Abstract
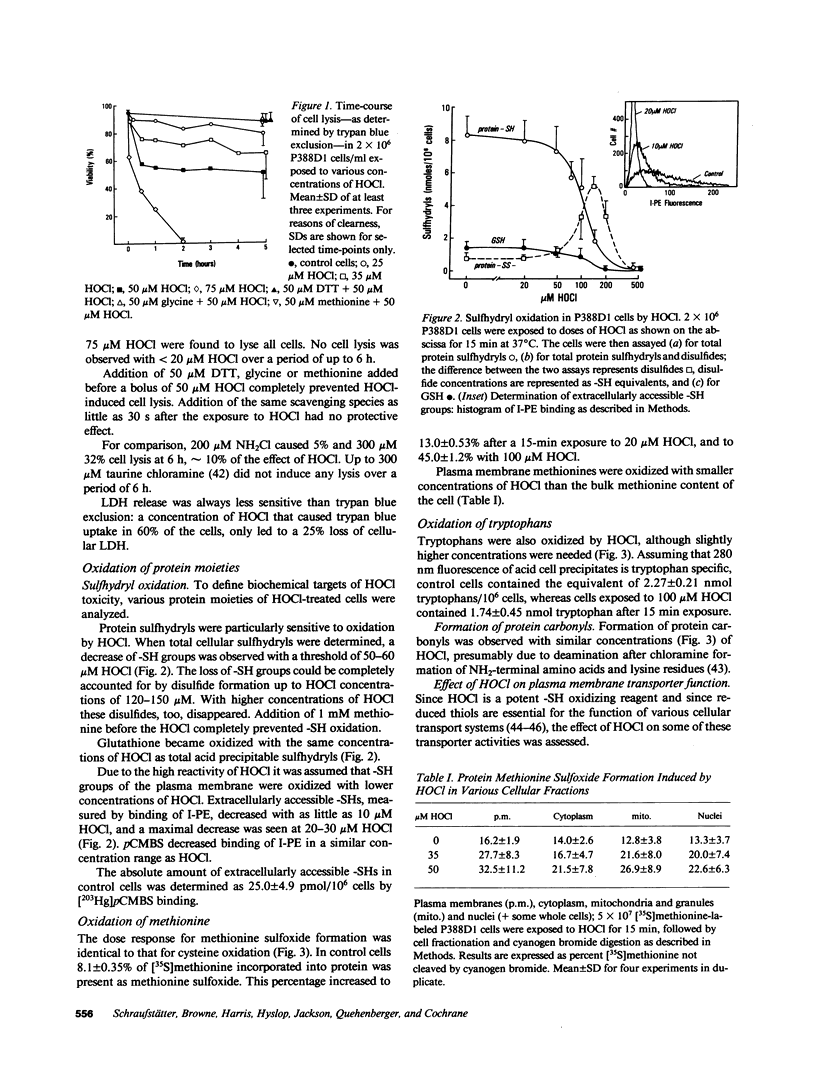
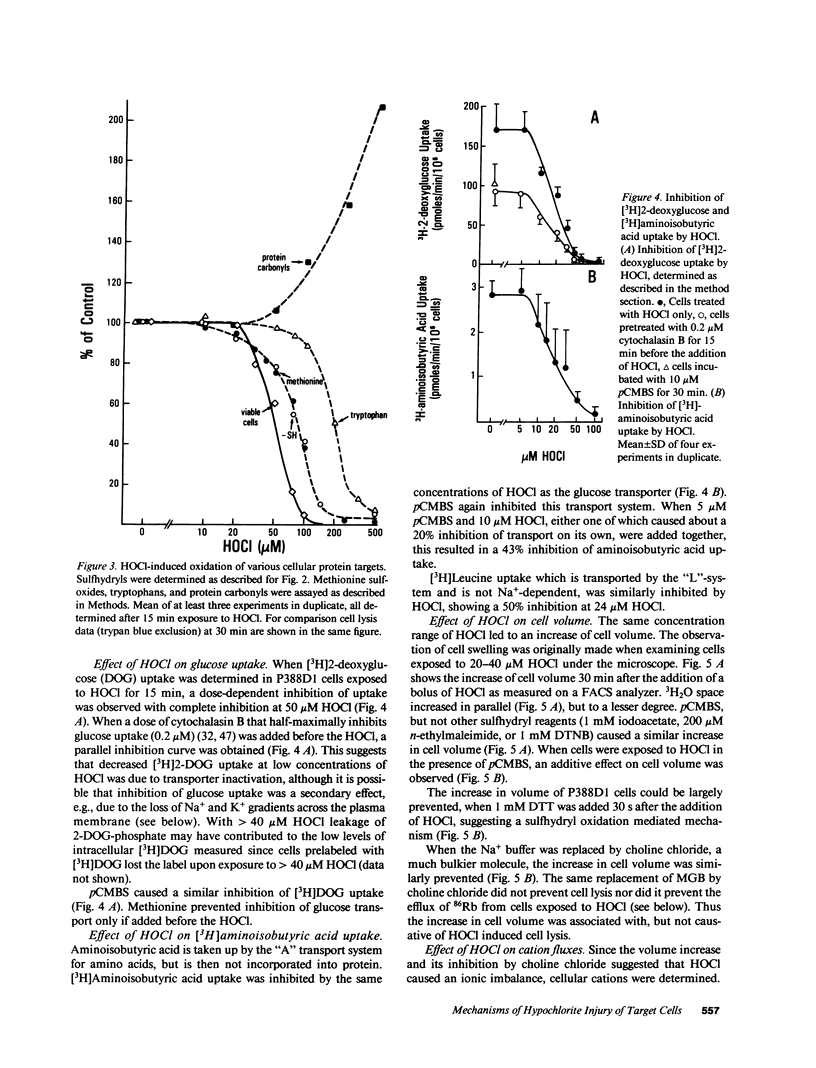
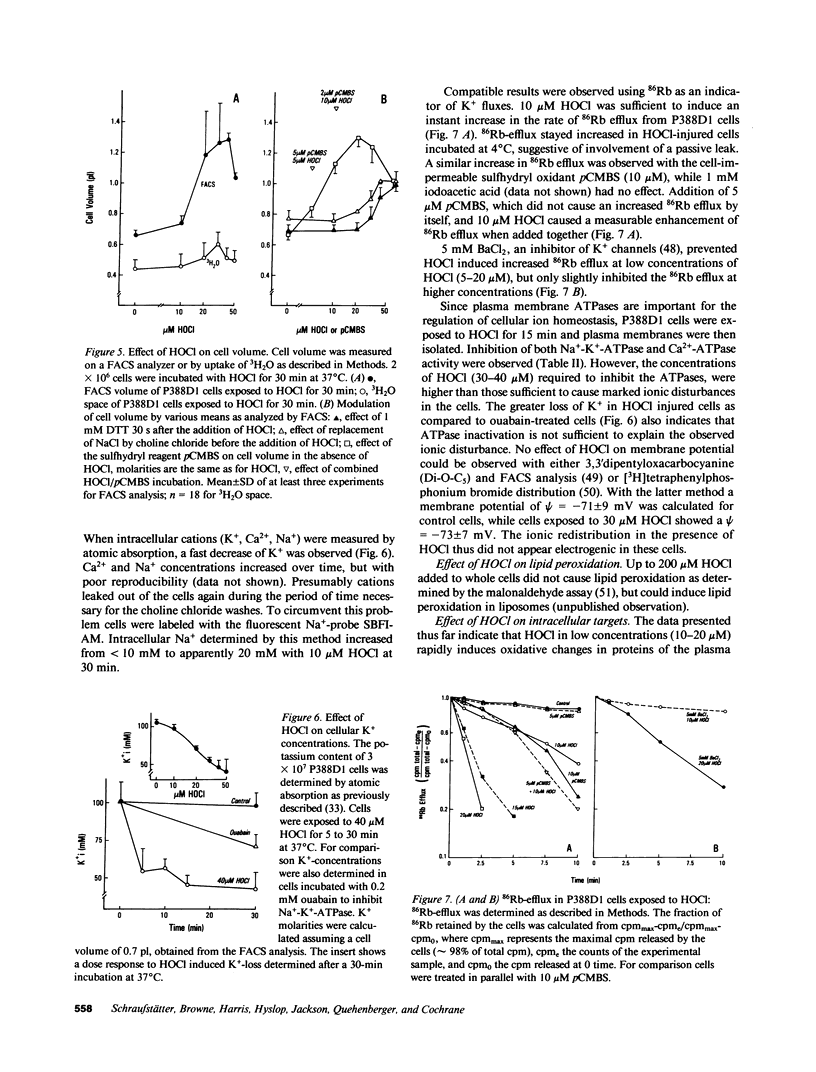
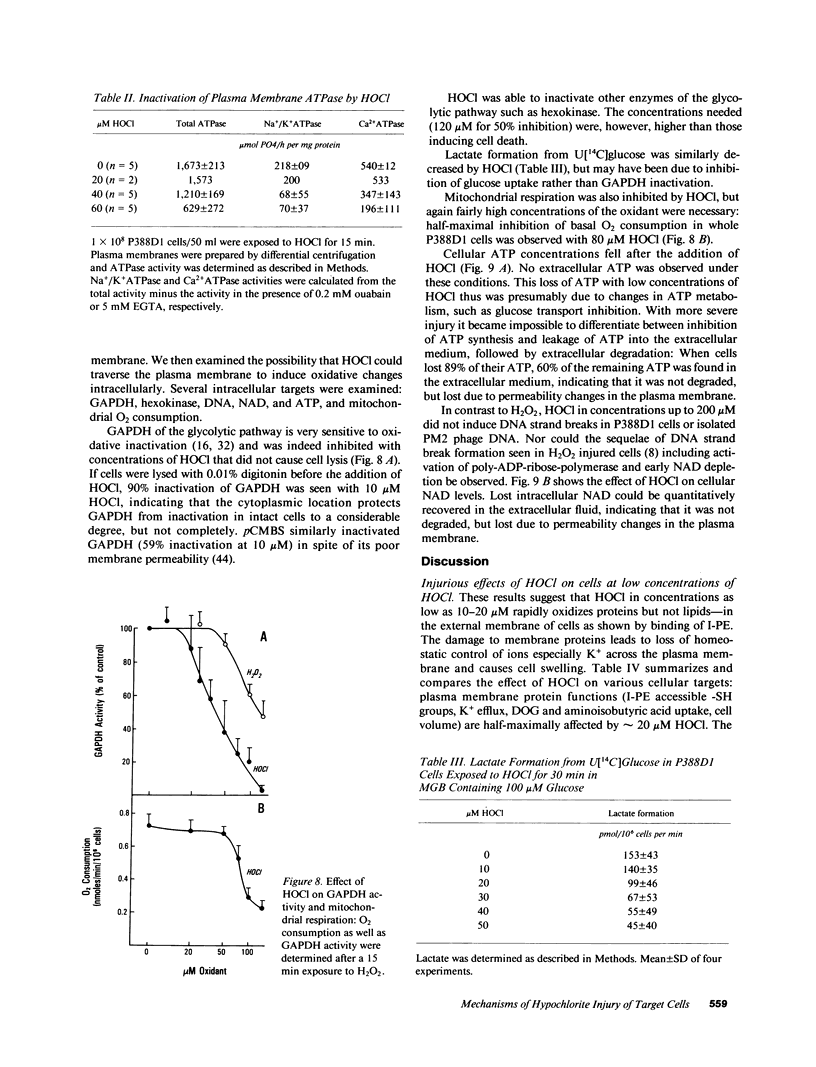
HOCl, which is produced by the action of myeloperoxidase during the respiratory burst of stimulated neutrophils, was used as a cytotoxic reagent in P388D1 cells. Low concentrations of HOCl (10-20 microM) caused oxidation of plasma membrane sulfhydryls determined as decreased binding of iodoacetylated phycoerythrin. These same low concentrations of HOCl caused disturbance of various plasma membrane functions: they inactivated glucose and aminoisobutyric acid uptake, caused loss of cellular K+, and an increase in cell volume. It is likely that these changes were the consequence of plasma membrane SH-oxidation, since similar effects were observed with para-chloromercuriphenylsulfonate (pCMBS), a sulfhydryl reagent acting at the cell surface. Given in combination pCMBS and HOCl showed an additive effect. Higher doses of HOCl (greater than 50 microM) led to general oxidation of -SH, methionine and tryptophan residues, and formation of protein carbonyls. HOCl-induced loss of ATP and undegraded NAD was closely followed by cell lysis. In contrast, NAD degradation and ATP depletion caused by H2O2 preceded cell death by several hours. Formation of DNA strand breaks, a major factor of H2O2-induced injury, was not observed with HOCl. Thus targets of HOCl were distinct from those of H2O2 with the exception of glyceraldehyde-3-phosphate dehydrogenase, which was inactivated by both oxidants.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adunyah S. E., Dean W. L. Effects of sulfhydryl reagents and other inhibitors on Ca2+ transport and inositol trisphosphate-induced Ca2+ release from human platelet membranes. J Biol Chem. 1986 Oct 5;261(28):13071–13075. [PubMed] [Google Scholar]
