Abstract
The ASPP (apoptosis-stimulating protein of p53) family of proteins can function in the nucleus to modulate the transcriptional activity of p53, with ASPP1 and ASPP2 contributing to the expression of apoptotic target genes. In this study, we describe a new function for cytoplasmic ASPP1 in controlling YAP (Yes-associated protein)/TAZ. ASPP1 can inhibit the interaction of YAP with LATS1 (large tumor suppressor 1), a kinase that phosphorylates YAP/TAZ and promotes cytoplasmic sequestration and protein degradation. This function of ASPP1 therefore enhances nuclear accumulation of YAP/TAZ and YAP/TAZ-dependent transcriptional regulation. The consequence of YAP/TAZ activation by ASPP1 is to inhibit apoptosis, in part through the down-regulation of Bim expression, leading to resistance to anoikis and enhanced cell migration. These results reveal a potential oncogenic role for cytoplasmic ASPP1, in contrast to the tumor-suppressive activity described previously for nuclear ASPP1.
Keywords: ASPP1, YAP, LATS, apoptosis
The ASPP (apoptosis-stimulating protein of p53) family of proteins (each containing ankyrin repeats, an SH3 domain, and a polyproline-rich region) was initially identified through interaction with the tumor suppressor protein p53 (Iwabuchi et al. 1994). Three ASPP proteins have been identified in mammals: ASPP1, ASPP2 (the first to be identified as a p53-binding protein and initially named p53BP2) (Samuels-Lev et al. 2001), and iASPP (Bergamaschi et al. 2003). The ASPP family is conserved in all organisms that possess a p53 ortholog, with a single ASPP gene identified in lower organisms such as Caenorhabditis elegans and Drosophila (Trigiante and Lu 2006). In experimental systems, each of the mammalian ASPP proteins has been shown to bind p53 and modulate the ability of p53 to function as a transcription factor (Samuels-Lev et al. 2001; Bergamaschi et al. 2003, 2004). The binding of iASPP to the proline-rich domain of p53 inhibits the ability of p53 to bind to the promoters of apoptotic target genes, thereby inhibiting the activation of cell death in response to p53 (Bergamaschi et al. 2006; Ahn et al. 2009). Dissociation of iASPP can be mediated by the prolyl isomerase Pin1 (Mantovani et al. 2007) or interaction with ASPP1 and ASPP2, resulting in the enhanced binding of p53 to apoptotic promoters. Importantly, ASPP2 can be found in complex with p53 bound to these promoters (Samuels-Lev et al. 2001), illustrating how nuclear ASPP1 and ASPP2 function to promote the induction of apoptosis.
The reduced expression of ASPP1 and ASPP2 in human tumors supports the tumor-inhibiting role for these proteins (Sullivan and Lu 2007). Studies in mice clearly show that ASPP2 can function as a tumor suppressor, with evidence that this reflects, in part, a functional interaction with p53 (Vives et al. 2006; Kampa et al. 2009a). However, deletion of ASPP1 in mice revealed a defect in lymphatic vessel assembly that was not p53-dependent (Hirashima et al. 2008). In this study, no evidence for a tumor suppressor function of ASPP1 was noted in the ASPP1-deficient mice, although down-regulation of ASPP1 expression has been reported in human cancers (Roman-Gomez et al. 2005; Agirre et al. 2006). The inability of ASPP1 to substitute for ASPP2 in mice and the clear differences in the phenotype of ASPP1 and ASPP2 knockouts suggests that that these two proteins do not have interchangeable activities. Furthermore, despite the well-documented interplay between ASPP and p53, it also seems clear that both ASPP1 and ASPP2 have p53-independent activities (Kampa et al. 2009b). Some of this activity may be related to the ability of the ASPP proteins to bind and regulate the function of the p53-related proteins p63 and p73 (Bergamaschi et al. 2004). However, ASPP1 and ASPP2 have been shown to interact with numerous other proteins in both the nucleus and the cytoplasm, and ASPP2 has been shown recently to bind the PAR complex protein Par-3 at cell junctions and contribute to the maintenance of polarity (Cong et al. 2010; Sottocornola et al. 2010). In Drosophila, the dASPP protein forms a complex with dRASSF8 to regulate C-terminal Src kinase activity (Csk) and adherens junction formation through direct binding to dCsk (Langton et al. 2007, 2009). It is therefore clear that the function of the ASPP proteins is strongly influenced by their subcellular localization. Interestingly, ASPP1 is predominantly cytoplasmic in most mammalian cells, but shows nuclear localization in germ cells (Thornton et al. 2006).
One of the ASPP-binding proteins identified through a yeast two-hybrid screen is YAP (Yes-associated protein) (Espanel and Sudol 2001). YAP and the closely related TAZ protein shuttle between the cytoplasm and nucleus, where they can control cell proliferation or apoptosis via the regulation of many transcription factors (Wang et al. 2009). These two proteins are structurally very similar—containing a PDZ domain, coiled–coiled domain, and WW domain involved in their interaction with other proteins—and function in a very similar way as transcriptional cofactors to control gene expression (Wang et al. 2009). YAP/TAZ activity is controlled by the LATS (large tumor suppressor) kinases, which function in the Hippo tumor suppression pathway (Hergovich and Hemmings 2009). Phosphorylation of YAP/TAZ by LATS promotes binding to 14-3-3 and cytoplasmic sequestration, and also targets YAP for degradation (B Zhao et al. 2010b). Although the function of YAP as a transcriptional coactivator is well established, the consequences of YAP-dependent gene regulation are complex. In Drosophila, YAP plays an important role in the regulation of organ size, and several lines of evidence suggest that YAP functions as an oncogene, promoting cell proliferation, survival, loss of contact inhibition, and increased organ size (Overholtzer et al. 2006; Zender et al. 2006; Dong et al. 2007; Zhao et al. 2008a). However, YAP has also been shown to participate in a tumor suppressor pathway, cooperating with p73 to promote the expression of apoptotic genes (Strano et al. 2005; Oka et al. 2008). The apparently opposing activities of YAP appear to reflect which transcriptional cofactors are available, such that YAP can contribute to the activation of proapoptotic genes through interaction with p73 or prosurvival/anti-apoptotic genes through the interaction with TEAD (Zhao et al. 2008b).
In this study, we examined activities of ASPP1 under conditions in which the protein remains cytoplasmic, describing a new function for ASPP1 in the regulation of YAP/TAZ.
