Constant regions of antibodies influence toxin neutralization in a manner dependent on FcγR.
Abstract
One important function of humoral immunity is toxin neutralization. The current view posits that neutralization results from antibody-mediated interference with the binding of toxins to their targets, a phenomenon viewed as dependent only on antibody specificity. To investigate the role of antibody constant region function in toxin neutralization, we generated IgG2a and IgG2b variants of the Bacillus anthracis protective antigen–binding IgG1 monoclonal antibody (mAb) 19D9. These antibodies express identical variable regions and display the same specificity. The efficacy of antibody-mediated neutralization was IgG2a > IgG2b > IgG1, and neutralization activity required competent Fcγ receptor (FcγR). The IgG2a mAb prevented lethal toxin cell killing and mitogen-activated protein kinase/extracellular signal-regulated kinase kinase cleavage more efficiently than the IgG1 mAb. Passive immunization with IgG1 and IgG2a mAb protected wild-type mice, but not FcγR-deficient mice, against B. anthracis infection. These results establish that constant region isotype influences toxin neutralization efficacy of certain antibodies through a mechanism that requires engagement of FcγR. These findings highlight a new parameter for evaluating vaccine responses and the possibility of harnessing optimal FcγR interactions in the design of passive immunization strategies.
mAbs have become an important therapeutic strategy in toxin neutralization. A historically established role of antibody-mediated immunity includes the ability to interfere with toxins by binding and interfering with its interactions with host cells. However, despite the fact that toxin neutralization was first described in the 1890s (von Behring and Kitasato, 1991), key elements of this process remain poorly understood. For example, the role, if any, of antibody constant regions and Fc receptors (FcRs) on antibody-mediated toxin neutralization remains largely unexplored for most toxin–antitoxin systems.
Understanding the role of FcR is particularly important for the currently available anthrax vaccine, which is believed to mediate protection by eliciting antibodies that neutralize the protective antigen (PA) component of anthrax toxin yet is poorly immunogenic and does not protect all hosts against experimental anthrax (Wang and Roehrl, 2005). The neutralizing antibody response to PA is the best established correlate of vaccine-mediated protection against anthrax (Little et al., 1997; Reuveny et al., 2001). Established mechanisms of antibody-mediated neutralization of PA are blocking PA binding to its receptor (Little et al., 1997) and slowing down the proteolytic digestion of this protein by furin (Rivera et al., 2006). Hence, each mechanism is currently thought to depend only on the interaction of antibody and toxin. Consistent with this notion, several studies have shown that protection against an anthrax challenge is based on antibody-neutralizing toxin components and that Fab fragments of antibodies induced by vaccination are sufficient for protection (Maynard et al., 2002; Wild et al., 2003; Laffly et al., 2005; Mabry et al., 2005; Harvill et al., 2008). These findings can be interpreted as indicating that neither FcR binding nor the Fc domain is essential for toxin neutralization. However, a role for FcR in anthrax toxin neutralization was suggested by the recent observations that polyclonal serum was more effective in the presence of competent receptor function (Verma et al., 2009) and that a neutralizing mAb lost efficacy in hosts with blocked FcRs (Vitale et al., 2006). In contrast, a subset of mAbs to anthrax toxin was suggested to potentiate toxin activity through their interaction with FcRs (Mohamed et al., 2004). These observations hint at a complex role for FcR in antibody-mediated toxin neutralization.
Four different classes of FcRs for IgG have been defined on murine and human immune effector cells, including the high-affinity FcγRI and the low-affinity FcγRII and FcγRIII (for review see Nimmerjahn and Ravetch, 2006). In mice, these receptors are categorized into two groups: the activating receptors FcγRI, FcγRIII, and FcγRIV and the inhibitory receptor FcγRIIB. Antibody-antigen binding events lead to effector functions that mediate antibody-dependent cytotoxicity or complement activation by FcR engagement on macrophages, dendritic cells, natural killer cells, neutrophils, and other cell types. Receptor assembly and signal transduction for all activating FcγRs in mice is mediated by the γ chain (Ra et al., 1989; Kurosaki et al., 1991). Deletion of the γ chain leads to loss of the ability to phagocytose antibody-coated particles, despite retaining the ability to bind (Takai et al., 1994).
In this paper, we report that IgG1, IgG2a, and IgG2b mAbs derived from one B cell precursor, sharing identical variable regions, differ in toxin neutralization capacity. In addition, none of the IgG subclasses was effective in altering lethal toxin (LeTx) cytotoxicity in FcRγ−/− and FcRγ chain/FcγRII double knockout (FcRγ−/−/RIIB−/−) BM-derived macrophages (BMMs) and in Bacillus anthracis infection in mice, suggesting that, for this mAb set, protection is modulated by Fc-dependent functions. These results imply that antibody isotype is an important variable in toxin neutralization through effects mediated by Fc domain interactions with FcR.
RESULTS
Generation of IgG2a and IgG2b switch variants
The 19D9 hybridoma makes a PA-neutralizing IgG1 antibody. We used the ELISA spot assay to detect spontaneously arising variant cells producing new downstream isotypes of IgG1. Hybridoma 19D9 spawned IgG2a- and IgG2b-producing cells at rates of 10−5 and 4 × 10−5, respectively, which is a typical isotype switching rate for a hybridoma (Spira and Scharff, 1992; Spira et al., 1994). We then attempted to enrich switch variant–producing cells by sib selection (Spira et al., 1984; Spira and Scharff, 1992). Despite successive rounds of enrichment and increased switching frequency rates, the variants remained too rare to recover. Consequently, we used FACS in combination with immunofluorescence staining for surface-associated antibody to identify a cell fraction significantly enriched in isotype variant cells. Viable cells staining for surface-associated IgG2a or IgG2b were isolated by cell sorting on the basis of surface fluorescence intensity, a step which enriched the fraction of hybridomas producing variant cells and reduced the fraction of IgG1 producers. Two rounds of soft agar cloning further stabilized the isotype variant cells. Analysis of the supernatants of the 19D9-derived IgG2a- and IgG2b-secreting clones revealed no trace of IgG1. DNA sequence analysis confirmed that the heavy and light chain variable VH7183 and VKBD2 regions of mAbs 19D9 IgG1, IgG2a, and IgG2b were identical.
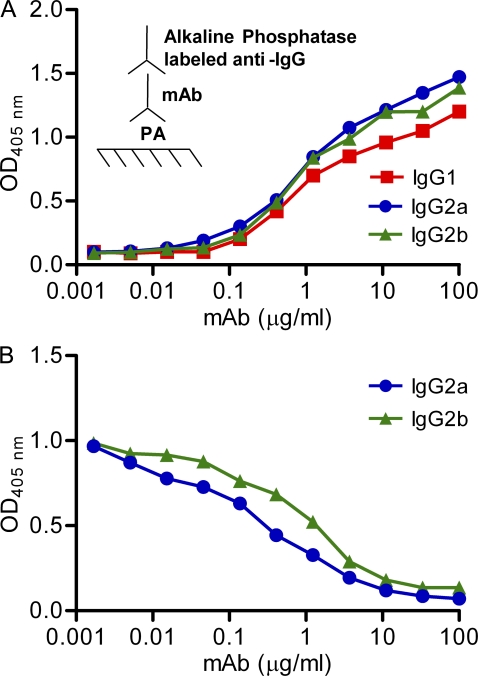
Given that isotype can affect fine specificity (Torres and Casadevall, 2008), the IgG2a and IgG2b switch variants were compared with the parent 19D9 IgG1 for their antigen binding characteristics. The observed IgG1, IgG2a, and IgG2b binding to PA was the same (Fig. 1 A), and IgG1 binding was inhibited similarly by the addition of IgG2a or IgG2b (Fig. 1 B). Furthermore, reactivity of the three IgG subclasses with a panel of mutated peptide mimetics that represented the epitope revealed similar binding (unpublished data). To further highlight that the constant regions did not affect fine specificity of the antibodies, we performed plasmon resonance analysis of IgG subclass binding to PA, which revealed that the dissociation constants (Kds) of IgG1, IgG2a, and IgG2b were 0.109, 0.149, and 0.108 µM, respectively, using the Langmuir fitting model to analyze the data. Similar results were obtained if the plasmon resonance data were analyzed by the two-state reaction-fitting model. These Kd values are essentially identical and were considered to be within the experimental error of the method.