- Albrich J. M., Gilbaugh J. H., 3rd, Callahan K. B., Hurst J. K. Effects of the putative neutrophil-generated toxin, hypochlorous acid, on membrane permeability and transport systems of Escherichia coli. J Clin Invest. 1986 Jul;78(1):177–184. doi: 10.1172/JCI112548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrich J. M., McCarthy C. A., Hurst J. K. Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc Natl Acad Sci U S A. 1981 Jan;78(1):210–214. doi: 10.1073/pnas.78.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althaus F. R., Lawrence S. D., Sattler G. L., Pitot H. C. ADP-ribosyltransferase activity in cultured hepatocytes. Interactions with DNA repair. J Biol Chem. 1982 May 25;257(10):5528–5535. [PubMed] [Google Scholar]
- Bellomo G., Orrenius S. Altered thiol and calcium homeostasis in oxidative hepatocellular injury. Hepatology. 1985 Sep-Oct;5(5):876–882. doi: 10.1002/hep.1840050529. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Jevcak J. J. Fluorometric method for rapid detection of DNA strand breaks in human white blood cells produced by low doses of radiation. Cancer Res. 1981 May;41(5):1889–1892. [PubMed] [Google Scholar]
- Blumenthal R., Weinstein J. N., Sharrow S. O., Henkart P. Liposome--lymphocyte interaction: saturable sites for transfer and intracellular release of liposome contents. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5603–5607. doi: 10.1073/pnas.74.12.5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. J Exp Med. 1975 Jun 1;141(6):1442–1447. doi: 10.1084/jem.141.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochrane C. G., Spragg R., Revak S. D. Pathogenesis of the adult respiratory distress syndrome. Evidence of oxidant activity in bronchoalveolar lavage fluid. J Clin Invest. 1983 Mar;71(3):754–761. doi: 10.1172/JCI110823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amore T., Lo T. C. Hexose transport in L6 rat myoblasts. II. The effects of sulfhydryl reagents. J Cell Physiol. 1986 Apr;127(1):106–113. doi: 10.1002/jcp.1041270114. [DOI] [PubMed] [Google Scholar]
- Davies K. J., Delsignore M. E., Lin S. W. Protein damage and degradation by oxygen radicals. II. Modification of amino acids. J Biol Chem. 1987 Jul 15;262(20):9902–9907. [PubMed] [Google Scholar]
