Abstract
Amyloid molecules harboring pyroglutamate (pGlu) residue at the N-termini are considered to be important for the development of cerebral amyloidosis such as Alzheimer’s disease and thought to be either spontaneously generated or being catalyzed by glutaminyl cyclase. Familial British dementia (FBD) is an autosomal dominant form of dementia neuropathologically characterized by parenchymal amyloid and preamyloid deposits, extensive cerebral amyloid angiopathy, and neurofibrillary tangles. FBD is caused by a stop to Arg mutation in the BRI2 gene, generating de novo created amyloid molecule ABri which accumulates in FBD brains but is not present in the normal population. Soluble ABri molecules present in the circulation of carriers of the BRI2 mutation are 34 amino acids long exclusively harboring Glu residue at the N-termini (ABri1-34E), whereas water- and formic acid-soluble ABri molecules extracted from FBD brains have abundant ABri species bearing pGlu residue (ABri1-34pE), suggesting that pyroglutamate formation occurs at the site of deposition. In order to further clarify the mechanism (s) of ABri deposition, we studied whether pyroglutamate formation indeed occurs outside the central nervous system taking advantage that FBD is also a systemic amyloidosis. Soluble and fibrillar ABri molecules extracted from systemic organs and analyzed biochemically using a combination of immunoprecipitation, mass spectrometry, and western blot analysis were oligomeric in size and contained a large proportion of ABri1-34pE. The data indicate that pyroglutamate formation at the N-termini of ABri molecules is an early step in the process of FBD amyloid deposition, and its formation is not restricted to the central nervous system.
Keywords: familial British dementia, ABri, pyroglutamate, amyloid, post-translational modification
Familial British dementia (FBD) is an autosomal dominant neurodegenerative disorder caused by a genetic defect in the BRI2 gene1 with striking neuropathological similarities to Alzheimer’s disease (AD), including neurofibrillary degeneration and ample cerebral amyloid deposition. FBD was first described by Worster-Drought et al. in 19332, and is clinically characterized by progressive dementia, spastic tetraparesis, and cerebellar ataxia with an age of onset in the fifth decade. Brain MRI of FBD patients has revealed periventricular white matter hyperintensities3,4. Neuropathological examination of several FBD cases revealed, in addition to neurofibrillary tangles in hippocampal neurons, a widespread severe amyloid angiopathy with perivascular deposits, amyloid plaques, and non-fibrillar preamyloid lesions affecting the cerebral cortex3,5,6. The main component of the parenchymal and vascular amyloid is the 34-amino acid ABri peptide1, which has no homology to Aβ, the major constituent of the brain deposits in AD.
The importance of the Aβ C-terminal heterogeneity in AD pathogenesis is today well established. Aβ42 shows the highest aggregation propensity, and its deposition precedes that of Aβ40 in vivo7. N-terminally truncated Aβ molecules also show an enhanced aggregation propensity, and are deposited in the brain earlier than intact molecules7–10. Among these N-terminally truncated species, Aβ molecules with pyroglutamate (pGlu) at position 3 are the major species found in AD and have been postulated to initiate amyloid plaque formation11,12. In spite of its relevance to the disease process, the mechanism of pGlu formation from Glu residues is poorly investigated. Recently, it was demonstrated that glutaminyl cyclase (QC) can catalyze the conversion of N-terminal Glu to pGlu in Aβ molecules13. Reinforcing the importance of pGlu species in disease pathogenesis, the suppression of pGlu formation with a QC inhibitor attenuated Aβ accumulation and the progression of AD pathology in transgenic mouse models of AD14. The conversion of N-terminal Glu residue to pGlu in amyloid molecules is not a feature restricted to AD. Amyloid molecules harboring pGlu at N-termini are also predominant species in FBD as well as in familial Danish dementia (FDD), another disorder related to a different BRI2 gene defect15.
