Abstract
The present work was aimed at formulation development, evaluation and comparative study of the effects of superdisintegrants in Cefixime 50 mg oral disintegrating tablets. The superdisintegrants used for the present study were sodium starch glycolate and crosscarmellose sodium. The formulated tablets were evaluated for various tableting properties, like hardness, thickness, friability, weight variation, disintegration time and dissolution rate. Comparative evaluation of the above-mentioned parameters established the superiority of the tablets formulated with crosscarmellose sodium to those formulated with sodium starch glycolate.
Keywords: Cefixime, superdisintegrants, oral disintegrating tablets
INTRODUCTION
Disintegrants are agents added to tablet formulations to promote the break-up of the tablet into smaller fragments in an aqueous environment, thereby increasing the available surface area and promoting a more rapid release of the drug substance. In more recent years, several newer disintegrants have been developed, often called “super disintegrants.” These newer substances can be used at lower levels than conventionally used disintegrants. Three major mechanisms and factors affecting tablet disintegration are suggested as swelling, porosity and capillary action and deformation. Three major group of compounds that have been developed as superdisintegrants are modified starches, cross-linked polyvinylpyrrolidone and modified cellulose.
One of the major streams of application of superdisintegrants is in the formulation of oral disintegrating tablets/mouth dissolving tablets. An oral disintegrating tablet is a solid dosage form that disintegrates and dissolves in the mouth, either on or beneath the tongue or in the buccal cavity without water, within 60 s or lower. The US Food and Drug Administration (FDA) Center for Drug Evaluation and Research (CDER) defines, in the orange book, an oral disintegrating tablet as, “A solid dosage form containing medicinal substances, which disintegrates rapidly, usually within a matter of seconds, when placed upon the tongue.” At present, oral disintegrating tablets are the only quick-dissolving dosage form recognized by FDA and listed in the approved drug products with therapeutic equivalence evaluations.[1,2]
The drug selected for the study was cefexime trihydrate, which is used in the treatment of uncomplicated urinary tract infections.[3,4] The aim of the study was to formulate an oral disintegrating tablet of cefixime trihydrate using two superdisintegrants separately (crosscarmellose sodium and sodium starch glycolate), and to select the best among the two based on the disintegration time and other tableting properties.
MATERIALS AND METHODS
Materials
Cefixime trihydrate was procured from Aurobindo Pharma Ltd. Sodium starch glycolate and croscarmellose sodium were procured from DK Enterprises, while magnesium stearate and talc was from Nice Chemicals.
Methodology
Preformulation studies[5–7]
Preformulation study is defined as an investigation of the physical and chemical properties of drug substance alone and when combined with the excipients. The overall objective of preformulation testing is to generate information useful to the formulator in developing a stable and bioavailable dosage form that can be mass produced. The commonly investigated preformulation parameters include angle of repose, bulk density/tapped density, pour density, Carr’s compressibility index and Hausner ratio.
Angle of repose
It is determined by allowing a powder to flow through a funnel and fall freely on to a surface. Further addition of powder is stopped as soon as the pile touches the tip of the funnel. A circle is drawn around the pile without disturbing it. The height and diameter of the resulting cone are measured. The same procedure is repeated three times and the average value is taken. Angle of repose is calculated by using the following equation:
Where, h = height of the powder cone; r = radius of the powder
Bulk density
Unless otherwise specified, pass a quantity of material sufficient to complete the test through a 1.00-mm (no. 18) screen to break up agglomerates that may have formed during storage. Into a dry 250-ml cylinder introduce, without compacting, approximately 100 g of the test sample (M) weighed with 0.1% accuracy. If it is not possible to use 100 g, the amount of the test sample and the volume of the cylinder may be modified. Select a sample mass having an untapped apparent volume of 150–250 ml. A 100-ml cylinder is used for apparent volumes between 50 and 100 ml. Fill the cylinder carefully. Carefully level the powder without compacting, if necessary, and read the unsettled apparent volume (Vo). Calculate the bulk density, in g/ml, using the formula,
Tapped density
Accurately weighed quantity of powder is introduced into a measuring cylinder. Mechanically tap the cylinder containing the sample by raising the cylinder and allowing it to drop under its own weight using a suitable mechanical tapped density tester at a nominal rate of 300 drops/min. Tap the cylinder 500 times and measure the tapped volume (Va). Repeat the operation for an additional 750 tappings and again measure the tapped volume as (Vb).
