2,600 | <1 | 95 |
| None | A-SIY + A-HEL | 1 | 18 | 1,300 | <1 | 94 |
| None | A-SIY + A-HEL | 2 | 18 | 1,200 | <1 | 95 |
| Experiment II | ||||||
| A-SIY/HEL | A-SIY | 1 | 26 | 520 | 80 | NA |
| A-SIY/HEL | A-SIY | 2 | 26 | 644 | 83 | NA |
| A-SIY/HEL | A-SIY | 3 | 29 | 520 | 82 | NA |
NA, not applicable. 5 × 105 A-SIY/HEL or A-SIY cancer cells or 2.5 × 105 A-SIY mixed with 2.5 × 105 A-HEL cancer cells were injected subcutaneously into opposite flanks of B6C3 mice as indicated. Tumor growth was monitored. Tumors on side B were readapted to culture and analyzed by flow cytometry for expression of the SIY and HEL antigens. Data are representative of at least two independent experiments.
Figure 5.
Insufficient systemic immune responses are induced in mice rejecting A-SIY/HEL cancers. (A) 5 × 105 A-SIY and 5 × 105 A-SIY/HEL cancer cells were injected into opposite flanks of B6C3 mice (n = 5). Tumor volume was monitored. Each line represents an individual mouse. Although 2/5 mice rejected the contralateral A-SIY inocula, 3/5 mice showed progressive growth of A-SIY tumors, albeit at a slower rate than A-SIY tumors growing in mice without a contralateral A-SIY/HEL inocula (*, P = 0.0449 vs. A-SIY without contralateral A-SIY/HEL inocula). The three A-SIY tumors were readapted to culture, and a summary of the flow analysis is shown in Table I (experiment II). Data are representative of two independent experiments. (B) 5 × 105 A-SIY/HEL and 5 × 105 A-HEL cancer cells were injected into opposite flanks of B6C3 mice (n = 4). Tumor volume was monitored. Error bars show SEM (*, P = 0.0007). Data are representative of two independent experiments.
CD4+ and CD8+ T cells eliminate antigen-loss variants (ALVs) embedded in the tumor microenvironment of A-SIY/HEL cancer cells
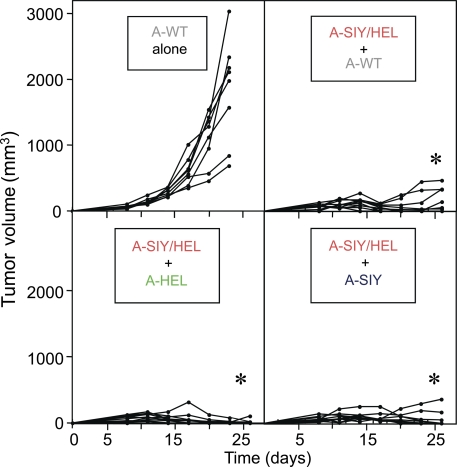
Cancer cells can evade immune-mediated destruction through mechanisms such as down-regulation of MHC or antigen-processing machinery. Another strategy is down-regulation or deletion of the targeted tumor antigens. The escape of ALVs is a major obstacle to T cell–based immunotherapy for cancer. Because the indirect destruction of A-SIY/HEL cancer cells was so powerful, we asked whether stromal targeting coordinated by CD4+ T cells and CD8+ T cells would eliminate ALVs embedded within the tumor stroma. Thus, naive B6C3 mice were injected with subcutaneous inocula of 2.5 × 105 Ag104A cancer cells (A-WT) either alone or mixed with 2.5 × 105 A-SIY/HEL cancer cells. As shown in Fig. 6 (top), A-WT inocula alone (8/8) grew progressively and killed the host within 4 wk. However, when A-WT cells were mixed with A-SIY/HEL cancer cells, tumor growth was significantly repressed, and tumors did not exceed a tumor volume of 250 mm3 after 28 d. Only 3/10 inocula developed larger tumors at later times. We also tested mixtures of A-HEL or A-SIY with A-SIY/HEL cells. Only 1/10 inocula of A-HEL and A-SIY/HEL cancer cells and 2/10 inocula of A-SIY and A-SIY/HEL cancer cells formed small tumors (Fig. 6, bottom).
