The interaction between Tim3 on Th1 cells and galectin-9 on Mycobacterium tuberculosis–infected macrophages restricts the bacterial growth by stimulating caspase-1–dependent IL-1β secretion.
Abstract
T cell immunoglobulin and mucin domain 3 (Tim3) is a negative regulatory molecule that inhibits effector TH1-type responses. Such inhibitory signals prevent unintended tissue inflammation, but can be detrimental if they lead to premature T cell exhaustion. Although the role of Tim3 in autoimmunity has been extensively studied, whether Tim3 regulates antimicrobial immunity has not been explored. Here, we show that Tim3 expressed on TH1 cells interacts with its ligand, galectin-9 (Gal9), which is expressed by Mycobacterium tuberculosis–infected macrophages to restrict intracellular bacterial growth. Tim3–Gal9 interaction leads to macrophage activation and stimulates bactericidal activity by inducing caspase-1–dependent IL-1β secretion. We propose that the TH1 cell surface molecule Tim3 has evolved to inhibit growth of intracellular pathogens via its ligand Gal9, which in turn inhibits expansion of effector TH1 cells to prevent further tissue inflammation.
TH1 cells are critical for the control of many intracellular pathogens because cell contact–dependent signals and the secretion of cytokines, including IFN-γ and TNF, activate infected cells to produce antimicrobial effector molecules. After resolution and clearance of infection, effector T cells need to be deleted to prevent excessive tissue inflammation and development of immunopathology. Tim3, a member of the recently discovered T cell Ig and mucin domain–containing molecule (Tim) superfamily, is a negative regulator of TH1 immunity (Monney et al., 2002). As such, Tim3 acts to suppress autoimmunity in the mouse EAE and arthritis models (Sabatos et al., 2003; Sánchez-Fueyo et al., 2003; Zhu et al., 2005; Seki et al., 2008). The human Tim3 orthologue appears to function similarly in people (Yang et al., 2008).
The only known ligand of Tim3 is galectin-9 (Gal9), a ubiquitously expressed soluble β-galactoside–binding protein (Zhu et al., 2005; Cao et al., 2007). Both IFN-γ and IL-1β induce Gal9, which enhances Tim3–Gal9 interaction during TH1-type immunity and tissue inflammation (Yoshida et al., 2001; Imaizumi et al., 2002). Binding of Gal9 to Tim3 transduces a signal into T cell that triggers apoptosis, resulting in clonal contraction of effector TH1 cells (Kashio et al., 2003; Zhu et al., 2005). Similarly, Tim3 expression by effector CD4+ and CD8+ T cells during HIV and hepatitis C virus (HCV) infection is associated with T cell dysfunction and correlates with disease progression (Jones et al., 2008; Golden-Mason et al., 2009). Under these conditions, Tim3–Gal9 interaction may lead to inappropriate exhaustion or apoptosis of Tim3+ effector T cells (Kashio et al., 2003). Although such a response may be an adaptation to chronic infection or inflammation that limits the development of immunopathology, disrupting T cell responses against pathogenic microbes may be detrimental to the host.
The ability of pathogenic Mycobacterium tuberculosis (Mtb) to adapt to the hostile intracellular environment of the macrophage has been instrumental to its success as a pathogen. Host resistance against Mtb relies on TH1-mediated immunity (Flynn and Chan, 2001). Importantly, effector TH1 cells are understood to mediate protective immunity by depriving the bacterium of its intracellular niche. The production of IFN-γ and TNF by Mtb-specific TH1 cells serves to activate infected macrophages, which induces intracellular mediators such as nitric oxide (NO) and promotes changes in intracellular physiology including phagolysosome fusion (Cooper et al., 1993; Flynn et al., 1993; MacMicking et al., 2003; Harris et al., 2008). Although IFN-γ is necessary for optimum antimycobacterial immunity, studies in humans indicate that IFN-γ levels do not necessarily predict disease progression (Zhang et al., 1995; Bhattacharyya et al., 1999; Flynn, 1999).
Because control of Mtb requires TH1 cells, we hypothesized that Tim3 would play a critical role in modulating host resistance to tuberculosis. Although Tim3 expression on T cells negatively regulates their expansion, how Tim3–Gal9 binding affects the Gal9-expressing APCs has not previously been addressed. We show that Mtb-infected macrophages express high levels of Gal9 and that Tim3 binding to its ligand Gal9 expressed by Mtb-infected macrophages activates innate pathways and inhibit intracellular bacterial replication. These data support a model in which Tim3–Gal9 interact as a bidirectional regulatory circuit that activates innate immune cells to clear intracellular pathogens, which in turn further up-regulate Tim3 ligand and Gal9 and promote termination of TH1 responses.
RESULTS
Tim3 and Gal9 expression after low-dose aerosol Mtb infection in mice
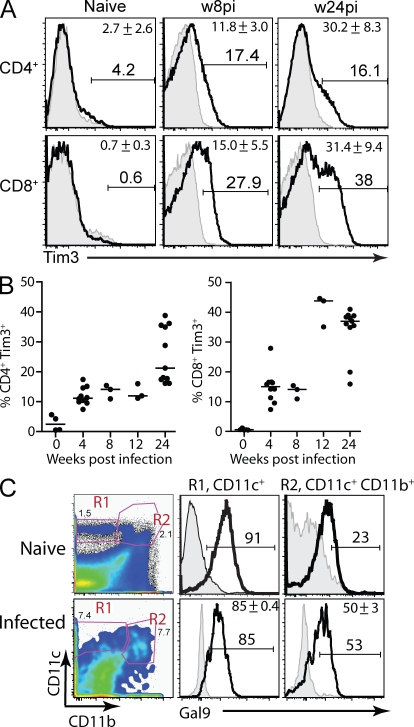
To determine the potential of Tim3 to modulate immunity to Mtb, its expression on T cells from uninfected and Mtb-infected mice was analyzed. In uninfected mice, Tim3 is expressed by only 1–2% of lung T cells. In contrast, after low-dose aerosol Mtb infection, the percentage of pulmonary CD4+ and CD8+ T cells that express Tim3 gradually increases (Fig. 1 A). During the chronic phase of infection, nearly 40% of all pulmonary CD8+ T cells and 20% of all pulmonary CD4+ T cells express Tim3 (Fig. 1 B). Similarly the frequency of splenic CD4+ and CD8+ T cells that express Tim3 increases after Mtb infection (unpublished data). The Tim3+ CD4+ and CD8+ T cells have an effector phenotype and secrete higher levels of TH1 cytokines, such as IFN-γ and TNF, than Tim3− T cells (unpublished data). In addition, we determined the expression of the Tim3 ligand, Gal9, by myeloid cells in the lung. To discriminate alveolar macrophages from other cell subsets, we used a cocktail of antibodies recognizing F4/80, CD11b, and CD11c. Alveolar macrophages are F4/80+ CD11c+ CD11blow in uninfected mice and can become F4/80+ CD11c+ CD11bhi after infection. We measured intracellular levels of Gal9 in CD11c+ CD11bhi/− cells in the lungs of uninfected and Mtb-infected mice and found high levels of Gal9 in both of these myeloid cell subsets (Fig. 1 C). The other myeloid cell subsets in the lungs of infected mice also expressed Gal9 (Fig. S1 A). The accumulation of Tim3+ T cells in the lungs after Mtb infection and the pulmonary expression of Gal9 supports a function for Tim3 and Gal9 during host immunity to infection.
Figure 1.
Tim3 and Gal9 expression in the lungs of uninfected and Mtb-infected mice. (A) Total lung cells were prepared from uninfected or Mtb-infected (8 and 24 wk after aerosol infection) C57BL/6 mice. Lymphocytes were gated based on size and on CD8+ or CD4+ staining, and the expression of Tim3 by CD4+ or CD8+ T cells is shown in the histograms (thick line). Shaded histograms represent isotype control. Representative FACS plots from 10 independent experiments performed 4–24 wk after infection is shown for a single animal at 8 and 24 wk after Mtb infection is shown. (B) The percentage of CD4+ and CD8+ T cells that express Tim3 is represented in graphs. Data represent 3–12 determinations from 10 independent experiments, done 4–24 wk after infection (some of the symbols are superimposed). The horizontal bar represents the median. (C) Myeloid cell subsets in the lung were identified based on CD11c and CD11b staining, and the expression of intracellular Gal9 (thick line) by CD11c+ (R1) or CD11b+ CD11c+ (R2) cells were determined. Shaded histograms represent isotype control. Representative histograms are shown for a cohort of mice (n = 4–5 for infected mice; n = 3 for naive mice). Data represent three independent experiments performed 4–40 wk after infection. Numbers indicate the percentage of cells in each gate, and mean values ± SD are shown in the top right corner of each plot.
