Abstract
Neurons display a myriad of dendritic architectures, reflecting their diverse roles in information processing and transduction in the nervous system. Recent findings suggest that neuronal signals may not account for all aspects of dendrite morphogenesis. Observations from C. elegans and other organisms suggest that glial cells can affect dendrite length and guidance, as well as localization and shapes of dendritic receptive structures, such as dendritic spines and sensory cilia. Thus, besides direct roles in controlling neuronal activity, glia contribute to neuron function by ensuring that neurons attain their proper shapes.
Introduction
The enormous diversity of dendritic shapes has been well documented [1]. This diversity is in no small part a result of each dendrite's unique task: to gather information from specific synaptic partners or from the environment, and to transmit this information to the axon. In mammals, dendritic arbors can be highly branched, and individual dendrite branches may possess numerous small protrusions termed dendritic spines, which represent postsynaptic terminals of excitatory synapses. The shapes of dendrites and their substructures can affect synaptic partner choice as well as the strength and efficacy of the connections that are made.
Intimately associated with neurons are glial cells, which comprise the most abundant cell type of the mammalian brain, and which, like neurons, exhibit dizzying morphological complexity and specialization [1-3]. Glia are well positioned to regulate dendritic morphology as they are not only in close proximity to neurons but also ensheath neuronal processes and synapses. Despite possible roles in controlling various aspects of nervous system function, in vivo studies of vertebrate glia and their roles in the nervous system have been complicated, primarily because the ablation or manipulation of glia often results in neuronal death [4,5]. By contrast, in the invertebrate nematode Caenorhabditis elegans, neurons survive following glia ablations, opening a unique in vivo arena in which to investigate the effects of glia on neuron function and shape [6*,7]. Like mammals, the dendrites of C. elegans neurons come in many shapes and sizes, from the single long extensions of amphid sensory neurons in the head of the animal [8], to the complex tiled branches of PVD and FLP mechanosensory neurons that cover the body [9*]. Associated with C. elegans neurons are 50 glial cells, which ensheath sensory endings, synapses, and neuron processes [10,11]. In this review, we describe recent findings that highlight the important roles of glia in dendritic morphogenesis, with a focus on recent studies of C. elegans.
Growing dendrites: anchors aweigh
The most prominent morphological features of neurons are their complex and highly stereotyped dendritic arbors [12]. Some of the signals controlling arbor shapes are neuron intrinsic. For example, the nuclear protein HAMLET is transiently expressed in external sensory neurons of Drosophila during the initial phases of dendrite outgrowth, and hamlet mutants display altered dendritic branching patterns [13]. In C. elegans, mutations that disrupt intrinsic activity of the transmembrane fusogen EFF-1 result in excessive and disorganized branching of PVD mechanosensory neuron dendrites, suggesting that EFF-1 may function to dictate membrane shape and curvature of the growing neurites [9*]. However, extrinsic signals seem to play important roles as well. External signals may be systemic [14], may emanate from other neurites, as in the case of activity-dependent dendritic shape determination [15] or dendritic tiling of da neurons of Drosophila [16,17], or may be provided by glia.
Glia have been implicated in directing process orientation in the developing vertebrate brain. Neurons generated by subventricular zone radial glia stem cells often contain a single process, resembling a dendrite [1,18], which is dynamically remodeled as neurons migrate to populate the brain. Neuronal migration is guided in part by the radial glia to which migrating neurons adhere and upon which they travel [19,20]. The dendrite-like processes that emanate from these migrating neurons are oriented along the radial glial tracks, suggesting specific adhesion. The basis of the adhesion is not well understood; however, astrotactin, a neuronal protein suggested to promote neuron-glia adhesion, is required for granule neuron migration and process adhesion in the cerebellum [21]. In the neocortex, recognition and adhesion of migrating neurons to radial glia requires integrins [22] and the gap junction proteins connexin 26 and connexin 43 [23].