If the difference between Va and Vb is <2%, Vb is the final tapped volume (Vf). If the difference is higher, repeat the tapings for an additional 1,250 times, and then the tapped density can be calculated using the following formula (United States pharmacopoeia, 2004)
Where, M = weight of the sample taken; Vf = final tapped volume
Carr’s index
The compressibility index of granules can be determined using Carr’s compressibility index, and can be determined by the following formula:
Hausner ratio
The Hausner ratio can be determined using the following formula:
Compatibility studies
IR studies of drug and drug with superdisintegrants were carried out in order to check the compatibility between the drug and the excipients.
Formulation development
The methodology selected for the preparation of cefixime oral disintegrating tablets is direct compression. About 10 formulations were prepared, of which five formulations included varying concentrations of the superdisintegrant sodium starch glycolate and five of crosscarmellose sodium. The list of ingredients is given in Tables 1 and 2.
Table 1.
Formulation of cefixime with sodium starch glycolate
| Ingredients | S 1(mg) | S2(mg) | S3(mg) | S4(mg) | S5(mg) |
|---|---|---|---|---|---|
| Cefixime trihydrate | 55.90 | 55.90 | 55.90 | 55.90 | 55.90 |
| Microcrystalline cellulose | 214.30 | 211.50 | - | - | 217.10 |
| Pregelatinised starch | - | - | 214.30 | 211.50 | - |
| Sodium starch glycolate | 5.60 | 8.40 | 5.60 | 8.40 | 2.80 |
| Magnesium stearate | 1.40 | 1.40 | 1.40 | 1.40 | 1.40 |
| Talc | 2.80 | 2.80 | 2.80 | 2.80 | 2.80 |
| Average weight (mg) | 280.00 | 280.00 | 280.00 | 280.00 | 280.00 |
Table 2.
Formulation of cefixime with cross carmellose sodium
| Ingredients | C1(mg) | C2(mg) | C3(mg) | C4(mg) | C5(mg) |
|---|---|---|---|---|---|
| Cefixime trihydrate | 55.90 | 55.90 | 55.90 | 55.90 | 55.90 |
| Microcrystalline cellulose | 214.30 | 211.50 | - | - | 217.10 |
| Pregelatinised starch | - | - | 214.30 | 211.50 | - |
| Sodium starch glycolate | 5.60 | 8.40 | 5.60 | 8.40 | 2.80 |
| Magnesium stearate | 1.40 | 1.40 | 1.40 | 1.40 | 1.40 |
| Talc | 2.80 | 2.80 | 2.80 | 2.80 | 2.80 |
| Average weight (mg) | 280.00 | 280.00 | 280.00 | 280.00 | 280.00 |
Tablet evaluation[8–10]
The selected batches made in bulk were subjected to evaluations as per Indian pharmacopoeia.
Weight variation
Twenty tablets were selected at random, weighed and the average weight was calculate. Not more than two of the individual weights should deviate from the average weight by more than 5%.
Friability
For each formulations, preweighed tablet samples (20 tablets) were placed on the friabilator, which is then operated for 100 revolutions. The tablets were then dusted and reweighed. Conventional compressed tablets that loose <0.5–1.0% of their weight are considered acceptable.
Hardness
Tablet hardness of each formulation was determined using a Monsanto hardness tester. Results were calculated from the average results of six tablets.
Thickness
Tablet thickness is determined using vernier calipers. Six tablets were evaluated to determine the average thickness.
Disintegration test
Introduce one tablet into each tube and add a disc to each tube. Suspend the assembly in the beaker containing the specified liquid and operate the apparatus for a specified period of time. The tablet passes the test if all tablets have disintegrated. If one or two tablets fail to disintegrate, repeat the test on 12 additional tablets, such that not <16 of the total of 18 tablets tested disintegrate. If the tablets adhere to the disc, repeat the test by omitting the disc. The preparation complies with the test if all the tablets in the repeat test disintegrate.