Figure 6.
ALVs lacking the SIY and HEL antigens are arrested or eliminated when mixed with A-SIY/HEL cancer cells in the same inoculum. (top) Naive B6C3 mice received bilateral subcutaneous inocula of 2.5 × 105 Ag104A WT (A-WT) cells either alone (n = 4) or mixed with A-SIY/HEL cancer cells (n = 5). (bottom) Mice received A-HEL (n = 5) or A-SIY (n = 5) mixed with A-SIY/HEL cancer cells. Tumor growth was monitored. Each line represents an individual mouse (*, P < 0.0001 vs. A-WT alone). Data are representative of two independent experiments.
These results are in contrast to those seen with the A-SIY + A-HEL mix tumors in which large tumors developed that consisted of >90% A-HEL. The mix tumor is analogous to a tumor made up of two different ALVs without any parental cancer cells expressing both the CD4+ and CD8+ T cell antigens. Because both antigens could be presented in the draining lymph nodes, a CD8+ T cell response was induced that could eliminate most of the A-SIY cancer cells, but because these are mosaic tumors (Fig. 4 C), the lack of consistent, concomitant CD4+ T cell antigen throughout the tumor meant that the immune response was not sufficient to prevent outgrowth of the CD8+ T cell ALV (A-HEL). Because ALVs arise from expansion of mutated clones within the parental tumor, this scenario in which the tumor starts with two ALVs and no parental cancer cells is unlikely. But even when 50% of the tumor consisted of cancer cells lacking CD4+ or CD8+ T cell antigens or both antigens (Fig. 6), concomitant expression of CD4+ and CD8+ T cell antigens in the other 50% of the cancer cells was sufficient to prevent or control their growth.
DISCUSSION
In conclusion, we have shown that aggressive cancer cells lacking MHC molecules for direct presentation can be eradicated by antigen-specific T cell targeting tumor stroma, and thus immune stromal targeting has enormous potential as a tool for cancer immunotherapy. Stromal cells do not mutate to evade detection but can present tumor antigens, making them T cell targets and resulting in bystander destruction of cancer cells. Using naive, immunocompetent hosts, we found that this bystander killing of cancers through stromal targeting requires the cooperation of CD4+ and CD8+ T cell not only during the induction phase, but during the effector phase. Although the role of CD4+ T cells in immune induction and memory T cell formation has been extensively studied, only recently was it shown that CD4+ T cells are also important in mobilizing effector CTL to the peripheral sites of infection (Nakanishi et al., 2009). The study presented here together with the findings of Nakanishi et al. (2009) reveal that CD4+ T cell help is not only restricted to priming or memory formation of CD8+ T cells and imply a new role for CD4+ T cells for the effector phase that has not been previously described. The exact role of CD4+ T cells during the effector phase in our tumor model remains to be elucidated. It is possible that the cooperation of CD8+ and CD4+ T cell during the effector phase leads to direct killing of stromal cells cross-presenting the antigen, which could result in the destruction of cancer cells as bystanders. Another equally plausible explanation is that T cell–stromal cell interactions lead to the release of IFN-γ and TNF that then act on the stroma and/or the cancer cells directly or indirectly, for example by the induction of inducible nitric oxide synthase, monokine induced by IFN-γ, and/or IP-10. Although this study has demonstrated bystander killing of cancer cells expressing tumor-specific antigens, it is likely that this mechanism of cancer cell elimination is also applicable to cancers expressing immunogenic self-antigens. Nonetheless, our results suggest that cancer immunotherapy strategies targeting stroma by T cells must ensure that CD4+ T cells provide help for CD8+ T cells during the effector phase in the tumor microenvironment.
MATERIALS AND METHODS
Mice and cell lines.
Ag104A, a spontaneous fibrosarcoma isolated from an aging mouse (female, C3H/HeN), was previously described (Ward et al., 1989) and is recognized by the tumor-specific monoclonal antibody 237mAb (Ward et al., 1989; Schietinger et al., 2006). B6C3 F1 mice were obtained from Charles River, 2C Rag1−/− mice were provided by J. Chen (Massachusetts Institute of Technology, Cambridge, MA), and HEL transgenic mice (TgN[TcrHEL3A9]Mmd) were obtained from The Jackson Laboratory. All mice were maintained in a specific pathogen-free barrier facility at the University of Chicago in accordance with the Institutional Animal Care and Use Committee guidelines.