Tim3-Ig reduces the mycobacterial burden in vivo
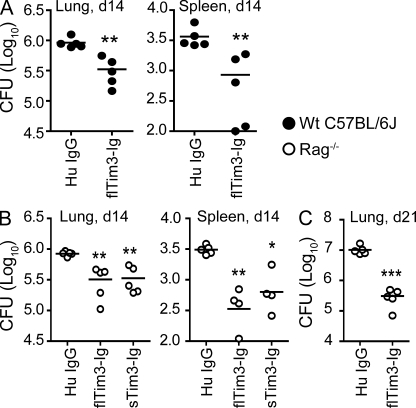
Accumulating evidence indicates that T cells expressing Tim3 during chronic viral infection are dysfunctional and Tim3 blockade can reverse exhaustion and restore normal effector function (Jones et al., 2008; Golden-Mason et al., 2009). The accumulation of Tim3+ T cells in the lungs after Mtb infection raised the possibility that Tim3 contributes to T cell dysfunction. To determine whether blocking Tim3 would enhance pulmonary immunity to Mtb, mice were infected with low-dose aerosolized virulent Mtb, and then treated with fusion protein expressing full-length or soluble forms of mouse Tim3 and human Ig Fc tail (flTim3-Ig, sTim3-Ig) or control, human Ig-γ (HuIgG; Sabatos et al., 2003; Zhu et al., 2005). Mice that received flTim3-Ig had a lower bacterial burden in the lung and spleen compared with mice treated with HuIgG (Fig. 2 A). To establish that the beneficial effect of Tim3-Ig on control of Mtb infection was a consequence of blocking Tim3 signaling on T cells, the experiment was repeated using RAG−/− mice. RAG−/− mice were similarly infected and treated with sTim3-Ig, flTim3-Ig, or control HuIgG. Treatment with either sTim3-Ig or flTim3-Ig led to a significant reduction in CFU in both the lung and spleen within 2 wk after infection (Fig. 2 B), and improved control of the bacterial burden by Tim3-Ig was sustained in the lung (Fig. 2 C) but not the spleen (not depicted) 3 wk after infection. This suggests that the antimicrobial effect of Tim3-Ig was independent of acquired immunity. Furthermore, these data raised the possibility that Tim3-Ig was acting as an agonist and that its binding to host macrophage-activated innate antimicrobial pathways.
Figure 2.
Tim3-Ig controls Mtb infection in vivo. WT C57BL/6J (A) and RAG−/− (B and C) mice were infected by the aerosol route with Mtb. The next day, 500 µg of full-length Tim3-Ig (flTim3-Ig), soluble Tim3-Ig (sTim3-Ig), or HuIgG (control) was administered. Additional protein was administered (100 µg) 5, 8, and 12 d after infection. On days 14 (A and B) and 21 (C), mice were sacrificed and CFUs were determined in their lungs and spleens. Closed and open symbols represent WT B6 and RAG−/− mice, respectively. Data are representative of three independent experiments, with each experiment having n = 5 mice per group. Each point represents an individual mouse. *, P < 0.05; **, P < 0.01; ***, P < 0.001, one-way ANOVA compared with HuIgG control for the respective mouse strain.
Tim3–Gal9 interaction leads to macrophage activation and stimulation of innate immunity
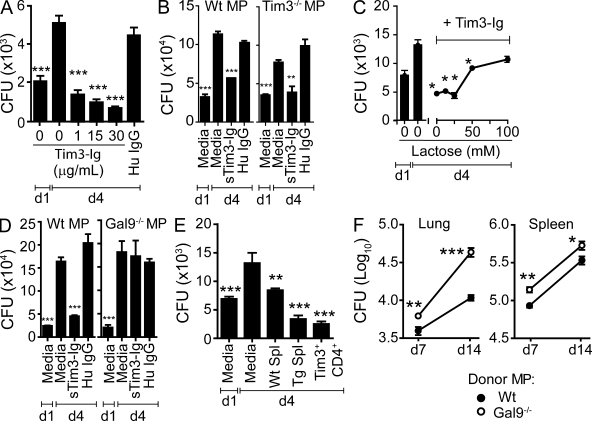
We hypothesized that the ability of Tim3-Ig to limit Mtb growth in the lungs of infected mice was caused by its direct interaction with infected macrophages. To test this hypothesis, Mtb-infected macrophages were treated with purified Tim3-Ig fusion protein or human IgG in vitro. The CFU recovered from Mtb-infected macrophages on day 4 was lower than the day 1 inoculum at all concentrations of Tim3-Ig tested (Fig. 3 A). At a dose of 30 µg/ml, 68% of the day 1 bacteria are killed (P < 0.05, one-way analysis of variance [ANOVA]). Tim3-Ig treatment of infected WT and Tim3−/− macrophages induced similar control of bacterial replication, which indicated that Tim3 expression by the macrophages was not necessary (Fig. 3 B). These results demonstrate that Tim3-Ig acts as an agonist and induces intracellular Mtb killing in the absence of T cells.
Figure 3.
Tim3–Gal9 interaction stimulates antimycobacterial activity. (A) H37Rv-infected macrophages (MP) were cultured alone or in the presence of increasing amounts of Tim3-Ig fusion protein or with 30 µg/ml of HuIgG as a control. Data are representative of three independent experiments. Error bars indicate mean ± SEM from three to six replicate cultures. (B) Mtb-infected WT or Tim3−/− macrophages were cultured alone or in the presence of 10 µg/ml of sTim3-Ig or HuIgG (control). (C) Mtb-infected macrophages were cultured alone or treated with 10 µg/ml Tim3-Ig in the presence of increasing amounts of lactose. Data are representative of three independent experiments. Error bars indicate mean ± SEM from three replicate cultures. (D) Mtb-infected WT or Gal9−/− macrophages were cultured alone or in the presence of 10 µg/ml Tim3-Ig or HuIgG (control). Data are representative of three independent experiments. Bars indicate mean ± SEM from three replicate cultures. (E) Mtb-infected macrophages cultured alone or with splenocytes from uninfected WT (WT Spl) or Tim3tg (Tg Spl) mice, or with Tim3+CD4+ T cells from uninfected Tim3tg mice. In all experiments (unless otherwise noted), day 1 (d1) is the CFU in infected macrophages alone 24 h after infection, before the addition of cells or Tim3-Ig or HuIgG and represents initial inoculum, whereas day 4 (d4) is the CFU recovered from macrophages 4 d after infection in the absence of any treatment. Data are representative of 3–11 independent experiments. Error bars indicate mean ± SEM from three to six replicate cultures. **, P < 0.01; ***, P < 0.001, one-way ANOVA compared with day 4 macrophages alone. (F) Pulmonary and splenic bacterial burden 7 and 14 d after intravenous transfer of H37Rv-infected WT or Gal9−/− macrophages into naive Tim3tg mice. Bacteria in lungs and spleen on day 1 after adoptive transfer were 60 and 1088, respectively. Data are from a single experiment with two time points (n = 5 mice per group per time point; error bars represent the SEM). **, P < 0.01; ***, P < 0.001, unpaired two-tailed Student’s t test.
The only known ligand of Tim3 is Gal9 (Zhu et al., 2005; Cao et al., 2007). Gal9 is expressed by peritoneal macrophages, and Mtb infection induces its expression in a manner that is synergistic with IFN-γ (Fig. S1, B and C). Because Gal9 is a β-galactoside binding S-type lectin that recognizes Tim3 via carbohydrates on the IgV domain, the Tim3–Gal9 interaction can be competitively blocked using lactose (Zhu et al., 2005). High concentrations of lactose inhibit the antimicrobial activity of Tim3-Ig, indicating that Tim3 interaction with Gal9 is required to induce macrophage antimicrobial activity (Fig. 3 C). To confirm these results, WT and Gal9−/− macrophages were infected with Mtb and treated with Tim3-Ig. Tim3-Ig treatment of Mtb-infected Gal9−/− macrophages did not affect Mtb growth, confirming that Tim3–Gal9 interaction is required to stimulate macrophage bactericidal activity (Fig. 3 D).
We next hypothesized that Tim3+ T cells could recapitulate the antimicrobial activity of Tim3-Ig by binding to Gal9 on infected macrophages, which would activate bactericidal pathways to kill Mtb. Because T cells from Mtb-infected mice have multiple effector functions that also control bacterial growth, we wished to determine whether Tim3–Gal9 limits bacterial growth in the absence of other T cell functions. Therefore, we used Tim3 transgenic (Tim3tg) mice, which were generated on the C57BL/6J background and express the Tim3 transgene (the BALB/c allele) under the control of the hCD2 promoter. Although 1–3% of splenic T cells from WT C57BL/6J mice express Tim3, Tim3 is constitutively expressed by ∼40–60% of T cells from Tim3tg mice (Fig. S2). Splenocytes from uninfected WT mice suppress intracellular Mtb growth in primary macrophages by 36% by day 4 (Fig. 3 E; Sada-Ovalle et al., 2008). In contrast, Tim3tg splenocytes suppress intracellular Mtb growth by 74% compared with day 4, and 51% compared with the day 1 initial inoculum. Purified Tim3+CD4+ T cells were sufficient to mediate control of Mtb replication and induce a 64% reduction in CFU compared with day 1. Splenocytes from Tim3−/− mice were unable to restrict intracellular bacterial replication, as well as WT splenocytes (Fig. S3). Thus, Tim3 expression by T cells can induce killing of intracellular Mtb.