Glia-derived cues are known to play important roles in axon guidance, affecting the shapes of axons by defining axonal extension paths [24]. Recent evidence suggests that these same glia-derived axon guidance cues can also act on dendrites [25]. For example, the extracellular matrix (ECM) protein Slit is expressed by specialized midline glia of the Drosophila central nervous system [26,27], and acts to repel axon growth cones that express the Slit receptor Robo [28,29]. In robo mutants, the dendrites of some neurons inappropriately migrate towards or cross the midline [30], and proper guidance of these dendrites requires cell autonomous expression of Robo (Figure 1a,b; [30]). In C. elegans, ventral cephalic sheath (CEPsh) glia that ensheath the nerve ring, a dense neuropil analogous to the brain of higher organisms, express the chemotropic protein Netrin/UNC-6. In unc-6 mutant animals [31] or in animals lacking CEPsh glia [7], axon paths are severely disrupted, demonstrating a role for these glial cells in axon guidance. RIA nerve ring neurons possess a single neurite whose proximal end is postsynaptic, resembling postsynaptic sites on dendrites. In unc-6 mutants this neurite also exhibits severe guidance defects, and fails to navigate towards the CEPsh glia [32]. Thus, glia can contribute to dendrite guidance via the secretion of chemotropic factors.
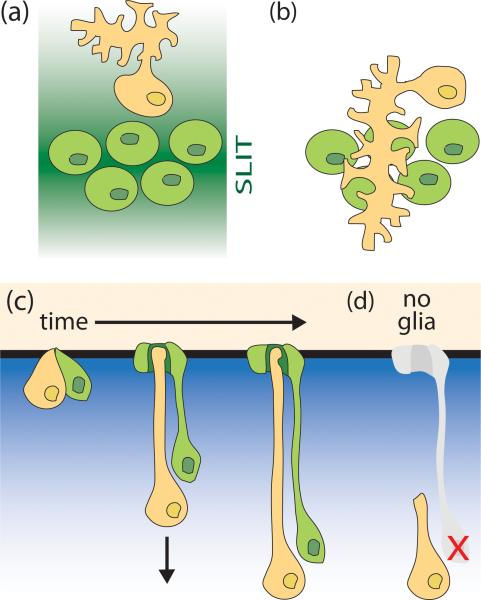
Figure 1.
Glia affect guidance and length of dendrite growth. (a) In Drosophila, midline glia (green) secrete the guidance molecule SLIT, shown as a dark green gradient in the extracellular environment. The dendrites of the RP2 motor neuron (orange) are repelled from the midline. The RP2 axon is not shown. (b) Same as (a), except in a SLIT receptor mutant background (robo). In these animals, the neurons no longer perceive SLIT (indicated by a loss of green shading). The RP2 dendrites inappropriately move towards and cross the midline [30]. (c,d) A model for glial involvement in dendrite extension of C. elegans sensory neurons. (c) The single, unbranched dendrite of a C. elegans amphid neuron (orange) extends a process via retrograde extension. The presumptive dendritic tip of the neuron is anchored to its local environment (black, horizontal line). The dendrite is then extended by posterior migration of the cell body (indicated by arrow). The direction of migration is likely driven by a gradient of a chemotropic factor (blue gradient). The neuron is associated with a glial cell (green), which extends a process that ensheaths the dendritic ending. The glial process likely develops by retrograde extension also [33**]. (d) When the amphid sheath glial precursor cell (Bacaj and Shaham, unpublished results) or the sheath glial precursor found in cephalic sensory structures [7] is ablated, the dendrite of the associated sensory neuron is too short.
