Abstract
We have examined the effects of acute administration of the cannabinoid receptor type 1 (CB1) antagonist AM251 on the rat hypothalamic-pituitary-adrenal (HPA) axis with respect to both gender and time of day. Blood samples were collected from conscious male and female rats every 5 min using an automated blood sampling system, and corticosterone concentrations were determined. In male rats, there was a distinct diurnal effect of AM251 with a greater activation of the HPA axis in the morning (diurnal trough) compared with the evening (diurnal peak). At both times of the day, circulating corticosterone concentrations were elevated for approximately 4 h after AM251 administration. In female rats, there was also diurnal variation in the activation of the HPA axis; however, these effects were not as profound as those in males. Corticosterone concentrations were only slightly elevated at the diurnal trough and for a shorter time period than in males (2 compared with 4 h). Moreover, there was no effect of AM251 on corticosterone concentrations when administered at the diurnal peak. Subsequent studies, only in males, in which both ACTH and corticosterone were measured, confirmed that the effects of AM251 on corticosterone were mediated by ACTH. Moreover, the elevation of both ACTH and corticosterone could be replicated using another CB1 antagonist, AM281. These data demonstrate that the extent and duration of HPA axis activation after CB1 blockade are clearly dependent on both gender and time of day.
Summary
The extent and duration of HPA axis activation following CB1 receptor blockade are clearly dependent on both sex of rat and time of day.
The hypothalamic-pituitary-adrenal (HPA) axis is the principal neuroendocrine system involved in the maintenance of homeostasis after stressful stimuli. HPA axis activity is regulated via the negative feedback actions of the glucocorticoid hormones (1). The HPA axis displays a characteristic circadian pattern of glucocorticoid hormone release (2) with the peak of HPA axis drive coinciding with the onset of activity. Pertubation of this diurnal variation results in the dysregulation of many physiological processes, which can lead to metabolic and immune disorders (3,4). In addition to the direct actions of the glucocorticoid hormones, its diurnal rhythm synchronizes peripheral clocks (4,5) and reinforces regulation of the suprachiasmatic nucleus (6,7). Despite this vital role, however, the underlying pathways and mechanisms controlling the rhythmic activity of the HPA axis are poorly understood.
Recent studies have suggested that the endocannabinoid system is directly involved in the modulation of the HPA axis. Di et al. (8) have shown that glucocorticoid feedback suppression of corticotropin-releasing factor (CRF) release from the paraventricular nucleus of the hypothalamus (PVN) can be blocked by cannabinoid receptor type 1 (CB1) antagonists, an action accomplished via the blockade of inhibitory endocannabinoid-mediated retrograde transmission (9). Both Manzanares et al. (10) and Cota (11) have postulated that the endocannabinoid system exerts an inhibitory tone on the HPA axis. A potential mechanism could be the constitutive synthesis and release of endocannabinoids, activation of inhibitory presynaptic CB1, and thus suppression of neuronal excitation. CB1 blockade under these conditions would result in an increase in neuronal activity and, in the model suggested by Di et al. (8), an increase in CRF release from the PVN and increased HPA axis drive. There is evidence for this inhibitory endocannabinoid-mediated tone from both pharmacological and receptor knockout studies. Studies in rodents using selective CB1 antagonists have clearly demonstrated that the blockade of endocannabinoid signaling results in an increase in basal and stress-induced HPA axis activity (10,12,13). Furthermore, CB1 knockout studies have demonstrated increased basal ACTH and corticosterone levels (14) and elevated dark phase corticosterone levels accompanied by elevated CRF mRNA expression in the PVN (15) in CB1 knockout animals. These observations suggest that an intact endocannabinoid system is required to maintain normal HPA axis function.
Most previous studies into the effects of CB1 blockade in rodents have relied on single time-point measurements of corticosterone and/or ACTH. The limitations of using single-point samples to interpret changes in hormone concentrations that display marked rhythmicity are well known. The aim of this study was to elucidate the effects of CB1 blockade with respect to both ultradian and circadian variation in HPA axis activity. Circulating corticosterone concentrations were measured over an extended time course using an automated blood-sampling system. Given that both ultradian and circadian variation in the HPA axis is known to vary between the genders, it was also our aim to elucidate how the effects of CB1 blockade varied between males and females.
Materials and Methods
Animals
Adult male and female Sprague Dawley rats were obtained from Harlan, UK (Oxon, UK), and maintained under standard housing conditions with lights on at 0500 h (14 h light, 10 h dark). Food and water were available ad libitum. Animals were allowed to adapt to our animal house conditions for at least 1 wk before surgery. All animal procedures were carried out in accordance with the United Kingdom Home Office animal welfare regulations.