Dissolution studies/in vitro release studies
Medium
0.05 M potassium phosphate buffer, pH 7.2, prepared by dissolving 6.8 g of monobasic potassium phosphate in 1,000 ml of water and adjusting with 1N sodium hydroxide to a pH of 7.2, 900 ml.
Apparatus
Dissolution apparatus with 100 rpm.
Time
Forty-five minutes.
Procedure
The amount of cefixime released was determined by measuring the absorbance of the sample withdrawn at 280 nm in comparison with a standard solution having a known concentration of USP cefixime reference standard (RS) in the same medium.
RESULTS AND DISCUSSION
Preformulation studies
Values of the preformulation studies are given in Table 3, from which it is evident that all the parameters analyzed showed satisfactory flow properties and compression characteristics.
Table 3.
Physicochemical evaluation of the formulations
| Parameters | Formulation with cross carmellose mean value of (C1 – C5) | Formulation with sodium starch glycollate (S1 – S5) |
|---|---|---|
| Angle of repose | 24 ° 37’ 2.1” | 27° 25’ 13.02” |
| Bulk density / tapped density | 0.6517 | 0.7117 |
| Pour density | 0.5140 | 0.5761 |
| Carr’s compressibility index | 21.12 | 19.05 |
| Hausner ratio | 1.26 | 1.23 |
Compatibility studies
IR spectra of cefixime, sodium starch glycolate, crosscarmellose sodium and combinations are given in Figures 1–5. The results of the FTIR spectral analysis showed that the peaks and the pattern of the spectra were similar in all cases, which indicated that there was no chemical interaction or decomposition of cefixime during the preparation of the tablets.
Figure 1.
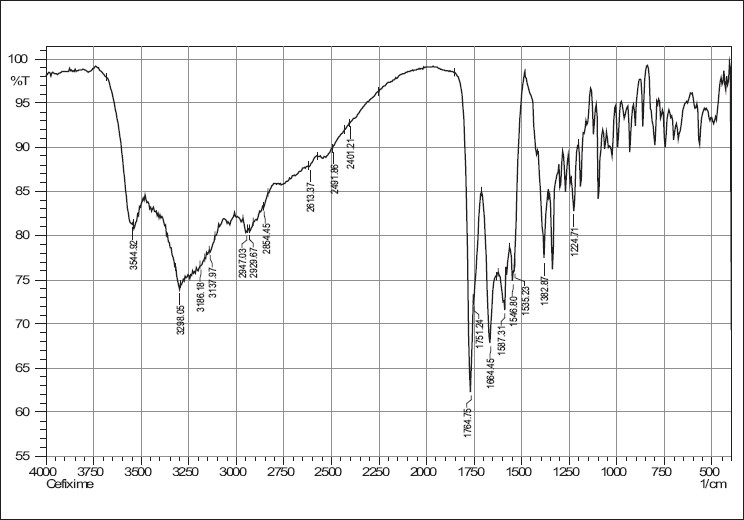
IR spectrum of cefixime
Figure 5.
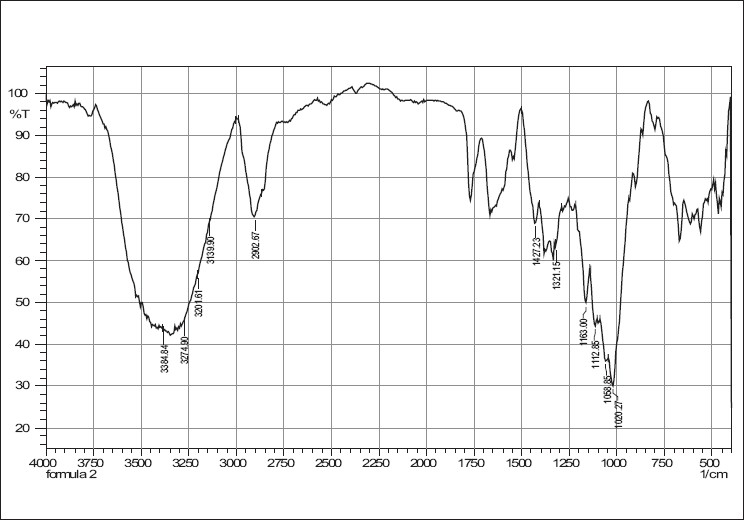
IR spectrum of cefixime tablet blend with sodium starch glycolate
Figure 2.