Results
Like ASPP2, ASPP1 has been shown to function in the nucleus, where it can bind p53 and directly influence p53-mediated gene expression. However, we found that, in several wild-type p53-expressing cell lines (HCT116, MRC5, U2OS, RKO, and RPE), ASPP1 was localized predominantly to the cytoplasm, with only low levels of nuclear ASPP1 (Fig. 1A; Supplemental Fig. 1A). As shown by Aylon et al. (2010), oncogenic stress (e.g., RAS activation) can provoke the relocalization of ASPP1 to the nucleus, where it contributes to p53-mediated regulation of gene expression and p53-mediated tumor suppression. However, this relocalization is dependent on the nature of the stress signals; treatment of cells with p53-inducing levels of hydroxyurea (HU) or with Nutlin (a stabilizer of p53), for example, did not change the localization of ASPP1 in these cells (Fig. 1A; data not shown). Using pools or individual siRNAs to deplete ASPP1 expression (Fig. 1B; Supplemental Fig. 1B), we examined the effect of ASPP1 down-regulation on p53 target gene expression. While nuclear ASPP1 is known to play a role in regulating p53-driven activation of apoptotic target genes, we did not detect a clear contribution of ASPP1 to the expression of this group of genes in cells in which ASPP1 is predominantly cytoplasmic. Pig3, Puma, and Bax expression, which was induced following activation of p53 by treatment of cells with Nutlin or HU, was very slightly increased by siRNA-mediated depletion of ASPP1 levels at the mRNA level (Fig. 1C), with no difference in expression at the protein level (Fig. 1D). This result was confirmed in another cell line (Supplemental Fig. 1C). Although there was no clear effect of ASPP1 modulation on p53-dependent gene expression, we extended our analysis to 130 genes regulated by different transcription factors under various stress condition. These included groups of genes that have been shown to be under the control of YAP/TAZ, Smad, HIF, p53, and NF-kB. Interestingly, we found that depletion of ASPP1 altered the expression of a subgroup of these genes (Fig. 1E), suggesting that ASPP1 can indirectly regulate transcription without overt relocalization to the nucleus. Previous studies have shown that the ASPP2 protein can interact with YAP (Espanel and Sudol 2001), and a closer examination of the expression profile generated by ASPP1 modulation showed a clear effect on genes previously described to be modulated by YAP (Fig. 1E; Supplemental Table 1). Since YAP/TAZ proteins can integrate cytoplasmic and nuclear functions, these data indicate a possible role for YAP/TAZ in mediating the response to depletion of cytoplasmic ASPP1.
Figure 1.
Cytoplasmic ASPP1 regulates gene expression. (A) Nuclear and cytoplasmic expression of ASPP1 in different cell lines detected by Western blot in control conditions or after treatment for 24 h with 2 mM HU. Lamin expression was used as loading and purity control for fractionation. (B) HCT116 cells were transfected with a pool of siRNA against ASPP1 or control siRNA, and ASPP1 expression was assessed by Western blot in control conditions or after 2 mM HU or 10 μM Nutlin treatment. (C) mRNA expression of the indicated genes was then determined by RT-qPCR with specific primers. The results were normalized against two different standard genes, and the graphs represent the mean of five independent experiments. (D) Expression of Pig3, Puma, and Bax assessed by Western blot in U2OS cells treated with the indicated siRNAs with or without 10 μM Nutlin for 30 h. Actin expression was used as a loading control. (E) mRNA expression of 130 genes quantified by RT-qPR in the same conditions as in B, shown as a dot plot. Results following control siRNA treatment are shown in the X-axis, and ASPP1 siRNA treatment is shown in the Y-axis. The YAP/TAZ target genes are highlighted by a red X.
To examine the relationship between ASPP1 and YAP/TAZ more closely, we first wished to confirm that depletion of ASPP1 would affect known YAP/TAZ target genes. We therefore compared the effect of ASPP1 and YAP/TAZ knockdown (using siRNAs that target both YAP and TAZ) on the expression of CTGF and PHLDB2 (activated by YAP/TAZ) and Bim (repressed by YAP/TAZ) (Zhao et al. 2008b). In both control cells and cells treated with HU, depletion of ASPP1 or YAP/TAZ by siRNA reduced the expression of CTGF and PHLDB2 and enhanced expression of Bim (Fig. 2A). A similar effect was seen using individual siRNA against ASPP1 or an siRNA targeting only TAZ (Supplemental Fig. 2A–C). Depletion of YAP/TAZ by siRNA resulted in an elevation of Bim protein levels similar to that seen following knockdown of ASPP1 (Fig. 2B). These effects were not dependent on p53, since a similar regulation of gene expression was also seen in p53-deleted HCT116 cells (HCT116 p53−/−) (Fig. 2C), and this correlated with an increased apoptotic rate in these cells (Fig. 2D). Importantly, the effects of the ASPP1 siRNA were reversed by ectopic expression of ASPP1 to levels similar to those seen in control cells (Supplemental Fig. 2D,E), indicting that these are not off-target effects of the siRNA. These results are consistent with a p53-independent function for cytoplasmic ASPP1 in the control of YAP/TAZ activity and the inhibition of apoptosis. This role for cytoplasmic ASPP1 is distinct from the proapoptotic role of nuclear ASPP1 (Samuels-Lev et al. 2001; Aylon et al. 2010).
Figure 2.
ASPP1 controls YAP target genes. (A) CTGF, PHLDB2, and Bim mRNA expression measured by RT-qPCR with specific primer in HCT116 cells transfected with control or ASPP1 siRNA, and treated or not treated with 400 μM HU. The results were normalized against two different standard genes, and the graphs represent the mean of five independent experiments. (B) Expression of YAP, TAZ, and Bim assessed by Western blot in HCT116 cells treated with the indicated siRNAs, with or without 400 μM HU, for 24 h. (C) Same experiment as in A in the HCT116 p53−/− cell line. (D) Apoptosis in HCT116p53−/− cells was quantified under the same conditions as in C by DNA staining and flow cytometry analysis. The graph is representative of three independent experiments and shows the percentage of cell in the sub-G1 fraction.