The antimicrobial effect of Tim3-Ig in Rag−/− mice is consistent with Gal9 signaling activating innate cells to control intracellular pathogens. To show that Tim3 and Gal9 interact in vivo, WT and Gal9−/− macrophages were infected with Mtb in vitro and intravenously transferred into uninfected Tim3tg recipients. Mice that received Mtb-infected Gal9−/− macrophages had a significantly higher bacterial burden in the lungs and spleen compared with mice that received Mtb-infected WT (Gal9+/+) macrophages 7 and 14 d after infection (Fig. 3 F). These results show that interaction between Tim3 and Gal9 in vivo leads to control of intracellular Mtb.
A soluble factor induced by Tim3–Gal9 interaction leads to control of bacterial infection
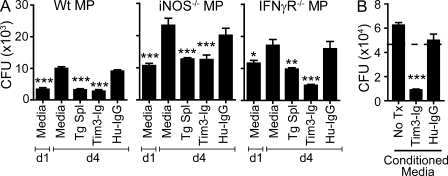
The best described mechanism that restricts intracellular Mtb replication is the induction of inducible NO synthase (iNOS) and subsequent NO production, which occurs after the activation of macrophages by IFN-γ (Denis, 1991; Flynn et al., 1993). Other cellular mechanisms that reduce Mtb viability, such as autophagy and phagolysosomal fusion mediated by LRG-47 family of GTPases, are also dependent on activation of infected macrophages with IFN-γ (MacMicking et al., 2003; Gutierrez et al., 2004). We assumed that the action of Tim3-Ig was IFN-γ–independent because there is no source of IFN-γ (such as T cells or NK cells) in our culture system, nor do we see induction of IFN-γ RNA or IFN-γ secretion (unpublished data). To definitively rule out the involvement of IFN-γ and iNOS in Tim3–Gal9–mediated Mtb killing, IFN-γR−/− and iNOS−/− macrophages were infected with Mtb and treated with Tim3-Ig. Tim3-Ig suppressed the intracellular growth of Mtb in both IFN-γR−/− and iNOS−/− macrophages (Fig. 4 A). In addition, iNOS inhibitors did not affect killing mediated by Tim3tg splenocytes (Fig. S4). These data indicate that Tim3–Gal9 interaction leads to killing of Mtb independently of IFN-γ receptor signaling and iNOS production and strongly suggest that Tim3 activates a novel antibacterial pathway in Mtb-infected macrophages.
Figure 4.
The action of Tim3-Ig is independent of iNOS and IFN-γ and is mediated by a soluble factor. (A) WT, iNOS−/−, and IFN-γR−/− macrophages (MP) were infected with H37Rv and CFUs determined on day 1 (d1) and day 4 (d4). On day 1, Tim3tg splenocytes (Tg Spl), 10 µg/ml Tim3-Ig, or 10 µg/ml HuIgG (control) were added to the macrophages. Data are representative of three independent experiments. Bars indicate mean ± SEM from three replicate cultures. (B) Conditioned media from Mtb-infected macrophages cultured alone for 24 h (No Tx) or treated with 10 µg/ml Tim3-Ig or HuIgG for 24 h were added to infected macrophages on day 1 after infection. CFUs were enumerated 3 d later on day 4 after infection. Dotted line represents the initial inoculums determined on day 1 (4.6 × 104). Data are from one experiment with three replicate cultures per condition. Error bars indicate mean ± SEM from three replicate cultures. *, P < 0.05; **, P < 0.01; ***, P < 0.001, one-way ANOVA compared with day 4 macrophages alone in A and to No Tx in B.
We next considered whether soluble mediators secreted after Tim3–Gal9 interaction act in an autocrine manner to restrict intracellular mycobacterial growth. To evaluate this possibility, supernatants were collected from infected macrophages 24 h after infection alone, or after treatment with Tim3-Ig or HuIgG. Supernatants from Tim3-Ig–treated infected macrophages induced Mtb killing, indicating that a soluble mediator secreted after Tim3–Gal9 interaction was sufficient to activate macrophages to reduce intracellular Mtb viability (Fig. 4 B).
Mtb killing requires caspase-1 and IL-1β
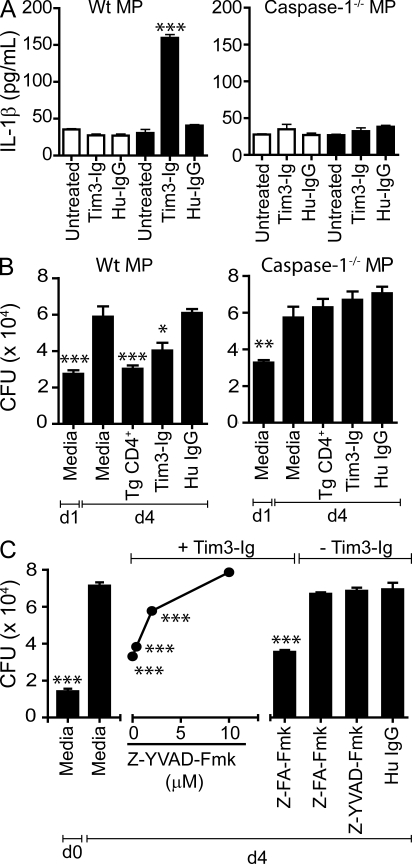
To determine which soluble mediators are produced by Mtb-infected macrophages after Tim3–Gal9 interaction, we profiled the cytokines in supernatants from Mtb-infected macrophages that were treated with or without Tim3-Ig. Under our in vitro conditions, uninfected macrophages constitutively produce significant amounts of IP10, TIMP-1, KC, JE, IL-1R antagonist (IL-1RA), and MIP-2 (Fig. S5). A similar pattern of cytokines and chemokines is detected after Tim3-Ig treatment of uninfected macrophages. In contrast, Tim3-Ig treatment of Mtb-infected macrophages stimulates significant production of IL-1α, IL-1β, IL-6, TNF, MIP1αβ, G-CSF, and increased level of MIP-2 and RANTES (Fig. S5). Although IL-18 was not included in this analysis, Tim3-Ig treatment of Mtb-infected macrophages increases IL-1β and IL-18 transcription (Fig. S6). The appearance of IL-1β in the culture supernatants indicates that active caspase-1 is expressed by Mtb-infected cells treated with Tim3-Ig.
To verify the results of the proteomic blot and to confirm whether the secretion of active IL-1β is caspase-1 dependent, IL-1β was measured in supernatants from uninfected and Mtb-infected WT and caspase-1−/− macrophages treated with and without Tim3-Ig. IL-1β was detected only in supernatants from Mtb-infected WT macrophages treated with Tim3-Ig (Fig. 5 A). We next addressed whether caspase-1 is required for the antimicrobial action mediated by Tim3-Ig. WT and caspase-1−/− macrophages treated with Tim3-Ig fusion protein were compared for their ability to suppress mycobacterial replication. Both Tim3-Ig and Tim3tg CD4+ T cells suppressed intracellular bacterial growth in WT macrophages. However, treatment with Tim3-Ig or co-culture of Tim3tg CD4+ T cells with Mtb-infected caspase-1−/− macrophages had no effect on the intracellular growth of Mtb (Fig. 5 B). To confirm these results, infected WT macrophages were treated with a caspase-1 inhibitor. Increasing concentrations of Z-YVAD-Fmk, but not a control peptide, abrogated Tim3-Ig mediated control of Mtb infection (Fig. 5 C). These results demonstrate that the secretion of IL-1β and control of Mtb replication induced by Tim3-Ig is dependent on macrophage caspase-1 activity.
Figure 5.
Tim3–Gal9 interaction induces caspase-1–dependent secretion of IL-1β and Mtb killing. (A) Mtb-infected WT C57BL/6J macrophages (MP) or Caspase-1−/− macrophages were cultured alone or with 10 µg/ml of Tim3-Ig fusion protein or HuIgG (control). 24 h later, culture supernatants from triplicate wells were assayed for IL-1β. Open bars indicate uninfected macrophages, and closed bars indicate Mtb-infected macrophages. Data are representative of seven independent experiments. Error bars indicate mean ± SEM from three replicate cultures. ***, P < 0.001, one-way ANOVA compared with untreated macrophages alone. (B) WT C57BL/6J or caspase-1−/− macrophages were infected with H37Rv in parallel. On day 1, Tim3Tg CD4+ T cells, Tim3-Ig, or HuIgG (control) were added to the macrophages. CFUs were determined on day 1 and day 4 (d4) after infection. Data are representative of three independent experiments. Bars indicate mean ± SEM from three replicate cultures. (C) WT C57BL/6J macrophages infected with H37Rv was co-cultured with Tim3-Ig in the presence or absence of Z-YVAD-Fmk (caspase-1 inhibitor) titrated fivefold. CFUs were determined on day 0, 2 h after Mtb infection, and on day 4. Z-FA-Fmk, negative control peptide for caspase-1 inhibitor. HuIgG, control for Tim3-Ig. Macrophages were also treated with 10 µM caspase-1 inhibitor and 10 µM negative peptide control in the absence of Tim3-Ig. *, P < 0.05; **, P < 0.01; ***, P < 0.001, one-way ANOVA compared with day 4 macrophages alone. Data are representative of three to four independent experiments. Error bars indicate mean ± SEM from three replicate cultures.