Surgery
Animals were anesthetized with a combination of Hypnorm (0.32 mg/kg fentanyl citrate and 10 mg/kg fluanisone, im; Janssen Pharmaceuticals, Oxford, UK) and diazepam (2.6 mg/kg ip; Phoenix Pharmaceuticals, Gloucester, UK). The right jugular vein was exposed, and a polythene cannula (Portex, Hythe, UK) tipped with SILASTIC brand (Dow Corning, Midland, MI) (inner diameter 0.50 mm, outer diameter 0.93 mm; Merck, Darmstadt, Germany) was inserted into the vessel until it lay close to the entrance of the right atrium. The cannula was prefilled with pyrogen-free heparinized (10 IU/ml) isotonic saline. The free end of the cannula was exteriorized through a scalp incision and then tunneled through a protective spring that was anchored to the parietal bones using two stainless steel screws and self-curing dental acrylic. After recovery, animals were housed in individual cages in the automated blood sampling room. The end of the protective spring was attached to a mechanical swivel that rotated through 360° in a horizontal plane and 180° through a vertical plane, allowing the animals maximum freedom of movement. The cannulas were flushed daily with the heparinized saline to maintain patency. Animals also received an additional sc cannula at the time of surgery. The sc cannula passed through the protective spring and was tunneled along a sc pocket above the scapular so that the tip of the cannula was approximately 3–4 cm distal to the scalp incision. The sc cannula was filled with saline, and a small amount of saline (0.1–0.2 ml) was flushed into the cannula daily.
Automated blood sampling
The collection of blood with an automated blood-sampling system has previously been described in detail (2,16,17). Briefly, 5 d after surgery, the jugular vein cannula of each animal was connected to the automated blood-sampling system. Blood samples were collected every 5 min using the automated system for a period of up to 14 h. Blood samples were collected at a dilution of 1:3 in heparinized saline for corticosterone measurement. Each sample from the automated system contained no more than 40 μl of whole blood. The concentration of corticosterone in the diluted whole blood was measured by RIA.
Manual blood sampling
Blood samples from separate groups of animals were collected manually for the determination of both ACTH and corticosterone. Blood samples of 0.2 ml were collected on ice into tubes containing aprotinin and EDTA and then centrifuged. The plasma was stored at −80 C until hormone measurement.
Antagonists
AM251 and AM281 were obtained from Tocris (Bristol, UK). Stock solutions were prepared in ethanol and dimethyl sulfoxide, respectively. Immediately before injection, the stock solutions were diluted with Cremophor and then saline to give a final injection dilution of (1:1:18).
Experimental protocols
Study 1: acute blockade of CB1 in male and female rats
Five days after the jugular vein was implanted, rats were connected to the automated blood-sampling system. Separate groups of rats were given an acute bolus (0.5 ml) of AM251 (1 mg/kg) or vehicle (5% ethanol, 5% Cremophor, 90% saline) near their diurnal trough or diurnal peak. The drugs were administered via the previously implanted sc cannula and therefore did not interfere with blood sampling. Blood sampling commenced at 0602 h for the morning groups and at 1402 h for the evening groups, and samples were collected every 5 min for up to 14 h. In the morning groups, AM251 or vehicle was administered at 0915 h in males and 1000 h in females. In the evening groups, AM251 or vehicle was administered at 1630 h in males and 1700 h in females.
Study 2: diurnal effects of AM251 in male rats
Two groups of male rats were cannulated as described for study 1, one group for treatment during the diurnal trough (0930 h) and the other for treatment at the diurnal peak (1700 h). Five days after surgery, blood samples were collected manually at 15 min before and 15 min and 1, 2, 3, 4, 5, and 6 h after the administration of AM251 (1 mg/kg) or vehicle (5% ethanol, 5% Cremophor, 90% saline) in a 0.5-ml bolus.
Study 3: diurnal effects of AM281 in male rats
The design of this study was identical to that of study 2, except that rats were treated with AM281 (1 mg/kg), and the vehicle was 5% dimethyl sulfoxide, 5% Cremophor, and 90% saline.
Hormone measurement
Total corticosterone in whole blood and plasma was measured by RIA using a citrate buffer (pH 3.0) to denature the binding globulin. Antisera was supplied by Prof. G. Makara (Institute of Experimental Medicine, Budapest, Hungary), and [125I]corticosterone was from OBI-DSL (Oxford, UK). ACTH in plasma (100 μl/tube) was measured using a commercial immunoradiometric assay (DiaSorin, Stillwater, MN).