YAP/TAZ are regulated through several mechanisms that depend, in part, on their phosphorylation, which can promote binding to 14-3-3 and cytoplasmic sequestration (Kanai et al. 2000; Zhao et al. 2007). We therefore examined the effect of ectopic ASPP1 expression on the subcellular localization of YAP/TAZ in U2OS cells. In control cells, YAP/TAZ was mainly nuclear until cells reached confluence, when YAP/TAZ became more equally distributed between the nucleus and the cytoplasm (Fig. 3A). However, in ASPP1-overexpressing cells, YAP/TAZ were found almost exclusively in the nucleus regardless of the cell confluence (Fig. 3A). Conversely, knockdown of ASPP1 in HCT116 cells resulted in a reduction in the number of cells containing only nuclear YAP/TAZ under sparse growth conditions (Fig. 3B). The ability of ASPP1 to promote nuclear localization of YAP/TAZ was also accompanied by a decrease in the phosphorylation of YAP on Ser127 (Fig. 3C), consistent with a role for this phosphorylation event in retaining cytoplasmic YAP. Conversely, knockdown of ASPP1 enhanced the phosphorylation of cytoplasmic YAP (Fig. 3D), an effect that correlated with a decrease in the levels of chromatin-bound YAP and TAZ (Fig. 3E).
Figure 3.
ASPP1 increases YAP and TAZ nuclear localization. (A) Immunofluorescence staining of endogenous YAP/TAZ in U2OS cells infected with a control or ASPP1 expression vector. Cells were counterstained with DAPI to localize the presence of YAP/TAZ in the nucleus. More than 10,000 cells were analyzed using an Operetta screening system, and the graph represents the percentage of cells expressing YAP/TAZ in the cytoplasm in three different experiments. ASPP1 overexpression was confirmed by immunoblot using actin as a loading control. (B) The same experiment as in A repeated in HCT116 transfected with control or ASPP1 siRNA. The graph represents the percentage of cells expressing YAP/TAZ in the cytoplasm in three different experiments. (C) YAP Ser127 phosphorylation analyzed by Western blot in U2OS cells transiently transfected with a YAP expression vector and an increasing concentration of a vector coding for ASPP1. (D) YAP Ser127 phosphorylation analysis by Western blot in HCT116 cells transfected with control or ASPP1 siRNA, with or without HU. The loading was normalized for total YAP to allow a comparison of the phosphorylation levels. (E) Unbound and chromatin-bound YAP and TAZ was fractionated by detergent and DNase treatment in HCT116 cells under the same conditions as in D. YAP phosphorylated on Ser127, total YAP, and TAZ levels were assessed by immunoblot. The high-mobility group protein HMGA2 was used as a control for the chromatin-bound fraction.
Recently, phosphorylation of YAP has also been linked to its enhanced degradation through the proteasome (B Zhao et al. 2010b). Knockdown of ASPP1 resulted in a slight but reproducible decrease in YAP protein levels in control and HU-treated cells that was not reflected at the mRNA level, suggesting that ASPP1 may regulate YAP/TAZ degradation (Fig. 4A). This was confirmed by showing that ASPP1 overexpression led to an increase in protein half-life of both YAP (Fig. 4B) and TAZ (Fig. 4C).
Figure 4.
Stabilization of YAP and TAZ proteins in response to ASPP1. (A) Expression of YAP and ASPP1 assessed by Western blot or RT-qPCR in HCT116 cells treated with control or ASPP1 siRNA, with or without 400 μM HU. Actin was used as a loading control. mRNA expression quantification was normalized against two different standard genes and represents five independent experiments. (B) YAP protein stability was tested in U2OS cells infected with a control or ASPP1 coding vector and treated with cyclohexamide for the indicated time. YAP expression was measured by Western blot using actin as a loading control. The graph represents YAP protein expression normalized to its initial level, and represents the mean of three independent experiments. (C) TAZ protein stability was tested in U2OS under the same conditions as described in B.
YAP phosphorylation is mediated by several kinases, including LATS1 and LATS2. Interestingly, we were able to detect an interaction between ASPP1 and LATS1 and LATS2. The LATS2/ASPP1 interaction is enhanced by RAS activation, resulting in the phosphorylation and nuclear localization of ASPP1 (Aylon et al. 2010). However, in the absence of oncogene activation, overexpression of ASPP1 can inhibit the interaction between exogenous YAP and LATS1 (Fig. 5B) and LATS2 (Fig. 5C). We generated a series of LATS1 mutants deleting the P-stretch (ΔP), the P-stretch and PPPY domains (ΔPΔPPPY), or the kinase domain (ΔKD), or point-mutating the phosphorylated tyrosine 376 (Y376F) (Fig. 5D). Coimmunoprocipitation after expression of these LATS1 proteins in cells showed that the ability of ASPP1 to bind LATS1 required the kinase domain (Fig. 5E,F) but did not need the presence of the P-stretch and PPPY regions. Interestingly, previous studies have shown that the interaction of LATS1 with YAP depends on the PPPY region but does not require the kinase domain (Hao et al. 2008). This suggests that the ability of ASPP1 to prevent YAP binding to LATS1 reflects a more complex structural interaction than simply steric inhibition. Examination of the endogenous proteins showed that, in these cells, YAP was associated mainly with LATS1 and more weakly with LATS2 (Fig. 5G), and these interactions were lost following depletion of YAP/TAZ by siRNA. Knockdown of ASPP1 resulted in a slight but reproducible increase in YAP/LATS1 interaction (Fig. 5H). The binding of ASPP1 to the LATS kinases therefore appears to modulate their ability to bind YAP/TAZ, allowing YAP and TAZ to escape LATS-mediated phosphorylation.
Figure 5.