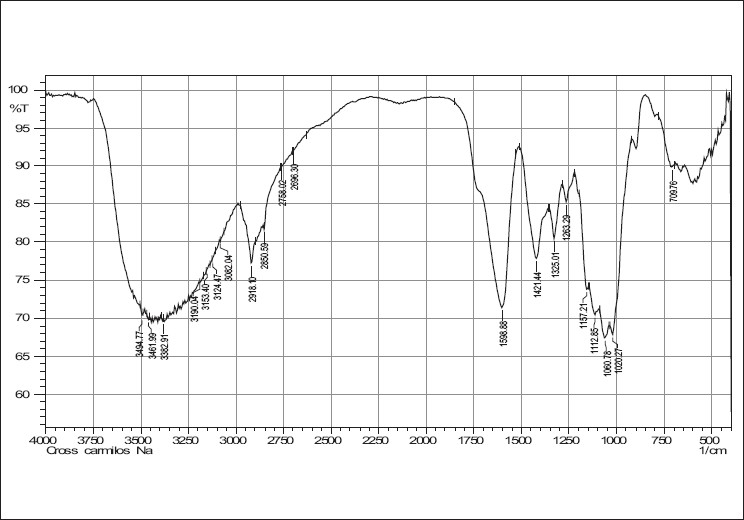
IR spectrum of crosscarmellose sodium
Figure 3.
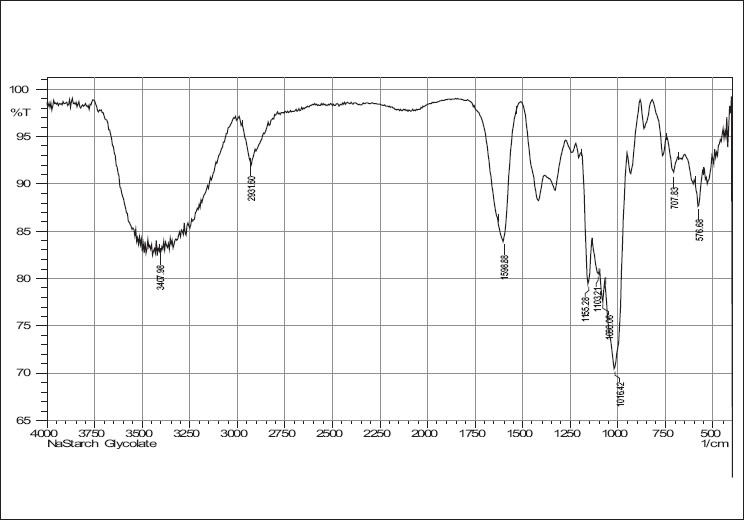
IR spectrum of sodium starch glycollate
Figure 4.
IR spectrum of cefixime tablet blend with crosscarmellose sodium
Formulation
Because the aim of the project was to formulate cefixime 50 mg DT tablet, which disintegrated within 1 min, priority was given so as to get good results with cost-effectiveness of the product.
From the tables and histograms, it was evident that the formulations C1 and S3 stood ahead in all the tableting properties and in disintegration performance. Both the batches were prepared in bulk [Table 4] and confirmatory evaluation tests were carried out.
Table 4.