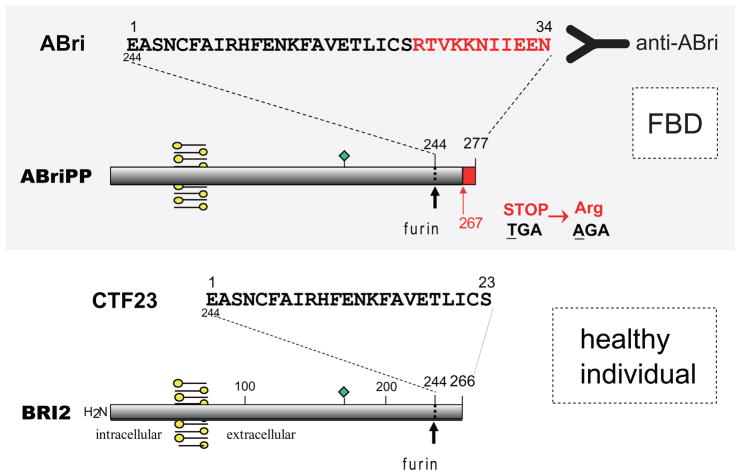
As illustrated in Figure 1, ABri is a 4-kDa peptide derived from a type II transmembrane precursor protein, BRI2, codified by a single multiexonic gene BRI2 (also known as ITM2B) located on the long arm of chromosome 131. A single substitution in the BRI2 gene, TGA to AGA at codon 267, results in an Arg residue replacing the normally occurring stop codon in the BRI2 gene, therefore generating a longer open reading frame and translating into the 277-amino acid protein ABriPP1. Furin-like proteolytic processing releases the 34-amino acid ABri peptide from the C-terminus of ABriPP, instead of the normal 23-amino acid CTF23 peptide present in healthy individuals16.
Figure 1. Genetic and biochemical mechanisms generating ABri from ABriPP.
FBD is associated with a Stop to Arg mutation in the BRI2 gene, which generates the de novo created amyloid molecule ABri after processing by furin-like proteases. Antibodies raised versus the C-terminal residues of ABri, not-existing in normal individuals, (Ab 338) allowed further immunohistochemical characterization of the deposits and WB probing. Circulating ABri molecules consist of 34 amino acids, and harbor a Glu residue at the N-terminus. Furin-like cleavage of BRI2 in normal individuals results in the production of CTF23, a peptide comprising the 23 first residues of ABri.
Amyloid isolated from FBD leptomeningeal fibrillar deposits shows a high degree of polymerization and a post-translationally modified N-terminus (pGlu)1,17. In contrast to AD, in which amyloid deposition is limited to the central nervous system (CNS), FBD is a systemic amyloidosis18. In the present study, we biochemically analyzed the ABri molecules deposited in the brain and systemic organs in FBD cases and demonstrate that pGlu formation in addition to the CNS also occurs at an early stage in peripheral tissues.
Materials and Methods
Sequential Tissue Fractionation
Systemic organs obtained from an FBD case (female, 68 years old at autopsy) as well as brains obtained from a series of autopsied FBD cases were subjected to biochemical analysis. ABri molecules were sequentially extracted from frozen FBD brains and systemic organs such as uterus, skeletal muscles and pancreas with water-based buffers followed by 70% formic acid (FA) which is known to completely solubilize the fibrillar amyloid deposits. Frozen tissues were homogenized in cold PBS [10 mM phosphate buffer, pH 7.4, containing 137 mM NaCl and 2.7 mM KCl and protease inhibitor cocktail (Complete; Roche)] using a Dounce glass homogenizer immersed on ice. After ultracentrifugation [Beckman Coulter; 70.1 Ti rotor] at 112,000 × g for 1 hour at 4°C, the resultant supernatants were analyzed as PBS-extracted fractions, and the pellets were re-homogenized in FA. Following centrifugation at 14,000 rpm in a 5417R microcentrifuge (Eppendorf) the resulting supernatants were analyzed as FA-extracted fractions. The pertinent extracted materials were biochemically analyzed with the combination of immunoprecipitation (IP), western blot (WB) and matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) mass spectrometry (MS) analyses as reported and briefly described below19.
Immunoprecipitation Experiments
Tissue-extracted ABri Peptides
Fifty microliters of paramagnetic beads coated with goat anti-rabbit IgG (Dynabeads M-280) were allowed to interact with 6 μg of IgG purified from rabbit antiserum 338, which is specific for the 10 C-terminal residues of ABri, TVKKNIIEEN18. After blocking in 0.1% (w/v) bovine serum albumin in PBS, antibody-coated beads were incubated with either FA-extracted amyloid fractions previously neutralized in 0.5 M Tris-base, pH 11, or PBS-extracted fractions. Elution of the bound material from the beads was performed by different methods, according to the techniques used for the studies that followed, as described19.