ASPP1–LATS1 interaction impedes LATS1–YAP complex formation. (A) Endogenous ASPP1 was immunoprecipitated from HCT116 cells. The immunoprecipitation was then resolved by SDS-PAGE and immunoblotted for LATS1, LATS2, YAP, and ASPP1. Five percent of the lysate was loaded onto the gel to assess input protein levels. (B) U2OS cells were transiently transfected with vectors encoding LATS1 and YAP and an increasing concentration of a vector encoding ASPP1. LATS1 was then immunoprecipitated, resolved by SDS-PAGE, and immunoblotted for LATS1, ASPP1, and YAP. Five percent of the lysate was loaded onto the gel to assess the input levels of ASPP1 and YAP. (C) Same experiment as in B examining LATS2. (D) Diagram showing the different domains of LATS1. (E) U2OS cells infected with ASPP1 were transiently transfected with vectors encoding Myc-tagged wild-type or mutant LATS1 as indicated. ASPP1 immunoprecipitations were resolved by SDS-PAGE and immunoblotted for ASPP1 using the anti-Myc antibody. Ten percent of the lysate was loaded onto the gel to assess the input levels of the different LATS1 constructs. (F) Immunofluorescence staining of ASPP1 and LATS1 expressed in U2OS cells, showing the colocalization of ASPP1 with wild-type LATS1 but not LATS-ΔKD. (G) Endogenous YAP was immunoprecipitated from HCT116 cells treated with control or YAP siRNAs. The immunoprecipitation was then resolved by SDS-PAGE and immunoblotted for LATS1 and LATS2. Five percent of the lysate was loaded onto the gel to assess input protein levels. (H) Endogenous YAP was immunoprecipitated from HCT116 cells treated with control or ASPP1 siRNAs. The immunoprecipitation was then resolved by SDS-PAGE and immunoblotted for LATS1 and YAP. Five percent of the lysate was loaded onto the gel to assess input protein levels.
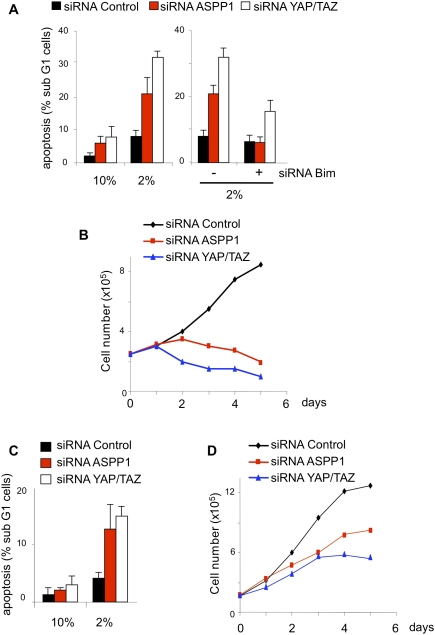
Our results suggest that cytoplasmic ASPP1 plays a role in impeding YAP/TAZ phosphorylation, thus enhancing their nuclear accumulation and transcriptional activity. We showed that ASPP1 down-regulation resulted in enhanced apoptosis in response to HU (Fig. 2D), and a similar increase in apoptosis in response to depletion of YAP/TAZ, TAZ, or ASPP1 was seen in HCT116 cells grown in 2% serum (Fig. 6A; Supplemental Fig. 3A,B). Re-expression of YAP or a phosphorylation site mutant of YAP (YAPS127A) rescued this apoptotic response to ASPP1 depletion (Supplemental Fig. 3C,D), supporting a role for YAP as a mediator of the response to ASPP1. As seen in the HU response, this cell death is accompanied by an increase in Bim expression (Fig. 6A; Supplemental Fig. 4A–C), and inhibition of Bim largely prevented the increase in apoptosis seen in response to YAP/TAZ or ASPP1 down-regulation (Fig. 6A; Supplemental Fig. 4A). While YAP/TAZ have been shown to regulate the expression of cell growth and survival genes, YAP can also induce apoptosis in cooperation with p73. Importantly, although cisplatin treatment efficiently induced p73 expression in these cells, very little p73 expression could be detected in cells grown in 2% serum, accounting for the absence of YAP-associated proapoptotic signaling (Supplemental Fig. 4D). The modulation of apoptosis in 2% serum was also reflected by a failure of cells depleted of ASPP1 or YAP/TAZ to increase in number over several days, in contrast to control cells that continued to expand (Fig. 6B). Importantly, this effect of ASPP1 or YAP/TAZ depletion was also seen in cells lacking p53 (Fig. 6C,D), although the effects on cell growth and apoptosis are weaker than in the p53 wild-type cells. The specificity of the siRNA was demonstrated by re-expression of ASPP1, which rescued the effect on apoptosis (Supplemental Fig. 5).
Figure 6.
ASPP1 and YAP inhibit apoptosis under low-serum conditions. (A) HCT116 cells were transfected with different combinations of control siRNA or siRNAs directed against ASPP1, YAP, and Bim. Cells were then incubated for 48 h in 10% or 2% serum and apoptosis was measured by the analysis of the sub-G1 fraction. The graph represents the mean of three independent experiments. (B) HCT116 cells were plated at low confluence and transfected with control, ASPP1, or YAP siRNAs. Cells were maintained in 2% serum and harvested each day for 6 d. Live cells were counted by Trypan blue exclusion; the graph represents the mean of three independent experiments. (C) Same experiments as in A in the HCT116 p53−/− cell line. (D) Same experiments as in B in the HCT116 p53−/− cell line.
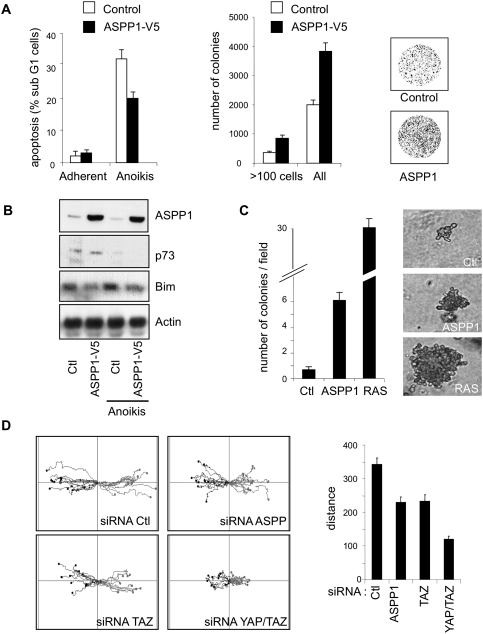
The ability of ASPP1 to modulate YAP/TAZ signaling suggests that ASPP1 may also regulate other cell responses to YAP/TAZ, including anchorage-independent growth, migration, and invasion (Chan et al. 2008). Overexpression of ASPP1 expression resulted in a decreased cell death under conditions of matrix detachment (Fig. 7A), leading to a higher proportion of cells that survived and recovered from transient periods of detachment (Fig. 7A). As seen in cell growth in 2% serum or cells treated with HU, this effect of ASPP1 correlated with decreased Bim levels (Fig. 7B) and very low p73 expression. Consistent with an effect of YAP in promoting anchorage-independent growth, we found that overexpression of ASPP1 allowed growth of U2OS cells in soft agar, increasing both the number and size of colonies, although this effect was significantly less profound than that seen following expression of activated RAS (Fig. 7C). Finally, we also found a significant effect of ASPP1 depletion in reducing cell motility in a wound-healing assay. While, in this case, the effect of YAP/TAZ knockdown was stronger than ASPP1 depletion, a specific reduction of TAZ expression gave an effect very similar to that seen after ASPP1 knockdown (Fig. 7D).
