Abstract
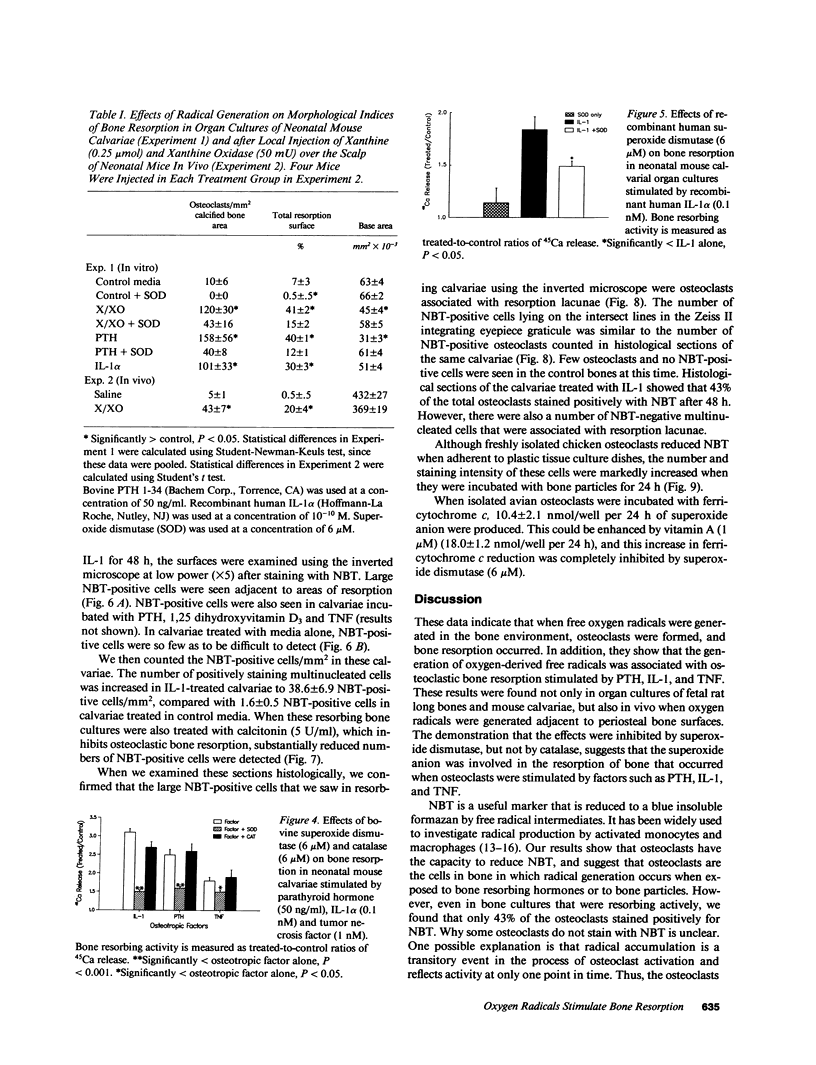
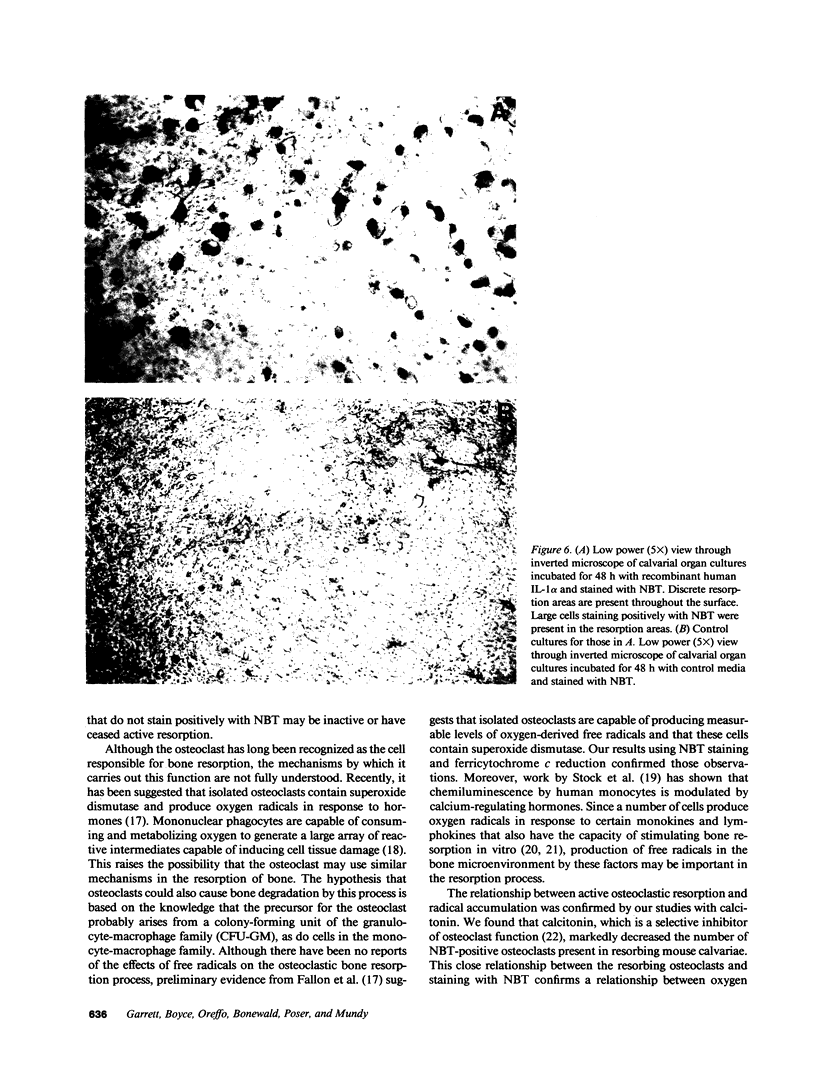
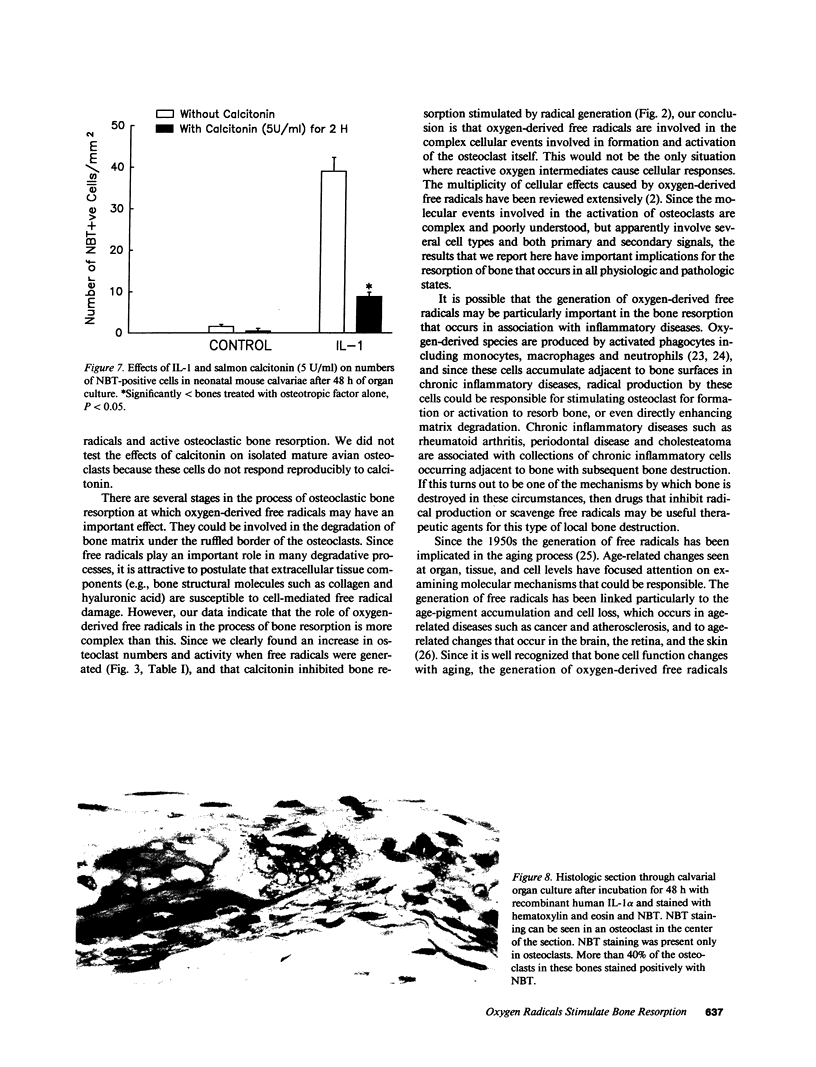
The mechanisms by which bone resorbing osteoclasts form and are activated by hormones are poorly understood. We show here that the generation of oxygen-derived free radicals in cultured bone is associated with the formation of new osteoclasts and enhanced bone resorption, identical to the effects seen when bones are treated with hormones such as parathyroid hormone (PTH) and interleukin 1 (IL-1). When free oxygen radicals were generated adjacent to bone surfaces in vivo, osteoclasts were also formed. PTH and IL-1-stimulated bone resorption was inhibited by both natural and recombinant superoxide dismutase, an enzyme that depletes tissues of superoxide anions. We used the marker nitroblue tetrazolium (NBT) to identify the cells that were responsible for free radical production in resorbing bones. NBT staining was detected only in osteoclasts in cultures of resorbing bones. NBT staining in osteoclasts was decreased in bones coincubated with calcitonin, an inhibitor of bone resorption. We also found that isolated avian osteoclasts stained positively for NBT. NBT staining in isolated osteoclasts was increased when the cells were incubated with bone particles, to which they attach. We confirmed the formation of superoxide anion in isolated avian osteoclasts using ferricytochrome c reduction as a method of detection. The reduction of ferricytochrome c in isolated osteoclasts was inhibited by superoxide dismutase. Our results suggest that oxygen-derived free radicals, and particularly the superoxide anion, are intermediaries in the formation and activation of osteoclasts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst of phagocytes. J Clin Invest. 1984 Mar;73(3):599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement L. T., Lehmeyer J. E. Regulation of the growth and differentiation of a human monocytic cell line by lymphokines. I. Induction of superoxide anion production and chemiluminescence. J Immunol. 1983 Jun;130(6):2763–2766. [PubMed] [Google Scholar]
