Summary
Surfactant protein A (SP-A), the most abundant protein in the lung alveolar surface, has multiple activities, including surfactant-related functions. SP-A is required for the formation of tubular myelin and the lung surface film. The human SP-A locus consists of two functional SP-A genes, SP-A1 and SP-A2, with a number of alleles characterized for each gene. We have found that the human in vitro expressed variants, SP-A1 (6A2) and SP-A2 (1A0), and the coexpressed SP-A1/SP-A2 (6A2/1A0) protein have a differential influence on the organization of phospholipid monolayers containing surfactant protein B (SP-B). Lipid films containing SP-B and SP-A2 (1A0) showed surface features similar to those observed in lipid films with SP-B and native human SP-A. Fluorescence images revealed the presence of characteristic fluorescent probe-excluding clusters coexisting with the traditional lipid liquid-expanded and liquid-condensed phase. Images of the films containing SP-B and SP-A1 (6A2) showed different distribution of the proteins. The morphology of lipid films containing SP-B and the coexpressed SP-A1/SP-A2 (6A2/1A0) combined features of the individual films containing the SP-A1 or SP-A2 variant. The results indicate that human SP-A1 and SP-A2 variants exhibit differential effects on characteristics of phospholipid monolayers containing SP-B. This may differentially impact surface film activity.
Introduction
Pulmonary surfactant is essential for normal lung function. Structurally, it is dynamic and a number of morphologic forms have been characterized for surfactant. These, among others, include lamellar bodies found both intracellulaly and extracellularly, and tubular myelin and the surface film found extracellularly. Its composition is about 90% lipids and 10% proteins. The surfactant-associated proteins (SPs) include SP-A, SP-B, SP-C, and SP-D. The primary lipid is dipalmitoylphosphatidylcholine (DPPC), which comprises over 40% of the material. Other lipids present in relatively substantial amounts are unsaturated phosphatidylcholine (PC), phosphatidylglycerol (PG), and cholesterol [1]. SP-A, an asialoglycoprotein of complex architecture, plays an important role in the structure, metabolism, and the surface-tension lowering activity of surfactant [2–7], host defense [8–10], and in parturition, serving as a hormone [11].
The human SP-A locus consists of two functional genes, SP-A1 and SP-A2, in opposite transcriptional orientation, and a pseudogene between the SP-A1 and SP-A2 genes [12]. Both, the cDNAs and the genomic sequences have been cloned and characterized [13–15]. Based on nucleotide differences within the coding region) more than 30 alleles (or variants) for both genes have been characterized in part or fully [16, 17]. Ten of these variants are found in the general population at a frequency of 0.01. Four of these are for SP-A1 (i.e. 6A, 6A2, 6A3, 6A4), and six are for SP-A2 (i.e. 1A, 1A0, 1A1, 1A2, 1A3, 1A5). Some of the nucleotide polymorphisms result in amino acid substitutions [18], and collectively SP-A1 and SP-A2 variants differ at 10 amino acid residues, including a cysteine at position 85. Native SP-A is an octadecamer that consists of six trimers, and it has been suggested that each trimer consists of two SP-A1 and one SP-A2 gene products [19]. However, the mRNA ratio of SP-A1 and SP-A2 differs from the 2:1 ratio proposed for protein structure [20], indicating that single gene products may exist in homo-oligomeric structures. Moreover, recent evidence indicates that the relative amounts of SP-A1 and SP-A2 gene products differ as a function of lung health status [21].
In vitro expressed single gene products of human SP-A1 or SP-A2 have been shown to be functional, and with differences in biochemical and biophysical properties, as well as in other functions [22–26]. Previous findings on the expressed protein products of the SP-A1 and SP-A2 alleles [24] have indicated that the two gene products exhibit the following differences: in oligomerization of the protein under appropriate conditions; in the structural stability of their collagen domains, with the SP-A2 products being more stable than those of SP-A1; in ability to cause aggregation of lipopolysaccharides and liposomal lipids, with SP-A2 products being more potent in causing aggregation than SP-A1 products. In addition, a number of SP-A variants have been shown to associate with several pulmonary diseases including respiratory distress syndrome in the prematurely born infant [22, 27–31]. A synergistic effect between SP-A and SP-B variants in RDS susceptibility has also been observed [31–34].
SP-A and SP-B are necessary for the formation of tubular myelin [5, 6], and SP-A has been shown, in vitro, to have an impact on the regional organization of phospholipid monolayers containing SP-B or SP-C [35, 36]. The question addressed in this report is whether the two human SP-A1 and SP-A2 gene products have a differential influence in the organization of phospholipid monolayers, especially monolayers containing phospholipids and the surfactant protein SP-B [36]. To explore this, we studied the interaction of human in vitro expressed SP-A variants (i.e. the 6A2 of SP-A1, the 1A0 of SP-A2, and the coexpressed 6A2/1A0 product of SP-A1/SP-A2) with SP-B, in phospholipid monolayers. The SP-A variants used in the study, were expressed in vitro from stably transfected mammalian Chinese Hamster Ovary (CHO) cells. The results indicated differences between the two SP-A variants in their interaction with SP-B.
Materials and Methods
1. Experimental Materials
DPPC and egg PG were obtained from Avanti Polar Lipids (Birmingham, AL), and were used as received after verification of their purity by thin layer chromatography. Water was deionized and doubly distilled, and the second distillation being from dilute potassium permanganate solution. Other chemicals were obtained from Fisher Scientific Co. (Ottawa, ON, Canada) or Sigma (St. Louis, Mo).