Figure 7.
ASPP1 protects cells from anoikis. (A) U2OS cells infected with a control or ASPP1 coding vector were incubated for 4 d in a dish coated with 1% agarose. Apoptosis induction was then measured by DNA staining and flow cytometry. The graph is representative of three independent experiments and shows the percentage of cells in the sub-G1 fraction. Alternatively, surviving cells were replated in normal conditions to allow them to recover from the matrix detachment period. Colonies formed 1 wk later were stained with Giemsa and quantified with ImageJ software. The graph shows the mean of three different experiments, and a dish from each condition is shown as an example. (B) p73, Bim, and ASPP1 expression in U2OS cells treated in the same way as in A were assessed by Western blot. Actin was used as a loading control. (C) Anchorage-independent growth of U2OS cells infected with a control or ASPP1 retroviral vector. Cells were embedded in 0.3% agar and left growing for 2 wk. The number of colonies was counted in a minimum of 50 different microscopic fields per condition and plotted as a mean of colonies per field. RAS-overexpressing U2OS cells were used as a positive control, and results show the mean of three different experiments. A representative colony for each condition is shown: for ASPP1- and RAS-expressing cells, an average-sized colony is shown; for the control cells, the largest colony seen is shown. (D) HCT116 cells transfected with control, ASPP1, TAZ, or YAP siRNA were observed by time-lapse video microscopy, and the movement of individual cells was followed using cell-tracking software. The results are presented as overlays of representative trajectories described by cells during their migration into the wound. The total distance of migration was extracted from the track plots. Values are mean ± SEM of >100 track plots from three independent experiments.
Discussion
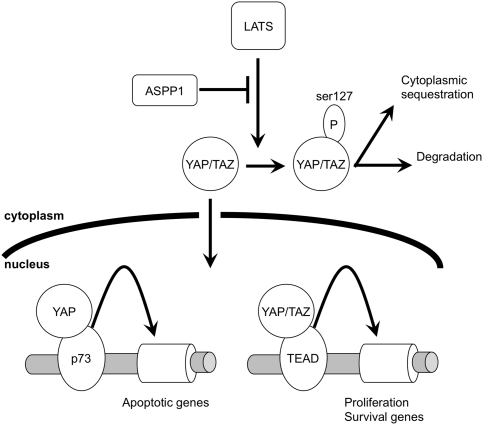
Nuclear ASPP1 functions to regulate p53 and promotes p53-mediated activation of target genes that promote apoptosis. The accompanying study by Aylon et al. (2010) in this issue of Genes & Development described a key role for LATS2 in the phosphorylation of ASPP1 in response to RAS activation, leading to its nuclear localization and contribution to apoptosis. In the present study, we show that cytoplasmic ASPP1 also functions in a p53-independent manner to regulate YAP/TAZ activity. Our results suggest that cytoplasmic ASPP1 can reduce the phosphorylation of YAP by inhibiting the interaction with LATS kinases, thereby promoting nuclear localization and activation of transcriptional control by YAP/TAZ (Fig. 8). Through this mechanism, ASPP1 can help to support the induction of YAP/TAZ-dependent suppression of Bim expression and a reduction in apoptosis induced by HU, low serum, or matrix detachment (anoikis). ASPP1 regulation of YAP/TAZ is also reflected in the control of cell motility. Taken together with the accompanying study (Aylon et al. 2010), we describe roles for cytoplasmic and nuclear ASPP1 that may have different outcomes in terms of cell viability, and so may contribute differently to the inhibition or promotion of tumor development.
Figure 8.
Model of YAP/TAZ regulation by cytoplasmic ASPP1. Under normal conditions, the activation of LATS leads to the phosphorylation of YAP/TAZ and their inactivation through cytoplasmic sequestration and degradation. ASPP1 can function to inhibit this activity of LATS1, allowing the nuclear translocation of YAP/TAZ where they can regulate gene expression. While this study shows an effect of ASPP1 on the regulation of YAP/TAZ-mediated survival signaling, it is also possible that ASPP1 can modulate the induction of apoptotic target gene expression regulated by a YAP/p73 complex.
While the ASPP family of proteins clearly plays an important role in regulating p53-mediated apoptosis, there is also accumulating evidence for p53-independent functions of ASPP. In Drosophila, the ability of dASPP to control cell adhesion is not dependent on p53, and the ability of ASPP2 to function at cell junctions to regulate polarity in mammalian cells does not appear to require p53. p53-independent activities of ASPP1 are suggested by the phenotype of ASPP1 knockout mice. Our identification of a role for ASPP1 in the regulation of YAP places ASPP1 at the heart of a second important tumor suppressor pathway that functions to regulate organ size and tumor development (B Zhao et al. 2010a). Our results suggest that cytoplasmic ASPP1 can play an oncogenic role in counteracting signaling through this pathway, thereby preventing the inhibition of the YAP/TAZ oncogenic function.
The consequences of ASPP1 expression or loss are likely to be complex, however, and strongly influenced by other controlling factors in the cells. Nuclear ASPP1 clearly helps to promote apoptosis mediated by p53 and p73. Moreover, YAP activity has been described to provide tumor suppressor activities by binding to p73 to promote apoptosis (Bertini et al. 2009). So, it is possible that, under some circumstances, cytoplasmic ASPP1 will also help to induce cell death by activating this YAP/p73 function (Fig. 8). Such a function of ASPP1 would be expected to coordinate with the proapoptotic nuclear function of ASPP1 to contribute to tumor suppression. This function of YAP would clearly depend on the presence of p73 within the cell, and our studies show that, in cells with low p73 levels, ASPP1 regulation of YAP/TAZ does not result in the promotion of the apoptotic function of YAP. However, it is possible that signals that induce p73 (such as treatment with cisplatin) (Supplemental Fig. 2B) or cells in which p73 levels are constitutively expressed at higher levels would show a different response to modulation of cytoplasmic ASPP1 levels. Indeed, in agreement with previous studies (Samuels-Lev et al. 2001), we found that, in response to cisplatin, ASPP1 can enhance, rather than inhibit, the apoptotic response (data not shown). Similarly, the accompanying study by Aylon et al. (2010) shows a clear effect of LATS2 in phosphorylating and driving nuclear accumulation of ASPP1 in response to oncogene activation. While RAS activation promotes the LATS2/ASPP1 interaction and subsequent phosphorylation of ASPP1, our studies show that, in the absence of a RAS-stimulating signal, cytoplasmic ASPP1 plays a role in regulating the YAP/LATS interaction, and so controls the phosphorylation of YAP by LATS kinases.