Circulating Soluble ABri
Plasma from carriers and noncarriers of the FBD genetic defect was analyzed by IP. Fifty microliters of paramagnetic beads interacted with Ab 338 were incubated with 1 ml of a 1:1 dilution of plasma in radioimmunoprecipitation assay buffer (1% Triton X-100 in 50 mM Tris, pH 8.0, containing 150 mM NaCl, 0.5% cholic acid, 0.1% SDS, 5 mM EDTA, and Complete protease inhibitor). Bound peptides were eluted for MALDI-TOF MS or WB analysis as described above.
MALDI-TOF MS Analysis
Prior to MS analysis, extracted peptides from PBS- and FA-fractions were purified by IP. Due to the abundance of ABri in FA fractions, IP was not necessary for its identification by mass spectrometry. In some cases, FA extracts were alternatively purified by micro-reverse-phase chromatography using ZipTipC4, according to the manufacturer’s protocol, utilizing 90% (v/v) acetonitrile and 0.1% (v/v) trifluoroacetic acid in water for elution. MALDI-TOF MS analysis was performed at the New York University Protein Analysis Facility19. Under the experimental acidic conditions, which also render noncovalent binding unobservable, mostly monomeric species of ABri were detected. FindPept tool from the ExPASy Proteomics server (available on the World Wide Web at us.expasy.org/tools) was used to assign the experimental mass values to specific peptide sequences and to search for the presence of post-translational modifications.
Western Blot Analysis
Samples from each of the extracts, either before or after IP, were separated on a 16% Tris-Tricine SDS-PAGE and electrotransferred for 45 min at 400 mA onto polyvinylidene difluoride membranes (Immobilon-P) using 10 mM 3-cyclohexylamino-1-propanesulfonic acid buffer, pH 11 containing 10% (v/v) methanol. After blocking in 5% nonfat milk in PBS containing 0.1% Tween 20, the membranes were immunoreacted with the Ab 338 (0.5 μg/ml), followed by anti-rabbit horseradish peroxidase-labeled F (ab′)2. Signals were developed with SuperSignal and exposed to Hyperfilm ECL.
Immunohistochemical analysis
Tissue blocks from different organs were taken at post mortem, fixed in 10% buffered formalin, and embedded in paraffin. Seven-μm-thick sections pretreated with 99% formic acid were incubated at room temperature with Ab 338 followed by biotinylated anti-rabbit IgG (1:200) and ABC complex. Color was developed with diaminobenzidine/H2O2. Double staining with Ab 338 and thioflavin−S was carried out as described previously5.
Results
IP-WB/-MS analysis of soluble ABri peptides obtained from plasma and brain extracts
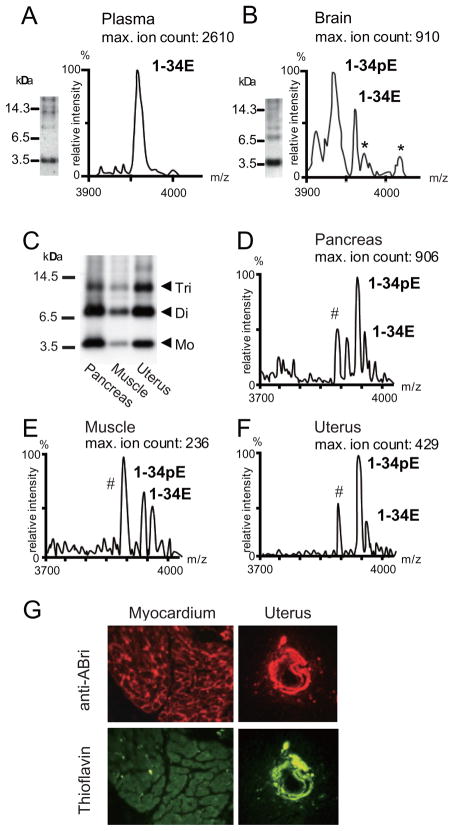
A striking difference was observed between soluble ABri in the circulation and water-soluble ABri extracted from brain homogenates. IP-WB and IP-MS analyses clearly showed that soluble ABri molecules obtained from plasma were monomeric, harboring Glu residues at their N-termini (ABri1-34E) with an observed average mass of 3953.5 ± 0.5 Da (expected: 3953.3 Da for ABri with oxidized Cys residues at positions 5 and 22). No obvious N- and/or C-terminal truncations were observed. The experimental average mass was close to the theoretical mass value of the oxidized ABri peptide, suggesting the presence of a single intrachain disulfide bond (Fig. 2, panel A). Water-extractable ABri molecules isolated from FBD brains showed different results. IP-WB analysis revealed ABri molecules to be oligomeric in size, showing monomers, dimers, and trimers. By IP-MS analysis, ABri molecules exhibited pGlu residue at their N-termini (ABri1-34pE) with an observed average mass of 3935.5 ± 0.5 Da (expected: 3935.3 Da for ABri with oxidized Cys residues at positions 5 and 22, smaller than ABri1-34E molecules by 18 mass units as a result of the loss of one molecule of water) (Fig. 2, panel B).