- Devés R., Krupka R. M. Cytochalasin B and the kinetics of inhibition of biological transport: a case of asymmetric binding to the glucose carrier. Biochim Biophys Acta. 1978 Jul 4;510(2):339–348. doi: 10.1016/0005-2736(78)90034-2. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fliss H., Weissbach H., Brot N. Oxidation of methionine residues in proteins of activated human neutrophils. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7160–7164. doi: 10.1073/pnas.80.23.7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foote C. S., Goyne T. E., Lehrer R. I. Assessment of chlorination by human neutrophils. Nature. 1983 Feb 24;301(5902):715–716. doi: 10.1038/301715a0. [DOI] [PubMed] [Google Scholar]
- Glaser J. H., Conrad H. E. Properties of chick embryo chondrocytes grown in serum-free medium. J Biol Chem. 1984 Jun 10;259(11):6766–6772. [PubMed] [Google Scholar]
- Grisham M. B., Jefferson M. M., Melton D. F., Thomas E. L. Chlorination of endogenous amines by isolated neutrophils. Ammonia-dependent bactericidal, cytotoxic, and cytolytic activities of the chloramines. J Biol Chem. 1984 Aug 25;259(16):10404–10413. [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Shalev O., Foker W., Rank B. H. Inhibition of erythrocyte Ca2+-ATPase by activated oxygen through thiol- and lipid-dependent mechanisms. Biochim Biophys Acta. 1986 Nov 6;862(1):8–16. doi: 10.1016/0005-2736(86)90463-3. [DOI] [PubMed] [Google Scholar]
- Hyslop P. A., Hinshaw D. B., Halsey W. A., Jr, Schraufstätter I. U., Sauerheber R. D., Spragg R. G., Jackson J. H., Cochrane C. G. Mechanisms of oxidant-mediated cell injury. The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J Biol Chem. 1988 Feb 5;263(4):1665–1675. [PubMed] [Google Scholar]
- Hyslop P. A., Hinshaw D. B., Schraufstätter I. U., Sklar L. A., Spragg R. G., Cochrane C. G. Intracellular calcium homeostasis during hydrogen peroxide injury to cultured P388D1 cells. J Cell Physiol. 1986 Dec;129(3):356–366. doi: 10.1002/jcp.1041290314. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968 Jun;95(6):2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learn D. B., Thomas E. L. Inhibition of tumor cell glutamine uptake by isolated neutrophils. J Clin Invest. 1988 Sep;82(3):789–796. doi: 10.1172/JCI113680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. T., Fein A. M., Lippmann M., Holtzman H., Kimbel P., Weinbaum G. Elastolytic activity in pulmonary lavage fluid from patients with adult respiratory-distress syndrome. N Engl J Med. 1981 Jan 22;304(4):192–196. doi: 10.1056/NEJM198101223040402. [DOI] [PubMed] [Google Scholar]