Finally, a study showing that, under some circumstances, the effect of LATS1 phosphorylation of YAP can also enhance YAP apoptotic activity suggests another scenario by which cytoplasmic ASPP1, through inhibition of LATS1/YAP binding, reduces the induction of cell death (Matallanas et al. 2007).
Taken together, our work and the study from Aylon et al. (2010) reveal a complex and intimate interdependent regulation between ASPP1, YAP/TAZ, and LATS. We showed that the outcome to ASPP1 function can switch between p53-mediated tumor suppression (when ASPP1 is driven to the nucleus by LATS phosphorylation) and p53-independent oncogenic activity (when cytoplasmic ASPP1 controls the ability of LATS to phosphorylate and activate nuclear accumulation of YAP/TAZ). Previous studies have shown that ASPP1 expression is down-regulated in certain cancer types (Agirre et al. 2006; J Zhao et al. 2010), which is more consistent with a role of ASPP1 in preventing tumor development. However, ASPP1−/− mice are not obviously tumor-prone, and it is possible that the opposing functions of cytoplasmic and nuclear ASPP1 in YAP and p53-mediated responses reflect changing roles of ASPP1 at different stages of tumor progression. It seems possible that a switch in ASPP1 activity to the nuclear tumor-suppressive role is manifest at later stages of malignant progression, following activation of oncogenes such as RAS, and so ASPP1 expression would be selected against in advanced tumors. Understanding how the eventual outcome to ASPP1 activity is determined will help to establish any role of ASPP1 in cancer progression.
Materials and methods
Cell culture, drug treatment, and vectors
HCT116 (colon carcinoma), HCT116p53−/− (Bunz et al. 1998), U2OS (osteosarcoma), hTERT-RPE (telomerase immortalized human retinal pigment epithelial cells; Clontech), and RKO (colon carcinoma) cell lines were cultured as described in the Supplemental Material. U2OS expressing the ecotropic receptor was made by infecting cells with a pBabe Ecotropic receptor vector, and hTERT-MRC5 was made by infecting cells with a pBabe HTERT vector. Both cell lines were then selected in 5 μg/mL puromycin. Treatment with HU, Nutlin3A, and Cisplatin (Sigma) was carried out as described in the figure legends.
V5-tagged ASPP1 and LATS2 vectors have been described previously (Samuels-Lev et al. 2001; Aylon et al. 2006). ASPP1 was subcloned in the retroviral vector pWZL-Blast. Myc-tagged LATS1 vector (a gift from E. O'Neill) was cloned in pRcCMV. LATS1 mutants were made by directed mutagenesis. The ΔP mutant was deleted of amino acids 236–266, the ΔPΔPPY was deleted of amino acids 236–560, and the ΔKD mutant introduced a stop codon at position 707. The Y376F mutation changes amino acid 376 from tyrosine to phenylalanine. Flag-tagged YAP and YAP S127A (a gift from W. Kolch) were cloned in pCDN1.
DNA and siRNA transfection
Cells were transfected with 6 μg total of the indicated plasmids per 10-mm dish using Genejuice reagent (Novagen) according to the manufacturer's instructions.
siRNAs, described in the Supplemental Material, were transfected at 25 nM using Hiperfect transfection reagent (Qiagen), according to the manufacturer's instructions. Efficient knockdown was detected between 24 h and 72 h after the transfection; for long-term knockdown, cells were retransfected every 3 d.
mRNA gene expression analysis
RNA was prepared using an extraction column (Qiagen) according to the manufacturer's instructions. Two micrograms of RNA was used for cDNA synthesis, and quantitative PCR (qPCR) analysis was performed with 5 μL of a 200× dilution of the cDNAs. Both were carried out with the DyNAmo SYBR Green two-step qRT–PCR kit (Finnzymes). Accumulation of fluorescent products was monitored by real-time PCR using a Chromo4 reader (Bio-Rad) and was analyzed with the Opticon Monitor3 software. The relative quantification of gene expression was performed using the comparative CT method, with normalization of the target gene to the endogenous housekeeping genes β2-microglobulin and RPLP0 (ribosomal protein, large, P0). Primers used for the PCR amplified only one product, which was fully sequenced to confirm gene specificity, and are available on request. qPCR cycling parameters were 15 min at 95°C; 40 cycles of 20 sec at 94°C, 30 sec at 60°C, and 30 sec at 72°C; and 10 min at 72°C. Results are presented as a percentage of variation rate or as a fold activation/repression between different conditions.
Cell fractionation, immunoprecipitation, immunoblotting, and protein stability
Chromatin fractionation was performed by treating cells for 10 min in a CSK buffer (100 mM NaCl, 0.3 M sucrose, 10 mM PIPES at pH 6.8, 3 mM MgCl2, 1 mM EGTA, 0.5% Triton X100) to obtain the unbound chromatin fraction. Cells were then washed twice with PBS and treated with the same buffer supplemented with 200 μg/mL DNase1 for 20 min to obtain the chromatin fraction.
For simple immunoblotting, cells were lysed in RIPA buffer (0.1% SDS, 1% deoxycholate, 1% nodidet P40 [NP40], 150 mM NaCl, and 25 mM Tris at pH 7.6). Cell extracts were then sonicated three times for 4 sec to solubilize the chromatin. For immunoprecipitation, cells were lysed in NP40 buffer (1% NP40, 150 mM NaCl, 50 mM Tris at pH 7.6), and the indicated proteins were immunoprecipitated for 6 h using 2–10 μg of specific antibodies and 30 μL of protein G coupled to magnetic Dynabeads (Invitrogen). Immunoprecipitations were washed three times with a buffer containing 0.05% Triton X-100, 150 mM NaCl, and 50 mM Tris (pH 7.6). In all cases, proteins were resolved by SDS-PAGE and analyzed by Western blot using specific antibodies (listed in the Supplemental Material).