Statistical analyses
Data are presented as individual profiles from a single animal or group mean ± se. For all statistical tests, significance at P < 0.05 was used. Details of the specific tests used are included in the text.
Results
Study 1: acute blockade of CB1 in male and female rats
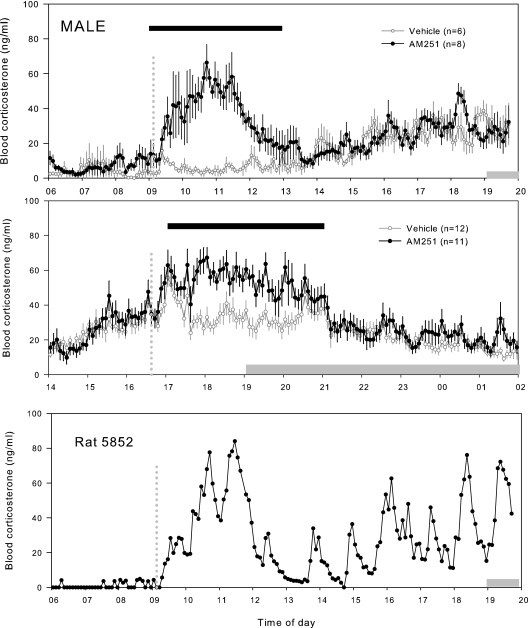
The blood corticosterone profiles for males treated with AM251 at either the peak or trough are presented in Fig. 1, and those for females are presented in Fig. 2. In males, acute blockade of the CB1 with a single sc bolus of AM251 elevated blood corticosterone concentrations when given during the trough or peak of the daily corticosterone rhythm. To compare groups, means of corticosterone concentrations were calculated for each hourly block (e.g. 0800–0900, 0900–1000, 1600–1700, and 1700–1800 h), and the hourly means for two groups (vehicle vs. AM251) were compared using a two-way repeated-measures ANOVA. During the morning, there was a significant effect of both treatment (P = 0.004) and time (P < 0.001) and a significant interaction between these factors (P < 0.001). During the evening, there was also a significant effect of both treatment (P = 0.017) and time (P < 0.001) and a significant interaction between these factors (P < 0.001). The Holm-Sidak post hoc test was then used to determine which times the drug treatments were significant (P < 0.05). In males, acute blockade of CB1 resulted in elevated hourly means for a total of 4 h from 0900–1300 h starting in the morning and in the evening for 4 h from 1700–2100 h.
Figure 1.
Blood corticosterone profiles from male rats treated with a cannabinoid receptor antagonist during the diurnal peak or trough. Top, Group means ± se from male rats treated with a sc bolus of AM251 (1 mg/kg) or vehicle (5% ethanol, 5% Cremophor, 90% saline) during the diurnal trough; middle, group means ± se from male rats treated with a sc bolus of AM251 (1 mg/kg) or vehicle (5% ethanol, 5% Cremophor, 90% saline) during the diurnal peak; bottom, profile from a male rat treated with AM251 during the diurnal trough. Note the pulsatile nature of corticosterone release. Vertical dotted lines indicate time of sc injection. Horizontal gray bars indicate the period of lights off. Horizontal black bars indicate where the hourly means of the AM251-treated rats were significantly greater than the vehicle-treated rats by repeated-measures ANOVA (see text for details).
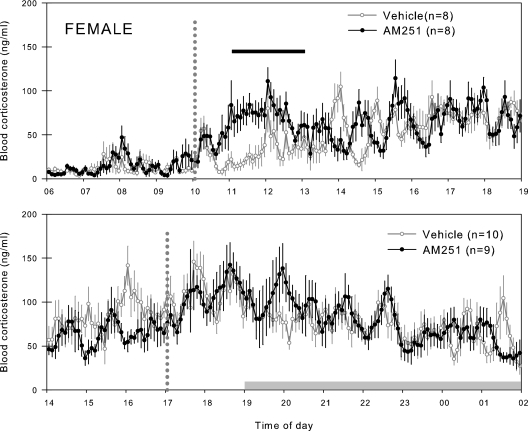
Figure 2.