- Lichtshtein D., Kaback H. R., Blume A. J. Use of a lipophilic cation for determination of membrane potential in neuroblastoma-glioma hybrid cell suspensions. Proc Natl Acad Sci U S A. 1979 Feb;76(2):650–654. doi: 10.1073/pnas.76.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F., Silverstein S. C., Brukner L. H., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. II. Hydrogen peroxide as a mediator of cytotoxicity. J Exp Med. 1979 Jan 1;149(1):100–113. doi: 10.1084/jem.149.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogolotti A. L., Jr, Santi D. V. High-pressure liquid chromatography--ultraviolet analysis of intracellular nucleotides. Anal Biochem. 1982 Nov 1;126(2):335–345. doi: 10.1016/0003-2697(82)90524-3. [DOI] [PubMed] [Google Scholar]
- Reglinski J., Hoey S., Smith W. E., Sturrock R. D. Cellular response to oxidative stress at sulfhydryl group receptor sites on the erythrocyte membrane. J Biol Chem. 1988 Sep 5;263(25):12360–12366. [PubMed] [Google Scholar]
- Rosen H., Klebanoff S. J. Oxidation of microbial iron-sulfur centers by the myeloperoxidase-H2O2-halide antimicrobial system. Infect Immun. 1985 Mar;47(3):613–618. doi: 10.1128/iai.47.3.613-618.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen H., Rakita R. M., Waltersdorph A. M., Klebanoff S. J. Myeloperoxidase-mediated damage to the succinate oxidase system of Escherichia coli. Evidence for selective inactivation of the dehydrogenase component. J Biol Chem. 1987 Nov 5;262(31):15004–15010. [PubMed] [Google Scholar]
- Schoner W., Hasselberg M., Kison R. Irreversible and reversible modification of SH groups and effect on catalytic activity. Methods Enzymol. 1988;156:302–312. doi: 10.1016/0076-6879(88)56031-7. [DOI] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hinshaw D. B., Hyslop P. A., Spragg R. G., Cochrane C. G. Oxidant injury of cells. DNA strand-breaks activate polyadenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J Clin Invest. 