- Freund M., Pick E. The mechanism of action of lymphokines. IX. The enzymatic basis of hydrogen peroxide production by lymphokine-activated macrophages. J Immunol. 1986 Aug 15;137(4):1312–1318. [PubMed] [Google Scholar]
- Fridovich I. Quantitative aspects of the production of superoxide anion radical by milk xanthine oxidase. J Biol Chem. 1970 Aug 25;245(16):4053–4057. [PubMed] [Google Scholar]
- Friedman J., Au W. Y., Raisz L. G. Responses of fetal rat bone to thyrocalcitonin in tissue culture. Endocrinology. 1968 Jan;82(1):149–156. doi: 10.1210/endo-82-1-149. [DOI] [PubMed] [Google Scholar]
- Gowen M., Mundy G. R. Actions of recombinant interleukin 1, interleukin 2, and interferon-gamma on bone resorption in vitro. J Immunol. 1986 Apr 1;136(7):2478–2482. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968 Nov 10;243(21):5753–5760. [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Macrophage oxygen-dependent antimicrobial activity. III. Enhanced oxidative metabolism as an expression of macrophage activation. J Exp Med. 1980 Dec 1;152(6):1596–1609. doi: 10.1084/jem.152.6.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray H. W., Cohn Z. A. Mononuclear phagocyte antimicrobial and antitumor activity: the role of oxygen intermediates. J Invest Dermatol. 1980 May;74(5):285–288. doi: 10.1111/1523-1747.ep12543457. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAISZ L. G. BONE RESORPTION IN TISSUE CULTURE. FACTORS INFLUENCING THE RESPONSE TO PARATHYROID HORMONE. J Clin Invest. 1965 Jan;44:103–116. doi: 10.1172/JCI105117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Steele J., Umar S., Dockrell H. M. A simple method for the solubilisation of reduced NBT, and its use as a colorimetric assay for activation of human macrophages by gamma-interferon. J Immunol Methods. 1985 Sep 3;82(1):161–167. doi: 10.1016/0022-1759(85)90235-2. [DOI] [PubMed] [Google Scholar]