Figure 1.
Specific and competitive binding of IgG1, IgG2a, and IgG2b mAbs to PA. (A) IgG1, IgG2a, and IgG2b mAbs were added at different concentrations to PA bound to polystyrene plates. Binding was measured by ELISA. (B) A constant amount of IgG1 (2 µg/ml) and increasing concentrations of IgG2a and IgG2b were allowed to compete for binding to PA bound to polystyrene plates. Data shown are representative of three independent experiments.
Protective efficacy of 19D9 IgG1, IgG2a, and IgG2b mAbs on LeTx-mediated macrophage cytotoxicity
The cellular intoxication process is initiated by PA binding to cell surface receptors known as TEM8 (tumor endothelial marker 8; Bradley et al., 2001) and CMG2 (human capillary morphogenesis protein 2; Scobie et al., 2003). After cleavage at its N-terminal domain by a furin-like protease and heptamerization, the heptamer–receptor complex then acts as a docking platform that subsequently translocates the enzymatic components LF and EF into the cytosol via acidified endosomes (Gordon et al., 1988; Abrami et al., 2003, 2004). LF is a zinc metalloprotease that has a very specific and limited set of substrates (Klimpel et al., 1994) and specifically cleaves the N terminal of mitogen-activated protein kinase kinases (MAPKKs) or ERK (extracellular signal-regulated kinase; Duesbery et al., 1998; Vitale et al., 1998). Although the anthrax toxin receptors are present in high concentrations on numerous cell types, macrophages are considered key cells in the toxin pathogenic effects because macrophage depletion can protect mice against toxin infusion (Welkos et al., 1986). Consequently, we focused on macrophages when evaluating the cytotoxic effects of LeTx and the efficacy of antibody-mediated protection. As reported previously (Abboud et al., 2009), the 19D9 IgG1 mAb protected macrophages from LeTx-mediated cytotoxicity. However, when the activity of 19D9 IgG2a and 19D9 IgG2b was compared with the parent IgG1, it was apparent that isotype switching had generated more effective neutralizing antibodies such that their relative efficacy was IgG2a > IgG2b > IgG1 (Fig. 2 A). To examine the concentration-dependent effects of LeTx in the presence of a constant amount of mAb (10 µg/ml), RAW 264.7 macrophages were treated with varying concentrations of LF and PA as indicated (Fig. 2, B and C). In the presence of a constant PA concentration (100 ng/ml), the IgG1 mAb half-maximal blockade of cell death was observed at a LF concentration of 6.25 ng/ml, whereas in the presence of nonlimiting concentrations of LF the half-maximal blockade of cell death was measured at a PA concentration of 25 ng/ml. Overall, RAW 264.7 macrophages were protected against LeTx in the presence of IgG2a mAb. The latter was able to protect cells at all concentrations of PA and LF, further distinguishing the enhanced efficacy of this isotype relative to IgG1.
Figure 2.
LeTx-neutralizing activity of IgG subclasses on RAW264.7 macrophages. Cells were treated with or without LeTx (100 ng/ml PA and 100 ng/ml LF) in the presence or absence of varying amounts of mAb (A), or treated with or without 10 µg/ml mAb in the presence or absence of varying amounts of LF (PA at fixed saturating concentrations, 100 ng/ml; B) or PA (LF at fixed saturating concentrations, 100 ng/ml; C). Viability was established by MTT assay 4 h after LT treatment. Cells treated with IgG2a and IgG2b mAbs showed statistically significant elevation in protection with cells treated with IgG1 mAb. Significance was determined by Student’s t test. Data shown are representative of three independent experiments. Means and standard deviations of triplicates are shown from one representative experiment out of three (A).
Protective efficacy of 19D9 IgG1, IgG2a, and IgG2b mAbs requires FcRs
The observations on antibody-mediated LeTx neutralization made with RAW 264.7 macrophage-like cells were repeated with macrophages from C57BL/6 mice to ascertain their relevance with primary cells. Furthermore, we used additional measures of toxicity by analyzing MAPKK cleavage, which is performed by LF once it is internalized with the result that three MPKK signaling pathways are disrupted (Duesbery et al., 1998; Chopra et al., 2003). LeTx uptake and subsequent MAPKK cleavage also occur in cells that are resistant to LeTx killing (Duesbery et al., 1998; Schaeffer and Weber, 1999), suggesting that cells differ in their response to MAPKK cleavage (Moayeri et al., 2004; Bonuccelli et al., 2005). To determine whether BMMs from C57BL/6 mice were killed by LeTx, these cells were treated with or without LeTx (100 ng/ml PA and 100 ng/ml LF) and analyzed for cell viability at various time intervals after LeTx exposure. Consistent with previous results (Muehlbauer et al., 2007), C57BL/6-derived BMMs were killed within 72 h of LeTx treatment (unpublished data). Next, we tested LeTx susceptibility of C57BL/6-derived BMMs in the presence of mAb. Consistent with our data, IgG2a mAb exhibited increased neutralization activity as compared with IgG1 and IgG2b mAbs (P < 0.01; Fig. 3 A). Our findings indicate that antibody-mediated protection against anthrax toxin is dependent on the isotype of the antibody.
Figure 3.
LeTx-neutralizing activity of IgG1, IgG2a, and IgG2b mAbs is dependent on FcR interactions. (A and B) BMMs from C57BL/6 (A) and FcRγ−/−/RIIB−/− (B) mice were plated in 96-well plates at a density of 8 × 104 cells per well in maintenance medium supplemented with FCS, 2 h before treatment with LeTx (100 ng/ml PA and 100 ng/ml LF). The indicated concentrations of mAb were added at the same time with LeTx. After 72 h, cell death was assayed with MTT (3,(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide; controls were cells treated with LeTx only or with media alone). Representative data are shown from three independent experiments. Significance was determined by a Student’s t test. (C) BMMs from C57BL/6 mice were plated as described in A and B, and the indicated dilutions of each mAb were used to neutralize LeTx in the presence of anti-FcRII/III (mAb 2.4G2). (D) RAW264.7 macrophages were treated with toxin, as described in A and B, and 10 µg/ml IgG2a mAb in the presence of blocking mAb to FcγRIII (2.4G2), FcγRIV (9E9), and/or a competitor inhibitor to block FcγRI (IgG2a Std.). (E) RAW264.7 macrophages were gated and analyzed by flow cytometric analysis for FcγRI, FcγRII, FcγRIII, and FcγRIV expression with specific antibodies. Histograms shaded in gray represent the specific FcγR, and histograms shaded in black indicates unstained cells used as a control. (F and G) C57BL/6 and FcRγ−/−/RIIB−/− were treated with 100 ng/ml LeTx and 10 µg/ml mAb. MEK-3 cleavage was determined by Western blotting with anti–MEK-3. Data shown are representative of three independent experiments. Means and standard deviations of triplicates are shown from one representative experiment out of three (A and D).
