Abstract
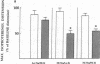
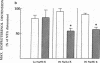
Hypertensive patients have reduced lymphocyte beta-adrenergic responsiveness which is corrected by a low sodium (Na) diet. To determine if this represents a more generalized abnormality in beta adrenoceptor response, we studied beta adrenergic-mediated vasodilation in hand veins of borderline hypertensive subjects and controls. Subjects received a 5-d diet containing high Na/low potassium (K), high Na/high K, or low Na/high K. Venous distension, as evaluated by a linear variable differential transformer, was measured in relation to infusion of phenylephrine followed by isoproterenol and nitroglycerin. On both the high Na/high K and high Na/low K diets, hypertensive subjects had significantly decreased isoproterenol-mediated vasodilation (47% decrease, P less than 0.01 and 36% decrease, P less than 0.01, respectively). On the low Na/high K diet, isoproterenol-mediated vasodilation in hypertensive subjects increased 41% (P less than 0.01) to a level not different from controls. Nitroglycerin-mediated vasodilation was not different in normotensive and hypertensive subjects, nor was it altered with Na intake. Phenylephrine-mediated vasoconstriction did not differ between normotensive and hypertensive groups. Venous beta-adrenergic response correlated with lymphocyte beta adrenoceptor density in normotensive (r = 0.53, P less than 0.005) but not hypertensive subjects. This study demonstrates that beta-adrenergic responsiveness is selectively reduced in peripheral veins of borderline hypertensive subjects, and this is corrected by a low Na diet. In view of our previous findings of reduced lymphocyte beta-adrenergic responsiveness in borderline hypertension, these studies suggest a generalized defect of beta adrenoceptor responsiveness in human hypertension. Further, dietary Na may play an important role in regulating this abnormality.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aellig W. H. A new technique for recording compliance of human hand veins. Br J Clin Pharmacol. 1981 Mar;11(3):237–243. doi: 10.1111/j.1365-2125.1981.tb00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aellig W. H. Investigation of the venoconstrictor effect of 8' hydroxydihydroergotamine, the main metabolite of dihydroergotamine, in man. Eur J Clin Pharmacol. 1984;26(2):239–242. doi: 10.1007/BF00630292. [DOI] [PubMed] [Google Scholar]
- Alradi A. O., Carruthers S. G. Evaluation and application of the linear variable differential transformer technique for the assessment of human dorsal hand vein alpha-receptor activity. Clin Pharmacol Ther. 1985 Nov;38(5):495–502. doi: 10.1038/clpt.1985.214. [DOI] [PubMed] [Google Scholar]
- Ambrosioni E., Costa F. V., Borghi C., Boschi S., Mussi A. Cellular and humoral factors in borderline hypertension. J Cardiovasc Pharmacol. 1986;8 (Suppl 5):S15–S22. doi: 10.1097/00005344-198608005-00004. [DOI] [PubMed] [Google Scholar]
- Ambrosioni E., Costa F. V., Borghi C., Montebugnoli L., Giordani M. F., Magnani B. Effects of moderate salt restriction on intralymphocytic sodium and pressor response to stress in borderline hypertension. Hypertension. 1982 Nov-Dec;4(6):789–794. doi: 10.1161/01.hyp.4.6.789. [DOI] [PubMed] [Google Scholar]
- Borkowski K. R. Pre- and postjunctional beta-adrenoreceptors and hypertension. J Auton Pharmacol. 1988 Jun;8(2):153–171. doi: 10.1111/j.1474-8673.1988.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- De Lean A., Hancock A. A., Lefkowitz R. J. Validation and statistical analysis of a computer modeling method for quantitative analysis of radioligand binding data for mixtures of pharmacological receptor subtypes. Mol Pharmacol. 1982 Jan;21(1):5–16. [PubMed] [Google Scholar]
- Egan B., Panis R., Hinderliter A., Schork N., Julius S. Mechanism of increased alpha adrenergic vasoconstriction in human essential hypertension. J Clin Invest. 1987 Sep;80(3):812–817. doi: 10.1172/JCI113138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler H. G., Ford G. A., Blaschke T. F., Swislocki A., Hoffman B. B. Responsiveness of superficial hand veins to phenylephrine in essential hypertension. Alpha adrenergic blockade during prazosin therapy. J Clin Invest. 1989 Jan;83(1):108–112. doi: 10.1172/JCI113845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler H. G., Hiremath A., Katzir D., Blaschke T. F., Hoffman B. B. Absence of age-related changes in venous responsiveness to nitroglycerin in vivo in humans. Clin Pharmacol Ther. 1987 Nov;42(5):521–524. doi: 10.1038/clpt.1987.191. [DOI] [PubMed] [Google Scholar]