Although dendrite tips may often need to be told where to go, this is not always the case. In C. elegans, most environmental signals are detected by neurons of the bilateral amphid sensilla. Each amphid consists of 12 neurons, each of which extends a single dendrite from the cell body to the nose-tip, a length of ~100 μm. Associated with these neurons is an amphid sheath (AMsh) glial cell, which also extends a process to the nose where it ensheaths the ciliated receptive endings of the dendrites [8]. Unlike some dendrites that elongate by growing a process out of a stationary cell body, time-lapse microscopy studies demonstrate that the dendrites of C. elegans amphid sensory neurons develop by first anchoring the presumptive dendritic tip to the surrounding environment at the nose [33**]. Posterior migration of the cell body then stretches out a dendritic process (Figure 1c; [33**]). The length of the dendrite and glial processes are correlated: in mutant backgrounds where the dendrites are too short, the glial process is also truncated [33**]. At least one component of the dendritic-tip anchor, DYF-7, is expressed by the sensory neurons [33**], suggesting that anchoring is in part determined by the neurons themselves. However, a second anchor component, DEX-1, is supplied by non-neuronal hypodermal cells surrounding the dendrite tip, raising the possibility that a number of cell types may contribute to creating the anchor.
The DYF-7/DEX-1 anchor seems to be an example of a structurally unique ECM of diverse functions in different systems. DYF-7 is a secreted zona pellucida (ZP) domain protein localized near the tips of anchored sensory dendrites, while DEX-1 is a secreted zonadhesin (zonad) domain protein. ZP domains form the ECM surrounding vertebrate oocytes (the zona pellucida), while zonadhesin is a sperm protein required for fertilization [34*]. Both domains are also present in α-tectorin, a major component of the tectorial membrane, a highly organized proteinaceous ECM that anchors the ciliated outer hair cells of the inner ear [35]. Mutations in dyf-7 and dex-1 exhibit genetic interactions suggestive of physical binding. Furthermore, polymerization of DYF-7 may be required for sensory dendrite anchoring [33**], suggesting that proper matrix formation is required for attachment.
The observation that a single AMsh glial cell ensheaths all 12 amphid sensory neurons suggests that glia are also in a position to contribute to the common anchoring matrix. Indeed, AMsh glia express several ZP domain proteins as well as other predicted extracellular proteins that could potentially contribute to the ECM anchor [6*]. Furthermore, ablation in early development of the precursor cells of the AMsh glia results in unanchored, short dendrites (Bacaj and Shaham, unpublished results), but does not affect sensory neuron cell migration. Similarly, the dendrites of CEP sensory neurons, which are part of another C. elegans anterior sensory organ, are shortened when their glial precursors are ablated, or when genes affecting differentiation of the associated glia are mutated (Figure 1d; [7]). Strengthening the notion that glia contribute to the ECM required for dendrite anchoring is the observation that the ECM that tethers Drosophila mechanosensory neurons is, at least in part, secreted by glia-like cells associated with these neurons [36]. In Drosophila type I mechanosensory organs, the ciliated endings of sensory neuron dendrites are ensheathed by cells analogous to C. elegans glia. These glia-like cells produce the dendritic cap, a specialized ECM that covers the cilia tip and connects it to a stimulating structure, either a mechanosensory bristle or an attachment cell. One of the components of this ECM is NompA, a ZP domain protein made by the thecogen and scolopale support cells [36]. In the absence of NompA, sensory neuron dendrite endings fail to form connections to the dendritic cap, and as a result, animals exhibit mechanosensory defects [36].
The studies reviewed here suggest that sensory organ glia may produce local ECM to which dendrite endings attach. This ECM, in turn, plays a key role in determining dendrite length. However, components of this specialized ECM may have other functions besides process anchoring. Indeed, recent studies suggest the involvement of glia-secreted proteins in controlling the shapes of dendritic receptive structures as well.