1986 Apr;77(4):1312–1320. doi: 10.1172/JCI112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstatter I. U., Hinshaw D. B., Hyslop P. A., Spragg R. G., Cochrane C. G. Oxidant injury of cells. DNA strand-breaks activate polyadenosine diphosphate-ribose polymerase and lead to depletion of nicotinamide adenine dinucleotide. J Clin Invest. 1986 Apr;77(4):1312–1320. doi: 10.1172/JCI112436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstätter I. U., Halsey W. A., Jr, Hyslop P. A., Cochrane C. G. In vitro models for the study of oxidant-induced injury of cells in inflammation. Methods Enzymol. 1988;163:328–339. doi: 10.1016/0076-6879(88)63031-x. [DOI] [PubMed] [Google Scholar]
- Schraufstätter I. U., Hinshaw D. B., Hyslop P. A., Spragg R. G., Cochrane C. G. Glutathione cycle activity and pyridine nucleotide levels in oxidant-induced injury of cells. J Clin Invest. 1985 Sep;76(3):1131–1139. doi: 10.1172/JCI112068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufstätter I., Hyslop P. A., Jackson J. H., Cochrane C. G. Oxidant-induced DNA damage of target cells. J Clin Invest. 1988 Sep;82(3):1040–1050. doi: 10.1172/JCI113660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro H. M., Natale P. J., Kamentsky L. A. Estimation of membrane potentials of individual lymphocytes by flow cytometry. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5728–5730. doi: 10.1073/pnas.76.11.5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R. H., Scoggin C. H., Patterson D. Hydrogen peroxide causes the fatal injury to human fibroblasts exposed to oxygen radicals. J Biol Chem. 1981 Jul 25;256(14):7181–7186. [PubMed] [Google Scholar]
- Slivka A., LoBuglio A. F., Weiss S. J. A potential role for hypochlorous acid in granulocyte-mediated tumor cell cytotoxicity. Blood. 1980 Feb;55(2):347–350. [PubMed] [Google Scholar]
- Smedly L. A., Tonnesen M. G., Sandhaus R. A., Haslett C., Guthrie L. A., Johnston R. B., Jr, Henson P. M., Worthen G. S. Neutrophil-mediated injury to endothelial cells. Enhancement by endotoxin and essential role of neutrophil elastase. J Clin Invest. 