Tim3–Gal9 does not increase death of Mtb-infected macrophages
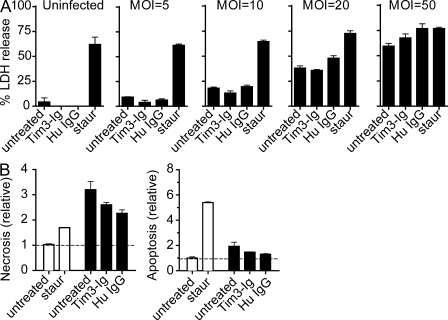
Certain pathogens induce pyroptosis, a form of host cell necrosis that is dependent on caspase-1 activity (Bergsbaken and Cookson, 2007). Our finding that control of Mtb replication after Tim3-Ig treatment is associated with caspase-1–dependent IL-1β production raised the possibility that restriction of intracellular growth was mediated by pyroptosis. Tim3-Ig treatment did not appear to induce necrosis under our in vitro infection conditions, as measured by the lactate dehydrogenase (LDH) release assay (unpublished data). However, the actual multiplicity of infection (MOI) of the infected macrophages is very low, and only <1% of cells are infected. The low frequency of infected cells could make it difficult to sensitively detect cell death. To address this issue, overnight infections were performed instead of our standard 2 h infection, and the MOI was varied. Necrosis was measured after 3 d, and cells were treated with staurosporine served as a control. No increase in LDH release was caused by Tim3-Ig treatment, although the overall necrosis paralleled the increase in MOI (Fig. 6 A). Because apoptosis is also associated with control of intracellular Mtb replication, we determined whether Tim3-Ig treatment induced apoptosis. An ELISA was used to measure intracellular and extracellular DNA–histone complexes, which is indicative of apoptosis and necrosis, respectively (Divangahi et al., 2009). Again, Tim3-Ig treatment did not increase the relative amount of necrosis or apoptosis observed in Mtb-infected macrophages (Fig. 6 B). Thus, Tim-Ig treatment does not induce pyroptosis and necrosis does not correlate with Tim3–Gal9–induced antimicrobial activity.
Figure 6.
Tim3–Gal9 interaction does not induce cell death in Tim3-Ig–treated macrophages. (A) WT macrophages were infected overnight at MOIs of 5, 10, 20, and 50. Uninfected and Mtb-infected macrophages were either cultured alone or with 10 µg/ml of Tim3-Ig fusion protein or human IgG (control). 1 µM staurosporine was added 24 h before LDH assay to induce necrosis and serve as positive control. 3 d after infection, culture supernatants were assayed for LDH release. (B) Enzyme-linked immunosorbent assay of apoptosis (right) and necrosis (left) 3 d after infection of WT macrophages cultured either alone or treated with 10 µg/ml of Tim3-Ig fusion protein or human IgG (control). Open bars indicate uninfected macrophages and closed bars indicate Mtb-infected macrophages. 1 µM staurosporine was either added 24 h before to induce necrosis or 3 h before assay to induce apoptosis. Data in A is representative of three independent experiments. Data in B is representative of two independent experiments.
IL-1β is required for the antimicrobial activity induced by Tim3
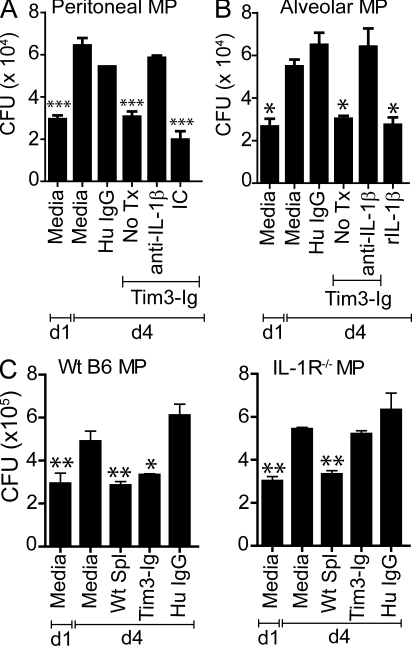
Our finding that bacterial killing can be transferred by a caspase-1–dependent secreted factor raised the possibility that IL-1β is the critical mediator induced by Tim3–Gal9 binding on Mtb-infected macrophages. Neutralizing antibody to IL-1β was added to Tim3-Ig–treated Mtb-infected peritoneal macrophages. Treatment with anti-IL-1β, but not an isotype control abrogated the antimicrobial effect of Tim3-Ig (Fig. 7 A). To show that this pathway is relevant for lung macrophages, alveolar macrophages were obtained from uninfected mice and infected in vitro. Treatment with Tim3-Ig led to control of Mtb growth and blockade of IL-1 abrogated the induction of antimicrobial activity of Tim3-Ig (Fig. 7 B). IL-1β treatment of Mtb-infected alveolar macrophages was sufficient to control growth of intracellular Mtb (Fig. 7 B). These results indicate that exogenously added IL-1β recapitulates the effects of Tim3-Ig, indicating that IL-1β is both necessary and sufficient to activate the mycobactericidal activity of infected macrophages. Tim3-Ig treatment did not modulate expression of IL-1RA or IL-1R (CD121α, IL-1R; Fig. S7). The action of IL-1β is mediated by binding to its cognate receptor, IL-1R, which forms a proximal signaling complex with MyD88 through TIR domains (Weber et al., 2010). We therefore hypothesized that the antimicrobial action of IL-1β is mediated through its receptor. To confirm the specificity of its action, we compared the protective efficacy of Tim3-Ig using infected WT and IL-1R−/− macrophages. As a control, we show that WT naive splenocytes suppress intracellular bacterial growth in both WT and IL-1R−/− macrophages. In contrast, Tim3-Ig treatment suppresses Mtb growth in WT macrophages but not in IL-1R−/− macrophages (Fig. 7 C). These results show that autocrine IL-1β signaling via the IL-1R is an important mechanism by which Tim3–Gal9 interaction induces antimicrobial activity in infected macrophages.
Figure 7.
IL-1β is necessary and sufficient to mediate Mtb killing. Mtb-infected WT peritoneal (A) or alveolar (B) macrophages (MP) were cultured either alone or in the presence of 10 µg/ml Tim3-Ig fusion protein with and without 25 µg/ml anti–IL-1β neutralizing antibody or isotype control (IC). No Tx, treatment with Tim3-Ig alone in the absence of neutralizing antibodies. Data in A is representative of 5–11 independent experiments. Data in B is from one experiment. Bars indicate mean ± SEM from three to six replicate cultures. (C) WT C57BL/6J or IL-1R−/− macrophages were infected with H37Rv in parallel. On day 1 (d1), WT splenocytes, Tim3-Ig, or HuIgG (control) were added to the macrophages. CFUs were determined on d1 and day 4 (d4) after infection. Representative data from three independent experiments are shown. CFUs were determined on day 1 and day 4 after infection. Data are from one experiment. Error bars indicate mean ± SEM from three to six replicate cultures. *, P < 0.05; **, P < 0.01; ***, P < 0.001, one-way ANOVA compared with day 4 macrophages alone.
DISCUSSION
Since its discovery in 2001, much has been learned about the role of Tim3 as a negative regulator of effector TH1 immunity in peripheral tolerance and during autoimmune disease (Monney et al., 2002; Sabatos et al., 2003; Sánchez-Fueyo et al., 2003; Zhu et al., 2005; Koguchi et al., 2006). Consistent with its capacity to inhibit T cell function, recent evidence indicates that Tim3 is a marker of dysfunctional T cells in patients with chronic HIV and HCV infection (Jones et al., 2008; Golden-Mason et al., 2009). Blockade with Tim3-Ig or anti-Tim3 mAb can augment immunity against these pathogens, indicating that Tim3 signaling may regulate T cell death or exhaustion (Jones et al., 2008; Golden-Mason et al., 2009). Although the consequences of Tim3 signaling on T cells have been the focus of numerous studies, whether there is a reciprocal signal transmitted to the participating Gal9-expressing APC is unknown. Here, we provide the first data that Tim3–Gal9 binding activates the Gal9+ APC. Using in vitro assays and in vivo models with the human pathogen Mtb, we show that a positive activating signal is transduced in a Gal9-dependent manner into infected macrophages after its interaction with Tim3. Tim3–Gal9 binding stimulates bactericidal activity in infected macrophages that is independent of IFN-γ and iNOS. Instead, the innate pathways activated by Tim3–Gal9 binding lead to caspase-1-dependent secretion of IL-1β. Other soluble factors such as TNF are secreted after Tim3-Ig treatment of Mtb-infected macrophages and may enhance control of intracellular Mtb replication (Fig. S5). However, our finding that neutralizing antibodies to IL-1β completely inhibit Tim3-Ig–mediated suppression of Mtb growth suggests that IL-1β activates infected macrophages and recruits additional antimicrobial effector functions. IL-1β signaling is both necessary and sufficient to restrict bacterial replication.