2. Human SP-A1 and SP-A2 variants from in vitro expression CHO cells
The mammalian Chinese Hamster Ovary (CHO)-K1 cell line (American Type Culture Collection, Manassas, VA, Cat. CCL 61) was used as the host to express human SP-A variants. The cell culture techniques and the culture media were previously described [37]. Stably transfected CHO cell lines that expressed human SP-A1 (6A2) or SP-A2 (1A0) were obtained through transfection and selection, as described previously [37]. To express SP-A variants, cells were grown to confluence in the growth medium with fetal bovine serum, then the growth medium was removed and expression medium, which did not contain fetal bovine serum but had 0.5 mM ascorbic acid, and 40 mg of proline per liter medium was added. The medium containing secreted SP-A protein was harvested after 5 days in culture, and SP-A variants were recovered and purified from the culture medium using mannose-affinity chromatography, as described previously [26]. Purified SP-A was concentrated using Amicon Centriprep-10 concentrators (Amicon, Beverly, MD).
3. Native human SP-A from BAL fluid
The native human SP-A was purified from BAL fluid obtained from alveolar proteinosis patients using a butanol-extraction method as described [38, 39] with slight modification. In brief, after complete extraction of whole BAL surfactant with butanol, the mixture was centrifuged at 5,000 × g at 15°C for 30 min and the supernatant, which contains the butanol, was discarded. The pellet was then completely dried with a flux of nitrogen gas. The dry pellet was homogenized in 24 ml of the OBG buffer (20 mM n-Octyl ß-D-Glucopyranoside, 10 mM Hepes, 150 mM NaCl, pH 7.4) and centrifuged at 210,000 × g at 15°C for 30 min, and then the supernatant containing detergent-soluble proteins and potential trace amount of butanol was discarded. The above procedure was repeated twice. After a final pelleting, the detergent-insoluble protein was dissolved in 5 ml of buffer (5mM Tris/HCl, pH 7.4) and dialyzed extensively for 48 hours against 4 liters of the same buffer with four changes of buffer. The dialyzed solution was centrifuged at 155,000 × g at 4°C for 30 min and the supernatant containing SP-A was collected and kept at −80°C.
With regards to the potential surface activity of butanol that was used early in the purification procedure, we have always been particularly cautious about avoiding any effects of residual surface active material by diluting and dialyzing our samples quite extensively. In this case, the butanol used in the early step was removed immediately by centrifugation and pellet dryness procedure. The pellet was then resuspended in 24 ml OBG buffer (see above) and centrifuged to remove any contaminating trace of butanol (this procedure was repeated 3 times). The final pellet was dissolved in 5 ml of buffer (see above) and dialysed against 0.8 × 103 fold buffer with four changes of the buffer. Therefore, the dilution factor is estimated to be about 1015. We have never observed abnormal effects that we could attributed to butanol or other materials in our studies with SP-A.
All procedures were performed at 4°C or on ice. Protein concentration of SP-A was determined using the Micro-BCA method of Smith et al [40] (Pierce, Rockford, IL) with RNase A as a standard. SP-A was aliquoted and stored at −80°C, until use.
4. Porcine SP-A and porcine SP-B preparation
Porcine SP-A and SP-B were prepared as described before [41]. In brief, pig lungs were lavaged with 150 mM NaCl, and the lavage was centrifuged at 800 × g for 10 min. The pellet was removed and the supernatant was centrifuged again at 7000 × g for 60 min. The pellet was used for isolation of either SP-A or SP-B. SP-A was obtained from the pellet by extraction with 1-butanol and further purified as described above [38, 42]. SP-B was prepared from the pellet using chloroform-methanol as described previously [43, 44].
5. Monolayer fluorescence analysis
Monolayer fluorescence measurements were performed in the following way. DPPC and SP-B were mixed in chloroform: methanol and 1 mol% of NBD-PC (based on phospholipid content) was added. Monolayers were formed by spreading of the lipidprotein mixture containing 17 wt% SP-B on subphases of 145 mM NaCl, 5 mM Tris, 5 mM CaCl2 (pH 6.9) in the presence of SP-A (subphase concentration 0.68 μg/ml). The apparatus used in these experiments has been described previously [45]. A modified monolayer trough was employed to reduce the subphase volume and surface area by about 10-fold from that of the original trough in order to study the small amounts of gene products that were available at subphase concentrations appropriate to those employed in previous studies of SP-A in monolayers systems [35, 36]. Experiments were performed in a small Teflon trough with a volume of 12.5 ml [36], at a temperature of 21–23°C. The initial spreading surface pressure was about 5 mN/m. After spreading, one hour was allowed for adsorption of SP-A to the spread monolayers during which time the subphase was stirred continuously. To increase the surface pressure of the films small aliquots of SP-B/DPPC were added to the surface and five minutes were allowed for evaporation of solvent and for equilibration of the films. At each surface pressure the monolayers were observed through the fluorescence of NBD-PC which partitions into the liquid-expanded (LE) phase of the lipid monolayer [36, 46]. Each experiment was repeated at least twice.
Results
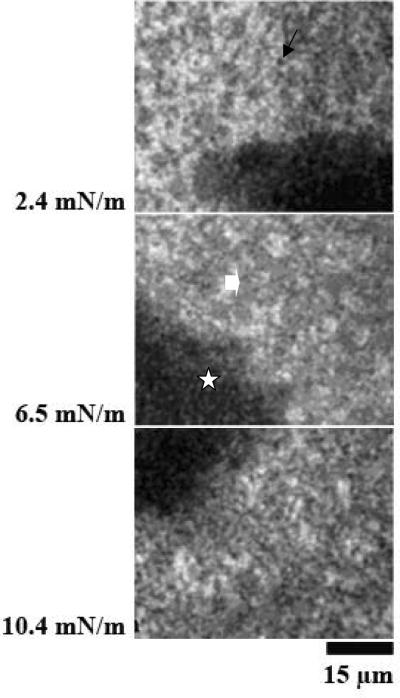
1. Effect of porcine SP-A in DPPC monolayers with porcine SP-B
Figure 1, panel A shows epifluorescence images at different surface pressures of monolayers of DPPC and porcine SP-B spread on subphases containing porcine SP-A. The images show the presence of three phases: lipid liquid expanded (LE) phase (bright regions, circles), lipid liquid-condensed (LC) phase (small black domains, arrows), and surface clusters characteristic of SP-A/SP-B complexes (grey regions, block arrows). In similar experiments with SP-A and SP-B labeled with two different fluorophores we have shown that the surface clusters, seen as grey phase in the images, are specific for the combination of SP-A and SP-B, and they are comprised of the two proteins and possibly some lipids [36].