Flow cytometric analysis.
Flow cytometric analysis was performed using FACSCalibur, FACScan, LSRII, and DakoCytomation CyanADP and analyzed with FlowJo software (BD). Cells were sorted using Cytomation MoFlo HTS (Dako) and FACS Aria (BD).
Antibodies, plasmids, and retroviral infections.
Anti-EGFP monoclonal antibody (clone JL-8; BD) was used for immunoblotting. PE-anti–mouse H-2Kb (AF6-88.5), PE-anti–mouse H-2Kk (36-7-5), PE-anti–mouse I-Ak (11-5.2), PE-anti–mouse Vβ8.1, 8.2 TCR (MR5-2), APC-anti–mouse CD4 (L3T4), and APC-rat anti–mouse CD8a (53-6.7) antibodies used for flow cytometry were obtained from BD. The retroviral vector pMFG was obtained from R.C. Mulligan (Whitehead Institute for Biomedical Research, Cambridge, MA), and retroviral infections were performed as described previously (Pear et al., 1993).
Tumor challenge and tumor reisolation and CD8+ and CD4+ T cell depletion.
Cultured cancer cells were trypsinized and washed with serum-free DME. Cancer cells in suspension were injected subcutaneously into the back of B6C3 F1 mice. Tumor volumes were measured along three orthogonal axes (a, b, and c) and calculated as tumor volume = abc/2. For confocal microscopy analysis, pieces of established tumors were excised and fixed for 2 d in 4% paraformaldehyde solution, embedded in OCT, and stored at −80°C. 15–20-µm-thick frozen sections were analyzed by confocal microscopy. A-SIY/HEL–challenged mice that had been tumor free for 2 yr were treated with anti-CD4–depleting antibody (clone GK1.5), followed by repeated injections of anti-CD8–depleting antibody (clone YTS 169.4.2).
Adoptive transfer of CFSE-labeled transgenic T cells.
B6C3 F1 mice were challenged with 1 × 107 cancer cells. The next day, splenocytes were isolated from 2C and HEL mice. Red blood cells were lysed with NH4Cl for 10 min, and the remaining lymphocytes at a concentration of 5 × 107/ml were labeled with 10 µM CFSE in HBSS at 37°C for 10 min. Cells were washed twice, and CFSE-labeled cells were injected intravenously into the retro-orbital plexus of B6C3 F1 mice in a 0.1-ml vol. 5–6 d later, inguinal lymph nodes were isolated, and single-cell suspensions were prepared. Flow cytometric analysis for CFSE proliferation was performed on CD8+ (for 2C) or CD4+ (for HEL) gated T cells.
IFN-γ treatment.
1–2 × 105 Ag104A cells were plated in 6 wells, and 20 ng/ml IFN-γ was added. 48 h later, cells were analyzed by flow cytometry for I-Ak expression. The tumor cell line B16 F10 was used as a positive control for IFN-γ–induced up-regulation of MHC class I (H-2Kb).
Preparation of single cell suspensions from murine tumors.
1–2-wk-old Ag104A tumors were surgically excised, minced into 1–2-mm pieces, and incubated for 30 min at 37°C in DME and 1% FCS, containing 1 mg/ml collagenase D, and 0.25 mg/ml DNase I. The digested tissue was filtered through a 70-µm nylon filter mesh, resulting in a single-cell suspension. Cells were washed twice with cold DME and stained with antibodies for subsequent flow cytometric analysis.
Generation of pMFG (SIY)3-ECFP and pMFG EGFP-HEL44–68-6xHis vectors.