Blood corticosterone profiles from female rats treated with a cannabinoid receptor antagonist during the diurnal peak or trough. Top, Group means ± se from female rats treated with a sc bolus of AM251 (1 mg/kg) or vehicle (5% ethanol, 5% Cremophor, 90% saline) during the diurnal trough; bottom, group means ± se from female rats treated with a sc bolus of AM251 (1 mg/kg) or vehicle (5% ethanol, 5% Cremophor, 90% saline) during the diurnal peak. Vertical dotted lines indicate time of sc injection. Horizontal gray bars indicate the period of lights off. Horizontal black bars indicate where the hourly means of the AM251-treated rats were significantly greater than the vehicle-treated rats by repeated-measures ANOVA (see text for details).
The maximal mean concentrations of corticosterone achieved after receptor blockade were similar at both times of the day. The highest mean corticosterone concentrations were 66.4 ng/ml during the morning (95 min after sc administration) and 67.2 ng/ml during the evening (85 min after sc administration). Visual inspection of the individual profiles (see Fig. 1, lower panel) revealed that corticosterone pulsatility per se was not abolished by CB1 blockade and that the pulse amplitudes were increased in AM251-treated rats. Excluding the first hour of corticosterone elevation, because it appears to be due to a minor stress response to drug administration, the period of corticosterone elevation was insufficient for pulse detection algorithms (such as PULSAR) to confirm these effects on pulse amplitude.
The blood corticosterone profiles for females treated with AM251 at either the peak or trough are presented in Fig. 2 and were analyzed exactly as described for the males above. In females, elevation of corticosterone concentrations after acute CB1 blockade was evident only in the morning and for a shorter duration than in males. The hourly means were significantly increased after acute CB1 blockade for 2 h from 1100–1300 h [(by two-way repeated-measures ANOVA with Holm-Sidak post hoc test (P < 0.05)].
Study 2
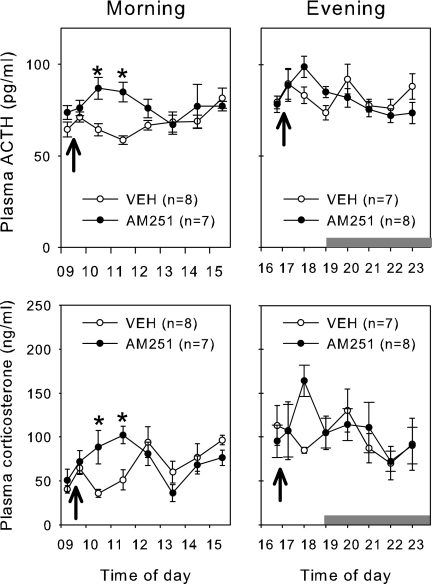
Group means for the circulating ACTH and corticosterone concentrations in male rats treated with AM251 or vehicle are presented in Fig. 3. During the morning (i.e. diurnal trough), both plasma ACTH and corticosterone were elevated in rats treated with AM251 compared with vehicle controls. There were significant interactions by repeated-measures two-way ANOVA for ACTH (P = 0.024) and corticosterone (P < 0.001). Post hoc tests revealed that both hormones were elevated at 15 min and 1 h after antagonist administration. During the evening, there were no significant differences between the animals treated with AM251 or vehicle for either hormone as determined by repeated-measures two-way ANOVA. This is not unexpected given the limited number of samples collected during manual sampling compared with automated sampling in study 1. There was, however a trend for increased values with antagonist treatment as determined by individual t tests at each time point (ACTH at 1800 h P = 0.057; ACTH at 1900 h P = 0.04; corticosterone at 1800 P = 0.02).
Figure 3.
Effect of the AM251 on plasma ACTH and corticosterone in male rats treated during the diurnal peak of trough. Male rats were given a single sc bolus of AM251 (1 mg/kg) or vehicle (VEH) in either the morning (left panels) or the evening (right panels). Plasma concentrations of both ACTH (top panels) and corticosterone (lower panels) are presented. Values are group means ± se. Horizontal gray bars indicate the period of lights off. Arrows indicate time of sc administration. *, Time points in AM251-treated animals that are significantly greater than for controls by repeated-measures ANOVA (see text for details).