Receptive ending shapes: a little help from my glia
All dendrites possess receptive structures that receive information, either from other neurons at synapses, or in the case of sensory neurons, from the environment. For example, in the mammalian brain, dendrites receive information at most excitatory synapses through specialized structures termed dendritic spines, which appear as small protrusions on the dendrite process. Dendritic spines can be remodeled by environmental experience [37], and changes in spine shape are correlated with neuronal function [38]. Likewise, the shapes of sensory neuron dendritic endings are important, as mutations that affect sensory cilia morphology perturb the ability of a neuron to respond correctly to environmental stimuli [39]. These sensory receptive endings are also morphologically malleable. The dendritic receptive structures that receive information at synapses and those that receive environmental input share many similarities in function, shape and molecular components [40]. Intriguingly, both structures are frequently ensheathed by glia [40-42].
Studies of cultured purified mammalian retinal ganglion cell (RGC) neurons have been particularly informative in uncovering details of glia-neuron interactions during synapse formation, as these neurons form far fewer synapses when cultured in vitro in the absence of glia than in their presence [43]. A recent study suggests that physical contact between RGC neurons and astrocytic glia may allow these neurons to become competent for synapse formation. Glia-neuron contact reduces dendritic localization of the axonal protein neurexin [44], which reduces synapse formation when expressed in postsynaptic structures [45]. In C. elegans, Netrin/UNC-6 may play a similar role in excluding presynaptic elements from postsynaptic compartments [46]. Synapse formation between RGC neurons is further induced by secretion of the ECM molecule thrombospondin (TSP) from glia [47]. TSP interacts postsynaptically with the Ca2+ channel subunit α2δ-1 on neurons [48]. Interestingly, the AMsh glia of C. elegans also secrete a TSP-domain protein, called FIG-1, which is required for sensory neuron properties and function [6*]. Sensory neurons in fig-1 mutants are no longer able to accumulate the membrane dye DiI, suggesting the speculative possibility that the synaptogenic effects of TSP on RGC neurons may reflect a role in setting up postsynaptic architecture.
Studies in C. elegans also provide evidence for roles of non-neuronal cells in determining the locations of synapses. The presynaptic HSN neurons form synapses onto the postsynaptic VC neuron to create part of the circuit controlling egg-laying behavior in the animal. The positions of these synapses is determined not by the neurons, but by guidepost epithelial cells [49]. These guidepost cells express the transmembrane, immunoglobulin superfamily protein SYG-2, which interacts with and localizes the SYG-1 immunoglobulin protein on the HSN neurons [49,50]. Synapses form where SYG-1 is localized [49]. Similarly, C. elegans CEPsh glia may affect the location of synapse formation between the presynaptic interneuron AIY and its postsynaptic partner RIA. The Netrin receptor DCC/UNC-40 is expressed in AIY and localizes near the site where the CEPsh glia contact the neuron and secrete Netrin/UNC-6 [32].
In addition to regulating the formation and localization of the receptive structures on dendrites, glia also affect the shapes of these structures. During development of the mammalian cerebellum, the extension of processes from Bergmann glia is intimately correlated with changes in Purkinje cell dendritic spine shapes [51], suggesting that glia might influence spine shape dynamics. One way they may do this is via ephrin-Eph signaling. The astrocytic glia that ensheath hippocampal excitatory synapses express ephrin A3, while the receptor EphA4 is expressed in neurons and localizes to dendritic spines [52]. When EphA4 is activated by adding exogenous ephrin A3, the dendritic spines retract [52]. By contrast, mice lacking either ligand or receptor tend to exhibit elongated dendritic spines (Figure 2a,b; [52,53*]). The analysis of EphA4 mutant mice suggests that the consequences of these spine shape abnormalities may include defects in hippocampus-dependent learning [53*].