To generate pMFG-ECFP, ECFP was amplified from pECFP N1 (Takara Bio Inc.) using the primers 5′-GCGCCATGGTGAGCAAGGGCGAGGAGC-3′ and 5′-GCGGATCCTTACTTGTACAGCTCGTCCATGCCG-3′, digested with NcoI and BamHI (New England Biolabs, Inc.), and ligated into pMFG digested with NcoI–BamHI. To generate pMFG (SIY)3-ECFP, the minigene SIYRYYGL-AAY trimer was cut from pLEGFP-SIY as previously described (Yu et al., 2003) with NcoI and ligated into pMFG-ECFP. To generate pMFG EGFP-HEL44–68-6xHis, a minigene encoding a 25-mer peptide sequence [N44RNTDGSTDYGILQINSRWWCNDGR68] encompassing the HEL epitope peptide sequence with known stimulatory capability was synthesized by annealing the partially complementary single-stranded oligonucleotides 5′-CCCTGTACAAGAACCGTAACACCGATGGGAGTACCGACTACGGAATCCTACAGATC-3′ and 5′-CCCGGATCCTTACCTGCCATCGTTGCACCACCAGCGGCTGTTGATCTGTAGGATTC-3′, converting into blunt double-stranded DNA using DNA polymerase I Large (Klenow) fragment, digested, and subsequently ligated into the vector pTAC2-EGFP (Philip et al., 2010) to generate pTAC2-EGFP-HEL44–68. EGFP-HEL44–68 was then cut and ligated into pMFG to generate pMFG-EGFP-HEL44–68. Next, a 6xHis tag was added to the C terminus of the EGFP-HEL44–68 antigen to generate the vector pMFG EGFP-HEL44–68-6xHis.
Confocal microscopy.
EGFP (HEL antigen) and ECFP (SIY antigen) fluorescence of tumors (15–20-µm frozen sections) were imaged with a spectral 2-photon confocal microscope (SP5 AOBS; Leica).
Statistical analyses.
Statistical analyses were performed with Prism version 5.0 (GraphPad Software, Inc.) using unpaired two-tailed Student’s t tests. A p-value of <0.05 was considered statistically significant.
Acknowledgments
We thank H. Booras for technical assistance and B. Engels, A. Arina, and D.A. Rowley for advice and helpful discussions. We thank the University of Chicago Cancer Research Center Core facilities, especially C. Labno for expert assistance with microscopy and R. Duggan, D. Leclerc, and M. Olson for expert assistance with cell sorting and flow cytometric analysis.
This work was supported by National Institutes of Health grants P01-CA97296, R01-CA22677, and R01-CA37516 to H. Schreiber and HD 07009 to M. Philip.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- ALV
- antigen-loss variant
- ECFP
- enhanced CFP
- EGFP
- enhanced GFP
- HEL
- hen egg lysozyme
References
- Allen P.M., Matsueda G.R., Evans R.J., Dunbar J.B., Jr, Marshall G.R., Unanue E.R. 1987. Identification of the T-cell and Ia contact residues of a T-cell antigenic epitope. Nature. 327:713–715 10.1038/327713a0 [DOI] [PubMed] [Google Scholar]
- Allinen M., Beroukhim R., Cai L., Brennan C., Lahti-Domenici J., Huang H., Porter D., Hu M., Chin L., Richardson A., et al. 2004. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 6:17–32 10.1016/j.ccr.2004.06.010 [DOI] [PubMed] [Google Scholar]
- Andersson M.L., Stam N.J., Klein G., Ploegh H.L., Masucci M.G. 1991. Aberrant expression of HLA class-I antigens in Burkitt lymphoma cells. Int. J. Cancer. 47:544–550 10.1002/ijc.2910470412 [DOI] [PubMed] [Google Scholar]
- Broderick L., Yokota S.J., Reineke J., Mathiowitz E., Stewart C.C., Barcos M., Kelleher R.J., Jr, Bankert R.B. 2005. Human CD4+ effector memory T cells persisting in the microenvironment of lung cancer xenografts are activated by local delivery of IL-12 to proliferate, produce IFN-gamma, and eradicate tumor cells. J. Immunol. 174:898–906 [DOI] [PubMed] [Google Scholar]
- Carson R.T., Vignali K.M., Woodland D.L., Vignali D.A. 1997. T cell receptor recognition of MHC class II-bound peptide flanking residues enhances immunogenicity and results in altered TCR V region usage. Immunity. 7:387–399 10.1016/S1074-7613(00)80360-X [DOI] [PubMed] [Google Scholar]