To determine whether FcγRs played a role in antibody-mediated LeTx neutralization, we compared the ability of antibody to protect BMMs derived from FcRγ−/− and FcRγ−/−/RIIB−/− mice. None of the mAbs protected macrophages deficient in FcγR from LeTx-mediated cytotoxicity (Fig. 3 B). The same result was obtained when wild-type C57BL/6-derived BMMs were pretreated with mAb 2.4G2 that blocks FcγRII/III (Fig. 3 C). To further establish that the IgGs mediate their effector activities in vitro through their engagement of FcγRs and to determine the relative contributions of these FcRs to toxin neutralization, we treated RAW macrophages with a blocking mAb (9E9) to FcγRIV alone and in combination with a blocking mAb to FcγRIIIs or soluble IgG2a (used as a competitor inhibitor to block FcγRI) and measured the effect of 19D9 IgG2a effect on LeTx toxicity. We note that it is possible to compete IgG immune complexes with sufficient soluble IgG, where this inhibitory capacity is a function of the relative affinities of soluble IgG and the IgG immune complex for the FcγR as well as of the concentrations of both ligands (Furriel et al., 1992). As expected, macrophages were not protected when all three activating FcγRs were blocked. Similarly, simultaneous blockade of both FcγRI and FcγRIV and FcγRIII and FcγRIV substantially decreased the protective activity of IgG2a (Fig. 3 D). Monolayers treated with only the mAb to FcγRIV rendered the cells less susceptible to LeTx cytotoxicity, suggesting that the IgG2a–PA complex was taken up via FcγRI or FcγRIII. The expression pattern of all four FcγRs was determined and FcγRI, FcγRII, FcγRIII, and FcγRIV expression was detected on RAW macrophages (Fig. 3 E).
To determine whether mAb interfered with the uptake or proteolytic activity of LF, we treated C57BL/6 and FcRγ−/−/RIIB−/− macrophages with LeTx and analyzed the amount of MAPK/ERK kinase (MEK)3 found in lysates of cells after various treatments by immunoblot (Fig. 3 G). The two groups were normalized based on the media-only condition, and Fig. 3 F illustrates that toxin leads to a 99 and 78% decrease of MEK-3 expression in wild-type and FcRγ−/−/RIIB−/−, respectively. In addition, mAb 19D9 IgG1 was less able to indirectly inhibit the LF-mediated rapid cleavage and degradation of MEK-3 in C57BL/6 cells than the IgG2a mAb (1–2 h), as demonstrated by the loss of uncleaved proteins and more cleaved MEK-3. In wild-type macrophages, IgG2a rescued 67% of the protein expression and was ∼10-fold more effective than that in IgG1. As measured by the MTT assay (Fig. 4 A), the decrease in cell death coincided with the decrease in MEK cleavage by IgG2a. Collectively, our findings confirm that IgG2a is more effective at toxin neutralization relative to IgG1 using MEK cleavage as the readout for protection.
Figure 4.
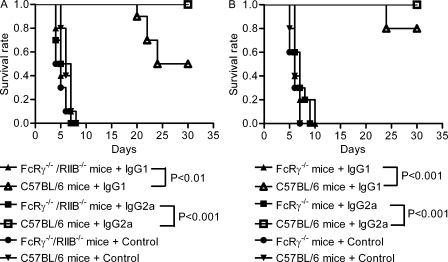
IgG1 and IgG2a mAbs protect wild-type but not FcRγ−/−/RIIB−/− or FcRγ−/− mice from B. anthracis infection. (A) Kaplan-Meier analysis of survival rate of FcRγ−/−/RIIB−/− or wild-type mice treated with IgG1 and IgG2a mAbs or isotype control antibodies (n = 10). (B) Kaplan-Meier analysis of survival rate of FcRγ−/− or wild-type mice treated with IgG1 and IgG2a mAbs or isotype control antibodies (n = 10). The log-rank test was used to determine significance between wild-type and FcRγ−/−/RIIB−/− and FcRγ−/− mouse groups treated with IgG1 and IgG2a mAbs. Data shown are representative of two independent experiments.
In FcRγ−/−/RIIB−/−, IgG1 and IgG2a rescued 22.7 and 33.2% of the MEK-3 expression, respectively, whereas IgG2a was ∼1.5-fold more effective than IgG1. MEK-3 cleavage was evident within 1 h of treatment with LeTx, illustrating that mAb failed to inhibit the internalization of the PA–LF toxin complex and ultimate LF-mediated proteolytic inactivation of MAPKKs. These results were consistent with the MTT results and show that activating FcγRs play a critical and necessary role in antibody-mediated toxin neutralization, such that neutralization requires both mAb binding to the toxin and activation of macrophages through engagement of the FcγRs.
Protective efficacy of 19D9 IgG1 and IgG2a mAbs on infected FcRg−/−/RIIB−/− mice
To ascertain whether the in vitro observations translated into in vivo effects, we evaluated the efficacy of the IgG1 and IgG2a isotype in protecting against B. anthracis on wild-type FcRγ−/−/RIIB−/− mice and FcRγ−/− mice infected with B. anthracis Sterne. Consistent with the in vitro results, the administration of both IgG1 and IgG2a reduced mortality in wild-type mice, with IgG2a being the more potent of the two, but neither reduced mortality in FcRγ−/−/RIIB−/− or FcRγ−/− mice (P < 0.001; Fig. 4).
Uptake of IgG2a-PA by BMMs
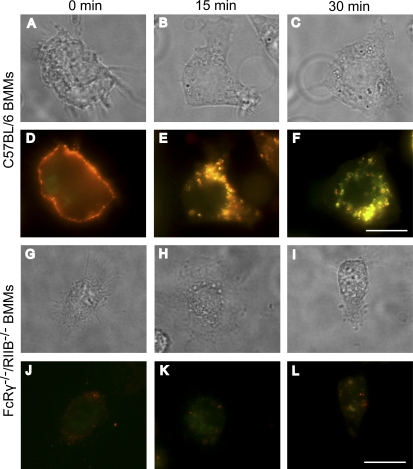
We examined the uptake of PA bound to IgG2a by BMMS from wild-type and FcRγ−/−/RIIB−/− mice using fluorescent microscopy. Cells were cooled to 4°C and labeled with low concentrations of IgG2a and PA. Cells were washed to remove unbound IgG2a-PA. Internalization was then initiated by incubation at 37°C for varying time intervals and the cells were then fixed. PA colocalized with IgG2a in wild-type BMMs (Fig. 5, D–F). IgG2a-PA were initially located on the cell surface and, with time, there was an increase in the amount of IgG2a-PA found in the cell interior such that the majority was intracellular after 15 min. As expected, BMMs lacking FcRs were unable to efficiently internalize IgG2a bound to PA. In these mice, there was very little colocalization of IgG2a with PA, which is most likely a result of PA binding and entering through its receptors TEM8 or CMG2 (Fig. 5, J–L).
Figure 5.
Colocalization between IgG2a mAb and PA. Wild-type and FcRγ−/−/RIIB−/− BMMs were cooled to 4°C, incubated with Alexa Fluor 488–conjugated IgG2a (red) and Alexa Fluor 568–conjugated PA (green), washed, and warmed to 37°C for the indicated time points. The cells were then fixed and analyzed by fluorescence microscopy. Images in A–C were acquired in a bright field and correspond to D–F, respectively, whereas G–I correspond to J–L, respectively. Data shown are representative of three independent experiments. Bars, 10 µm.