Study 3
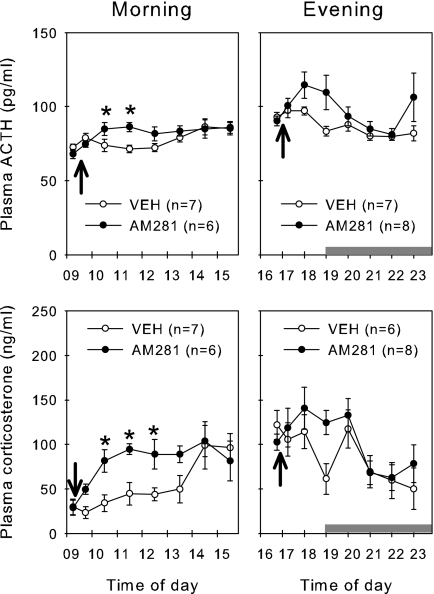
Group means for the circulating ACTH and corticosterone concentrations in male rats treated with AM281 or vehicle are presented in Fig. 4. During the morning (i.e. diurnal trough), both plasma ACTH and corticosterone were elevated in rats treated with AM281 compared with vehicle controls when compared by repeated-measures two-way ANOVA. For ACTH, plasma concentrations were elevated at 1030 and 1130 h (antagonist administered at 0930 h), and for corticosterone, plasma concentrations were elevated at 1030, 1130, and 1230 h. During the evening, there were no significant differences between the animals treated with AM281 or vehicle for either hormone as determined by repeated-measures two-way ANOVA. For ACTH, however, there was a significant difference between AM281 and vehicle by Mann-Whitney U test at 1900 h (P < 0.01).
Figure 4.
Effect of the AM281 on plasma ACTH and corticosterone in male rats treated during the diurnal peak of trough. Male rats were given a single sc bolus of AM281 (1 mg/kg) or vehicle (VEH) in either the morning (left panels) or the evening (right panels). Plasma concentrations of both ACTH (top panels) and corticosterone (lower panels) are presented. Values are group means ± se. Horizontal gray bars indicate the period of lights off. Arrows indicate time of sc administration. *, Time points in AM281-treated animals that are significantly greater than for controls by repeated-measures ANOVA (see text for details).
Discussion
This is the first study to systematically look at the effects of CB1 blockade on basal HPA activity with respect to both time of day and gender. We have shown distinct diurnal changes in the endocannabinoid effect on basal HPA axis activity in the rat. This system provides greater inhibitory tone during the diurnal nadir (i.e. lights on) compared with the diurnal acrophase (i.e. lights off). Moreover, this regulation is sexually diergic in that it is more pronounced in males than in females.
In the present study, blockade of the CB1 via sc administration of AM251 increased basal HPA activity both in the morning and the evening in male rats. The effect of a single dose was to elevate corticosterone concentrations at both times of day, with the relative increase in steroid concentrations greater in the morning than the evening. Interestingly there appeared to be a ceiling effect with similar maximal corticosterone concentrations achieved at both times of day.
Other studies have shown administration of either AM251 or SR141716 (a structurally related CB1 antagonist) to rats or mice result in elevated corticosterone in some (10,12,18) but not all studies (19,20,21). Most studies concentrate on the stress responses of the HPA axis with descriptions of basal HPA activity limited to single samples or at best a few samples and typically look within the first hour after drug administration. In the current study, we have used automated frequent blood sampling to characterize the effects of CB1 blockade on basal HPA activity. We have shown that the effect of a single dose can last for 4 h and, more importantly, that corticosterone pulsatility is maintained. From visual inspection of the profiles, it appears that only the amplitude of pulses was affected by AM251 treatment. It was not possible to statistically analyze this increase in amplitude with PULSAR or DECON due to the limited number of pulses. The duration of this effect was approximately three to four pulses, of which the first may have been a minor stress response (because it was also seen in the vehicle-treated animals). It should be possible to confirm these observations in future studies using a constant infusion of AM251 for a longer time period.
Diurnal variation is one of the major characteristics of the HPA axis, and despite the number of studies that have examined the endocannabinoid regulation of the HPA axis, the majority of the studies focus on the inactive phase of diurnal rodents. Interestingly, it has been shown that the concentrations of the endocannabinoids anandamide and 2-arachidonoyl-glycerol vary diurnally in multiple brain regions of the rat, suggesting circadian variation in this system may not only be of importance for HPA activity but may also have much more global effects in the central nervous system (22).