Figure 1.
Image of monolayers of DPPC containing 17% SP-B spread over a subphase of 0.68 μg/ml of porcine SP-A (Panel A) and human SP-A (Panel B). Surface pressures at which images were obtained are given at the sides of the Figure. Three phases in the images were observed, including lipid liquid expanded (LE) phase (bright regions, circles), lipid liquid-condensed (LC) phase (small black domains, arrows), and surface clusters characteristic of SP-A and SP-B complexes (grey regions, block arrows). The morphology of the DPPC monolayers containing SP-B and human SP-A in panel B was similar to that of the lipid monolayers plus SP-B and porcine SP-A in panel A.
These control studies showed that the different methodology used to form and compress the monolayer that was used in these experiments gave results which were consistent with monolayers formed on a larger trough and compressed mechanically using a ribbon barrier, the more “conventional” way to carry out monolayer compression.
2. Effect of human SP-A in DPPC or DPPC:PG monolayers with porcine SP-B
Figure 1B shows images at different surface pressures of monolayers of DPPC/SP-B spread on solutions of human SP-A. A surface phase (grey clusters in the images, block arrows), in addition to the conventional lipid LE and LC phases, is detectable. The morphology of the DPPC monolayers containing SP-B and human SP-A was similar to that of the lipid monolayers plus SP-B and porcine SP-A (Fig. 1A). These experiments showed that human SP-A and porcine SP-B formed the same type of protein-rich complexes as were seen with porcine SP-A in previous studies [36].
Many of the previous studies on the SP-A and SP-B interactions in monolayer experimental systems had been carried out in the presence of DPPC plus 20% PG [36]. The influence of PG on the interactions of human SP-A and porcine SP-B was therefore investigated. Figure 2 represents images of monolayers of DPPC:eggPG (8:2, mol/mol) plus SP-B spread on human SP-A. The data showed that the interactions between SP-A and SP-B produced the protein-rich phase (grey clusters, block arrows) in the monolayers regardless of the presence of 20 % unsaturated PG.
Figure 2.
Images of films of DPPC: egg PG (8:2, mol/mol) containing 17% SP-B spread over human SP-A at 0.68 μg/ml. Surface pressures are given at the side of each image. In these images of monolayers of DPPC:eggPG plus SP-B spread on human SP-A show that the interactions between SP-A and SP-B produced the protein-rich phase (grey clusters, block arrows) in the monolayers regardless of the presence of 20 mol% unsaturated PG.
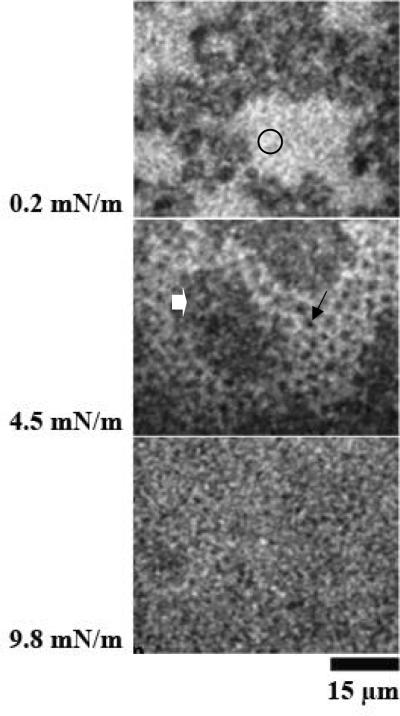
3. Effect of SP-A1 variant (6A2) in DPPC monolayers with porcine SP-B
Figure 3 A and B shows images at different surface pressures of two experiments with monolayers of SP-B/DPPC spread on subphases containing SP-A1 variant (6A2). The images reveal three characteristic features typical for the monolayers: a fine network of grey phase (block arrows), which extended throughout the LE phase, large protein-rich patches (large dark grey regions, stars), and LC phase (small black domains, arrows). The SP-A1 molecules were likely localized in the network in the lipid LE phase and in the large characteristic patches seen in the monolayers. The distribution of the grey network throughout the LE phase was different than any of the images seen before with SP-A and SP-B in any of these monolayer systems, suggesting that the 6A2 variant of SP-A1 had a different type or extent of interaction with SP-B, and possibly lipids, than did “native” SP-A or the allele 1A0 of SP-A2 protein (see below).
Figure 3.
Images from films of DPPC with SP-B (17%) spread over the product of the 6A2 of SP-A1 (0.68 μg/ml). Surface pressures are given at the side of each image. Panels A and B are from separate experiments. Both panel A and B show three characteristic features typical for the monolayers: a fine network of grey phase (block arrows) which extended throughout the LE phase, large protein-rich patches (large dark grey regions, stars), and LC phase (small black domains, arrows).