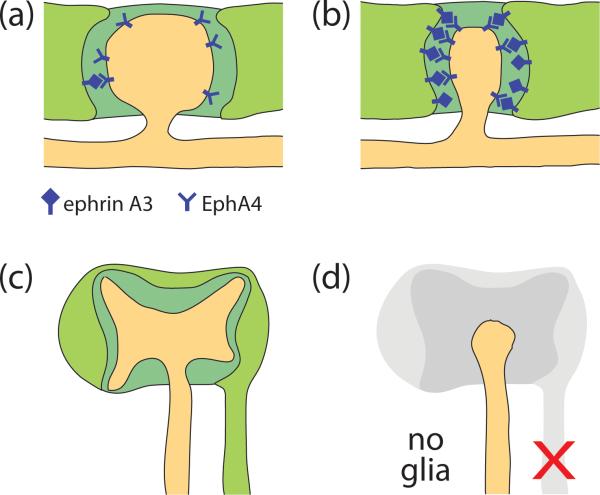
Figure 2.
Glia affect the shapes of dendritic receptive structures. (a,b) Mammalian astrocytic glia (light green) ensheath dendritic spines (orange protrusion) at excitatory synapses (a). For simplicity, the presynaptic specialization is not shown. An increase in ephrin A3/EphA4 signaling between the glia and dendritic spine results in spine retraction (b) [52,53*]. (c,d) The C. elegans amphid neuron AWC (orange) has a fan-shaped sensory cilium at its dendritic tip (c). The cilium is ensheathed by the amphid sheath glia (light green). When the glia is ablated late in development (d), the AWC cilium fails to maintain its proper shape [6*]. Extracellular matrix in (a)-(c) is colored dark green.
Similar roles for glia in controlling receptive structure shapes are also seen in C. elegans sensory organs. Late-stage ablations of the AMsh glia result in changes in the morphology of the sensory endings of the ensheathed amphid neurons (Figure 2c,d; [6*]). These changes correlate with behavioral defects of the animals in response to specific environmental stimuli [6*]. The molecules contributed by the AMsh glia to maintain dendrite ending shape are not yet known; however, the identification of a large number of glia-enriched mRNAs encoding secreted and transmembrane proteins by microarray analysis [6*] may provide candidates for mediating shape determination.
In addition to a maintenance role, the AMsh glia are also required for plasticity of sensory dendrite receptive endings. In response to environmental stressors, C. elegans enters a protective, developmentally-arrested stage termed dauer, in which the dendritic sensory endings of the AWC amphid neurons change shape [54]. This remodeling correlates with expansion and fusion of the two bilateral AMsh glia where they ensheath the AWC sensory endings [54]. By using mutations that specifically block glial fusion, we have shown that the changes in AWC shape are delimited by concomitant, dauer-dependent remodeling of glial shape (Procko and Shaham, submitted). Thus, sensory receptive ending plasticity in C. elegans depends on glial plasticity.
Conclusions
Dendrite length and guidance, as well as the formation, placement, and shapes of dendritic receptive structures can all be affected by glia, suggesting that these cells, once thought of as merely support cells, play key roles in shaping the nervous system. The implications of these studies are profound, as in all nervous systems, neuronal shape determines circuitry, and the shapes of receptive structures affect signal strength. Thus, exploration of glial roles in controlling neuron shape and activity is essential for understanding how the nervous system is put together and how it functions. A major unanswered question that must now be tackled is whether glial roles are permissive or regulatory. Are glia the sites of control, or a necessary background? Although the answer to this question is still unclear, and is likely to be complex, the advent of new model systems in which to study glia may help in tackling this important question. Studies of the nematode C. elegans may prove particularly useful in understanding gliadendrite interactions. C. elegans has a small, invariant number of neurons and glia, which have stereotyped shapes and connections. Importantly, C. elegans glia are not essential for neuronal survival. Furthermore, the facile genetics of C. elegans provides a powerful setting for gene discovery, which may prove useful for uncovering the molecular basis of glial actions in the nervous system. The conserved functional, morphological, and molecular features of mammalian and C. elegans glia [6*,7] suggest that this ‘simple’ nematode may be able to teach us something about the role of glia in the development and function of the most complex of organs: the human brain.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