Figure 2. Biochemical and histopathological analysis of CNS and systemic ABri deposits.
Panel A. IP-WB and IP-MS analysis of soluble ABri in FBD plasma. Panel B. IP-WB and IP-MS analysis of ABri in the PBS-extracted fractions of the brain of an FBD patient. *: represents non-specific peaks appearing in control samples in which the pertinent brain extracts were immunoprecipitated with paramagnetic beads un-coated with the primary anti-ABri antibody. Panel C. WB analysis of immunoprecipitated PBS-extracted fractions from the pancreas, skeletal muscles, and uterus of an autopsied FBD patient. Mo, monomers; Di, dimers; Tri, trimers. Panels D to F. IP-MS analysis of PBS-extracted fractions from FBD pancreas (panel D), skeletal muscles (panel E) and uterus (panel F). #: indicates non-specific peaks found in control samples in which tissue extracts were replaced by PBS during the IP-MS analysis procedure. Panel G. Fluorescent microscopy images illustrating ABri deposition in FBD myocardium and uterus. Upper panels show anti-ABri antibody immunoreactivity; lower panels illustrate thioflavin-S staining. Please note that parenchymal lesions are thioflavin-negative non-fibrillar preamyloid deposits whereas vascular lesions are thioflavin-positive fibrillar ABri deposits.
IP-WB/-MS analysis of soluble ABri peptides obtained from systemic organs
PBS-extracted fractions obtained from systemic organs were analyzed to determine whether water-extractable ABri molecules in peripheral tissues are similar to those in the CNS or in the circulation. IP-WB analysis showed ABri molecules as monomers, dimers, trimers, and even tetramers (Fig. 2, panel C). IP-MS revealed that the predominant ABri specie was ABri1-34pE although variable quantities of ABri1-34E were also observed in all the organs analyzed: pancreas (observed average mass of 3936.5 Da and 3955.1 Da, for ABri1-34pE and ABri1-34E, respectively); skeletal muscles (3936.0 Da and 3953.6 Da); and uterus (3936.5 Da and 3954.7 Da) (Fig. 2, panels D to F). Thus, these results indicated that water-extractable ABri molecules from systemic organs have similar characteristics to water-extractable molecules obtained from the brain.
Analysis of insoluble ABri peptides isolated from brain and systemic organs
Figure 2 panel G illustrates ABri deposits in the myocardium and uterus as visualized by fluorescence microscopy. Vessels in both organs were labeled by anti-338 ABri antibody (upper panels; red signal) and thioflavin-S (lower panels; green signal). Notably, the majority of the parenchymal systemic lesions immunoreactive with anti-ABri antibody were thioflavin-S negative, non-fibrillar, pre-amyloid deposits, whereas vascular lesions were consistently thioflavin-S positive, fibrillar ABri deposits. Similar results were obtained from the pancreas, skeletal muscles and uterus (not shown). Insoluble amyloid molecules extracted from the brain and systemic organs into FA and tested by WB analysis using anti-ABri antibody revealed the presence of monomers, dimers, trimers, and highly aggregated oligomers appearing as smears (not shown), as reported elsewhere18. IP-MS analysis revealed that, in all organs, the predominant ABri molecule was ABri1-34pE, although it co-localized with the non-pyroglutamate modified species, ABri1-34E (not shown), identical to the findings observed in the brain and pancreas18.