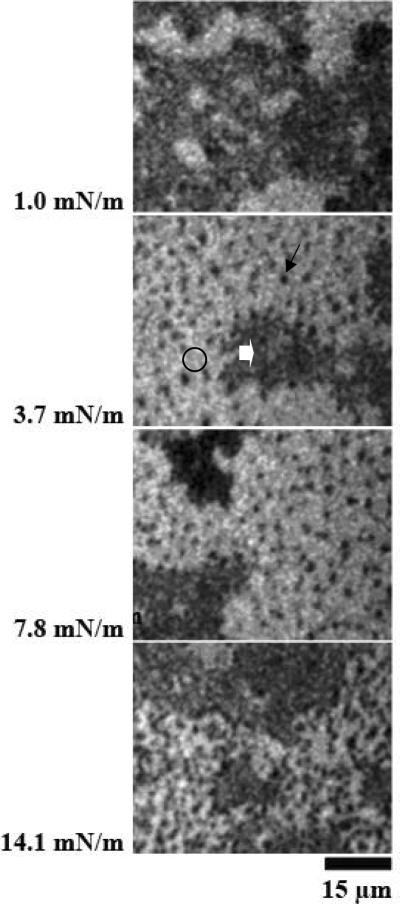
4. Effect of SP-A2 variant (1A0) in DPPC monolayers with porcine SP-B
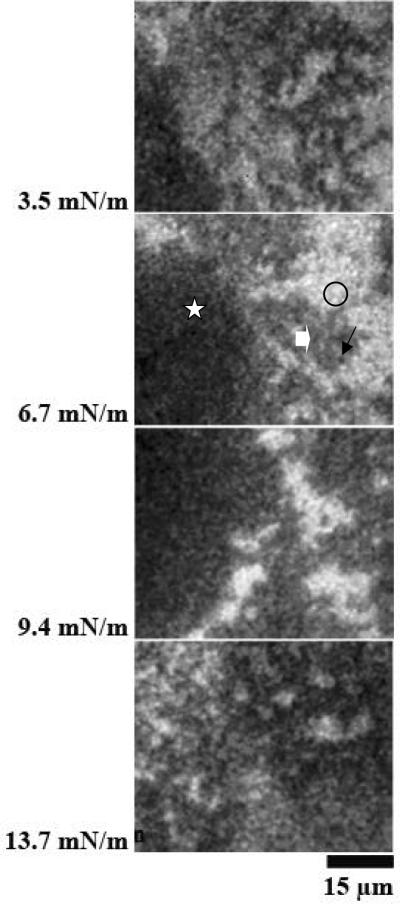
Figure 4 A and B shows results at different surface pressures from two experiments with monolayers of SP-B/DPPC spread on subphases containing the protein product of allele 1A0 of SP-A2. The morphology of the films was very similar to that observed in the films containing combinations of SP-B and porcine SP-A (Figure 1A) or human SP-A (Figures 1B and 2). In this case, the probe-excluding phase (block arrows), which was characteristic for the phospholipid monolayers containing combinations of SP-B and native SP-A, co-existed with, but was separate from, the lipid LE (bright regions, circles) and LC (black domains, arrows) phases.
Figure 4.
Images from films of DPPC/SP-B (17%) spread over the product of the 1A0 of SP-A2 (0.68 μg/ml). Surface pressures are given at the side of each image. Panels A and B are from separate experiments. The morphology of the films was very similar to that observed in the films containing combinations of SP-B and porcine SP-A (Figure 1A) or human SP-A (Figure 1B).
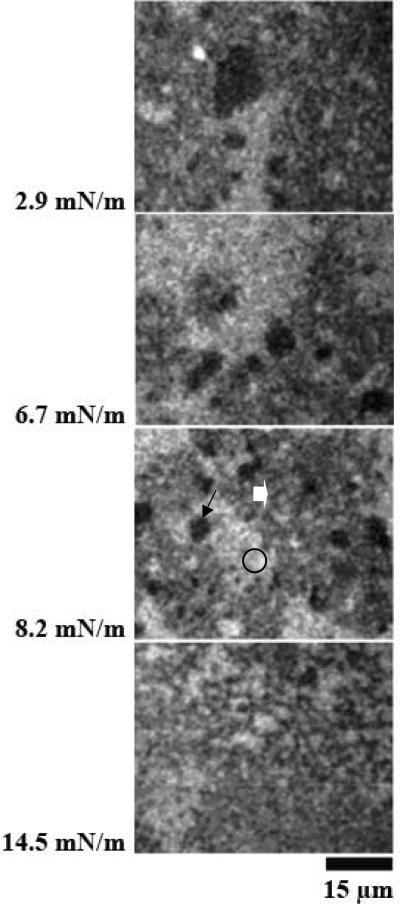
5. Effect of the coexpressed SP-A1/SP-A2 (6A2/1A0) protein in DPPC monolayers with porcine SP-B
Figure 5 A and B represents images at different surface pressures from two experiments with monolayers of SP-B/DPPC spread on subphases containing the coexpressed SP-A1/SP-A2 (6A2/1A0) protein. The morphology of the films containing the coexpressed SP-A1/SP-A2 (6A2/1A0) protein appears to combine features of the lipid-protein monolayers containing SP-A1 (Figure 3) and SP-A2 (Figure 4). Large likely protein-rich patches (stars), similar to those seen in the monolayers spread on SP-A1 (Figure 3) and a network of grey phase (block arrows) extended throughout the LE phase in the films containing the coexpressed SP-A1/SP-A2 (6A2/1A0) protein. In some cases, however, the grey phase appeared to segregate from the LE phase and regions of the traditional lipid LE phase were seen in the films (bright regions (circle) in Figure 5B). In this property the morphology of the system resembled that of the films of SP-B and DPPC spread on SP-A2 (Figure 4).
Figure 5.
Images of films of DPPC/SP-B (17%) spread over a coexpressed product (1A0/6A2) of SP-A1 and SP-A2 (0.68 μg/ml). Surface pressures are given at the side of each image. Panels A and B are from different experiments. The morphology of the films containing SP-A1 and SP-A2 variants appears to combine features of the lipid-protein monolayers containing SP-A1 (Figure 3) and SP-A2 (Figure 4). Large likely protein-rich patches (stars), similar to those seen in the monolayers spread on SP-A1 (Figure 3), and a network of grey phase (block arrows), similar to those seen in the monolayer spread on SP-A2 (Figure 4), are observed in the films containing SP-A1 and SP-A2.