Although blockade of CB1 with AM251 did elevate basal corticosterone in female rats, this effect was much less profound than in males. Furthermore, the corticosterone concentrations were significantly elevated only in the morning and not the evening, and this effect was of shorter duration in the females, 2 h compared with 4 h in males. This may be a result of different drug metabolism in males vs. females or reflect differential modulation of basal HPA activity by cannabinoids between the sexes. Although there are no data on the differences in AM251 metabolism in male and female rodents, the related CB1 antagonist SR141716 (rimonabant) has been extensively used in humans, and there are no known gender-related differences in its metabolism. It is therefore more likely that HPA-related endocannabinoid activity differs in males and females. This may be an important factor in the underlying differences in HPA activity in males and females (23). In rats, females have a greater number of pulses than males. More specifically, females have pulses during the diurnal nadir, a time of day when corticosterone pulses in male rats are not measurable. During the nadir, treatment with AM251 has a greater effect stimulating (or disinhibiting) basal corticosterone in males than in females. We have shown that corticosterone pulse profile can be manipulated by androgen and estrogens. Ovariectomy in a female rat can change the females’ corticosterone profile to that of a male, and likewise, gonadectomy in a male rat can change the male’s corticosterone profile to that of a female (24). It is quite possible that diergic patterns of endocannabinoid tone may be a factor in the sex hormone-mediated differential circadian patterns of basal corticosterone in male and female rats. This may not be the only neuroendocrine system regulated in this manner, because the inhibitory effect of anandamide on LHRH secretion has also been shown to be an estrogen-sensitive response (25).
Various studies have examined HPA activity in CB1 knockout mice, with most focusing on stress responsiveness rather than basal activity. Basal corticosterone concentrations were elevated in males during the light phase (14), in males but not in females during the dark phase (18), and in diestrous females at lights off but not lights on (15). All of these studies rely on single time points for the determination of basal circulating corticosterone. Taken together, the CB1 knockout studies show that basal HPA activity is elevated at some time points.
Sex differences in the cannabinoid regulation of the HPA axis are presumably mediated by gonadal status and or estrous cycle stage. Other studies have demonstrated estrous cycle-related changes in the cannabinoid system. Endocannabinoid content of the hypothalamus was greater at diestrus compared with estrus (26). Cannabinoid receptor density in the medial basal hypothalamus is also greater at diestrus compared with estrus (27). The distribution of cannabinoid receptors in the brain is quite extensive and controls many functions. These estrous cycle changes may be associated with the regulation of the hypothalamic-pituitary-gonadal axis and sexual behavior rather than the HPA axis (for review see Ref. 28).
In the first study, AM251 was administered peripherally, and corticosterone was the only hormone of the HPA axis that was measured. Although ACTH is the major mechanism through which corticosterone secretion is controlled, splanchnic nerve activity also inhibits basal HPA activity during the diurnal nadir by actions at the level of the adrenal gland. Indeed, a recent study (29) has shown that the orphan receptor GPR55 is a novel cannabinoid receptor (which also binds to AM251) and that this receptor is highly expressed in the adrenal. It was therefore important to assess whether the effect of AM251 was centrally or peripherally mediated. We therefore conducted two additional studies, one with AM251 and one with AM281 (a CB1 antagonist that does not activate the adrenal GPR55 receptor) in which both ACTH and corticosterone were measured.
These studies demonstrated that administration of both AM251 and AM281 resulted in elevated circulating ACTH concentrations. This strongly infers that the effects of CB1 antagonism are mediated via ACTH and not at the level of the adrenal gland. We cannot determine the precise site of action of the CB1 antagonists, but CB1 are widespread in the central nervous system and have been found in key regions of the brain that regulate the HPA axis, namely the hippocampus and hypothalamus (see review see Ref. 30).
Regulation of the HPA axis predominately involves a complex interplay between inhibitory GABAergic neurons and the activation of glutamatergic neurons at multiple sites (31,32). CB1 blockade could be acting to inhibit the GABAergic neurons, activate the glutamatergic neurons, or both. There is in vitro evidence that CB1 inhibit PVN activity via the suppression of glutamatergic inputs to parvocellular neurons (8).
In humans, SR141716 (rimonabant) was widely tested as a treatment for obesity, although the potential side effects of depression have limited its clinical use (33). Depression is frequently characterized by increased basal HPA activity (34). It is tempting to speculate that increased HPA activity in male users of rimonabant may be associated with increased susceptibility to depression. Indeed, the odds ratios for depressive systems were greater in those Rimonabant in Obesity (RIO) studies with the greatest percentage of males (35,36,37,38). To our knowledge, no studies have explored whether there is a sex difference in the susceptibility to depression in users of rimonabant. This may be a more difficult question to answer in humans because sex differences in HPA axis function are not as pronounced as they are in rodents (39,40). Moreover, the users of rimonabant are obese, and this in itself alters HPA regulation (41).
We have shown that acute blockade of the CB1 results in hyperactivity of the rat HPA axis as demonstrated by elevated corticosterone and ACTH. Our data demonstrate that endocannabinoid regulation of this axis exhibits both circadian variation and sexual diergism and suggests that endocannabinoids may regulate the HPA axis via amplitude modulation of corticosterone pulses. In conclusion, we propose that a dynamic endocannabinoid-mediated inhibitory tone regulates the HPA axis and that this tone is modulated by both gender and time of day.