- Castellino F., Germain R.N. 2006. Cooperation between CD4+ and CD8+ T cells: when, where, and how. Annu. Rev. Immunol. 24:519–540 10.1146/annurev.immunol.23.021704.115825 [DOI] [PubMed] [Google Scholar]
- Chace J.H., Cowdery J.S., Field E.H. 1994. Effect of anti-CD4 on CD4 subsets. I. Anti-CD4 preferentially deletes resting, naive CD4 cells and spares activated CD4 cells. J. Immunol. 152:405–412 [PubMed] [Google Scholar]
- Egilmez N.K., Hess S.D., Chen F.A., Takita H., Conway T.F., Bankert R.B. 2002. Human CD4+ effector T cells mediate indirect interleukin-12- and interferon-gamma-dependent suppression of autologous HLA-negative lung tumor xenografts in severe combined immunodeficient mice. Cancer Res. 62:2611–2617 [PubMed] [Google Scholar]
- Frey A.B. 1995. Rat mammary adenocarcinoma 13762 expressing IFN-gamma elicits antitumor CD4+ MHC class II-restricted T cells that are cytolytic in vitro and tumoricidal in vivo. J. Immunol. 154:4613–4622 [PubMed] [Google Scholar]
- Fukino K., Shen L., Patocs A., Mutter G.L., Eng C. 2007. Genomic instability within tumor stroma and clinicopathological characteristics of sporadic primary invasive breast carcinoma. JAMA. 297:2103–2111 10.1001/jama.297.19.2103 [DOI] [PubMed] [Google Scholar]
- Gao F.G., Khammanivong V., Liu W.J., Leggatt G.R., Frazer I.H., Fernando G.J. 2002. Antigen-specific CD4+ T-cell help is required to activate a memory CD8+ T cell to a fully functional tumor killer cell. Cancer Res. 62:6438–6441 [PubMed] [Google Scholar]
- Greenberg P.D. 1991. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv. Immunol. 49:281–355 10.1016/S0065-2776(08)60778-6 [DOI] [PubMed] [Google Scholar]
- Greenberg P.D., Cheever M.A., Fefer A. 1981. Eradication of disseminated murine leukemia by chemoimmunotherapy with cyclophosphamide and adoptively transferred immune syngeneic Lyt-1+2- lymphocytes. J. Exp. Med. 154:952–963 10.1084/jem.154.3.952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg P.D., Kern D.E., Cheever M.A. 1985. Therapy of disseminated murine leukemia with cyclophosphamide and immune Lyt-1+,2- T cells. Tumor eradication does not require participation of cytotoxic T cells. J. Exp. Med. 161:1122–1134 10.1084/jem.161.5.1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho W.Y., Cooke M.P., Goodnow C.C., Davis M.M. 1994. Resting and anergic B cells are defective in CD28-dependent costimulation of naive CD4+ T cells. J. Exp. Med. 179:1539–1549 10.1084/jem.179.5.1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H.M., Winter H., Urba W.J., Fox B.A. 2000. Divergent roles for CD4+ T cells in the priming and effector/memory phases of adoptive immunotherapy. J. Immunol. 165:4246–4253 [DOI] [PubMed] [Google Scholar]
- Hung K., Hayashi R., Lafond-Walker A., Lowenstein C., Pardoll D., Levitsky H. 1998. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med. 188:2357–2368 10.1084/jem.188.12.2357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaklamanis L., Gatter K.C., Hill A.B., Mortensen N., Harris A.L., Krausa P., McMichael A., Bodmer J.G., Bodmer W.F. 1992. Loss of HLA class-I alleles, heavy chains and beta 2-microglobulin in colorectal cancer. Int. J. Cancer. 51:379–385 10.1002/ijc.2910510308 [DOI] [PubMed] [Google Scholar]
- Keene J.A., Forman J. 1982. Helper activity is required for the in vivo generation of cytotoxic T lymphocytes. J. Exp. Med. 155:768–782 10.1084/jem.155.3.768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marincola F.M., Jaffee E.M., Hicklin D.J., Ferrone S. 2000. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv. Immunol. 74:181–273 10.1016/S0065-2776(08)60911-6 [DOI] [PubMed] [Google Scholar]