Discussion
Recent work has provided evidence for specific interaction of the porcine proteins SP-A and SP-B in monolayers of lipids [35, 36]. The ability of monolayers containing SP-B to attract SP-A into the surface were much greater than those containing SP-C where little or no interaction with SP-A occurred [36, 41]. The interaction between SP-B and SP-A was dependent on the presence of lipid, especially phosphatidylcholine [36]. In this paper, we investigated interactions between SP-B and in vitro expressed SP-A1 and SP-A2 gene variants in the formation of lipid monolayers in the presence of DPPC. The results, a) confirmed the interactions between SP-B and human SP-A from alveolar proteinosis fluid in monolayers containing DPPC with or without the presence of unsaturated PG; b) showed differences in the interaction of the protein products of each of the two human SP-A genes. These differences were manifested in the organizational states in the monolayer in which the SP-A and SP-B complex is found; c) the 1A0 variant of SP-A2 formed complexes similar to those seen before, with porcine SP-A or with native human SP-A from alveolar proteinosis fluid; d) while the 6A2 variant from SP-A1 also formed a surface complex, its organization and distribution differed from that formed by native SP-As or by the 1A0 variant. In the case of 6A2, the complex observed was distributed in large patches and an extensive network rather than in clusters in the LE phase as they were when the 1A0 or native SP-A was present; e) coexpressed 1A0/6A2 SP-A showed a surface complex formation with characteristics of both complexes (i.e. when the 1A0 or 6A2 protein was used alone). These observations indicate a differential impact of SP-A1 and SP-A2 products in film formation and film regional organization including the organization of film lipid-protein complexes that may influence overall surface activity and other characteristics of the surface in the lung.
The network formed, when the 6A2 product was present, appeared to extend throughout the region of LE phase (that is the fluid or mobile phase) of the lipid. It is unlikely that the material was “dissolved” in the LE phase, but that the LE phase provided a convenient (fluid) environment for the material to form and lodge. While previous studies have shown that for porcine SP-A the clustered complex formed contained both SP-A and SP-B [36], this has not been shown definitively for human SP-A and the SP-A variants. Nevertheless, it seems to be a very reasonable assumption that all the grey phase complexes seen in the monolayers described here contain both SP-A and SP-B. One cannot obtain a reliable estimate of the extent of the phases with the current technology. Therefore, one cannot tell if the extent or strength of interaction of SP-B with one allelic product is different than with the other. Nor can one tell the stoichiometry of the complexes. There is, however, a remarkable difference observed in the shapes and locations of the complexes in the monolayer.
Previous studies indicate that the two SP-A variants (1A0 and 6A2) differ in six amino acid residues within the coding region [16–18, 47]. These amino acid substitutions, based on the SP-A precursor numbering are shown in the Table. Recent work has shown that removal of the signal peptide results in molecules with various N-terminal sequences [26]. Some molecules may and others may not contain amino acid 19. However, in either case, alanine and valine are relatively small molecules and represent conservative amino acid differences, and therefore it is unlikely that changes in amino acid 19 have a major impact on SP-A function. The remaining amino acid changes are located within the collagen-like domain of SP-A and, as such, may play a role in the structural stability of SP-A [24], or other functions [48–50].
| Amino Acid No. | 19 | 66 | 73 | 81 | 85 | 91 |
|---|---|---|---|---|---|---|
| 1A0 | Ala | Thr | Asn | Val | Arg | Ala |
| 6A2 | Val | Met | Asp | lle | Cys | Pro |
We have shown previously that SP-A1 has a lower thermal stability than SP-A2 [24] and suggested that this difference is due to differences in amino acid 85. A cysteine at residue 85 may affect stability in the triple helix resulting in local micro-unfolding. Studies on the stability of the tripeptide unit (Gly-X-Y)n with different substitutions at the X and Y positions have shown that the collagen triple-helix with guest triplets of Gly-Glu-Arg had a higher stability than 40 other different guest triplets tested [51, 52]. We have also shown that in-vitro expressed SP-A1 is identified with higher size oligomers than SP-A2 [26], and speculated that amino acid 85 contributes to oligomerization differences. Whether SP-A1 and SP-A2 differences, in the structural stability, oligomerization, or both, contribute to the film differences observed in this study, is currently unknown. However, recent findings from our laboratory indicate that amino acid 85 plays indeed an important role in characteristics that distinguish SP-A1 and SP-A2 [53].
Moreover, differences in other biophysical and biochemical properties have been observed between SP-A1 and SP-A2 [22, 24, 26]. These include self-aggregation, phospholipid and lipopolysaccharide aggregation, differences in aggregation and oligomerization pattern in response to ozone oxidation, and others. The results described here extend the range of differences between SP-A1 and SP-A2 products observed previously in solution [24] to the influence of the SP-A1 and SP-A2 variants on lipid monolayers containing SP-B. The lipid-protein rearrangements caused by SP-A1 and SP-A2 seen in this work are consistent with the influence of these products on aggregation [24] in that the SP-A2 product (1A0) had a greater influence on the monolayers than did the SP-A1 product (6A2). Also, as in the previous observations in solution [24] the SP-A2 product behaved more like native SP-A in lipid-protein monolayers than did the SP-A1 product. Functional differences between SP-A1 and SP-A2 in proinflammatory cytokine production [25, 54], inhibition of surfactant secretion [26], and ability of SP-A variants to enhance phagocytosis [23, 55] have also been observed. These findings, along with the present observations indicate that the properties and function of the SP-A1 (6A2) and SP-A2 (1A0) differ, and the mechanisms through which these contribute to diseases such as RDS [28, 30–33] are likely to differ. However, we speculate that a normally functioning lung may depend on the relative levels of SP-A1 and SP-A2 and not the total levels of SP-A. Support for this postulate is provided by our recent findings where the ratio of SP-A1 to total SP-A differs as a function of lung health status [21].
In summary, this work indicates that differences in the nature of physical interaction between SP-A variants with SP-B exist. We speculate that these differences account for differences in physiological properties of the surface monolayer, and therefore an appropriate SP-A1 to SP-A2 content may be critical for lung health. Additional work is necessary to understand more fully the precise nature of these differences in physical properties and their consequences for surfactant function.
Acknowledgements
Supported by the Canadian Institutes of Health Research (K.M.W.K.) and NIH R37 HL-34788 (J.F.).