To measure protein stability, cells were treated for the indicated time with 25 μg/mL cyclohexamide (Sigma). Cells were harvested and processed for Western blot as described above. Actin was used as a loading control, and bands were quantified using ImageJ software by normalizing to the band intensity of the sample without any cyclohexamide treatment. Results are expressed as a percentage of intensity relative to the untreated sample.
Apoptosis assays
Cells were harvested, fixed in ice-cold methanol, treated with 1 mg/mL of RNaseA (Sigma) for 1 h, and analyzed by flow cytometry (FACScan, Becton Dickinson) for DNA fragmentation using propidium iodide staining at 50 μg/mL in PBS 0.1% Tween20. Cells with a sub-G1 DNA content were identified as apoptotic. Results are expressed as a percentage of apoptotic cells in the whole population.
Immunofluorescence and YAP localization analysis
Cells were plated in 12-well Borosilicate glass plates at low confluence (20%). After 3 d, cells were fixed with ice-cold 4% paraformaldehyde in PBS for 10 min at room temperature, then permeabilized in PBS containing 0.2% Triton X-100 for 5 min. The cells were washed three times with the blocking solution (PBS, 0.5% BSA, 0.1% Tween) and incubated for 30 min at room temperature with YAP antibody at 2 μg/mL in blocking solution. After three washes in PBS, the cells were incubated for 30 min at room temperature with a donkey anti-mouse Alexa488-conjugated antibody (Molecular Probes) in blocking solution containing 1 μg/mL DAPI (4′,6′-diamidino-2-phenylindole) (Sigma). The cells were washed three times with PBS, and fluorescence was monitored by the high-content screening Operetta system (Perkin Elmer). At least 10,000 cells were evaluated for the localization of YAP in the cytoplasm and the nucleus for each condition.
Anoikis and anchorage-independent growth
A total of 1 × 106 cells were seeded in 100-mm dishes coated with 5 mL of 1% agarose. Cells were allowed to grow without any matrix attachment for 4 d, and apoptosis was quantified as described previously. Alternatively, cells were replated after 4 d in a normal 100-mm dish and incubated for one more week to assess colony formation. Colonies were stained with Giemsa and counted using ImageJ software.
For the anchorage-independent growth assay, cells (5 × 105) were added to 6 mL of growth medium with 0.3% agar and layered onto 6 mL of 0.6% agar base in a 100-mm dish. Cells were left to grow for 2 wk, after which visible colonies were counted and photographed. Mean values per field were calculated from three independent experiments and a minimum of 50 different fields were used for each condition.
Scratch wound assay
Movement of HCT116 cells into a wound scratched into a monolayer and grown in 1% FCS was monitored with a phase-contrast 20× objective, using an inverted microscope (Nikon TE2000). Images were obtained every 10 min over 16 h. Cells were transfected on the previous day with the indicated siRNA.
Acknowledgments
We thank Xin Lu, Eric O'Neill, and Walter Kolch for plasmids and helpful discussion. This work was funded by Cancer Research-UK.
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1954310.
Supplemental material is available at http://www.genesdev.org.
References
- Agirre X, Roman-Gomez J, Jimenez-Velasco A, Garate L, Montiel-Duarte C, Navarro G, Vazquez I, Zalacain M, Calasanz MJ, Heiniger A, et al. 2006. ASPP1, a common activator of TP53, is inactivated by aberrant methylation of its promoter in acute lymphoblastic leukemia. Oncogene 25: 1862–1870 [DOI] [PubMed] [Google Scholar]
- Ahn J, Byeon IJ, Byeon CH, Gronenborn AM 2009. Insight into the structural basis of pro- and antiapoptotic p53 modulation by ASPP proteins. J Biol Chem 284: 13812–13822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylon Y, Michael D, Shmueli A, Yabuta N, Nojima H, Oren M 2006. A positive feedback loop between the p53 and Lats2 tumor suppressors prevents tetraploidization. Genes Dev 20: 2687–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylon Y, Ofir-Rosenfeld Y, Yabuta N, Lapi E, Nojima H, Lu X, Oren M 2010. The Lats2 tumor suppressor augments p53-mediated apoptosis by promoting the nuclear proapoptotic function of ASPP1. Genes Dev (this issue) doi: 10.1101/gad.1954410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi D, Samuels-Lev Y, O'Neil NJ, Trigiante G, Crook T, Hseih JK, O'Conner DJ, Zhong S, Compargue I, Tomlinson ML, et al. 2003. iASPP oncoprotein is a key inhibitor of p53 conserved from worms to humans. Nat Genet 33: 162–167 [DOI] [PubMed] [Google Scholar]
- Bergamaschi D, Samuels Y, Jin B, Duraisingham S, Crook T, Lu X 2004. ASPP1 and ASPP2: Common activators of p53 family members. Mol Cell Biol 24: 1341–1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergamaschi D, Samuels Y, Sullivan A, Zvelebil M, Breyssens H, Bisso A, Del Sal G, Syed N, Smith P, Gasco M, et al. 2006. iASPP preferentially binds p53 proline-rich region and modulates apoptotic function of codon 72-polymorphic p53. Nat Genet 38: 1133–1141 [DOI] [PubMed] [Google Scholar]
- Bertini E, Oka T, Sudol M, Strano S, Blandino G 2009. YAP: At the crossroad between transformation and tumor suppression. Cell Cycle 8: 49–57 [DOI] [PubMed] [Google Scholar]
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B 1998. Requirement for p53 and p21 to sustain G2arrest after DNA damage. Science 282: 1497–1501 [DOI] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Guo K, Ng CP, Lee I, Hunziker W, Zeng Q, Hong W 2008. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res 68: 2592–2598 [DOI] [PubMed] [Google Scholar]
- Cong W, Hirose T, Harita Y, Yamashita A, Mizuno K, Hirano H, Ohno S 2010. ASPP2 regulates epithelial cell polarity through the PAR complex. Curr Biol 20: 1408–1414 [DOI] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D 2007. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130: 1120–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espanel X, Sudol M 2001. Yes-associated protein and p53-binding protein-2 interact through their WW and SH3 domains. J Biol Chem 276: 14514–14523 [DOI] [PubMed] [Google Scholar]