* of special interest
** of outstanding interest
- 1.Cajal SR. Histologie du Système Nerveux de L'Homme et des Vertébrés. Maloine. Paris. 1911 [Google Scholar]
- 2.Awasaki T, Lai SL, Ito K, Lee T. Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J Neurosci. 2008;28:13742–13753. doi: 10.1523/JNEUROSCI.4844-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doherty J, Logan MA, Tasdemir OE, Freeman MR. Ensheathing glia function as phagocytes in the adult Drosophila brain. J Neurosci. 2009;29:4768–4781. doi: 10.1523/JNEUROSCI.5951-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delaney CL, Brenner M, Messing A. Conditional ablation of cerebellar astrocytes in postnatal transgenic mice. J Neurosci. 1996;16:6908–6918. doi: 10.1523/JNEUROSCI.16-21-06908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui W, Allen ND, Skynner M, Gusterson B, Clark AJ. Inducible ablation of astrocytes shows that these cells are required for neuronal survival in the adult brain. Glia. 2001;34:272–282. doi: 10.1002/glia.1061. [DOI] [PubMed] [Google Scholar]
- 6 *.Bacaj T, Tevlin M, Lu Y, Shaham S. Glia are essential for sensory organ function in C. elegans. Science. 2008;322:744–747. doi: 10.1126/science.1163074. [In addition to describing a role for C. elegans glia in the maintenance of sensory neuron shape and function, this study identified a large number of molecules potentially secreted by the glia to form a specialized ECM surrounding the dendritic sensory endings. One of these glia-secreted molecules, FIG-1, is related to thrombospondins (TSPs), and is required for neuronal properties and function. Interestingly, TSPs are also secreted by mammalian glia and are required for synaptogenesis [47].] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshimura S, Murray JI, Lu Y, Waterston RH, Shaham S. mls-2 and vab-3 Control glia development, hlh-17/Olig expression and glia-dependent neurite extension in C. elegans. Development. 2008;135:2263–2275. doi: 10.1242/dev.019547. [DOI] [PubMed] [Google Scholar]
- 8.Ward S, Thomson N, White JG, Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol. 1975;160:313–337. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- 9 *.Oren-Suissa M, Hall DH, Treinin M, Shemer G, Podbilewicz B. The Fusogen EFF-1 Controls Sculpting of Mechanosensory Dendrites. Science. 2010 doi: 10.1126/science.1189095. [Although the focus of this review is on extrinsic glia-derived signals that regulate dendrite morphogenesis, this particularly interesting study shows that intrinsic activity of the transmembrane fusogen EFF-1 is required for sculpting of complex dendritic arbors in C. elegans. The authors hypothesize that EFF-1 may function in this capacity by inducing changes in membrane shape and curvature of the growing dendrites.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 11.Shaham S. Glia-neuron interactions in the nervous system of Caenorhabditis elegans. Curr Opin Neurobiol. 2006;16:522–528. doi: 10.1016/j.conb.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Gao FB. Molecular and cellular mechanisms of dendritic morphogenesis. Curr Opin Neurobiol. 2007;17:525–532. doi: 10.1016/j.conb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore AW, Jan LY, Jan YN. hamlet, a binary genetic switch between single- and multiple- dendrite neuron morphology. Science. 2002;297:1355–1358. doi: 10.1126/science.1072387. [DOI] [PubMed] [Google Scholar]
- 14.Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parrish JZ, Emoto K, Kim MD, Jan YN. Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Annu Rev Neurosci. 2007;30:399–423. doi: 10.1146/annurev.neuro.29.051605.112907. [DOI] [PubMed] [Google Scholar]
- 16.Grueber WB, Jan LY, Jan YN. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development. 2002;129:2867–2878. doi: 10.1242/dev.129.12.2867. [DOI] [PubMed] [Google Scholar]