Abbreviations used
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphatidylcholine
- CHO
Chinese Hamster Ovary (CHO)-K1 cell line
- LE phase
liquid-expanded phase
- LC phase
liquid-condensed phase
- PG
L-α-phosphatidylglycerol (from egg PC, sodium salt)
- SP-A
surfactant protein A
- SP-B
surfactant protein B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Yu S, Harding PG, Smith N, Possmayer F. Bovine pulmonary surfactant: chemical composition and physical properties. Lipids. 1983;18:522–9. doi: 10.1007/BF02535391. [DOI] [PubMed] [Google Scholar]
- [2].Rice WR, Ross GF, Singleton FM, Dingle S, Whitsett JA. Surfactant-associated protein inhibits phospholipid secretion from type II cells. J Appl Physiol. 1987;63:692–8. doi: 10.1152/jappl.1987.63.2.692. [DOI] [PubMed] [Google Scholar]
- [3].Jain D, Dodia C, Bates SR, Hawgood S, Poulain FR, Fisher AB. SP-A is necessary for increased clearance of alveolar DPPC with hyperventilation or secretagogues. Am J Physiol Lung Cell Mol Physiol. 2003;284:L759–65. doi: 10.1152/ajplung.00200.2002. [DOI] [PubMed] [Google Scholar]
- [4].Benson BJ, Williams MC, Sueishi K, Goerke J, Sargeant T. Role of calcium ions the structure and function of pulmonary surfactant. Biochim Biophys Acta. 1984;793:18–27. doi: 10.1016/0005-2760(84)90048-1. [DOI] [PubMed] [Google Scholar]
- [5].Suzuki Y, Fujita Y, Kogishi K. Reconstitution of tubular myelin from synthetic lipids and proteins associated with pig pulmonary surfactant. Am Rev Respir Dis. 1989;140:75–81. doi: 10.1164/ajrccm/140.1.75. [DOI] [PubMed] [Google Scholar]
- [6].Williams MC, Hawgood S, Hamilton RL. Changes in lipid structure produced by surfactant proteins SP-A, SP-B, and SP-C. Am J Respir Cell Mol Biol. 1991;5:41–50. doi: 10.1165/ajrcmb/5.1.41. [DOI] [PubMed] [Google Scholar]
- [7].Yu SH, McCormack FX, Voelker DR, Possmayer F. Interactions of pulmonary surfactant protein SP-A with monolayers of dipalmitoylphosphatidylcholine and cholesterol: roles of SP-A domains. J Lipid Res. 1999;40:920–9. [PubMed] [Google Scholar]
- [8].Floros J, Phelps DS. In: Surfactant-Update of Intensive Care Medicine. Nakos G, Lekka ME, editors. University of Ioannina; Ioannina, Greece: 2002. pp. 87–102. [Google Scholar]
- [9].Crouch E, Wright JR. Surfactant proteins A and D and pulmonary host defense. Annu Rev Physiol. 2001;63:521–54. doi: 10.1146/annurev.physiol.63.1.521. [DOI] [PubMed] [Google Scholar]
- [10].Phelps DS. Surfactant regulation of host defense function in the lung: a question of balance. Pediatr Pathol Mol Med. 2001;20:269–92. [PubMed] [Google Scholar]
- [11].Condon JC, Jeyasuria P, Faust JM, Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A. 2004;101:4978–83. doi: 10.1073/pnas.0401124101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hoover RR, Floros J. Organization of the human SP-A and SP-D loci at 10q22–q23. Physical and radiation hybrid mapping reveal gene order and orientation. Am J Respir Cell Mol Biol. 1998;18:353–62. doi: 10.1165/ajrcmb.18.3.3035. [DOI] [PubMed] [Google Scholar]
- [13].Floros J, Steinbrink R, Jacobs K, Phelps D, Kriz R, Recny M, Sultzman L, Jones S, Taeusch HW, Frank HA, et al. Isolation and characterization of cDNA clones for the 35-kDa pulmonary surfactant-associated protein. J Biol Chem. 1986;261:9029–33. [PubMed] [Google Scholar]
- [14].White RT, Damm D, Miller J, Spratt K, Schilling J, Hawgood S, Benson B, Cordell B. Isolation and characterization of the human pulmonary surfactant apoprotein gene. Nature. 1985;317:361–3. doi: 10.1038/317361a0. [DOI] [PubMed] [Google Scholar]
- [15].Katyal SL, Singh G, Locker J. Characterization of a second human pulmonary surfactant-associated protein SP-A gene. Am. J. Respir. Crit. Care Med. 1992;6:446–52. doi: 10.1165/ajrcmb/6.4.446. [DOI] [PubMed] [Google Scholar]
- [16].Floros J, Hoover RR. Genetics of the hydrophilic surfactant proteins A and D. Biochim Biophys Acta. 1998;1408:312–22. doi: 10.1016/s0925-4439(98)00077-5. [DOI] [PubMed] [Google Scholar]
- [17].DiAngelo S, Lin Z, Wang G, Phillips S, Ramet M, Luo J, Floros J. Novel, non-radioactive, simple and multiplex PCR-cRFLP methods for genotyping human SP-A and SP-D marker alleles. Dis Markers. 1999;15:269–81. doi: 10.1155/1999/961430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Floros J, Wang G. A point of view: quantitative and qualitative imbalance in disease pathogenesis; pulmonary surfactant protein A genetic variants as a model. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:295–303. doi: 10.1016/s1095-6433(01)00325-7. [DOI] [PubMed] [Google Scholar]
- [19].Voss T, Melchers K, Scheirle G, Schafer KP. Structural comparison of recombinant pulmonary surfactant protein SP-A derived from two human coding sequences: implications for the chain composition of natural human SP-A. Am J Respir Cell Mol Biol. 1991;4:88–94. doi: 10.1165/ajrcmb/4.1.88. [DOI] [PubMed] [Google Scholar]