Interfering with actin and preventing phagocytosis does not eliminate antibody-mediated protection
Cross-linking of FcRs by multivalent ligands can initiate both phagocytosis and endocytosis; therefore, we sought to define if a phagocytic event was involved when these receptors are cross-linked by IgG-PA. Hence, we tested whether cells treated with cytochalasin d (concentrations from 2 to 10 µM), a classical inhibitor of phagocytosis by inhibiting actin polymerization, could prevent the uptake of antibody-PA, as measured by cell viability with the MTT assay. Blocking phagocytosis did not interfere with the uptake of antibody and PA, as the protective efficacy of both mAbs tested was unaltered (Fig. 6 A). To examine whether FcγR activation was sufficient for protection, we evaluated the toxicity of LeTx for RAW 264.7 cells placed on plates coated with a control irrelevant IgG1. Stimulation of FcγR alone was not sufficient to protect the cells from LeTx but did reduce their susceptibility when PA-neutralizing antibody was added, suggesting that prestimulation of FcγR enhanced antibody-mediated protection (Fig. 6 B). This data suggests that the mechanism by which FcγRs mediate protection involves a signaling pathway that activates these receptors and that the differences in efficacy between IgG1 and IgG2a strongly reflect a quantitative difference because IgG2a binds to a higher affinity receptor and can result in a greater Fc-mediated signal.
Figure 6.
Inhibition of phagocytosis by cytochalasin d and up-regulation of FcγRs by IgG1 control antibody on RAW264.7 macrophages. (A) RAW264.7 macrophages that were treated with LeTx and mAb or LeTx, mAb, and 2 µM cytochalasin d (Cyto D). Cells were preincubated for 45 min with cytochalasin d. Cell viability was determined as indicated in the Cell viability assays section of Materials and methods. (B) RAW264.7 macrophages were either not preincubated or preincubated overnight with an IgG1 control antibody (used to preactivate FcRs) and next-day treated with toxin and mAb. Data shown are representative of two independent experiments. Means and standard deviations of triplicates are shown from one representative experiment out of two (A).
DISCUSSION
The importance of antibody isotype in antibody-mediated toxin neutralization has not been explored. Perhaps this is not surprising because many Fab fragments have been shown to neutralize toxins. However, the interaction of FcRs with the antibody Fc fragment results in a variety of cellular functions depending on the cell in which they are expressed. Therefore, both the FcR type engaged and the antibody isotype can affect the responses of effector cells (Wirthmueller et al., 1992; Ravetch, 1997). Generating the IgG2a and IgG2b isotype switch variants of an IgG1 anti-PA mAb showed isotype differences in the ability of the mAbs to protect murine macrophages from LeTx-mediated cytotoxicity. The IgG2a and IgG2b mAbs were significantly more effective at reducing LeTx cytotoxic effects than the parent IgG1 mAb. This result revealed that isotype was pertinent for protecting against toxin and that the efficacy of a mAb can be enhanced by changing its constant region. For this antibody set, the V region amino sequences were identical and their serological behavior for peptides containing the epitope and for PA in plasmon resonance analysis was essentially indistinguishable, indicating conservation of fine specificity and affinity with isotype change. Hence, the V region identical set of isotype-switched variants behaves differently than two other mAbs where isotype change affects specificity (Torres and Casadevall, 2008) and suggests that this phenomenon is probably associated with only certain V region combinations. Consequently, we can ascribe differences in this antibody set to differences in the Fc region and its interaction with FcR.
Antibody responses to PA in the mouse are predominantly IgG1. Thus, the predominant natural isotype response to PA is not the most effective toxin neutralizing isotype. The major FcRs engaged by IgG1, IgG2a, and IgG2b on macrophages are different, suggesting at least one explanation for the differences in efficacy observed for the three isotypes. The details of these interactions have been extensively studied in the murine system (for review see Nimmerjahn and Ravetch, 2006). The high-affinity FcγRI exclusively binds IgG2a and the low-affinity FcγRIII binds IgG1, IgG2a, and IG2b (Ravetch and Kinet, 1991; Hulett et al., 1994). FcγRIV binds IgG2a and IgG2b with intermediate affinity but does not interact with IgG1 or IgG3 antibody isotypes (Nimmerjahn et al., 2005). Our results imply that FcγRI, FcγRIII, and FcγRIV can each play a role in FcγR-dependent neutralization. Furthermore, our data does exclude the possibility that FcγRIIB can contribute to this neutralization. It is noteworthy that for viral infections the IgG2a subclass plays a dominant role in antibody-mediated protection (Baldridge and Buchmeier, 1992; Markine-Goriaynoff et al., 2002). In this paper, we also illustrate that the IgG2a subclass is prominent in enhanced protection to experimental anthrax.
The observation that mAb failed to protect FcRγ−/− and FcRγ−/−/RIIB−/− mice from B. anthracis challenge while protecting mice with competent FcγRs demonstrates that the FcRγ chain is essential for antibody-mediated protection. The fact that IgG2a mAb was able to protect wild-type mice and not mice deficient in FcγRs illustrates that binding to PA is a necessary, but not sufficient, condition for complete protection. Western blot results showed that the mAbs indeed prevented LF-mediated MEK-3 cleavage in wild-type cells, yet the opposite was observed in the FcRγ−/−/RIIB−/− cells, which in turn illustrates that without FcγR engagement, mAbs are not able to decrease the LeTx-mediated cytotoxicity. Thus, the interaction of the Fc portion of the mAb with FcγR reduced LeTx cytotoxicity by eliminating PA on the cell surface, thereby decreasing the PA bound to the cell and the LF internalized into the cell cytosol.
Upon engagement of FcγRs, the mode of internalization of immune complexes is determined by the size of the bound complexes (Daëron, 1997). Small immune complexes are internalized by receptor-mediated endocytosis, whereas internalization of large opsonized particles occurs via phagocytosis (Mellman et al., 1984; Aderem and Underhill, 1999). The molecular mechanisms underlying FcγR-mediated phagocytosis and endocytosis differ markedly. Endocytosis requires assembly of clathrin at the site of receptor clustering, yet phagocytosis involves formation of an actin phagocytic cup and is blocked by inhibitors such as cytochalasin d (Koval et al., 1998). In our experiments, inhibition of actin polymerization by cytochalasin d did not interfere with the protective activity of IgG mAbs, suggesting that the IgG–PA complex was taken up via FcγR-mediated endocytosis. However, the prestimulation of FcγR significantly enhanced antibody-mediated protection of cells. Although the mechanism for this effect was not studied in detail, possible explanations include up-regulation of FcR to the cell surface, which consequently contributed to the antibody-dependent clearance of toxin. Alternatively, FcγR engagement with consequent signal transduction activation could alter the metabolism of the macrophage-like cell to reduce its susceptibility to LeTx. Although we could not prove a role for clathrin in the internalization of IgG2a–PA complexes because inhibiting assembly of clathrin would also interfere with toxin entry that occurs via a clathrin-dependent pathway, it is nevertheless likely that clathrin-mediated endocytosis is responsible for internalizing the bound complexes. Confocal microscopy revealed that cross-linking of surface-bound IgG2a–PA caused relocation of the receptor–ligand complex to an intracytoplasmic compartment within wild-type cells, with essentially completed internalization occurring within 15 min. We have also shown that this redistribution is affected in cells deficient in FcγR, revealing that it is necessary for FcγR to bind and mediate endocytosis of IgG2a–PA complex.
The results of this study established that protective anti-toxin antibodies can be made more protective by switching their constant regions to other isotypes and that toxin neutralization is FcγR dependent, at least for a subset to toxin-binding antibodies which includes those used in this study. These observations establish an important precedent because they suggest new options and complexity for antibody-mediated neutralization. For example, our results suggest that there may be optimal combinations of antibody specificity, constant region usage, and FcR type that would differ depending on the particular antibody and toxin pair. Nevertheless, important questions remain regarding the precise mechanism for toxin neutralization after FcγR engagement. In this regard, we hypothesize that mAb binding to PA and FcγRs may result in an immune complex that is endocytosed and sorted to lysosomes for degradation. The inability to clear toxin infection in mice deficient in FcγRs may reflect defective endocytosis and/or loss in functional effects on signal transduction pathways mediated by activating FcRs. The differences in efficacy between IgG1, IgG2a, and IgG2b strongly suggest a difference in Fc-mediated effector functions. Consequently, isotype selection and response are important considerations when designing passive antibody therapies and evaluating the response to toxin-neutralizing vaccines.