The ability of caspase-1 inhibitors and neutralizing antibodies specific for IL-1β to abrogate intracellular control of Mtb shows that the autocrine action of IL-1β is required to mediate the antimicrobial effects of Tim3–Gal9 signaling. Active IL-1β secretion depends on caspase-1 activity, which in turn requires the assembly and activation of the inflammasome, an innate response to pathogenic bacteria (Mariathasan and Monack, 2007; Yu and Finlay, 2008). Under some conditions, Mtb and Mycobacterium marinum activate the Nalp3 inflammasome leading to caspase-1 activation and secretion of IL-1β (Koo et al., 2008). High MOI infection with virulent Mtb, which favors necrosis, also stimulates IL-1β production. Under the conditions used for our studies (low MOI, <1% of cells infected), IL-1β secretion by infected macrophages was not detected. Only when infected macrophages were treated with Tim3-Ig was IL-1β secreted. Conversely, mycobacterial species produce caspase-1 inhibitors, such as the zinc metalloprotease 1, which prevents phagosome maturation (Master et al., 2008). We speculate that Tim3–Gal9 interaction may overcome zinc metalloprotease 1 inhibition of caspase-1 activity by increasing the amount of active caspase-1, which would promote IL-1β secretion and activate bactericidal pathways.
IL-1 is an essential cytokine for host resistance to Mtb. Both IL-1R−/− and IL-1αβ−/− mice rapidly succumb after infection with virulent Mtb infection (Fremond et al., 2007; Juffermans et al., 2000; Sugawara et al., 2001; Yamada et al., 2000). Furthermore, the discrepancy between the substantial susceptibility of MyD88−/− mice and the absent or modest phenotype of other Toll-like receptor knockout strains suggests that the critical role of MyD88 during Mtb infection is a manifestation of its requirement for IL-1R signaling (Fremond et al., 2007; Mayer-Barber et al., 2010). Although the greater susceptibility of caspase-1−/− mice than WT mice suggests a role for caspase-1 in the control of Mtb, Mayer-Barber et al. (2010) show that IL-1β−/− mice are significantly more susceptible than caspase-1−/− mice. This recent data suggest that alternate pathways exist that lead to the secretion and function of IL-1β in host immunity to tuberculosis. Although IL-1β production is generally acknowledged to require both Toll-like receptor signaling and caspase-1 activity, IL-1β function early during the host response to Mtb infection is independent of these two factors. IL-1β has several activities that could inhibit mycobacterial replication. IL-1β induces COX-2, PGE2, and EP4 in a variety of cell types (Takii et al., 1992; Tetsuka et al., 1994; Guan et al., 1997; Watanabe et al., 2009). Although it is unknown whether this occurs in Mtb-infected macrophages treated with Tim3-Ig, we have previously shown that these proinflammatory mediators play a key role in inhibiting necrosis of Mtb-infected macrophages by preventing mitochondrial inner membrane instability and repairing plasma membrane damage (Divangahi et al., 2009). Therefore, IL-1β secretion secondary to Tim3–Gal9 interaction might induce the prostanoid production and promote macrophage apoptosis instead of necrosis (Divangahi et al., 2009). Although further work is required to delineate the signaling pathways that culminate in the control of intracellular Mtb, our current studies provide the first evidence that IL-1β directly stimulates bactericidal activity in Mtb-infected macrophages.
Tim3 is an important negative regulatory molecule for TH1 and CD8+ T cells. It functions to maintain tolerance and to terminate effector T cell responses by inducing apoptosis (Kashio et al., 2003; Sabatos et al., 2003; Sánchez-Fueyo et al., 2003; Zhu et al., 2005). In the case of acute infection, Tim3 signaling may be an important mechanism that limits tissue damage and immunopathology from an uncontrolled immune response. On the other hand, during chronic infection, Tim3 signaling could be detrimental if it leads to suboptimal immunity because of premature clonal contraction or T cell exhaustion (Jones et al., 2008; Golden-Mason et al., 2009). However, the effect of Tim3–Gal9 signaling on antimicrobial immunity in vivo is likely to be a balance between its potential negative effects on T cells and its ability to stimulate bactericidal pathways in APCs to counter intracellular pathogens. This leads us to propose that Tim3, which predominantly appears on effector T cells, has evolved to inhibit intracellular pathogens by activating innate cells via Gal9, which, in turn, when expressed at high levels dampens effector T cells and prevents immunopathology. By providing a feedback-signaling loop involving cytokines including IFN-γ and IL-1β to up-regulate Gal9, Tim3 could play a central role in a bidirectional regulatory circuit that activates APCs to inhibit intracellular pathogens and the activated APCs in turn inhibit/terminate Tim3+ effector T cells (Fig. S8). IFN-γ and IL-1β may participate at different stages of infection to regulate the production of Gal9. Understanding how Gal9, which is a secreted molecule but also decorated on cell surface, signals and leads to macrophage activation is an important question. There is precedence for secreted proteins assembling on the cell surface with transmembrane proteins to form a signal transduction complex (Kirschning et al., 1998; Yang et al., 1998; Means et al., 1999). We propose that Tim3 and Gal9 form a complex that includes other membrane proteins and facilitates the activation of macrophages. There is likely to be strict regulation in the expression of Gal9 and a Gal9 dose-dependent regulation of T cell and macrophage activation that in turn determine their cellular fates.
In summary, our work provides the first evidence that during T cell–APC interaction, Gal9 signaling leads to activation of macrophages. We propose that although Tim3–Gal9 signaling regulates effector T cell expansion and tolerance induction, and prevents prolonged inflammation in target tissues under certain conditions, it may have primarily evolved to activate the innate immune system to control intracellular pathogens. How these two potentially opposing functions mediated by Tim3 and Gal9 are balanced in vivo during chronic infection requires further elucidation. Tim3 and Gal9 thus represent novel cell surface targets to modulate antimicrobial immunity and control infection in vivo.
MATERIALS AND METHODS
Materials.
The following reagents were used in this study: anti-CD3 (145-2C11; BD), anti-CD4 (GK1.5; BD), anti-CD8 (53–6.7; BD), rat anti–mouse CD16/CD32 (Fc-Block; BD), anti-Tim3 (5D12; V.K. Kuchroo), goat F(ab’)2 anti–human Ig (2012–01; SouthernBiotech), CD11b microbeads (Miltenyi Biotec), CD4 microbeads (Miltenyi Biotec), human IgG1κ (I5154; Sigma-Aldrich), human serum (Gemini Bioproducts), α-lactose (L-3625; Sigma-Aldrich), NG-monomethyl-l-arginine (L-NMMA; 475886; Calbiochem), L-N6-(1-Iminoethyl)lysine dihydrochloride (L-NIL; 482100; Calbiochem), caspase-1 inhibitor VI (Z-YVAD-Fmk; 218746; Calbiochem), caspase inhibitor negative control (Z-FA-Fmk; 342000; Calbiochem), recombinant mouse IL-1β (rIL-1β; R&D Systems), anti–IL-1β (B122; eBioscience), Armenian hamster IgG (eBio299Arm; eBioscience), proteome profiler mouse cytokine array (ARY006; R&D Systems), cytotoxicity detection kitplus (LDH; 04744926001; Roche), Cell Death Detection ELISAPLUS (11 920685 001; Roche), QuantiTect reverse transcription kit (QIAGEN), TRIzol (Invitrogen), TRIzol plus RNA purification system (Invitrogen), Brilliant SYBR Green QPCR master mix (Stratagene), Remel 7H10 M.tb plates (R01610; Thermo Fisher Scientific), full-length Tim3-Ig fusion protein (flTim3-Ig; V.K. Kuchroo), soluble Tim3-Ig fusion protein (sTim3-Ig; V. Kuchroo), and IL-1β ELISA antibody pairs (14–7012; 13–7112; eBioscience).
Mice.
6–10-wk-old C57BL/6J or Rag1−/− or IL-1R−/− were purchased from Jackson ImmunoResearch Laboratories; Galectin-9−/− was obtained from V. Kuchroo; Tim3tg and Tim3−/− mice (obtained from V. Kuchroo) were bred locally; Caspase-1−/− mice were obtained from M. Starnbach (Harvard Medical School, Boston, MA). All procedures were approved by the Institutional Animal Care and Use Committee of the Dana Farber Cancer Research Institute.
Bacteria, cells, and culture.
Virulent Mtb (H37Rv) was grown to mid-log phase in Middlebrook 7H9 broth (BD) with BBL Middlebrook OADC Enrichment (BD) and 0.05% Tween 80 (Difco). Aggregation was prevented by sonication for 10 s. CD11b+ peritoneal macrophages were harvested after being elicited with 3% thioglycollate followed by CD11b+ selection using MACS columns. Purified cells were >95% F4/80+ CD11b+, as determined by flow cytometry. Macrophages (0.5 × 106 cells per well) were seeded in a 24-well culture plate in complete RPMI 1640 medium (Invitrogen Life Technologies) supplemented with 10% FBS (HyClone), 1 mM pyruvate, 1% nonessential amino acids, 1% minimal essential amino acids, 2 mM l-glutamine, 7 mM NaOH, and 20 mM Hepes (all from Invitrogen). Cells were allowed to adhere for 2–24 h before in vitro infection with Mtb.