- Sabatini M., Boyce B., Aufdemorte T., Bonewald L., Mundy G. R. Infusions of recombinant human interleukins 1 alpha and 1 beta cause hypercalcemia in normal mice. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5235–5239. doi: 10.1073/pnas.85.14.5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. L., Coderre J. A., Levine P. H. Effects of calcium-regulating hormones and drugs on human monocyte chemiluminescence. J Clin Endocrinol Metab. 1982 Nov;55(5):956–960. doi: 10.1210/jcem-55-5-956. [DOI] [PubMed] [Google Scholar]
- Takahashi N., MacDonald B. R., Hon J., Winkler M. E., Derynck R., Mundy G. R., Roodman G. D. Recombinant human transforming growth factor-alpha stimulates the formation of osteoclast-like cells in long-term human marrow cultures. J Clin Invest. 1986 Oct;78(4):894–898. doi: 10.1172/JCI112677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teti A., Oreffo R. O., Zambonin Zallone A., Triffitt J. T., Francis M. J. Acid phosphatase activity is stimulated in isolated osteoclasts by vitamin A. Boll Soc Ital Biol Sper. 1986 Oct 30;62(10):1311–1314. [PubMed] [Google Scholar]
- Thomson B. M., Mundy G. R., Chambers T. J. Tumor necrosis factors alpha and beta induce osteoblastic cells to stimulate osteoclastic bone resorption. J Immunol. 1987 Feb 1;138(3):775–779. [PubMed] [Google Scholar]
- Weiss S. J., LoBuglio A. F. Phagocyte-generated oxygen metabolites and cellular injury. Lab Invest. 1982 Jul;47(1):5–18. [PubMed] [Google Scholar]
- Yates A. J., Gutierrez G. E., Smolens P., Travis P. S., Katz M. S., Aufdemorte T. B., Boyce B. F., Hymer T. K., Poser J. W., Mundy G. R. Effects of a synthetic peptide of a parathyroid hormone-related protein on calcium homeostasis, renal tubular calcium reabsorption, and bone metabolism in vivo and in vitro in rodents. J Clin Invest. 1988 Mar;81(3):932–938. doi: 10.1172/JCI113406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambonin Zallone A., Teti A., Primavera M. V. Isolated osteoclasts in primary culture: first observations on structure and survival in culture media. Anat Embryol (Berl) 1982 Dec;165(3):405–413. doi: 10.1007/BF00305576. [DOI] [PubMed] [Google Scholar]
- Zambonin Zallone A., Teti A. The osteoclasts of hen medullary bone under hypocalcaemic conditions. Anat Embryol (Berl) 1981;162(4):379–392. doi: 10.1007/BF00301864. [DOI] [PubMed] [Google Scholar]