Footnotes
This project was supported by a program grant from The Wellcome Trust (074112/Z/04/Z).
Disclosure Summary: The authors have nothing to disclose.
First Published Online June 9, 2010
Abbreviations: CB1, Cannabinoid receptor type 1; CRF, corticotropin-releasing factor; HPA, hypothalamic-pituitary-adrenal; PVN, paraventricular nucleus of the hypothalamus.
References
- Dallman MF, Yates FE 1969 Dynamic asymmetries in the corticosteroid feedback path and distribution-metabolism-binding elements of the adrenocortical system. Ann NY Acad Sci 156:696–721 [DOI] [PubMed] [Google Scholar]
- Lightman SL, Windle RJ, Julian MD, Harbuz MS, Shanks N, Wood SA, Kershaw YM, Ingram CD 2000 Significance of pulsatility in the HPA axis. Novartis Found Symp 227:244–257; discussion 257–260 [DOI] [PubMed] [Google Scholar]
- Chrousos GP 1998 Ultradian, circadian, and stress-related hypothalamic-pituitary-adrenal axis activity: a dynamic digital-to-analog modulation. Endocrinology 139:437–440 [DOI] [PubMed] [Google Scholar]
- Hastings M, O'Neill JS, Maywood ES 2007 Circadian clocks: regulators of endocrine and metabolic rhythms. J Endocrinol 195:187–198 [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U 2000 Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289:2344–2347 [DOI] [PubMed] [Google Scholar]
- Sage D, Ganem J, Guillaumond F, Laforge-Anglade G, François-Bellan AM, Bosler O, Becquet D 2004 Influence of the corticosterone rhythm on photic entrainment of locomotor activity in rats. J Biol Rhythms 19:144–156 [DOI] [PubMed] [Google Scholar]
- Becquet D, Girardet C, Guillaumond F, François-Bellan AM, Bosler O 2008 Ultrastructural plasticity in the rat suprachiasmatic nucleus. Possible involvement in clock entrainment. Glia 56:294–305 [DOI] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Halmos KC, Tasker JG 2003 Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci 23:4850–4857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di S, Malcher-Lopes R, Marcheselli VL, Bazan NG, Tasker JG 2005 Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and γ-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinology 146:4292–4301 [DOI] [PubMed] [Google Scholar]
- Manzanares J, Corchero J, Fuentes JA 1999 Opiod and cannabinoid receptor-mediated regulation of the increase in adrenocorticotropin hormone and corticosterone plasma concentrations induced by central administration of Δ9-tetrahydrcannabinol in rats. Brain Res 839:173–179 [DOI] [PubMed] [Google Scholar]
- Cota D 2008 The role of the endocannabinoid system in the regulation of hypothalamic-pituitary-adrenal axis activity. J Neuroendocrinol 20(Suppl 1):35–38 [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ 2004 Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology 145:5431–5438 [DOI] [PubMed] [Google Scholar]
- Wade MR, Degroot A, Nomikos GG 2006 Cannabinoid CB1 receptor antagonism modulates plasma corticosterone in rodents. Eur J Pharmacol 551:162–167 [DOI] [PubMed] [Google Scholar]
- Barna I, Zelena D, Arszovszki AC, Ledent C 2004 The role of endogenous cannabinoids in the hypothalamo-pituitary-adrenal axis regulation: in vivo and in vitro studies in CB1 receptor knockout mice. Life Sci 75:2959–2970 [DOI] [PubMed] [Google Scholar]
- Cota D, Steiner MA, Marsicano G, Cervino C, Herman JP, Grübler Y, Stalla J, Pasquali R, Lutz B, Stalla GK, Pagotto U 2007 Requirement of cannabinoid receptor type 1 for the basal modulation of hypothalamic-pituitary-adrenal axis function. Endocrinology 148:1574–1581 [DOI] [PubMed] [Google Scholar]
- Atkinson HC, Wood SA, Kershaw YM, Bate E, Lightman SL 2006 Diurnal variation in the responsiveness of the hypothalamic-pituitary-adrenal axis of the male rat to noise stress. J Neuroendocrinol 18:526–533 [DOI] [PubMed] [Google Scholar]
- Windle RJ, Wood SA, Shanks N, Lightman SL, Ingram CD 1998 Ultradian rhythm of basal corticosterone release in the female rat: dynamic interaction with the response to acute stress. Endocrinology 139:443–450 [DOI] [PubMed] [Google Scholar]
- Steiner MA, Marsicano G, Nestler EJ, Holsboer F, Lutz B, Wotjak CT 2008 Antidepressant-like behavioural effects of impaired cannabinoid receptor type 1 signaling coincide with exaggerated corticosterone secretion in mice. Psychoneuroendocrinology 33:54–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Hernández E, Muñoz RM, del Arco I, Villanúa MA, Carrera MR, Rodríguez de Fonseca F 1997 Acute administration of the CB1 cannabinoid receptor antagonist SR141716A induces anxiety-like responses in the rat. Neuroreport 8:491–496 [DOI] [PubMed] [Google Scholar]