Tablet ingredients for scale up batch
| Ingredients | Quantity/ tablet (mg) | Quantity for 50 tablets (g) (S3) | Quantity for 50 tablets (g) (C 1) |
|---|---|---|---|
| Cefixime trihydrate | 55.90 | 2.795 | 2.795 |
| Microcrystalline cellulose | 214.30 | 10.715 | 10.715 |
| Sodium starch glycolate | 5.60 | 0.280 | ---- |
| Cross carmellose sodium | 5.60 | ---- | 0.280 |
| Magnesium stearate | 1.40 | 0.070 | 0.070 |
| Talc | 2.80 | 0.140 | 0.140 |
| Pineapple flavor | q.s | q.s | q.s |
| Tarazarine lake | q.s | q.s | q.s |
| Average weight (mg) | 280.00 | ---- | ---- |
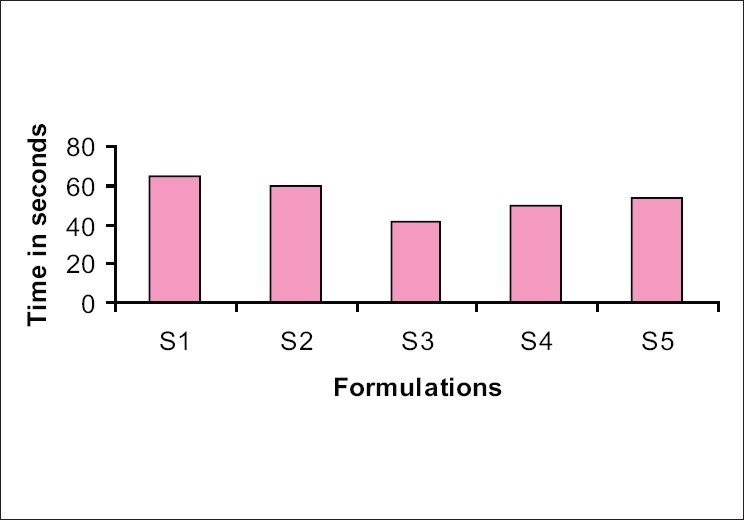
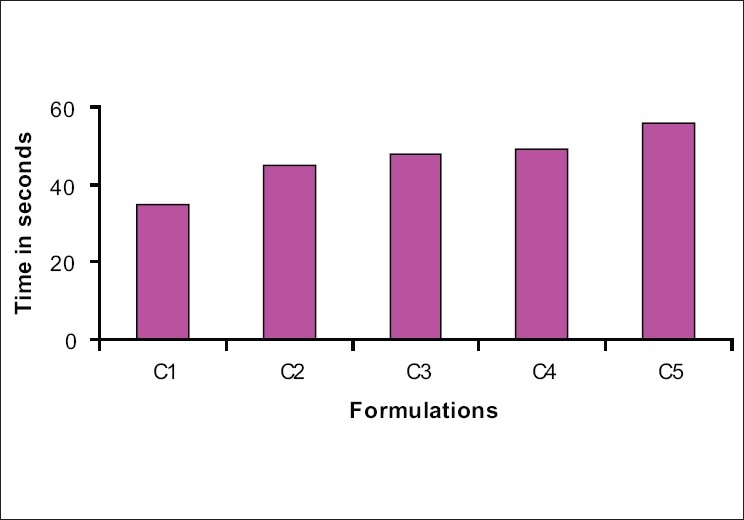
DT stands satisfactory with the time limit of <1 min for all the batches, as shown in Figures 6 and 7. Now, the question exists for fixing the best batch among the rest. Hence, for the comparative study, one with the same percentage was selected (2%). The batches S3 and C1 were selected to carry out further evaluation [Table 5].
Figure 6.
Disintegration profile of the selected batch
Figure 7.
Disintegration profile of the selected batch
Table 5.
Evaluation parameters of the best formulations
| Tablet evaluation parameters | Formula S3 | Formula C1 |
|---|---|---|
| Diameter | 0.96 | 0.95 |
| Thickness | 0.36 | 0.38 |
| Hardness | 3.0 | 3.5 |
| Friability | 0.751 | 0.725 |
| Weight variation | pass | pass |
| Disintegration time (sec) | 42 | 32 |
| Dissolution rate (%) | 94.56 | 96.37 |
Evaluation of tableting properties of the selected batches confirm the standards prescribed in Indian pharmacopoeia. The results are given in Tables 6 and 7.