- 17.Matthews BJ, Kim ME, Flanagan JJ, Hattori D, Clemens JC, Zipursky SL, Grueber WB. Dendrite self-avoidance is controlled by Dscam. Cell. 2007;129:593–604. doi: 10.1016/j.cell.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Hatten ME. New directions in neuronal migration. Science. 2002;297:1660–1663. doi: 10.1126/science.1074572. [DOI] [PubMed] [Google Scholar]
- 19.Rakic P. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus Rhesus. J Comp Neurol. 1971;141:283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- 20.Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- 21.Fishell G, Hatten ME. Astrotactin provides a receptor system for CNS neuronal migration. Development. 1991;113:755–765. doi: 10.1242/dev.113.3.755. [DOI] [PubMed] [Google Scholar]
- 22.Anton ES, Kreidberg JA, Rakic P. Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron. 1999;22:277–289. doi: 10.1016/s0896-6273(00)81089-2. [DOI] [PubMed] [Google Scholar]
- 23.Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- 24.Chotard C, Salecker I. Neurons and glia: team players in axon guidance. Trends Neurosci. 2004;27:655–661. doi: 10.1016/j.tins.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Kim S, Chiba A. Dendritic guidance. Trends Neurosci. 2004;27:194–202. doi: 10.1016/j.tins.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Rothberg JM, Hartley DA, Walther Z, Artavanis-Tsakonas S. slit: an EGF-homologous locus of D. melanogaster involved in the development of the embryonic central nervous system. Cell. 1988;55:1047–1059. doi: 10.1016/0092-8674(88)90249-8. [DOI] [PubMed] [Google Scholar]
- 27.Rothberg JM, Jacobs JR, Goodman CS, Artavanis-Tsakonas S. slit: an extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev. 1990;4:2169–2187. doi: 10.1101/gad.4.12a.2169. [DOI] [PubMed] [Google Scholar]
- 28.Battye R, Stevens A, Jacobs JR. Axon repulsion from the midline of the Drosophila CNS requires slit function. Development. 1999;126:2475–2481. doi: 10.1242/dev.126.11.2475. [DOI] [PubMed] [Google Scholar]
- 29.Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 30.Furrer MP, Kim S, Wolf B, Chiba A. Robo and Frazzled/DCC mediate dendritic guidance at the CNS midline. Nat Neurosci. 2003;6:223–230. doi: 10.1038/nn1017. [DOI] [PubMed] [Google Scholar]
- 31.Wadsworth WG, Bhatt H, Hedgecock EM. Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron. 1996;16:35–46. doi: 10.1016/s0896-6273(00)80021-5. [DOI] [PubMed] [Google Scholar]
- 32.Colon-Ramos DA, Margeta MA, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 2007;318:103–106. doi: 10.1126/science.1143762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33 **.Heiman MG, Shaham S. DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration. Cell. 2009;137:344–355. doi: 10.1016/j.cell.2009.01.057. [This study describes the coordinated growth of sensory neuron dendrites and glial processes by retrograde extension in C. elegans. The presumptive dendritic tips are anchored to a local ECM, and then migration of the cell bodies drags out the dendritic processes. DEX-1 and DYF-7, zonadhesin and zona pellucida domain-containing proteins, respectively, are part of the local ECM required for dendritic tip anchoring.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34 *.Monne M, Han L, Schwend T, Burendahl S, Jovine L. Crystal structure of the ZP-N domain of ZP3 reveals the core fold of animal egg coats. Nature. 2008;456:653–657. doi: 10.1038/nature07599. [This study describes the first high resolution structure of the amino-region of the zona pellucida (ZP) domain. The authors show that this region has a unique, immunoglobulin-like fold that is required for polymerization of ZP proteins into higher order structures to form a matrix.] [DOI] [PubMed] [Google Scholar]