MATERIALS AND METHODS
B. anthracis strains.
B. anthracis Sterne strain 34F2 (pXO1 positive, pXO2 negative) was obtained from A. Hoffmaster (Centers for Disease Control and Prevention, Atlanta, GA). Bacterial cultures were grown from frozen stocks in brain heart infusion broth (BD) at 37°C for 18 h (mid- to late-logarithmic phase) with shaking. Before experiments with the bacterial strain, the cells were washed. Recombinant PA and LF were obtained from Wadsworth Center (New York State Department of Health, Albany, NY).
Macrophages and hybridomas.
Hybridoma cell lines were grown in DME (H-21; Invitrogen) supplemented with 10% (vol/vol) FCS, 5% National Cancer Tissue Culture 109 (BioWhittaker), 1% nonessential amino acids (Invitrogen), and 1% penicillin. J774A.1 and RAW 264.7 macrophages (American Type Culture Collection) were maintained in DME and RPMI media, respectively, supplemented with 10% FCS and 1% penicillin (Mediatech). Murine BMMs were isolated by flushing femoral and tibial bones of C57BL/6, FcRγ chain knockout (FcRγ−/−), and FcRγ chain/FcγRII double knockout (FcRγ−/−/RIIB−/−) mice. Cells were differentiated into macrophages (BMMs) by incubation for 6 d in DME supplemented with 1% penicillin, 100 µg/ml streptomycin, 10 mM Hepes, 2.0 mM L-glutamine, 0.05 mM 2-mercaptoethanol, 1% mM of nonessential amino acids, 10% FCS, and 20% conditioned medium from a confluent culture of L929 fibroblasts as a source of CSF-1 (LCCM). After removal of nonadherent cells, macrophages were recovered by washing plates with cold PBS plus 5 mM EDTA. BMMs were maintained in DME supplemented with 2 mM L-glutamine, 0.05 mM 2-mercaptoethanol, 1% nonessential amino acids, 10 mM Hepes, 1% penicillin, 100 µg/ml streptomycin, 10% FCS, and 10% L929 preconditioned media.
Reagents.
Caspase inhibitors BOC-D-FMK and BOC-D-CMK (EMD) were reconstituted in DMSO and used at a concentration of 40 µM. Rat anti–mouse CD16/CD32 mAb clone 2.4G2 (FcγRII and FcγRIII blocking mAb) was obtained from BD. mAb 9E9, which specifically blocks FcγRIV, has been previously described (Kaneko et al., 2006). Cytochalasin d was obtained from Sigma-Aldrich.
Ab purification.
The 19D9 IgG1 was generated from mice immunized with a galactoxylomannan-PA conjugate made for other studies (De Jesus et al., 2009). For in vitro studies, antibodies were purified by protein G chromatography (Thermo Fisher Scientific) and sterilized by filtering through a 0.2-mm-pore-size membrane (Sigma-Aldrich).
Isolation of isotype-switched variants.
IgG2a and IgG2b isotype switch variants of mAb 19D9 were identified using the ELISA spot assay and isolated by the sib selection technique. The assays were performed as previously reported with minor modifications (Greene et al., 1990; Spira and Scharff, 1992). In brief, 103 cells were plated per well in a 96-well plate and grown for 3 d. Then, half of the cells were moved and subsequently examined for IgG2a or IgG2b switch variants using ELISA spot assay. The corresponding well with the highest number of spots was plated out in a new 96-well plate at progressively lower cell densities and duplicate plates were screened again for the frequency of switch variants. For the ELISA spot assay, 50 µl/well of the anti–mouse antibody against the corresponding isotype (SouthernBiotech) diluted 1:500 in coating buffer (20 mM K2HPO4, 10 mM KH2PO4, 1 mM Na-EDTA, 0.8% NaCl, and 0.01% NaN3) was added to coat polystyrene plates and blocked with 2% BSA in TBS at 4°C. Plates were washed in TBS with 0.1% Tween 20 (TBS-T), rinsed with DME, and 4 × 103 cells were plated and incubated at 37°C for 16–18 h. The plates were washed with TBS-T, and 50 µl/well of biotinylated antibody against the corresponding isotype (SouthernBiotech) diluted 1:500 in 2% BSA TBS was added for 2 h at 37°C, washed, and then treated with the Vectastain avidin phosphatase amplification system as per the manufacturer’s instructions (Vector Laboratories). The plates were washed and incubated with 1 mg/ml 5-bromo-4 chloro-3indoyl phosphate (Amresco) in AMP buffer (0.2 mg/mlCl2, 0.01% Triton X-405, and 9.6% 2-amino-2-methyl-1-propanol, pH 9.8). Plates were washed and rinsed with distilled H2O. The spots were counted with a dissecting microscope and the median frequencies of switching were calculated.
Cells for analysis or selection were centrifuged and washed once with FACS buffer supplemented with 1× PBS, 1% FCS, and 1 mM EDTA in sterile water. Cells were resuspended at 2.5 × 107 cells/200 µl. Isotype-specific FITC-conjugated goat anti–mouse antibody was added and allowed to react for 30 min at 4°C. Cells were resuspended at 5 × 107cells/ml for analysis and sorting. Fluorescence cell sorting was performed using a FACSAria (BD) equipped with four lasers and coherent sapphire with a 100-mm quartz nozzle. FITC-labeled detection reagents were excited by the 488-nm spectral line of a 100-mW diode-pumped solid-state laser. FITC-positive cells were collected using a 525-nm band pass filter. For acquisition and statistical analysis, 6.1.1 Diva software (BD) was used. Live cells were gated using the forward and side scatter parameters. Background fluorescence was determined using unlabeled cells as a control. Cells sorted for bulk enrichment were collected directly into a 12 × 75 mm Falcon tube (BD) containing 1 ml of culture medium supplemented with 20% FCS. Cells were centrifuged and resuspended in 1 ml of culture medium and then grown in a single well of a 24-well plate.
Cell subcloning was performed using soft agar–containing 0.3–0.4% SeaPlaque agarose (FMC Bioproducts). First, 4 ml IMDM cloning medium (IMDM with 20% FCS) containing 0.4% SeaPlaque agarose was introduced into a 60-mm culture plate (BD). After solidification at 4°C for 10 min, 1 ml of the same medium containing 1,000 cells was laid over the top of the soft agar and put at 4°C for 10 min. Cells were grown at 37°C for 5–7 d. Clones were randomly picked and placed into a 96-well plate.
RNA and RT-PCR.
Cells (5–10 × 106) were lysed with 1 ml Trizol reagent (Invitrogen) and RNA extracted according to the manufacturer’s instructions. About 1 µg of total RNA was reverse transcribed in a 20-µl reaction using the Superscript II kit (Invitrogen). Universal 5′ (sense) variable region and specific 3′ (antisense) constant region primers were used in a PCR reaction to generate cDNA encoding the variable domains of mAbs. The primers are as follows: 3′MsCγ, 5′-AGACCTATGGGGCTGTTGTTTTGGC-3′; 3′MsCµ, 5′-GACATTTGGGAAGGACTGACTCTC-3′; 3′MsCK, 5′-TGGATACAGTTGGTGCAGCATCAGC-3′; 5′VHuni, 5′-TGAGGTGCAGCTGGAGGAGTC-3′; and 5′VKuni, 5′-GACATTCTGATGACCCAGTCT-3′. PCR products were cloned and the heavy and light chain of the 19D9 IgG2a and IgG2b switch variants were sequenced to confirm that they were identical to those of the parent IgG1.
Peptide synthesis.