Table 6.
Evaluation of tabletting parameters
| Tablet parameters | Formula S1 | Formula S2 | Formula S3 | Formula S4 | Formula S5 |
|---|---|---|---|---|---|
| Thickness (cm) | 0.37 | 0.37 | 0.36 | 0.38 | 0.36 |
| Hardness (kg/cm2) | 2.6 | 2.8 | 3.0 | 2.6 | 2.6 |
| Friability (%) | 0.750 | 0.751 | 0.526 | 0.640 | 0.575 |
Table 7.
Evaluation of tabletting parameters
| Tablet parameters | Formula C1 | Formula C2 | Formula C3 | Formula C4 | Formula C5 |
|---|---|---|---|---|---|
| Thickness(cm) | 0.38 | 0.37 | 0.37 | 0.36 | 0.37 |
| Hardness (kg/cm2) | 2.8 | 2.8 | 2.6 | 2.4 | 2.6 |
| Friability (%) | 0.538 | 0.751 | 0.752 | 0.638 | 0.575 |
Disintegration time was found to be within 1 min and the percentage drug release, which confirms the in vitro bioavailability, was found to be 94.56 and 96.37, respectively, for S3 and C1, which proves the credibility of the selected products. The results are given in Table 8.
Table 8.
Disintegration profile of the formulations
| Formula | S1 | S2 | S3 | S4 | S5 | C1 | C2 | C3 | C4 | C5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Disintegration time in seconds | 65 | 60 | 42 | 50 | 54 | 35 | 45 | 48 | 49 | 53 |
With reference to Table 5, a comparison of the different parameters like hardness, friability, disintegration and dissolution was carried out and superiority of the tablets formulated with crosscarmellose was established.
CONCLUSION
The aim of the present project is formulation development, evaluation and comparative study of superdisintergrants in the Cefixime 50 mg oral disintegrating tablet.
With the proof of different evaluation parameters listed in Table 8, it was concluded that C3 (CCS) was the best formulation.
Comparative evaluation studies proved that crosscarmelose is superior to sodium starch glycolate.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Carter JC. The role of disintegrants in solid oral dosage manufacturing. Carter Pharmaceutical Consulting Inc. 2002:6. [Google Scholar]
- 2.Raymond C R, Paul JS, Sian CO. Hand book of Pharmaceutical Excipients. 5th ed. London: Pharmaceutical Press and American Pharmacist association; 2006. [Google Scholar]
- 3.Department of heath, social services and public safety, British Pharmacopoeia. London: The stationary office on behalf of MHRA; 2005. [Google Scholar]
- 4.McMillan A, Young H. The treatment of Pharyngeal Gonirrhoea with a single oral dose of Cefixime. Int J STD AIDS. 2007;18:253–4. doi: 10.1258/095646207780658971. [DOI] [PubMed] [Google Scholar]
- 5.Leon L, liberman, Kanig . The Theory and practice of Industrial pharmacy. 2nd ed. Bombay: Varghese publishing house; 1991. [Google Scholar]
- 6.Aulton ME, editor. Pharmaceutics, The science of dosage form design. 2nd ed. Sydney: Churchill livingstone; 2002. [Google Scholar]
- 7.Loyd VA, Nicholas GP, Howard CA. Ansel’s pharmaceutical sosage forms and drug delivery systems. 8th ed. London: Lippincott Williams and Willkins; 2005. [Google Scholar]
- 8.Indian Pharmacopoeia. Delhi: Controller of Publications; 1996. Ministry of health and family welfare; Government of India. [Google Scholar]
- 9.Shirsand SB, Suresh S, Para MS, Swamy PV, Kumar DN. Plantago ovata mucilage in the design of Fast disintegrating tablets. Indian J Pharm Sci. 2009;71:41–5. doi: 10.4103/0250-474X.51952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mundada AS, Meshram DR, Mishra M, Bhalekar MR, Avari JG. Formulation and evaluation of bitterless rapidly disintegrating tablet of Famotidine using ion exchange resins. Int J Pharm Excipients. 2008;7:23–5. [Google Scholar]