In vitro infections and co-cultures.
Peritoneal macrophages were infected with H37Rv at a MOI of 10 as previously described (Sada-Ovalle et al., 2008). In brief, Mtb were opsonized for 5 min using RPMI 1640 medium supplemented with 2% human serum/10% FBS/0.05% Tween 80, washed twice with complete medium without antibiotics. Bacteria were passed through a 5-µm syringe filter (Millipore), counted in a Petroff-Hausser chamber, and added to macrophages at the MOI indicated. The length of infection was 2 h for all experiments. Infected macrophages were cultured overnight before the addition of splenocytes, purified cell subsets, or other conditions. At days 1 and 4 after infection, cells were lysed with 1% Triton X-100 for 5 min and mycobacteria enumerated by plating serial dilutions of cell lysates on Middlebrook 7H10 agar plates and culture at 37°C. Colonies were counted after 21 d. WT and Tim3tg splenocytes were aseptically prepared, and CD4+ T cells were enriched by magnetic cell sorting using microbeads. CD4+ TIM3+ T cells from Tim3tg mice were sorted and cell purity was >90% as determined by flow cytometry. 2.5 × 106 splenocytes or T cells/well were added to Mtb-infected macrophages. In all the culture conditions, CFU was enumerated at day 1 (unless otherwise indicated) in untreated infected macrophages to determine initial inoculum and at day 4 after infection to determine growth of intracellular Mtb in untreated infected macrophages and relative suppression/killing mediated by various treatments. The “experimental” MOI is calculated by dividing the CFU recovered on day 1 by the number of macrophages per well. For example, recovery of 2,000 CFU in a well containing 500,000 macrophages indicates an experimental MOI of 0.004 (Fig. 3 A).
Alveolar macrophage isolation and infection.
Alveolar macrophages were isolated by bronchoalveolar lavage. Mice were sacrificed by CO2 inhalation. Lungs were lavaged with 1 ml sterile saline each time through an intratracheal catheter, and a total of 10 ml saline was instilled and recovered from each mouse. The lavage fluid was centrifuged at 300 g for 10 min to pellet cells, and 95–98% of cells recovered were macrophages (alveolar macrophages).The pelleted cells were resuspended and cultured in a 96-well culture plate at 37°C with 5% CO2 at a concentration of 5 × 104 cells per well in 200 µl RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% FBS, 1 mM pyruvate, 1% nonessential amino acids, 1% minimal essential amino acids, 2 mM l-glutamine, 7 mM NaOH, and 20 mM Hepes. After 24 h of incubation, nonadherent cells were washed off with PBS, and the medium was refreshed. Alveolar macrophages were infected with H37Rv according to methods mentioned in the previous section for peritoneal macrophages.
Tim3-Ig fusion protein treatment and Gal9, iNOS, caspase, IL-1β inhibition studies.
Tim3-Ig constructed as human IgG1 Fc tail fusion protein, is available as full-length or soluble Tim3-Ig based on the domains included in the fusion protein construct (Sabatos et al., 2003; Sánchez-Fueyo et al., 2003). Fusion proteins contained <0.1 EU/µg of LPS (Chimerigen Laboratories). In all in vitro infections, both flTim3-Ig and sTim3-Ig were used and data obtained were identical. However, for simplicity, data from either fusion protein is included in the figures. flTim3-Ig, sTim3-Ig, and HuIgG (control) were added to Mtb-infected macrophages at the concentrations indicated in the figure legends. After a 20-min incubation, goat F(ab’)2 anti–human Ig at a final concentration of 2.5 µg/ml was added to cross-link the fusion proteins or HuIgG. Tim3–Gal9 interaction was inhibited using differing final concentrations of α-lactose (12.5, 25, 50, and 100 mM) that was added to the culture media before 10 µg/ml Tim3-Ig treatment. To evaluate the role of iNOS in Tim3-mediated Mtb control, iNOS inhibitors L-NMMA (2 mM) and L-NIL (0.5 mM) were added to Mtb-infected macrophages 1 h before addition of WT and Tim3tg splenocytes. To determine whether caspase-1 was involved in Tim3-Ig–mediated control, caspase-1 inhibitor (Z-YVAD-Fmk; 0.08, 0.4, 2, and 10 µM final concentrations) was added to the culture media 20 min before Tim3-Ig treatment. Negative control peptide (Z-FA-Fmk) was added at 10 µM final concentration. As a control for cytoxicity, we treated macrophages with caspase-1 inhibitor and the negative control peptide at the highest concentration (10 µM) tested in the absence of Tim3-Ig. Our ability to recover similar levels of intracellular mycobacteria in caspase-1/negative peptide inhibitor–treated macrophages and untreated macrophages indicated that there was minimal cytotoxicity associated with these inhibitors at the concentrations used. To test control of Mtb by IL-1β, rIL-1β at a final concentration of 10 ng/ml was added directly to media containing infected macrophages. To determine whether IL-1β was required for Tim3-Ig–mediated Mtb killing, anti–IL-1β (25 µg/ml; final concentration) was added separately or in combination with anti–IL-1α (25 µg/ml; final concentration) to infected macrophages along with 10 µg/ml Tim3-Ig. To account for any cells dying after Tim3-Ig treatment and releasing mycobacteria into cell culture supernatant, leading to underestimation of the total amount of Mtb in infected macrophages, we measured CFU the following: (a) removal of supernatant, lysis of macrophages in 1% Triton X-100, and plating the supernatant and the macrophage lysate; or (b) lysing macrophages without the removal of cell culture supernatant by adding 10% Triton X-100 at 1/10th the cell culture volume. Under the standard in vitro infection conditions, we detected <10% of the total CFU in the cell culture supernatant. The CFU present in the supernatant was not statistically significant between macrophages treated with media, Tim3-Ig, or HuIgG.
Cytokine and chemokine detection.
Culture supernatants from uninfected and infected macrophages after Tim3-Ig treatment were filtered through 0.2 µM filter to remove any bacteria. Supernatants were assayed for cytokines and chemokines by either a sandwich ELISA or by mouse proteome cytokine profiler. ELISA was done in accordance with the manufacturer’s instructions, and absorbance was recorded at 405 nm on SoftMax Pro ELISA analysis software (Molecular Devices). IL-1β in culture supernatants was quantified by comparison with the appropriate recombinant standard (purchased from eBioscience). For the proteomic blots, culture supernatants from uninfected and infected macrophages treated with and without Tim3-Ig were mixed with a cocktail of biotinylated detection antibodies for various chemokines/cytokines provided with the kit and used to probe nitrocellulose membranes with spotted capture antibodies according to manufacturer’s instructions. Any cytokine/detection antibody complex present is bound to its cognate immobilized capture antibody on the membrane. Streptavidin-HRP detection agents were subsequently used to develop the blot with the intensity of the spot in direct proportion to the amount of cytokine bound.
Real-time reverse transcription-PCR.
Total RNA from equivalent macrophage numbers (typically 1–2 × 106 macrophages, pooled from 2–4 replicate wells) was isolated with Trizol plus RNA purification system, and 200–500 ng RNA was transcribed into cDNA using random hexamers and QuantiTect (QIAGEN) reverse transcription kit according to the manufacturer’s instructions. The cDNA was denatured for 10 min at 95°C. Specific DNA fragments were amplified using Brilliant SYBR Green QPCR Master Mix and Mx3000p qPCR Stratagene cycler for 40 cycles of 95°C for 15 s, 56°C for 60 s, and 72°C for 30 s. The oligonucleotide primers used were as follows: GAPDH, 5′-AGGTCGGTGTGAACGGATTTG-3′ (forward) and 5′-TGTAGACCATGTAGTTGAGGTCA-3′ (reverse); IL-1β, 5′-GCAACTGTTCCTGAACTCAACT-3′ (forward) and 5′-ATCTTTTGGGGTCCGTCAACT-3′ (reverse); IL-18, 5′-GACTCTTGCGTCAACTTCAAGG-3′ (forward) and 5′-CAGGCTGTCTTTTGTCAACGA-3′ (reverse); galectin-9, 5′-GTTGTCCGAAACACTCAGAT-3′ (forward) and 5′-ATATGATCCACACCGAGAAG-3′ (reverse). Threshold cycle numbers (Ct) were determined and transformed using ΔΔCt method as described by the manufacturer, using GAPDH as the calibrator. Expression of Gal9, IL-18, and IL-1β was calculated as fold increase over uninfected/untreated macrophages alone.
In vitro assays of necrosis and apoptosis.