1986 Apr;77(4):1233–1243. doi: 10.1172/JCI112426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spragg R. G., Hinshaw D. B., Hyslop P. A., Schraufstätter I. U., Cochrane C. G. Alterations in adenosine triphosphate and energy charge in cultured endothelial and P388D1 cells after oxidant injury. J Clin Invest. 1985 Oct;76(4):1471–1476. doi: 10.1172/JCI112126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starke P. E., Oliver C. N., Stadtman E. R. Modification of hepatic proteins in rats exposed to high oxygen concentration. FASEB J. 1987 Jul;1(1):36–39. doi: 10.1096/fasebj.1.1.2886388. [DOI] [PubMed] [Google Scholar]
- Stelmaszyńska T., Zgliczynski J. M. N-(2-Oxoacyl)amino acids and nitriles as final products of dipeptide chlorination mediated by the myeloperoxidase/H2O2/Cl- system. Eur J Biochem. 1978 Dec 1;92(1):301–308. doi: 10.1111/j.1432-1033.1978.tb12748.x. [DOI] [PubMed] [Google Scholar]
- Thannhauser T. W., Konishi Y., Scheraga H. A. Analysis for disulfide bonds in peptides and proteins. Methods Enzymol. 1987;143:115–119. doi: 10.1016/0076-6879(87)43020-6. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Grisham M. B., Jefferson M. M. Cytotoxicity of chloramines. Methods Enzymol. 1986;132:585–593. doi: 10.1016/s0076-6879(86)32043-3. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Grisham M. B., Jefferson M. M. Preparation and characterization of chloramines. Methods Enzymol. 1986;132:569–585. doi: 10.1016/s0076-6879(86)32042-1. [DOI] [PubMed] [Google Scholar]
- Thomas E. L. Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: nitrogen-chlorine derivatives of bacterial components in bactericidal action against Escherichia coli. Infect Immun. 1979 Feb;23(2):522–531. doi: 10.1128/iai.23.2.522-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANSTEVENINCK J., WEED R. I., ROTHSTEIN A. LOCALIZATION OF ERYTHROCYTE MEMBRANE SULFHYDRYL GROUPS ESSENTIAL FOR GLUCOSE TRANSPORT. J Gen Physiol. 1965 Mar;48:617–632. doi: 10.1085/jgp.48.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Young J., LoBuglio A. F., Slivka A., Nimeh N. F. Role of hydrogen peroxide in neutrophil-mediated destruction of cultured endothelial cells. J Clin Invest. 1981 Sep;68(3):714–721. doi: 10.1172/JCI110307. [DOI] [PMC free article] [PubMed] [Google Scholar]