To map the functional linear epitopes of PA, biotinylated soluble peptides representing the entire length of PA were synthesized as 15-mers, overlapping by 10 residues (total of 145 peptides) according to guidelines of the manufacturer (Invitrogen). Biotinylation of the peptides was performed by coupling d-biotine to the N terminus. The identity of each of the peptides was confirmed by mass spectral analysis. The peptides were >98% pure, as assessed by high-pressure liquid chromatography analysis, and peptides were supplied as a white powder soluble in water. All peptides were stored at a concentration of 1 mg/ml.
ELISAs for PA and peptides.
Antibody binding to PA and PA-derived peptides was studied by ELISAs. In brief, the wells in polystyrene plates (Costar) were coated overnight with 1 µg/ml of recombinant PA in PBS. The plates were then blocked with 2% BSA/PBS and incubated for 1 h at 37°C. After the wells were washed with 0.1% Tween 20 in PBS (PBST), serial dilutions of purified MAbs were added and incubated at 37°C for 1 h. After washing, bound antibodies were detected with isotype-specific alkaline phosphatase–conjugated goat anti–mouse reagent (SouthernBiotech). The plates were then washed and developed by adding substrate and determining absorbance at 405 nm.
For the peptide ELISAs, polystyrene plates were coated with 5 µg/ml streptavidin (100 µl/well) and kept overnight at 37°C. Then, plates were blocked with 2% BSA in PBS (200 µl/well) for 1 h at 37°C and washed with 0.1% PBST. Subsequently, 5 µg/ml biotinylated peptides were added and incubated at room temperature for 1 h. After three washes with PBST, mAb was added at a 1:100 dilution in blocking buffer and incubated for 2 h. The plates were again washed with PBST. Alkaline phosphatase–conjugated goat isotype-specific antibody was diluted 1:1,000 in blocking buffer, added to the plates, and incubated for 1 h at 37°C. After another wash, alkaline phosphatase substrate was added to each well, color was allowed to develop for 20 min, and the absorbance at 405 nm was measured. These experiments were performed at least three times for each mAb. The background of each individual serum or mAb was determined in parallel, using streptavidin-coated peptide-free wells. The cut-off value used for binding was an absorbance three times the mean background value.
Competition ELISAs.
mAb–mAb competition ELISAs were used to investigate the specificity of PA mAbs as previously described (Casadevall et al., 1992). In brief, we developed separate assays with different combinations of a variable amount of one mAb mixed with a constant amount of a second different isotype mAb and allowed them to bind to PA immobilized in a polystyrene plate. Binding of the mAbs was detected by isotype-specific alkaline phosphatase–conjugated goat anti–mouse reagent. In all instances, incubations were done at 37°C for 1 h and absorbances were measured at 405 nm.
Biacore analysis.
Surface plasmon resonance (SPR) analysis was performed using a Biacore 3000 instrument. The 19D9 IgG1 mAb and its IgG2a and IgG2b switch variants in 10 mM 2-(N-morpholino) ethanesulphonic acid (Sigma-Aldrich), pH 6.0, were immobilized on a CM5 chip using 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide N-hydroxy-succinimide chemistry. The mAb solutions were added as a 5 µg/ml solution until an amount equivalent to 1,000 response units was obtained. Recombinant PA (3.9–1,000 nM) in 0.2 mM K2HPO4/KH2PO4, pH 7.4, 130 mM KCl, and 0.005% Tween 20 was used at a flow rate of 5, 10, and 20 µl/min. A solution of 50 mM NaOH was used as the regeneration buffer between runs. Data were analyzed using BIAevaluation software (version 3.2; Biacore) to yield the Kd using the Langmuir and two-state reaction fitting models.
Cell viability assays.
Cell viability was analyzed by MTT (3,(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide) assay as described previously (Abboud and Casadevall, 2008). In brief, 6–8 × 104 cells/well were plated in 96-well plates. In the case of FcγR cross-linking assay, culture plates were coated with IgG1 mAb 18B7 diluted with PBS to 20 µg/ml, and then cells were plated. Plates were incubated at 37°C for 2 h or 4°C overnight. For all assays, cells were treated with LeTx (100 ng/ml PA and 100 ng/ml LF) in the presence or absence of mAb. After LeTx treatment, MTT solution (5 mg/ml MTT in PBS) was added directly to wells at different time points and incubated at 37°C for 4 h. The reaction was terminated by the addition of the extraction buffer (12% SDS and 45% DMF) and incubated overnight. Absorbance was measured at 570 nm.
Western blotting was performed on cell lysates. For collection of cell lysates, 5 × 105 cells were plated in 24-well plates in 500 µl of media, followed by treatment with LeTx in the presence or absence of mAb. At the indicated time points, culture medium was removed and the cells lysed in the wells with RIPA buffer (Boston BioProducts) supplemented with a cocktail of protease inhibitors (Roche). Lysates were then mixed with SDS buffer and denatured at 100°C for 3 min. Equivalent amounts of protein were separated on precast SDS-Tris HCl polyacrylamide gels (Bio-Rad Laboratories) and transferred to nitrocellulose membrane (0.20-µm pore size) by electrophoretic transfer. Membranes were probed with anti–MEK-3 polyclonal antibody (Santa Cruz Biotechnology, Inc.) and mAb to actin (Sigma-Aldrich; Ac-40). Polyclonal HRP-conjugated anti–rabbit antibodies were used as secondary antibodies (Santa Cruz Biotechnology, Inc.), and blots were developed using the ECL chemiluminescence kit (Thermo Fisher Scientific) according to the manufacturer’s instructions.
Animal experiments.
C57BL/6 (wild type) mice were obtained from The Jackson Laboratory. FcRγ chain knockout (FcRγ−/−) mice and FcRγ chain and FcγRII double knockout (FcRγ−/−/RIIB−/−) mice had been backcrossed to C57BL/6 for 10 generations. Mice used for protection experiments were 6–8 wk of age. 10 mice per group were given 0.5 mg of IgG2a or isotype control antibody. mAb was administered via intraperitoneal injection 3 h before intravenous challenge with 104 B. anthracis Sterne, and mouse deaths were recorded daily. All experiments were done in compliance with federal laws and institutional guidelines and have been approved by the Albert Einstein College of Medicine.
PA and IgG internalization assays.
BMMs from wild-type and FcRγ−/−/RIIB−/− mice were plated on MatTek plates, labeled with serum-free medium containing 25 µg/ml Alex Fluor 488–conjugated IgG2a and Alexa Fluor 568–conjugated PA and incubated at 4°C for 2 h to enable binding. The cells were washed, warmed to 37°C for varying amounts of time, washed, and then fixed with 2% paraformaldehyde in PBS for 10 min at room temperature. The cells were subsequently imaged by epifluorescence microscopy on an inverted microscope (Axioskop 200; Carl Zeiss, Inc.) equipped with a cooled charge-coupled device using a 63× 1.4-NA objective with a 1.6× optovar. Images were acquired using the same exposure times and microscopic settings and processed by AxioVision software (version 4.6; Carl Zeiss, Inc.).
Statistics.
The statistical significance of differences in antibody LeTx neutralization was determined using the Student’s t test and differences were considered significant at P < 0.01 (Prism software 5.0; GraphPad Software, Inc.). Survival rate was analyzed with Kaplan-Meier estimates, and the statistical significance of differences in antibody B. anthracis Sterne toxin neutralization was determined using the log-rank test and P < 0.05 was considered significant. SPSS version 11.0 for Windows (SPSS Inc.) was used for statistical analysis.
Acknowledgments
We thank Lee Jacobson for help with MTT assays. We thank the staff of the Analytical Imaging Facility of Albert Einstein College of Medicine for technical assistance with confocal microscopy.