- [20].Karinch AM, deMello DE, Floros J. Effect of genotype on the levels of surfactant protein A mRNA and on the SP-A2 splice variants in adult humans. Biochem J. 1997;321(Pt 1):39–47. doi: 10.1042/bj3210039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tagaram HR, Wang G, Umstead TM, Mikerov AN, Thomas NJ, Graff GR, Hess JC, Thomassen MJ, Kavuru MS, Phelps DS, Floros J. Characterization of a Human Surfactant Protein A1 (SP-A1) Gene-Specific Antibody; SP-A1 Content Variation among Individuals of Varying Age and Pulmonary Health. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1052–63. doi: 10.1152/ajplung.00249.2006. [DOI] [PubMed] [Google Scholar]
- [22].Selman M, Lin HM, Montano M, Jenkins AL, Estrada A, Lin Z, Wang G, DiAngelo S, Guo X, Umstead TM, Lang CM, Pardo A, Phelps DS, Floros J. Surfactant protein A and B genetic variants predispose to idiopathic pulmonary fibrosis. Hum Genet. 2003;113:542–50. doi: 10.1007/s00439-003-1015-4. [DOI] [PubMed] [Google Scholar]
- [23].Mikerov AN, Umstead TM, Huang W, Liu W, Phelps DS, Floros J. SP-A1 and SP-A2 variants differentially enhance association of Pseudomonas aeruginosa with rat alveolar macrophages. Am J Physiol Lung Cell Mol Physiol. 2005;288:L150–8. doi: 10.1152/ajplung.00135.2004. [DOI] [PubMed] [Google Scholar]
- [24].Garcia-Verdugo I, Wang G, Floros J, Casals C. Structural analysis and lipid-binding properties of recombinant human surfactant protein A derived from one or both genes. Biochemistry. 2002;41:14041–53. doi: 10.1021/bi026540l. [DOI] [PubMed] [Google Scholar]
- [25].Wang G, Phelps DS, Umstead TM, Floros J. Human SP-A protein variants derived from one or both genes stimulate TNF-alpha production in the THP-1 cell line. Am J Physiol Lung Cell Mol Physiol. 2000;278:L946–54. doi: 10.1152/ajplung.2000.278.5.L946. [DOI] [PubMed] [Google Scholar]
- [26].Wang G, Bates-Kenney SR, Tao JQ, Phelps DS, Floros J. Differences in biochemical properties and in biological function between human SP-A1 and SP-A2 variants, and the impact of ozone-induced oxidation. Biochemistry. 2004;43:4227–39. doi: 10.1021/bi036023i. [DOI] [PubMed] [Google Scholar]
- [27].Marttila R, Haataja R, Ramet M, Pokela ML, Tammela O, Hallman M. Surfactant protein A gene locus and respiratory distress syndrome in Finnish premature twin pairs. Ann Med. 2003;35:344–52. doi: 10.1080/07853890310006389. [DOI] [PubMed] [Google Scholar]
- [28].Ramet M, Haataja R, Marttila R, Floros J, Hallman M. Association between the surfactant protein A (SP-A) gene locus and respiratory-distress syndrome in the Finnish population. Am J Hum Genet. 2000;66:1569–79. doi: 10.1086/302906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Floros J, Lin HM, Garcia A, Salazar MA, Guo X, DiAngelo S, Montano M, Luo J, Pardo A, Selman M. Surfactant protein genetic marker alleles identify a subgroup of tuberculosis in a Mexican population. J Infect Dis. 2000;182:1473–8. doi: 10.1086/315866. [DOI] [PubMed] [Google Scholar]
- [30].Floros J, Fan R, Matthews A, DiAngelo S, Luo J, Nielsen H, Dunn M, Gewolb IH, Koppe J, van Sonderen L, Farri-Kostopoulos L, Tzaki M, Ramet M, Merrill J. Family-based transmission disequilibrium test (TDT) and case-control association studies reveal surfactant protein A (SP-A) susceptibility alleles for respiratory distress syndrome (RDS) and possible race differences. Clin Genet. 2001;60:178–87. doi: 10.1034/j.1399-0004.2001.600303.x. [DOI] [PubMed] [Google Scholar]
- [31].Kala P, Ten Have T, Nielsen H, Dunn M, Floros J. Association of pulmonary surfactant protein A (SP-A) gene and respiratory distress syndrome: interaction with SP-B. Pediatr Res. 1998;43:169–77. doi: 10.1203/00006450-199802000-00003. [DOI] [PubMed] [Google Scholar]
- [32].Floros J, Fan R, Diangelo S, Guo X, Wert J, Luo J. Surfactant protein (SP) B associations and interactions with SP-A in white and black subjects with respiratory distress syndrome. Pediatr Int. 2001;43:567–76. doi: 10.1046/j.1442-200x.2001.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Floros J, Fan R. Surfactant protein A and B genetic variants and respiratory distress syndrome: allele interactions. Biol Neonate 80 Suppl. 2001;1:22–5. doi: 10.1159/000047173. [DOI] [PubMed] [Google Scholar]
- [34].Haataja R, Ramet M, Marttila R, Hallman M. Surfactant proteins A and B as interactive genetic determinants of neonatal respiratory distress syndrome. Hum Mol Genet. 2000;9:2751–60. doi: 10.1093/hmg/9.18.2751. [DOI] [PubMed] [Google Scholar]
- [35].Nag K, Perez-Gil J, Ruano ML, Worthman LA, Stewart J, Casals C, Keough KMW. Phase transitions in films of lung surfactant at the air-water interface. Biophys J. 1998;74:2983–95. doi: 10.1016/S0006-3495(98)78005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Taneva SG, Keough KMW. Differential effects of surfactant protein A on regional organization of phospholipid monolayers containing surfactant protein B or C. Biophys J. 2000;79:2010–23. doi: 10.1016/S0006-3495(00)76449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wang G, Umstead TM, Phelps DS, Al-Mondhiry H, Floros J. The effect of ozone exposure on the ability of human surfactant protein A variants to stimulate cytokine production. Environ Health Perspect. 2002;110:79–84. doi: 10.1289/ehp.0211079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Haagsman HP, Hawgood S, Sargeant T, Buckley D, White RT, Drickamer K, Benson BJ. The major lung surfactant protein, SP 28–36, is a calcium-dependent, carbohydrate-binding protein. Journal of Biological Chemistry. 1987;262:13877–80. [PubMed] [Google Scholar]