- Matsumoto N., Yoshida T., Yamashita K., Numata Y., Okayasu I. 2003. Possible alternative carcinogenesis pathway featuring microsatellite instability in colorectal cancer stroma. Br. J. Cancer. 89:707–712 10.1038/sj.bjc.6601141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison N.A., O’Malley C. 1987. Three-cell-type clusters of T cells with antigen-presenting cells best explain the epitope linkage and noncognate requirements of the in vivo cytolytic response. Eur. J. Immunol. 17:1579–1583 10.1002/eji.1830171109 [DOI] [PubMed] [Google Scholar]
- Moinfar F., Man Y.G., Arnould L., Bratthauer G.L., Ratschek M., Tavassoli F.A. 2000. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer Res. 60:2562–2566 [PubMed] [Google Scholar]
- Momburg F., Degener T., Bacchus E., Moldenhauer G., Hämmerling G.J., Möller P. 1986. Loss of HLA-A,B,C and de novo expression of HLA-D in colorectal cancer. Int. J. Cancer. 37:179–184 10.1002/ijc.2910370203 [DOI] [PubMed] [Google Scholar]
- Monach P.A., Meredith S.C., Siegel C.T., Schreiber H. 1995. A unique tumor antigen produced by a single amino acid substitution. Immunity. 2:45–59 10.1016/1074-7613(95)90078-0 [DOI] [PubMed] [Google Scholar]
- Mumberg D., Monach P.A., Wanderling S., Philip M., Toledano A.Y., Schreiber R.D., Schreiber H. 1999. CD4(+) T cells eliminate MHC class II-negative cancer cells in vivo by indirect effects of IFN-gamma. Proc. Natl. Acad. Sci. USA. 96:8633–8638 10.1073/pnas.96.15.8633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muranski P., Boni A., Antony P.A., Cassard L., Irvine K.R., Kaiser A., Paulos C.M., Palmer D.C., Touloukian C.E., Ptak K., et al. 2008. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 112:362–373 10.1182/blood-2007-11-120998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi Y., Lu B., Gerard C., Iwasaki A. 2009. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 462:510–513 10.1038/nature08511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C.A., Viner N.J., Young S.P., Petzold S.J., Unanue E.R. 1996. A negatively charged anchor residue promotes high affinity binding to the MHC class II molecule I-Ak. J. Immunol. 157:755–762 [PubMed] [Google Scholar]
- Patocs A., Zhang L., Xu Y., Weber F., Caldes T., Mutter G.L., Platzer P., Eng C. 2007. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N. Engl. J. Med. 357:2543–2551 10.1056/NEJMoa071825 [DOI] [PubMed] [Google Scholar]
- Pear W.S., Nolan G.P., Scott M.L., Baltimore D. 1993. Production of high-titer helper-free retroviruses by transient transfection. Proc. Natl. Acad. Sci. USA. 90:8392–8396 10.1073/pnas.90.18.8392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Diez A., Joncker N.T., Choi K., Chan W.F., Anderson C.C., Lantz O., Matzinger P. 2007. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood. 109:5346–5354 10.1182/blood-2006-10-051318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip M., Schietinger A., Schreiber H. 2010. Ribosomal versus non-ribosomal cellular antigens: factors determining efficiency of indirect presentation to CD4+ T cells. Immunology. 130:494–503 10.1111/j.1365-2567.2010.03258.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z., Blankenstein T. 2000. CD4+ T cell—mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFN gamma receptor expression by nonhematopoietic cells. Immunity. 12:677–686 10.1016/S1074-7613(00)80218-6 [DOI] [PubMed] [Google Scholar]
- Qiu W., Hu M., Sridhar A., Opeskin K., Fox S., Shipitsin M., Trivett M., Thompson E.R., Ramakrishna M., Gorringe K.L., et al. 2008. No evidence of clonal somatic genetic alterations in cancer-associated fibroblasts from human breast and ovarian carcinomas. Nat. Genet. 40:650–655 10.1038/ng.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schietinger A., Philip M., Yoshida B.A., Azadi P., Liu H., Meredith S.C., Schreiber H. 2006. A mutant chaperone converts a wild-type protein into a tumor-specific antigen. Science. 314:304–308 10.1126/science.1129200 [DOI] [PubMed] [Google Scholar]