Necrosis/pyroptosis in macrophages was evaluated through the release of the intracellular enzyme LDH in cell culture supernatants. The in vitro infections performed were done at a low (actual) MOI, resulting in 1–2% of cells being infected. Under these standard in vitro infection conditions, we did not detect LDH release. To improve the sensitivity of the LDH assay we sought to increase the frequency of infected cells by infecting the macrophages overnight as opposed to 2 h. At the times indicated after infection, the LDH activity of the culture supernatants of infected cells was measured by using a cytotoxicity detection kit according to the manufacturer’s protocol. Percentage of LDH release was calculated according to the formula: (LDH activitytest sample, SN − LDH activityuntreated uninfected macrophage, SN)/(Maximal releasable LDHSN+lys − LDH activityuntreated uninfected macrophage, SN), where SN is the amount in the supernatant and Lys is the amount in the lysates. In some experiments, apoptosis and necrosis were measured by enzyme-linked immunosorbent assay cell (Cell Death Detection ELISAPLUS; Roche) for quantification of cytoplasmic (apoptosis) and extracellular (necrosis) histone-associated DNA fragments according to the specifications of the manufacturer. The relative amount of necrosis or apoptosis was calculated as a ratio of the absorbance of infected macrophages to that of uninfected control macrophages.
Aerosol infection of mice.
All in vivo infections were performed using virulent H37Rv (Erdman strain) by the aerosol route with a nose-only exposure unit (Intox Products) that delivers ∼100–200 CFU per mouse (Woodworth et al., 2008). At different times after infection, mice were euthanized by carbon dioxide inhalation and lungs and spleens were aseptically removed. Organs were individually homogenized in 0.9% NaCl/0.02% Tween 80 with MiniBead Beater 8 (BioSpec Products) and viable bacteria were enumerated by plating 10-fold serial dilutions of organ homogenates onto 7H10 agar plates. Colonies were counted after 21 d.
In vivo treatment with Tim3-Ig.
WT and Rag−/− mice were treated with flTim3-Ig, sTim3-Ig, or HuIgG (control). The antibodies (0.5 mg each) were administered intraperitoneally 1 d after aerosol infection, followed by administration of 0.1 mg of antibody 5, 8, and 12 d after infection. 2 and 3 wk after infection, mice were euthanized and CFU was enumerated in lungs and spleens.
Adoptive transfer model of infection.
Elicited peritoneal macrophages from WT and Gal9−/− mice were isolated and infected in vitro with H37Rv as previously described. Suspended macrophages were infected for 1 h in vitro at a MOI of 10:1 of H37Rv. Free bacteria were then removed by six washes with PBS, each followed by centrifugation for 10 min at 200 g and 4°C. Cells were resuspended in PBS at a density of 0.5E6 cells per 100 µL, and then transferred by the intravenous route into naive Tim3tg mice. At 7 and 14 d after adoptive transfer, mice were euthanized and CFU was enumerated in lungs and spleens.
Flow cytometry.
Pulmonary and splenic cells from infected and uninfected WT C57BL/6J mice were stained for 20 min at 4°C with 25 µg/ml of anti–CD3-FITC, anti–CD4-PE, anti–CD8-PerCP, and anti–Tim3-APC antibodies. To inhibit nonspecific staining, murine FC receptors were blocked with 25 µg/ml of Fc-block for 15 min at 4°C before staining with fluorochrome-conjugated antibodies and appropriate isotype controls. Splenic T cells from uninfected Tim3tg mice were stained for CD3/CD4/CD8/Tim3 in a similar manner to determine Tim3+ T cells in Tim3tg mice. Data were collected using a FACSCanto (BD) and analyzed with FlowJo (Tree Star, Inc.).
Statistical analysis.
The data from the in vivo Tim3-Ig treatment experiments were analyzed by the nonparametric Kruskal-Wallis test (95% confidence interval) with Dunnett’s post-test comparing. A one-way ANOVA was used to analyze the in vitro macrophage infections, and Dunnett’s post-test was used to compare the day 4 control condition (infected and/or untreated macrophages) to the other groups. Analysis was performed using Prism 5.0 software (GraphPad Software, Inc.).
Online supplemental material.
Fig. S1 shows intracellular Gal9 expression in distinct myeloid subsets in lungs of Mtb-infected mice, induction of intracellular Gal9 in peritoneal macrophages after LPS stimulation, and induction of Gal9 transcripts after treatment with IFN-γ and Mtb infection. Fig. S2 shows representative FACS plots of greater Tim3 expression in T cells in Tim3tg mice compared with WT mice. Fig. S3 shows that Tim3 expression by splenocytes is crucial for Mtb control in infected macrophages. Fig. S4 shows that the antimicrobial action of Tim3-mediated Mtb control is independent of iNOS. Fig. S5 shows that a novel activation state is induced after Tim3-Ig treatment of Mtb-infected macrophages and that this activation state is dependent on caspase-1. Fig. S6 shows the induction of IL-1β and IL-18 transcripts after Tim3-Ig treatment of macrophages. Fig. S7 shows that other molecules involved in IL-1β signaling such as IL-1R and IL-1RA is not affected after Tim3-Ig treatment. Fig. S8 proposes a model of a Tim3–Gal9–mediated bidirectional circuit and its potential consequences on participating activated effector T cells and infected macrophages. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20100687/DC1.
Acknowledgments
We thank I-C. Ho, H. Remold, M. Divangahi, and M. Brenner for critical reading of the manuscript, and M. Starnbach for caspase-1−/− mice. We appreciate the technical assistance of C. Speich and D. Desjardins.
This work was supported by the National Institutes of Health grant R01 AI067731 to S.M. Behar, R01 NS 045937 and TIM program project grant P01 AI 073748 to V. K. Kuchroo, the Parker B. Francis Foundation postdoctoral fellowship to I. Sada-Ovalle, and the American Lung Association postdoctoral research training fellowship (RT-123085-N) to P. Jayaraman.
The authors have no competing financial interests.
P. Jayaraman, I. Sada-Ovalle, and S.M. Behar conceived of and designed the experiments, analyzed the data, and wrote the paper; P. Jayaraman and I. Sada-Ovalle did the experiments with assistance from S. Beladi; and A.C. Anderson, V. Dardalhon, C. Hotta, and V.K. Kuchroo provided reagents and intellectual input.
Footnotes
Abbreviations used:
- ANOVA
- analysis of variance
- Gal9
- galectin-9
- HCV
- hepatitis C virus
- HuIgG
- human Ig-γ
- IL-1RA
- IL-1R antagonist
- iNOS
- inducible NO synthase
- LDH
- lactate dehydrogenase
- L-NIL
- L-N6-(1-Iminoethyl)lysine dihydrochloride
- MOI
- multiplicity of infection
- Mtb
- Mycobacterium tuberculosis
- NO
- nitric oxide
- TIM-3
- T cell Ig and mucin domain 3
- Tim3-Ig
- Tim3-Ig fusion protein
- TLR
- Toll-like receptor
References
- Bergsbaken T., Cookson B.T. 2007. Macrophage activation redirects yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 3:e161 10.1371/journal.ppat.0030161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S., Singla R., Dey A.B., Prasad H.K. 1999. Dichotomy of cytokine profiles in patients and high-risk healthy subjects exposed to tuberculosis. Infect. Immun. 67:5597–5603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao E., Zang X., Ramagopal U.A., Mukhopadhaya A., Fedorov A., Fedorov E., Zencheck W.D., Lary J.W., Cole J.L., Deng H., et al. 2007. T cell immunoglobulin mucin-3 crystal structure reveals a galectin-9-independent ligand-binding surface. Immunity. 26:311–321 10.1016/j.immuni.2007.01.016 [DOI] [PubMed] [Google Scholar]
- Cooper A.M., Dalton D.K., Stewart T.A., Griffin J.P., Russell D.G., Orme I.M. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243–2247 10.1084/jem.178.6.2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M. 1991. Interferon-gamma-treated murine macrophages inhibit growth of tubercle bacilli via the generation of reactive nitrogen intermediates. Cell. Immunol. 132:150–157 10.1016/0008-8749(91)90014-3 [DOI] [PubMed] [Google Scholar]
- Divangahi M., Chen M., Gan H., Desjardins D., Hickman T.T., Lee D.M., Fortune S., Behar S.M., Remold H.G. 2009. Mycobacterium tuberculosis evades macrophage defenses by inhibiting plasma membrane repair. Nat. Immunol. 10:899–906 10.1038/ni.1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J.L. 1999. Why is IFN-gamma insufficient to control tuberculosis? Trends Microbiol. 7:477–478, author reply :478–479 10.1016/S0966-842X(99)01611-X [DOI] [PubMed] [Google Scholar]
- Flynn J.L., Chan J. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93–129 10.1146/annurev.immunol.19.1.93 [DOI] [PubMed] [Google Scholar]