- Hao Y, Chun A, Cheung K, Rashidi B, Yang X 2008. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem 283: 5496–5509 [DOI] [PubMed] [Google Scholar]
- Hergovich A, Hemmings BA 2009. Mammalian NDR/LATS protein kinases in hippo tumor suppressor signaling. Biofactors 35: 338–345 [DOI] [PubMed] [Google Scholar]
- Hirashima M, Sano K, Morisada T, Murakami K, Rossant J, Suda T 2008. Lymphatic vessel assembly is impaired in Aspp1-deficient mouse embryos. Dev Biol 316: 149–159 [DOI] [PubMed] [Google Scholar]
- Iwabuchi K, Bartel PL, Li B, Marraccino R, Fields S 1994. Two cellular proteins that bind to wild-type but not mutant p53. Proc Natl Acad Sci 91: 6098–6102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa KM, Acoba JD, Chen D, Gay J, Lee H, Beemer K, Padiernos E, Boonmark N, Zhu Z, Fan AC, et al. 2009a. Apoptosis-stimulating protein of p53 (ASPP2) heterozygous mice are tumor-prone and have attenuated cellular damage-response thresholds. Proc Natl Acad Sci 106: 4390–4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampa KM, Bonin M, Lopez CD 2009b. New insights into the expanding complexity of the tumor suppressor ASPP2. Cell Cycle 8: 2871–2876 [DOI] [PubMed] [Google Scholar]
- Kanai F, Marignani PA, Sarbassova D, Yagi R, Hall RA, Donowitz M, Hisaminato A, Fujiwara T, Ito Y, Cantley LC, et al. 2000. TAZ: A novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J 19: 6778–6791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langton PF, Colombani J, Aerne BL, Tapon N 2007. Drosophila ASPP regulates C-terminal Src kinase activity. Dev Cell 13: 773–782 [DOI] [PubMed] [Google Scholar]
- Langton PF, Colombani J, Chan EH, Wepf A, Gstaiger M, Tapon N 2009. The dASPP-dRASSF8 complex regulates cell-cell adhesion during Drosophila retinal morphogenesis. Curr Biol 19: 1969–1978 [DOI] [PubMed] [Google Scholar]
- Mantovani F, Tocco F, Girardini J, Smith P, Gasco M, Lu X, Crook T, Del Sal G 2007. The prolyl isomerase Pin1 orchestrates p53 acetylation and dissociation from the apoptosis inhibitor iASPP. Nat Struct Mol Biol 14: 912–920 [DOI] [PubMed] [Google Scholar]
- Matallanas D, Romano D, Yee K, Meissl K, Kucerova L, Piazzolla D, Baccarini M, Vass JK, Kolch W, O'Neill E 2007. RASSF1A elicits apoptosis through an MST2 pathway directing proapoptotic transcription by the p73 tumor suppressor protein. Mol Cell 27: 962–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T, Mazack V, Sudol M 2008. Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP). J Biol Chem 283: 27534–27546 [DOI] [PubMed] [Google Scholar]
- Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA 2006. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci 103: 12405–12410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman-Gomez J, Jimenez-Velasco A, Agirre X, Prosper F, Heiniger A, Torres A 2005. Lack of CpG island methylator phenotype defines a clinical subtype of T-cell acute lymphoblastic leukemia associated with good prognosis. J Clin Oncol 23: 7043–7049 [DOI] [PubMed] [Google Scholar]
- Samuels-Lev Y, O'Conner DJ, Bergamaschi D, Trigiante G, Hsieh J-K, Zhong S, Campargue I, Naumovski L, Crook T, Lu X 2001. ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell 8: 781–794 [DOI] [PubMed] [Google Scholar]
- Sottocornola R, Royer C, Vives V, Tordella L, Zhong S, Wang Y, Ratnayaka I, Shipman M, Cheung A, Gaston-Massuet C, et al. 2010. ASPP2 binds Par-3 and controls the polarity and proliferation of neural progenitors during CNS development. Dev Cell 19: 126–137 [DOI] [PubMed] [Google Scholar]
- Strano S, Monti O, Pediconi N, Baccarini A, Fontemaggi G, Lapi E, Mantovani F, Damalas A, Citro G, Sacchi A, et al. 2005. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA damage. Mol Cell 18: 447–459 [DOI] [PubMed] [Google Scholar]
- Sullivan A, Lu X 2007. ASPP: A new family of oncogenes and tumour suppressor genes. Br J Cancer 96: 196–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JK, Dalgleish C, Venables JP, Sergeant KA, Ehrmann IE, Lu X, Saunders PT, Elliott DJ 2006. The tumour-suppressor protein ASPP1 is nuclear in human germ cells and can modulate ratios of CD44 exon V5 spliced isoforms in vivo. Oncogene 25: 3104–3112 [DOI] [PubMed] [Google Scholar]
- Trigiante G, Lu X 2006. ASPP and cancer. Nat Rev Cancer 6: 217–226 [DOI] [PubMed] [Google Scholar]
- Vives V, Su J, Zhong S, Ratnayaka I, Slee E, Goldin R, Lu X 2006. ASPP2 is a haploinsufficient tumor suppressor that cooperates with p53 to suppress tumor growth. Genes Dev 20: 1262–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Degerny C, Xu M, Yang XJ 2009. YAP, TAZ, and Yorkie: A conserved family of signal-responsive transcriptional coregulators in animal development and human disease. Biochem Cell Biol 87: 77–91 [DOI] [PubMed] [Google Scholar]
- Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et al. 2006. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell 125: 1253–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, et al. 2007. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev 21: 2747–2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Lei QY, Guan KL 2008a. The Hippo-YAP pathway: New connections between regulation of organ size and cancer. Curr Opin Cell Biol 20: 638–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, et al. 2008b. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 22: 1962–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Lei Q, Guan KL 2010a. The Hippo–YAP pathway in organ size control and tumorigenesis: An updated version. Genes Dev 24: 862–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang CY, Guan KL 2010b. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(β-TRCP). Genes Dev 24: 72–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Wu G, Bu F, Lu B, Liang A, Cao L, Tong X, Lu X, Wu M, Guo Y 2010. Epigenetic silence of ankyrin-repeat-containing, SH3-domain-containing, and proline-rich-region-containing protein 1 (ASPP1) and ASPP2 genes promotes tumor growth in hepatitis B virus-positive hepatocellular carcinoma. Hepatology 51: 142–153 [DOI] [PubMed] [Google Scholar]