This work was supported by grants from the Department of Defense (Proposal Log #07164001; Award No. W81XWH08-01-0011), from the Northeast Biodefense Center (5U54AI05715807 to W.I. Lipkin), and from the National Institutes of Health (2R01 CA102705, 2RO1CA72649, and U54 AI57158 to M.D. Scharff). In addition, A. Casadevall is also supported by HL059842-3, A1033774, A1052733, and AI033142. Alena Janda is supported by the Institutional AIDS training grant T32-AI007501.
The authors have no conflicting financial interests.
Footnotes
Abbreviations used:
- BMM
- BM-derived macrophage
- FcR
- Fc receptor
- LeTx
- lethal toxin
- MAPKK
- mitogen-activated protein kinase kinase
- MEK
- MAPK/ERK kinase
- PA
- protective antigen
References
- Abboud N., Casadevall A. 2008. Immunogenicity of Bacillus anthracis protective antigen domains and efficacy of elicited antibody responses depend on host genetic background. Clin. Vaccine Immunol. 15:1115–1123 10.1128/CVI.00015-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abboud N., De Jesus M., Nakouzi A., Cordero R.J., Pujato M., Fiser A., Rivera J., Casadevall A. 2009. Identification of linear epitopes in Bacillus anthracis protective antigen bound by neutralizing antibodies. J. Biol. Chem. 284:25077–25086 10.1074/jbc.M109.022061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrami L., Liu S., Cosson P., Leppla S.H., van der Goot F.G. 2003. Anthrax toxin triggers endocytosis of its receptor via a lipid raft-mediated clathrin-dependent process. J. Cell Biol. 160:321–328 10.1083/jcb.200211018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrami L., Lindsay M., Parton R.G., Leppla S.H., van der Goot F.G. 2004. Membrane insertion of anthrax protective antigen and cytoplasmic delivery of lethal factor occur at different stages of the endocytic pathway. J. Cell Biol. 166:645–651 10.1083/jcb.200312072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aderem A., Underhill D.M. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593–623 10.1146/annurev.immunol.17.1.593 [DOI] [PubMed] [Google Scholar]
- Baldridge J.R., Buchmeier M.J. 1992. Mechanisms of antibody-mediated protection against lymphocytic choriomeningitis virus infection: mother-to-baby transfer of humoral protection. J. Virol. 66:4252–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonuccelli G., Sotgia F., Frank P.G., Williams T.M., de Almeida C.J., Tanowitz H.B., Scherer P.E., Hotchkiss K.A., Terman B.I., Rollman B., et al. 2005. ATR/TEM8 is highly expressed in epithelial cells lining Bacillus anthracis’ three sites of entry: implications for the pathogenesis of anthrax infection. Am. J. Physiol. Cell Physiol. 288:C1402–C1410 10.1152/ajpcell.00582.2004 [DOI] [PubMed] [Google Scholar]
- Bradley K.A., Mogridge J., Mourez M., Collier R.J., Young J.A. 2001. Identification of the cellular receptor for anthrax toxin. Nature. 414:225–229 10.1038/n35101999 [DOI] [PubMed] [Google Scholar]
- Casadevall A., Mukherjee J., Scharff M.D. 1992. Monoclonal antibody based ELISAs for cryptococcal polysaccharide. J. Immunol. Methods. 154:27–35 10.1016/0022-1759(92)90209-C [DOI] [PubMed] [Google Scholar]
- Chopra A.P., Boone S.A., Liang X., Duesbery N.S. 2003. Anthrax lethal factor proteolysis and inactivation of MAPK kinase. J. Biol. Chem. 278:9402–9406 10.1074/jbc.M211262200 [DOI] [PubMed] [Google Scholar]
- Daëron M. 1997. Fc receptor biology. Annu. Rev. Immunol. 15:203–234 10.1146/annurev.immunol.15.1.203 [DOI] [PubMed] [Google Scholar]
- De Jesus M., Nicola A.M., Rodrigues M.L., Janbon G., Casadevall A. 2009. Capsular localization of the Cryptococcus neoformans polysaccharide component galactoxylomannan. Eukaryot. Cell. 8:96–103 10.1128/EC.00331-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesbery N.S., Webb C.P., Leppla S.H., Gordon V.M., Klimpel K.R., Copeland T.D., Ahn N.G., Oskarsson M.K., Fukasawa K., Paull K.D., Vande Woude G.F. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science. 280:734–737 10.1126/science.280.5364.734 [DOI] [PubMed] [Google Scholar]
- Furriel R.P., Lucisano Y.M., Mantovani B. 1992. Precipitated immune complexes of IgM as well as of IgG can bind to rabbit polymorphonuclear leucocytes but only the immune complexes of IgG are readily phagocytosed. Immunology. 75:528–534 [PMC free article] [PubMed] [Google Scholar]
- Gordon V.M., Leppla S.H., Hewlett E.L. 1988. Inhibitors of receptor-mediated endocytosis block the entry of Bacillus anthracis adenylate cyclase toxin but not that of Bordetella pertussis adenylate cyclase toxin. Infect. Immun. 56:1066–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene G., Hodous J., Dintzis R.Z., Dintzis H.M. 1990. Modification, optimization and simplification of the spot ELISA technique for the enumeration of cells secreting anti-hapten antibodies. J. Immunol. Methods. 129:187–197 10.1016/0022-1759(90)90438-2 [DOI] [PubMed] [Google Scholar]
- Harvill E.T., Osorio M., Loving C.L., Lee G.M., Kelly V.K., Merkel T.J. 2008. Anamnestic protective immunity to Bacillus anthracis is antibody mediated but independent of complement and Fc receptors. Infect. Immun. 76:2177–2182 10.1128/IAI.00647-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulett M.D., Witort E., Brinkworth R.I., McKenzie I.F., Hogarth P.M. 1994. Identification of the IgG binding site of the human low affinity receptor for IgG Fc gamma RII. Enhancement and ablation of binding by site-directed mutagenesis. J. Biol. Chem. 269:15287–15293 [PubMed] [Google Scholar]
- Kaneko Y., Nimmerjahn F., Madaio M.P., Ravetch J.V. 2006. Pathology and protection in nephrotoxic nephritis is determined by selective engagement of specific Fc receptors. J. Exp. Med. 203:789–797 10.1084/jem.20051900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimpel K.R., Arora N., Leppla S.H. 1994. Anthrax toxin lethal factor contains a zinc metalloprotease consensus sequence which is required for lethal toxin activity. Mol. Microbiol. 13:1093–1100 10.1111/j.1365-2958.1994.tb00500.x [DOI] [PubMed] [Google Scholar]
- Koval M., Preiter K., Adles C., Stahl P.D., Steinberg T.H. 1998. Size of IgG-opsonized particles determines macrophage response during internalization. Exp. Cell Res. 242:265–273 10.1006/excr.1998.4110 [DOI] [PubMed] [Google Scholar]
- Kurosaki T., Gander I., Ravetch J.V. 1991. A subunit common to an IgG Fc receptor and the T-cell receptor mediates assembly through different interactions. Proc. Natl. Acad. Sci. USA. 88:3837–3841 10.1073/pnas.88.9.3837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffly E., Danjou L., Condemine F., Vidal D., Drouet E., Lefranc M.P., Bottex C., Thullier P. 2005. Selection of a macaque Fab with framework regions like those in humans, high affinity, and ability to neutralize the protective antigen (PA) of Bacillus anthracis by binding to the segment of PA between residues 686 and 694. Antimicrob. Agents Chemother. 49:3414–3420 10.1128/AAC.49.8.3414-3420.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S.F., Ivins B.E., Fellows P.F., Friedlander A.M. 1997. Passive protection by polyclonal antibodies against Bacillus anthracis infection in guinea pigs. Infect. Immun. 65:5171–5175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabry R., Rani M., Geiger R., Hubbard G.B., Carrion R., Jr., Brasky K., Patterson J.L., Georgiou G., Iverson B.L. 2005. Passive protection against anthrax by using a high-affinity antitoxin antibody fragment lacking an Fc region. Infect. Immun. 73:8362–8368 10.1128/IAI.73.12.8362-8368.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markine-Goriaynoff D., Hulhoven X., Cambiaso C.L., Monteyne P., Briet T., Gonzalez M.D., Coulie P., Coutelier J.P. 2002. Natural killer cell activation after infection with lactate dehydrogenase-elevating virus. J. Gen. Virol. 83:2709–2716 [DOI] [PubMed] [Google Scholar]