- 35.Legan PK, Rau A, Keen JN, Richardson GP. The mouse tectorins. Modular matrix proteins of the inner ear homologous to components of the sperm-egg adhesion system. J Biol Chem. 1997;272:8791–8801. doi: 10.1074/jbc.272.13.8791. [DOI] [PubMed] [Google Scholar]
- 36.Chung YD, Zhu J, Han Y, Kernan MJ. nompA encodes a PNS-specific, ZP domain protein required to connect mechanosensory dendrites to sensory structures. Neuron. 2001;29:415–428. doi: 10.1016/s0896-6273(01)00215-x. [DOI] [PubMed] [Google Scholar]
- 37.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 38.Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Annu Rev Neurosci. 2008;31:47–67. doi: 10.1146/annurev.neuro.31.060407.125646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 40.Shaham S. Chemosensory organs as models of neuronal synapses. Nat Rev Neurosci. 2010;11:212–217. doi: 10.1038/nrn2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spacek J. Three-dimensional analysis of dendritic spines. III. Glial sheath. Anat Embryol (Berl) 1985;171:245–252. doi: 10.1007/BF00341419. [DOI] [PubMed] [Google Scholar]
- 42.Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- 44.Barker AJ, Koch SM, Reed J, Barres BA, Ullian EM. Developmental control of synaptic receptivity. J Neurosci. 2008;28:8150–8160. doi: 10.1523/JNEUROSCI.1744-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taniguchi H, Gollan L, Scholl FG, Mahadomrongkul V, Dobler E, Limthong N, Peck M, Aoki C, Scheiffele P. Silencing of neuroligin function by postsynaptic neurexins. J Neurosci. 2007;27:2815–2824. doi: 10.1523/JNEUROSCI.0032-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poon VY, Klassen MP, Shen K. UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature. 2008;455:669–673. doi: 10.1038/nature07291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 48.Eroglu C, Allen NJ, Susman MW, O'Rourke NA, Park CY, Ozkan E, Chakraborty C, Mulinyawe SB, Annis DS, Huberman AD, et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell. 2009;139:380–392. doi: 10.1016/j.cell.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen K, Bargmann CI. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell. 2003;112:619–630. doi: 10.1016/s0092-8674(03)00113-2. [DOI] [PubMed] [Google Scholar]
- 50.Shen K, Fetter RD, Bargmann CI. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell. 2004;116:869–881. doi: 10.1016/s0092-8674(04)00251-x. [DOI] [PubMed] [Google Scholar]
- 51.Lippman JJ, Lordkipanidze T, Buell ME, Yoon SO, Dunaevsky A. Morphogenesis and regulation of Bergmann glial processes during Purkinje cell dendritic spine ensheathment and synaptogenesis. Glia. 2008;56:1463–1477. doi: 10.1002/glia.20712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Murai KK, Nguyen LN, Irie F, Yamaguchi Y, Pasquale EB. Control of hippocampal dendritic spine morphology through ephrin-A3/EphA4 signaling. Nat Neurosci. 2003;6:153–160. doi: 10.1038/nn994. [DOI] [PubMed] [Google Scholar]
- 53 *.Carmona MA, Murai KK, Wang L, Roberts AJ, Pasquale EB. Glial ephrin-A3 regulates hippocampal dendritic spine morphology and glutamate transport. Proc Natl Acad Sci U S A. 2009;106:12524–12529. doi: 10.1073/pnas.0903328106. [Here, in conjunction with [52], the authors show that glial ephrin A3 activates neuronal EphA4 to regulate the morphology of dendritic spines of hippocampal pyramidal neurons. In addition, ephrin A3-EphA4 activity modulated glial glutamate transport. Animals with impaired ephrin A3-EphA4 signaling showed defects in hippocampus-based learning, suggesting that dendritic spine morphology and glial glutamate transport are required for correct hippocampal function.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Albert PS, Riddle DL. Developmental alterations in sensory neuroanatomy of the Caenorhabditis elegans dauer larva. J Comp Neurol. 1983;219:461–481. doi: 10.1002/cne.902190407. [DOI] [PubMed] [Google Scholar]