- [39].Huang W, Wang G, Phelps DS, Al-Mondhiry H, Floros J. Combined SP-A-bleomycin effect on cytokines by THP-1 cells: impact of surfactant lipids on this effect. Am J Physiol Lung Cell Mol Physiol. 2002;283:L94–L102. doi: 10.1152/ajplung.00434.2001. [DOI] [PubMed] [Google Scholar]
- [40].Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- [41].Taneva SG, Keough KMW. Adsorption of pulmonary surfactant protein SP-A to monolayers of phospholipids containing hydrophobic surfactant protein SP-B or SP-C: potential differential role for tertiary interaction of lipids, hydrophobic proteins, and SP-A. Biochemistry. 2000;39:6083–93. doi: 10.1021/bi992074x. [DOI] [PubMed] [Google Scholar]
- [42].Taneva S, McEachren T, Stewart J, Keough KMW. Pulmonary surfactant protein SP-A with phospholipids in spread monolayers at the air-water interface. Biochemistry. 1995;34:10279–89. doi: 10.1021/bi00032a023. [DOI] [PubMed] [Google Scholar]
- [43].Curstedt T, Johansson J, Barros-Soderling J, Robertson B, Nilsson G, Westberg M, Jornvall H. Low-molecular-mass surfactant protein type 1. The primary structure of a hydrophobic 8-kDa polypeptide with eight half-cystine residues. Eur J Biochem. 1988;172:521–5. doi: 10.1111/j.1432-1033.1988.tb13918.x. [DOI] [PubMed] [Google Scholar]
- [44].Taneva SG, Keough KMW. Calcium ions and interactions of pulmonary surfactant proteins SP-B and SP-C with phospholipids in spread monolayers at the air/water interface. Biochim Biophys Acta. 1995;1236:185–95. doi: 10.1016/0005-2736(95)00046-6. [DOI] [PubMed] [Google Scholar]
- [45].Nag K, Boland C, Rich N, Keough KM. Design and construction of an epifluoresence microscopic surface balance for the study of lipid monolayer phase transitions. Rev Sci Instrum. 1990;61:3425–3430. [Google Scholar]
- [46].Nag K, Taneva SG, Perez-Gil J, Cruz A, Keough KMW. Combinations of fluorescently labeled pulmonary surfactant proteins SP-B and SP-C in phospholipid films. Biophys J. 1997;72:2638–50. doi: 10.1016/S0006-3495(97)78907-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Karinch AM, Floros J. 5' splicing and allelic variants of the human pulmonary surfactant protein A genes. Am J Respir Cell Mol Biol. 1995;12:77–88. doi: 10.1165/ajrcmb.12.1.7811473. [DOI] [PubMed] [Google Scholar]
- [48].Ikegami M, Elhalwagi BM, Palaniyar N, Dienger K, Korfhagen T, Whitsett JA, McCormack FX. The collagen-like region of surfactant protein A (SP-A) is required for correction of surfactant structural and functional defects in the SP-A null mouse. J Biol Chem. 2001;276:38542–8. doi: 10.1074/jbc.M102054200. [DOI] [PubMed] [Google Scholar]
- [49].Rodriguez Capote K, McCormack FX, Possmayer F. Pulmonary surfactant protein-A (SP-A) restores the surface properties of surfactant after oxidation by a mechanism that requires the Cys6 interchain disulfide bond and the phospholipid binding domain. J Biol Chem. 2003;278:20461–74. doi: 10.1074/jbc.M212697200. [DOI] [PubMed] [Google Scholar]
- [50].Palaniyar N, Ikegami M, Korfhagen T, Whitsett J, McCormack FX. Domains of surfactant protein A that affect protein oligomerization, lipid structure and surface tension. Comp Biochem Physiol A Mol Integr Physiol. 2001;129:109–27. doi: 10.1016/s1095-6433(01)00309-9. [DOI] [PubMed] [Google Scholar]
- [51].Chan VC, Ramshaw JA, Kirkpatrick A, Beck K, Brodsky B. Positional preferences of ionizable residues in Gly-X-Y triplets of the collagen triple-helix. J Biol Chem. 1997;272:31441–6. doi: 10.1074/jbc.272.50.31441. [DOI] [PubMed] [Google Scholar]
- [52].Persikov AV, Ramshaw JA, Kirkpatrick A, Brodsky B. Peptide investigations of pairwise interactions in the collagen triple- helix. J Mol Biol. 2002;316:385–94. doi: 10.1006/jmbi.2001.5342. [DOI] [PubMed] [Google Scholar]
- [53].Wang G, Myers C, Mikerov A, Floros J. Effect of Cysteine 85 on biochemical properties and biological function of human surfactant protein A variants. Biochemistry. 2007 doi: 10.1021/bi7004569. (In press) [Epub ahead of print]10.1021/bi7004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Huang W, Wang G, Phelps DS, Al-Mondhiry H, Floros J. Human SP-A genetic variants and bleomycin-induced cytokine production by THP-1 cells: effect of ozone-induced SP-A oxidation. Am J Physiol Lung Cell Mol Physiol. 2004;286:L546–53. doi: 10.1152/ajplung.00267.2003. [DOI] [PubMed] [Google Scholar]
- [55].Mikerov AN, Wang G, Umstead TM, Zacharatos M, Thomas NJ, Phelps DS, Floros J. Surfactant protein A2 (SP-A2) variants expressed in CHO cells stimulate phagocytosis of Pseudomonas aeruginosa more than Do SP-A1 variants. Infect Immun. 2007;75:1403–12. doi: 10.1128/IAI.01341-06. [DOI] [PMC free article] [PubMed] [Google Scholar]