Discussion
The formation of pGlu is a common post-translational modification found in a series of hormones and neuropeptides, including neurotensin, thyrotropin, and gonadotropin-releasing hormones; –their biological activities largely depend on the existence of the N-terminal pGlu residue20. N-terminally truncated forms of Aβ molecules harboring pGlu residues (e.g., Aβ3-42pE or Aβ11-42pE) are considered to be important for the development of AD neuropathology. However, the mechanism by which the N-terminal pGlu is formed remains unclear. Although the final pGlu product is the same, the amino acid that serves as the substrate for the post-translational modification in neuropeptides and amyloid molecules differs. In neuropeptides, the N-terminal modification occurs at a Gln residue and the enzymatic reaction involves the nucleophilic attack of the α-amino group on the amidated carboxyl group and the release of NH3 catalyzed by glutaminyl cyclase (QC; EC 2.3.2.5) at a neutral pH21,22. In contrast, few examples are known for the post-translational modification of Glu to pGlu, which involves the loss of one molecule of water instead of deamidation. Since QC had not previously been considered capable of catalyzing pGlu formation from Glu residue in amyloid molecules, it was believed that the post-translational modifications at positions 3 and 11 of truncated Aβ were spontaneously generated23. However, a series of recent studies, using QC isolated from Arabidopsis thaliana, showed that the enzyme does have the capability to catalyze the conversion of N-terminal Glu to pGlu –but with about 100,000-fold lower specificity24. Notably, a QC inhibitor diminished the production of A β3-40/42pE in different cell lines in which the QC-catalyzed-formation of N-terminal pGlu is favored in the acidic environment of secretory compartments13. In vivo, QC expression was found up-regulated in the cortices of individuals with AD and was correlated with the appearance of pGlu-modified Aβ14. The oral application of a QC inhibitor was able to reduce Aβ3-42pE burden in 2 transgenic mouse models of AD and in a new Drosophila model14. Furthermore, treatment of these transgenic mice was associated with a reduction in Aβx-40/42 load, diminished plaque formation and gliosis, and improved performance in context memory and spatial learning tests14.
The introduction of pGlu at the N-terminus changes the biochemical nature of Aβ molecules in vitro, increasing their hydrophobicity, altering the pH-dependent solubility profile, and rendering the pGlu-modified Aβ species less soluble25. The presence of pGlu residues increases the aggregation propensity of Aβ –as evidenced by thioflavin-T fluorescence assays and dynamic light scattering– whereas far-UV CD spectroscopic analysis points reveal an enhanced β-sheet structure. These structural changes translated into the fibril morphology, resulting in short fibers frequently arranged in bundles25.
N-terminal pGlu formation in cerebral amyloidosis is a feature not restricted to AD. The majority of ABri and ADan molecules accumulated in FBD and FDD brain tissues are converted from N-terminal Glu species found in the circulation, ABri1-34E and ADan1-34E, to the pertinent pGlu-modified molecules, ABri1-34pE and ADan1-34pE. The data presented herein, clearly showed that ABri molecules deposited in peripheral tissues are indistinguishable from those found in CNS deposits and also carry the pGlu modification. The differential distribution of the post-translationally modified species with their unique presence in systemic and CNS tissues and complete absence from the circulation clearly indicates that the modification takes place at the site of deposition. Whether QC, an enzyme known to exist in human CNS and pituitary gland, also exists in systemic organs and is able to catalyze in situ pGlu generation in ABri molecules is yet to be determined. Certainly, the biochemical similarities between CNS and systemic deposits, together with the accessibility of the peripheral tissues, make them good candidates for diagnostic specimens and appropriate initial targets to evaluate novel therapeutic strategies for FBD –e.g., the inhibition of pGlu formation–. This may have further applicability for other neurodegenerative disorders, such as AD, caused by the same post-translational modification.
Acknowledgments
These studies were partially funded by NIH grants NS051715 and AG30539, the Alzheimer’s Association, and the American Heart Association.