- Flynn J.L., Chan J., Triebold K.J., Dalton D.K., Stewart T.A., Bloom B.R. 1993. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J. Exp. Med. 178:2249–2254 10.1084/jem.178.6.2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremond C.M., Togbe D., Doz E., Rose S., Vasseur V., Maillet I., Jacobs M., Ryffel B., Quesniaux V.F. 2007. IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection. J. Immunol. 179:1178–1189 [DOI] [PubMed] [Google Scholar]
- Golden-Mason L., Palmer B.E., Kassam N., Townshend-Bulson L., Livingston S., McMahon B.J., Castelblanco N., Kuchroo V., Gretch D.R., Rosen H.R. 2009. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J. Virol. 83:9122–9130 10.1128/JVI.00639-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Z., Baier L.D., Morrison A.R. 1997. p38 mitogen-activated protein kinase down-regulates nitric oxide and up-regulates prostaglandin E2 biosynthesis stimulated by interleukin-1beta. J. Biol. Chem. 272:8083–8089 10.1074/jbc.272.12.8083 [DOI] [PubMed] [Google Scholar]
- Gutierrez M.G., Master S.S., Singh S.B., Taylor G.A., Colombo M.I., Deretic V. 2004. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 119:753–766 10.1016/j.cell.2004.11.038 [DOI] [PubMed] [Google Scholar]
- Harris J., Hope J.C., Keane J. 2008. Tumor necrosis factor blockers influence macrophage responses to Mycobacterium tuberculosis. J. Infect. Dis. 198:1842–1850 10.1086/593174 [DOI] [PubMed] [Google Scholar]
- Imaizumi T., Kumagai M., Sasaki N., Kurotaki H., Mori F., Seki M., Nishi N., Fujimoto K., Tanji K., Shibata T., et al. 2002. Interferon-gamma stimulates the expression of galectin-9 in cultured human endothelial cells. J. Leukoc. Biol. 72:486–491 [PubMed] [Google Scholar]
- Jones R.B., Ndhlovu L.C., Barbour J.D., Sheth P.M., Jha A.R., Long B.R., Wong J.C., Satkunarajah M., Schweneker M., Chapman J.M., et al. 2008. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 205:2763–2779 10.1084/jem.20081398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juffermans N.P., Florquin S., Camoglio L., Verbon A., Kolk A.H., Speelman P., van Deventer S.J., van Der Poll T. 2000. Interleukin-1 signaling is essential for host defense during murine pulmonary tuberculosis. J. Infect. Dis. 182:902–908 10.1086/315771 [DOI] [PubMed] [Google Scholar]
- Kashio Y., Nakamura K., Abedin M.J., Seki M., Nishi N., Yoshida N., Nakamura T., Hirashima M. 2003. Galectin-9 induces apoptosis through the calcium-calpain-caspase-1 pathway. J. Immunol. 170:3631–3636 [DOI] [PubMed] [Google Scholar]
- Kirschning C.J., Wesche H., Merrill Ayres T., Rothe M. 1998. Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide. J. Exp. Med. 188:2091–2097 10.1084/jem.188.11.2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koguchi K., Anderson D.E., Yang L., O’Connor K.C., Kuchroo V.K., Hafler D.A. 2006. Dysregulated T cell expression of TIM3 in multiple sclerosis. J. Exp. Med. 203:1413–1418 10.1084/jem.20060210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo I.C., Wang C., Raghavan S., Morisaki J.H., Cox J.S., Brown E.J. 2008. ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell. Microbiol. 10:1866–1878 10.1111/j.1462-5822.2008.01177.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking J.D., Taylor G.A., McKinney J.D. 2003. Immune control of tuberculosis by IFN-gamma-inducible LRG-47. Science. 302:654–659 10.1126/science.1088063 [DOI] [PubMed] [Google Scholar]
- Mariathasan S., Monack D.M. 2007. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat. Rev. Immunol. 7:31–40 10.1038/nri1997 [DOI] [PubMed] [Google Scholar]
- Master S.S., Rampini S.K., Davis A.S., Keller C., Ehlers S., Springer B., Timmins G.S., Sander P., Deretic V. 2008. Mycobacterium tuberculosis prevents inflammasome activation. Cell Host Microbe. 3:224–232 10.1016/j.chom.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer-Barber K.D., Barber D.L., Shenderov K., White S.D., Wilson M.S., Cheever A., Kugler D., Hieny S., Caspar P., Núñez G., et al. 2010. Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 184:3326–3330 10.4049/jimmunol.0904189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means T.K., Lien E., Yoshimura A., Wang S., Golenbock D.T., Fenton M.J. 1999. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J. Immunol. 163:6748–6755 [PubMed] [Google Scholar]
- Monney L., Sabatos C.A., Gaglia J.L., Ryu A., Waldner H., Chernova T., Manning S., Greenfield E.A., Coyle A.J., Sobel R.A., et al. 2002. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 415:536–541 10.1038/415536a [DOI] [PubMed] [Google Scholar]
- Sabatos C.A., Chakravarti S., Cha E., Schubart A., Sánchez-Fueyo A., Zheng X.X., Coyle A.J., Strom T.B., Freeman G.J., Kuchroo V.K. 2003. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat. Immunol. 4:1102–1110 10.1038/ni988 [DOI] [PubMed] [Google Scholar]
- Sada-Ovalle I., Chiba A., Gonzales A., Brenner M.B., Behar S.M. 2008. Innate invariant NKT cells recognize Mycobacterium tuberculosis-infected macrophages, produce interferon-gamma, and kill intracellular bacteria. PLoS Pathog. 4:e1000239 10.1371/journal.ppat.1000239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Fueyo A., Tian J., Picarella D., Domenig C., Zheng X.X., Sabatos C.A., Manlongat N., Bender O., Kamradt T., Kuchroo V.K., et al. 2003. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 4:1093–1101 10.1038/ni987 [DOI] [PubMed] [Google Scholar]
- Seki M., Oomizu S., Sakata K.M., Sakata A., Arikawa T., Watanabe K., Ito K., Takeshita K., Niki T., Saita N., et al. 2008. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin. Immunol. 127:78–88 10.1016/j.clim.2008.01.006 [DOI] [PubMed] [Google Scholar]
- Sugawara I., Yamada H., Hua S., Mizuno S. 2001. Role of interleukin (IL)-1 type 1 receptor in mycobacterial infection. Microbiol. Immunol. 45:743–750 [DOI] [PubMed] [Google Scholar]
- Takii T., Akahoshi T., Kato K., Hayashi H., Marunouchi T., Onozaki K. 1992. Interleukin-1 up-regulates transcription of its own receptor in a human fibroblast cell line TIG-1: role of endogenous PGE2 and cAmacrophage. Eur. J. Immunol. 22:1221–1227 10.1002/eji.1830220517 [DOI] [PubMed] [Google Scholar]
- Tetsuka T., Daphna-Iken D., Srivastava S.K., Baier L.D., DuMaine J., Morrison A.R. 1994. Cross-talk between cyclooxygenase and nitric oxide pathways: prostaglandin E2 negatively modulates induction of nitric oxide synthase by interleukin 1. Proc. Natl. Acad. Sci. USA. 91:12168–12172 10.1073/pnas.91.25.12168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Namba A., Honda K., Aida Y., Matsumura H., Shimizu O., Suzuki N., Tanabe N., Maeno M. 2009. IL-1beta stimulates the expression of prostaglandin receptor EP4 in human chondrocytes by increasing production of prostaglandin E2. Connect. Tissue Res. 50:186–193 10.1080/03008200802588451 [DOI] [PubMed] [Google Scholar]
- Weber A., Wasiliew P., Kracht M. 2010. Interleukin-1beta (IL-1beta) processing pathway. Sci. Signal. 3:cm2 10.1126/scisignal.3105cm2 [DOI] [PubMed] [Google Scholar]
- Woodworth J.S., Wu Y., Behar S.M. 2008. Mycobacterium tuberculosis-specific CD8+ T cells require perforin to kill target cells and provide protection in vivo. J. Immunol. 181:8595–8603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H., Mizumo S., Horai R., Iwakura Y., Sugawara I. 2000. Protective role of interleukin-1 in mycobacterial infection in IL-1 alpha/beta double-knockout mice. Lab. Invest. 80:759–767 [DOI] [PubMed] [Google Scholar]
- Yang R.B., Mark M.R., Gray A., Huang A., Xie M.H., Zhang M., Goddard A., Wood W.I., Gurney A.L., Godowski P.J. 1998. Toll-like receptor-2 mediates lipopolysaccharide-induced cellular signalling. Nature. 395:284–288 10.1038/26239 [DOI] [PubMed] [Google Scholar]
- Yang L., Anderson D.E., Kuchroo J., Hafler D.A. 2008. Lack of TIM-3 immunoregulation in multiple sclerosis. J. Immunol. 180:4409–4414 [DOI] [PubMed] [Google Scholar]
- Yoshida H., Imaizumi T., Kumagai M., Kimura K., Satoh C., Hanada N., Fujimoto K., Nishi N., Tanji K., Matsumiya T., et al. 2001. Interleukin-1beta stimulates galectin-9 expression in human astrocytes. Neuroreport. 12:3755–3758 10.1097/00001756-200112040-00030 [DOI] [PubMed] [Google Scholar]
- Yu H.B., Finlay B.B. 2008. The caspase-1 inflammasome: a pilot of innate immune responses. Cell Host Microbe. 4:198–208 10.1016/j.chom.2008.08.007 [DOI] [PubMed] [Google Scholar]
- Zhang M., Lin Y., Iyer D.V., Gong J., Abrams J.S., Barnes P.F. 1995. T-cell cytokine responses in human infection with Mycobacterium tuberculosis. Infect. Immun. 63:3231–3234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Anderson A.C., Schubart A., Xiong H., Imitola J., Khoury S.J., Zheng X.X., Strom T.B., Kuchroo V.K. 2005. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 6:1245–1252 10.1038/ni1271 [DOI] [PubMed] [Google Scholar]