- Schreiber K., Rowley D.A., Riethmüller G., Schreiber H. 2006. Cancer immunotherapy and preclinical studies: why we are not wasting our time with animal experiments. Hematol. Oncol. Clin. North Am. 20:567–584 10.1016/j.hoc.2006.03.001 [DOI] [PubMed] [Google Scholar]
- Sha W.C., Nelson C.A., Newberry R.D., Kranz D.M., Russell J.H., Loh D.Y. 1988. Selective expression of an antigen receptor on CD8-bearing T lymphocytes in transgenic mice. Nature. 335:271–274 10.1038/335271a0 [DOI] [PubMed] [Google Scholar]
- Smith M.E., Bodmer W.F., Bodmer J.G. 1988. Selective loss of HLA-A,B,C locus products in colorectal adenocarcinoma. Lancet. 1:823–824 10.1016/S0140-6736(88)91682-0 [DOI] [PubMed] [Google Scholar]
- Spiotto M.T., Schreiber H. 2005. Rapid destruction of the tumor microenvironment by CTLs recognizing cancer-specific antigens cross-presented by stromal cells. Cancer Immun. 5:8. [PubMed] [Google Scholar]
- Spiotto M.T., Rowley D.A., Schreiber H. 2004. Bystander elimination of antigen loss variants in established tumors. Nat. Med. 10:294–298 10.1038/nm999 [DOI] [PubMed] [Google Scholar]
- Toes R.E., Ossendorp F., Offringa R., Melief C.J. 1999. CD4 T cells and their role in antitumor immune responses. J. Exp. Med. 189:753–756 10.1084/jem.189.5.753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vago L., Perna S.K., Zanussi M., Mazzi B., Barlassina C., Stanghellini M.T., Perrelli N.F., Cosentino C., Torri F., Angius A., et al. 2009. Loss of mismatched HLA in leukemia after stem-cell transplantation. N. Engl. J. Med. 361:478–488 10.1056/NEJMoa0811036 [DOI] [PubMed] [Google Scholar]
- Ward P.L., Koeppen H., Hurteau T., Schreiber H. 1989. Tumor antigens defined by cloned immunological probes are highly polymorphic and are not detected on autologous normal cells. J. Exp. Med. 170:217–232 10.1084/jem.170.1.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernert N., Löcherbach C., Wellmann A., Behrens P., Hügel A. 2001. Presence of genetic alterations in microdissected stroma of human colon and breast cancers. Anticancer Res. 21:2259–2264 [PubMed] [Google Scholar]
- Wick M., Dubey P., Koeppen H., Siegel C.T., Fields P.E., Chen L., Bluestone J.A., Schreiber H. 1997. Antigenic cancer cells grow progressively in immune hosts without evidence for T cell exhaustion or systemic anergy. J. Exp. Med. 186:229–238 10.1084/jem.186.2.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P., Spiotto M.T., Lee Y., Schreiber H., Fu Y.X. 2003. Complementary role of CD4+ T cells and secondary lymphoid tissues for cross-presentation of tumor antigen to CD8+ T cells. J. Exp. Med. 197:985–995 10.1084/jem.20021804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P., Lee Y., Liu W., Chin R.K., Wang J., Wang Y., Schietinger A., Philip M., Schreiber H., Fu Y.X. 2004. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat. Immunol. 5:141–149 10.1038/ni1029 [DOI] [PubMed] [Google Scholar]
- Zhang B., Bowerman N.A., Salama J.K., Schmidt H., Spiotto M.T., Schietinger A., Yu P., Fu Y.X., Weichselbaum R.R., Rowley D.A., et al. 2007. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J. Exp. Med. 204:49–55 10.1084/jem.20062056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Zhang Y., Bowerman N.A., Schietinger A., Fu Y.X., Kranz D.M., Rowley D.A., Schreiber H. 2008. Equilibrium between host and cancer caused by effector T cells killing tumor stroma. Cancer Res. 68:1563–1571 10.1158/0008-5472.CAN-07-5324 [DOI] [PubMed] [Google Scholar]