References
- 1.Vidal R, Frangione B, Rostagno A, Mead S, Revesz T, Plant G, Ghiso J. A stop-codon mutation in the BRI gene associated with familial British dementia. Nature. 1999;399:776–781. doi: 10.1038/21637. [DOI] [PubMed] [Google Scholar]
- 2.Worster-Drought C, Hill T, McMenemey W. Familial presenile dementia with spastic paralysis. J Neurol Psychopathol. 1933;14:27–34. doi: 10.1136/jnnp.s1-14.53.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plant G, Revesz T, Barnard R, Harding A, Gautier-Smith P. Familial cerebral amyloid angiopathy with nonneuritic plaque formation. Brain. 1990;113:721–747. doi: 10.1093/brain/113.3.721. [DOI] [PubMed] [Google Scholar]
- 4.Mead S, James Galton M, Revesz T, Doshi RB, Harwood G, Pan EL, Ghiso J, Frangione B, Plant G. Familial British dementia with amyloid angiopathy: Early clinical, neuropsychological and imaging findings. Brain. 2000;123:975–986. doi: 10.1093/brain/123.5.975. [DOI] [PubMed] [Google Scholar]
- 5.Holton J, Ghiso J, Lashley T, Rostagno A, Guerin C, Gibb G, Houlden H, Ayling H, Martinian L, Anderton B, Wood N, Vidal R, Plant G, Frangione B, Revesz T. Regional distribution of fibrillar and non-fibrillar ABri deposition and its association with neurofibrillary degeneration in Familial British Dementia. Am J Pathology. 2001;158:515–526. doi: 10.1016/S0002-9440(10)63993-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Revesz T, Holton JL, Doshi B, Anderton BH, Scaravilli F, Plant G. Cytoskeletal pathology in familial cerebral amyloid angiopathy (British type) with non-neuritic amyloid plaque formation. Acta Neuropathol. 1999;97:170–176. doi: 10.1007/s004010050970. [DOI] [PubMed] [Google Scholar]
- 7.Iwatsubo T, Saido T, Mann DM, Lee V, Trojanowski JQ. Full-length amyloid-beta 1-42 (43) and amino-terminally modified and truncated amyloid-β 1-42 (43) deposit in diffuse plaques. Am J Pathol. 1996;149:1823–1830. [PMC free article] [PubMed] [Google Scholar]
- 8.Pike CJ, Overman MJ, Cotman CW. Amino-terminal deletions enhance aggregation of β-amyloid peptides in vitro. J Biol Chem. 1995;270:23895–23898. doi: 10.1074/jbc.270.41.23895. [DOI] [PubMed] [Google Scholar]
- 9.Miravalle L, Calero M, Takao M, Roher AE, Ghetti B, Vidal R. Amino-terminally truncated Aβ peptide dpecies are the main component of cotton wool plaques. Biochemistry. 2005;44:10810–10821. doi: 10.1021/bi0508237. [DOI] [PubMed] [Google Scholar]
- 10.Saido T, Yamao-Harigaya W, Iwatsubo T, Kawashima S. Amino-and carboxyl-terminal heterogeneity of beta-amyloid peptides deposited in human brain. Neuroscience Letters. 1996;13:173–176. doi: 10.1016/0304-3940(96)12970-0. [DOI] [PubMed] [Google Scholar]
- 11.Harigaya Y, Saido TC, Eckman CB, Prada CM, Shoji M, Younkin SG. Amyloid beta protein starting pyroglutamate at position 3 is a major component of the amyloid deposits in the Alzheimer’s disease brain. Biochem Biophys Res Commun. 2000;276:422–427. doi: 10.1006/bbrc.2000.3490. [DOI] [PubMed] [Google Scholar]
- 12.Saido T, Iwatsubo T, Mann DM, Shimada H, Ihara Y, Kawashima S. Dominant and differential deposition of distinct β-amyloid peptide species, AβN3(pE), in senile plaques. Neuron. 1995;14:457–466. doi: 10.1016/0896-6273(95)90301-1. [DOI] [PubMed] [Google Scholar]
- 13.Cynis H, Scheele E, Saido TC, Schilling S, Demuth HU. Amyloidogenic processing of amyloid precursor protein: evidence of a pivotal role of glutaminyl cyclase in generation of pyroglutamate-modified amyloid-beta. Biochemistry. 2008;47:7405–7413. doi: 10.1021/bi800250p. [DOI] [PubMed] [Google Scholar]