- Maynard J.A., Maassen C.B., Leppla S.H., Brasky K., Patterson J.L., Iverson B.L., Georgiou G. 2002. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat. Biotechnol. 20:597–601 10.1038/nbt0602-597 [DOI] [PubMed] [Google Scholar]
- Mellman I., Plutner H., Ukkonen P. 1984. Internalization and rapid recycling of macrophage Fc receptors tagged with monovalent antireceptor antibody: possible role of a prelysosomal compartment. J. Cell Biol. 98:1163–1169 10.1083/jcb.98.4.1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moayeri M., Martinez N.W., Wiggins J., Young H.A., Leppla S.H. 2004. Mouse susceptibility to anthrax lethal toxin is influenced by genetic factors in addition to those controlling macrophage sensitivity. Infect. Immun. 72:4439–4447 10.1128/IAI.72.8.4439-4447.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed N., Li J., Ferreira C.S., Little S.F., Friedlander A.M., Spitalny G.L., Casey L.S. 2004. Enhancement of anthrax lethal toxin cytotoxicity: a subset of monoclonal antibodies against protective antigen increases lethal toxin-mediated killing of murine macrophages. Infect. Immun. 72:3276–3283 10.1128/IAI.72.6.3276-3283.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlbauer S.M., Evering T.H., Bonuccelli G., Squires R.C., Ashton A.W., Porcelli S.A., Lisanti M.P., Brojatsch J. 2007. Anthrax lethal toxin kills macrophages in a strain-specific manner by apoptosis or caspase-1-mediated necrosis. Cell Cycle. 6:758–766 [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F., Ravetch J.V. 2006. Fcgamma receptors: old friends and new family members. Immunity. 24:19–28 10.1016/j.immuni.2005.11.010 [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F., Bruhns P., Horiuchi K., Ravetch J.V. 2005. FcgammaRIV: a novel FcR with distinct IgG subclass specificity. Immunity. 23:41–51 10.1016/j.immuni.2005.05.010 [DOI] [PubMed] [Google Scholar]
- Ra C., Jouvin M.H., Blank U., Kinet J.P. 1989. A macrophage Fc gamma receptor and the mast cell receptor for IgE share an identical subunit. Nature. 341:752–754 10.1038/341752a0 [DOI] [PubMed] [Google Scholar]
- Ravetch J.V. 1997. Fc receptors. Curr. Opin. Immunol. 9:121–125 10.1016/S0952-7915(97)80168-9 [DOI] [PubMed] [Google Scholar]
- Ravetch J.V., Kinet J.P. 1991. Fc receptors. Annu. Rev. Immunol. 9:457–492 [DOI] [PubMed] [Google Scholar]
- Reuveny S., White M.D., Adar Y.Y., Kafri Y., Altboum Z., Gozes Y., Kobiler D., Shafferman A., Velan B. 2001. Search for correlates of protective immunity conferred by anthrax vaccine. Infect. Immun. 69:2888–2893 10.1128/IAI.69.5.2888-2893.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J., Nakouzi A., Abboud N., Revskaya E., Goldman D., Collier R.J., Dadachova E., Casadevall A. 2006. A monoclonal antibody to Bacillus anthracis protective antigen defines a neutralizing epitope in domain 1. Infect. Immun. 74:4149–4156 10.1128/IAI.00150-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer H.J., Weber M.J. 1999. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol. 19:2435–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scobie H.M., Rainey G.J., Bradley K.A., Young J.A. 2003. Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proc. Natl. Acad. Sci. USA. 100:5170–5174 10.1073/pnas.0431098100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spira G., Scharff M.D. 1992. Identification of rare immunoglobulin switch variants using the ELISA spot assay. J. Immunol. Methods. 148:121–129 10.1016/0022-1759(92)90165-P [DOI] [PubMed] [Google Scholar]
- Spira G., Bargellesi A., Teillaud J.L., Scharff M.D. 1984. The identification of monoclonal class switch variants by sib selection and an ELISA assay. J. Immunol. Methods. 74:307–315 10.1016/0022-1759(84)90298-9 [DOI] [PubMed] [Google Scholar]
- Spira G., Gregor P., Aguila H.L., Scharff M.D. 1994. Clonal variants of hybridoma cells that switch isotype at a high frequency. Proc. Natl. Acad. Sci. USA. 91:3423–3427 10.1073/pnas.91.8.3423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai T., Li M., Sylvestre D., Clynes R., Ravetch J.V. 1994. FcR gamma chain deletion results in pleiotrophic effector cell defects. Cell. 76:519–529 10.1016/0092-8674(94)90115-5 [DOI] [PubMed] [Google Scholar]
- Torres M., Casadevall A. 2008. The immunoglobulin constant region contributes to affinity and specificity. Trends Immunol. 29:91–97 10.1016/j.it.2007.11.004 [DOI] [PubMed] [Google Scholar]
- Verma A., Ngundi M.M., Meade B.D., De Pascalis R., Elkins K.L., Burns D.L. 2009. Analysis of the Fc gamma receptor-dependent component of neutralization measured by anthrax toxin neutralization assays. Clin. Vaccine Immunol. 16:1405–1412 10.1128/CVI.00194-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale G., Pellizzari R., Recchi C., Napolitani G., Mock M., Montecucco C. 1998. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macrophages. Biochem. Biophys. Res. Commun. 248:706–711 10.1006/bbrc.1998.9040 [DOI] [PubMed] [Google Scholar]
- Vitale L., Blanset D., Lowy I., O’Neill T., Goldstein J., Little S.F., Andrews G.P., Dorough G., Taylor R.K., Keler T. 2006. Prophylaxis and therapy of inhalational anthrax by a novel monoclonal antibody to protective antigen that mimics vaccine-induced immunity. Infect. Immun. 74:5840–5847 10.1128/IAI.00712-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Behring E., Kitasato S. 1991. [The mechanism of diphtheria immunity and tetanus immunity in animals. 1890]. Mol. Immunol. 28:1317: 1319–1320 10.1016/0161-5890(91)90032-F [DOI] [PubMed] [Google Scholar]
- Wang J.Y., Roehrl M.H. 2005. Anthrax vaccine design: strategies to achieve comprehensive protection against spore, bacillus, and toxin. Med. Immunol. 4:4 10.1186/1476-9433-4-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welkos S.L., Keener T.J., Gibbs P.H. 1986. Differences in susceptibility of inbred mice to Bacillus anthracis. Infect. Immun. 51:795–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild M.A., Xin H., Maruyama T., Nolan M.J., Calveley P.M., Malone J.D., Wallace M.R., Bowdish K.S. 2003. Human antibodies from immunized donors are protective against anthrax toxin in vivo. Nat. Biotechnol. 21:1305–1306 10.1038/nbt891 [DOI] [PubMed] [Google Scholar]
- Wirthmueller U., Kurosaki T., Murakami M.S., Ravetch J.V. 1992. Signal transduction by Fc gamma RIII (CD16) is mediated through the gamma chain. J. Exp. Med. 175:1381–1390 10.1084/jem.175.5.1381 [DOI] [PMC free article] [PubMed] [Google Scholar]