- Feldman R. D. Beta-adrenergic desensitization reduces the sensitivity of adenylate cyclase for magnesium in permeabilized lymphocytes. Mol Pharmacol. 1989 Mar;35(3):304–310. [PubMed] [Google Scholar]
- Feldman R. D. Beta-adrenergic receptor alterations in hypertension--physiological and molecular correlates. Can J Physiol Pharmacol. 1987 Aug;65(8):1666–1672. doi: 10.1139/y87-261. [DOI] [PubMed] [Google Scholar]
- Feldman R. D., Lawton W. J., McArdle W. L. Low sodium diet corrects the defect in lymphocyte beta-adrenergic responsiveness in hypertensive subjects. J Clin Invest. 1987 Jan;79(1):290–294. doi: 10.1172/JCI112797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. D., Limbird L. E., Nadeau J., Robertson D., Wood A. J. Leukocyte beta-receptor alterations in hypertensive subjects. J Clin Invest. 1984 Mar;73(3):648–653. doi: 10.1172/JCI111255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber E., Koerner T., Page L. B., Kliman B., Purnode A. Application of a radioimmunoassay for angiotensin I to the physiologic measurements of plasma renin activity in normal human subjects. J Clin Endocrinol Metab. 1969 Oct;29(10):1349–1355. doi: 10.1210/jcem-29-10-1349. [DOI] [PubMed] [Google Scholar]
- Hancock A. A., Bush E. N., Stanisic D., Kyncl J. J., Lin C. T. Data normalization before statistical analysis: keeping the horse before the cart. Trends Pharmacol Sci. 1988 Jan;9(1):29–32. doi: 10.1016/0165-6147(88)90239-8. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Minuth M., Aktories K. Sodium regulation of hormone-sensitive adenylate cyclase. J Recept Res. 1984;4(1-6):443–458. doi: 10.3109/10799898409042566. [DOI] [PubMed] [Google Scholar]
- Jennings G., Bobik A., Esler M., Korner P. Contribution of cardiovascular reflexes to differences in beta-adrenoceptor-mediated responses in essential hypertension. Clin Sci (Lond) 1981 Dec;61 (Suppl 7):177s–180s. doi: 10.1042/cs061177s. [DOI] [PubMed] [Google Scholar]
- Jie K., van Brummelen P., Vermey P., Timmermans P. B., van Zwieten P. A. Alpha 1- and alpha 2-adrenoceptor mediated vasoconstriction in the forearm: differences between normotensive and hypertensive subjects. J Hypertens Suppl. 1985 Dec;3(3):S89–S91. [PubMed] [Google Scholar]
- Korner P. I. The pathogenesis of hypertension: the Baker concerto. Clin Exp Hypertens A. 1984;6(1-2):565–586. doi: 10.3109/10641968409062584. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Alexieva S., Carruthers S. G. The influence of age on dorsal hand vein responsiveness to norepinephrine. Clin Pharmacol Ther. 1986 Sep;40(3):257–260. doi: 10.1038/clpt.1986.172. [DOI] [PubMed] [Google Scholar]
- Michel M. C., Beckeringh J. J., Ikezono K., Kretsch R., Brodde O. E. Lymphocyte beta 2-adrenoceptors mirror precisely beta 2-adrenoceptor, but poorly beta 1-adrenoceptor changes in the human heart. J Hypertens Suppl. 1986 Dec;4(6):S215–S218. [PubMed] [Google Scholar]
- Michel M. C., Pingsmann A., Beckeringh J. J., Zerkowski H. R., Doetsch N., Brodde O. E. Selective regulation of beta 1- and beta 2-adrenoceptors in the human heart by chronic beta-adrenoceptor antagonist treatment. Br J Pharmacol. 1988 Jul;94(3):685–692. doi: 10.1111/j.1476-5381.1988.tb11576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minuth M., Jakobs K. H. Sodium regulation of agonist and antagonist binding to beta-adrenoceptors in intact and Ns-deficient membranes. Naunyn Schmiedebergs Arch Pharmacol. 1986 Jun;333(2):124–129. doi: 10.1007/BF00506514. [DOI] [PubMed] [Google Scholar]
- Pan H. Y., Hoffman B. B., Pershe R. A., Blaschke T. F. Decline in beta adrenergic receptor-mediated vascular relaxation with aging in man. J Pharmacol Exp Ther. 1986 Dec;239(3):802–807. [PubMed] [Google Scholar]
- Safar M. E., London G. M. Arterial and venous compliance in sustained essential hypertension. Hypertension. 1987 Aug;10(2):133–139. doi: 10.1161/01.hyp.10.2.133. [DOI] [PubMed] [Google Scholar]
- Takeshita A., Mark A. L. Decreased venous distensibility in borderline hypertension. Hypertension. 1979 May-Jun;1(3):202–206. doi: 10.1161/01.hyp.1.3.202. [DOI] [PubMed] [Google Scholar]
- Thede-Reynolds K. R., Motulsky H. J., Feldman R. D. Temperature-dependent binding of hydrophilic beta-adrenergic receptor ligands to intact human lymphocytes. Life Sci. 1986 Oct 13;39(15):1325–1334. doi: 10.1016/0024-3205(86)90330-9. [DOI] [PubMed] [Google Scholar]