- 14.Cynis H, Rahfeld JU, Stephan A, Kehlen A, Koch B, Wermann M, Demuth HU, Schilling S. Isolation of an isoenzyme of human glutaminyl cyclase: retention in the Golgi complex suggests involvement in the protein maturation machinery. J Mol Biol. 2008;379:966–980. doi: 10.1016/j.jmb.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 15.Vidal R, Ghiso J, Revesz T, Rostagno A, Kim E, Holton J, Bek T, Bojsen-Moller M, Braendgaard H, Plant G, Frangione B. A decamer duplication in the 3′ region of the BRI gene originates a new amyloid peptide that is associated with dementia in a Danish kindred. Proc Natl Acad Sci USA. 2000;97:4920–4925. doi: 10.1073/pnas.080076097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S-H, Wang R, Gordon DJ, Bass J, Steiner D, Lynn DG, Thinakaran G, Meredith S, Sisodia SS. Furin mediates enhanced production of fibrillogenic ABri peptides in familial British dementia. Nature Neurosc. 1999;2:984–988. doi: 10.1038/14783. [DOI] [PubMed] [Google Scholar]
- 17.Ghiso J, Vidal R, Rostagno A, Miravalle L, Holton J, Revesz T, Plant G, Frangione B. Amyloidogenesis in Familial British Dementia is associated with a genetic defect on chromosome 13. In: Growdon J, Wurtman R, Corkin S, Nitsch R, editors. Molecular basis of dementia. New York Academy of Sciences; New York: 2000. pp. 84–92. [DOI] [PubMed] [Google Scholar]
- 18.Ghiso J, Holton J, Miravalle L, Calero M, Lashley T, Vidal R, Houlden H, Wood N, Neubert TA, Rostagno A, Plant G, Revesz T, Frangione B. Systemic amyloid deposits in Familial British Dementia. J Biol Chem. 2001;276:43909–43914. doi: 10.1074/jbc.M105956200. [DOI] [PubMed] [Google Scholar]
- 19.Tomidokoro Y, Lashley T, Rostagno A, Neubert TA, Bojsen-Moller M, Braendgaard H, Plant G, Holton J, Frangione B, Revesz T, Ghiso J. Familial Danish dementia: co-existence of Danish and Alzheimer amyloid subunits (ADan and Aβ) in the absence of compact plaques. J Biol Chem. 2005;280:36883–36894. doi: 10.1074/jbc.M504038200. [DOI] [PubMed] [Google Scholar]
- 20.Sykes PA, Watson SJ, Temple JS, Bateman RCJ. Evidence for tissue-specific forms of glutaminyl cyclase. FEBS Lett. 1999;455:159–161. doi: 10.1016/s0014-5793(99)00872-8. [DOI] [PubMed] [Google Scholar]
- 21.Busby WH, Quackenbush GE, Humm J, Youngblood WW, Kizer JS. n enzyme that converts glutaminyl-peptides into pyroglutamyl-peptides. J Biol Chem. 1987;262:8532–8536. [PubMed] [Google Scholar]
- 22.Fischer WH, Spiess J. Identification of a mammalian glutaminyl cyclase converting glutaminyl into pyroglutamyl peptides. Proc Natl Acad Sci U S A. 1987;84:3628–3632. doi: 10.1073/pnas.84.11.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hashimoto T, Wakabayashi T, Watanabe A, Kowa H, Hosoda R, Nakamura A, Kanazawa I, Arai T, Takio K, Mann DM, Iwatsubo T. CLAC: a novel Alzheimer amyloid plaque component derived from a transmembrane precursor, CLAC-P/collagen type XXV. EMBO J. 2002;21:1524–1534. doi: 10.1093/emboj/21.7.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schilling S, Stenzel I, von Bohlen A, Wermann M, Schulz K, Demuth HU, Wasternack C. Isolation and characterization of the glutaminyl cyclases from Solanum tuberosum and Arabidopsis thaliana: implications for physiological functions. Biol Chem. 2007;388:145–153. doi: 10.1515/BC.2007.016. [DOI] [PubMed] [Google Scholar]
- 25.Schlenzig D, Manhart S, Cinar Y, Kleinschmidt M, Hause G, Willbold D, Funke SA, Schilling S, Demuth HU. Pyroglutamate formation influences solubility and amyloidogenicity of amyloid peptides. Biochemistry. 2009;48:7072–7078. doi: 10.1021/bi900818a. [DOI] [PubMed] [Google Scholar]