- Wenger T, Jamali KA, Juanéda C, Léonardelli J, Tramu G 1997 Arachidonyl ethanolamide (anandamide) activates the parvocellular part of the hyposthalamic paraventricular nucleus. Biochem Biophys Res Commun 237:724–728 [DOI] [PubMed] [Google Scholar]
- Hill MN, Ho WS, Sinopoli KJ, Viau V, Hillard CJ, Gorzalka BB 2006 Involvement of the endocannabinoid system in the ability of long-term tricycllic antidepressant treatment to suppress stress-induced activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology 31:2591–2599 [DOI] [PubMed] [Google Scholar]
- Valenti M, Viganò D, Casico MG, Rubino T, Steardo L, Parolaro D, Di Marzo V 2004 Differential diurnal variations of anandamide and 2-arachidonoyl-glycerol levels in rat brain. Cell Mol Life Sci 61:945–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Bate E, Lightman SL, Ingram CD, Jessop DS, Harbuz MS 2004 Gonadectomy reverses the sexually diergic patterns of circadian and stress-induced hypothalamic-pituitary-adrenal axis activity in male and female rats. J Neuroendocrinol 16:516–524 [DOI] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL 2004 Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. J Neuroendocrinol 16:989–998 [DOI] [PubMed] [Google Scholar]
- Scorticati, D Scorticati C, Fernández-Solari J, De Laurentiis A, Mohn C, Prestifilippo JP, Lasaga M, Seilicovich A, Billi S, Franchi A, McCann SM, Rettori V 2004 The inhibitory effect of anandamide on luteinizing hormone-releasing hormone secretion is reversed by estrogen. Proc Natl Acad Sci USA 101:11891–11896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw HB, Rimmerman N, Krey JF, Walker JM 2006 Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am J Physiol Regul Integr Comp Physiol 291:R349–R358 [DOI] [PubMed] [Google Scholar]
- Rodríguez de Fonseca F, Cebeira M, Ramos JA, Martín M, Fernández-Ruiz JJ 1994 Cannabinoid receptors in rat brain areas: sexual differences, fluctuations during estrous cycle and changes after gonadectomy and sex steroid replacement. Life Sci 54:159–170 [DOI] [PubMed] [Google Scholar]
- López HH 2010 Cannabinoid-hormone interactions in the regulation of motivational processes. Horm Behav 58:100–110 [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ 2007 The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol 152:1092–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner MA, Wotjak CT 2008 Role of the endocannabinoid system in regulation of the hypothalamic-pituitary-adrenocortical axis. Prog in Brain Res 170:397–432 [DOI] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE 2003 Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24:151–180 [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK, Figueiredo H 2004 Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann NY Acad Sci 1018:35–45 [DOI] [PubMed] [Google Scholar]
- Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A 2007 Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 370:1706–1713 [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlöv E, Bissette G, Walléus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W 1984 Elevated concentrations of CRF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science 226:1342–1344 [DOI] [PubMed] [Google Scholar]
- Després JP, Golay A, Sjöström L 2005 Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 353:2121–2134 [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rössner S; RIO-Europe Study Group 2005 Effects of cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet 365:1389–1397 [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J 2006 Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors inoverweight or obese patients: RIO-North America: a randomized controlled trial. JAMA 295:761–775 [DOI] [PubMed] [Google Scholar]
- Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF for the RIO-Diabetes study group 2006 Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet 368:1660–1672 [DOI] [PubMed] [Google Scholar]
- Horrocks PM, Jones AF, Ratcliffe WA, Holder G, White A, Holder R, Ratcliffe JG, London DR 1990 Patterns of ACTH and cortisol pulsatility over twenty-four hours in normal males and females. Clin Endocrinol (Oxf) 32:127–134 [DOI] [PubMed] [Google Scholar]
- Young EA, Altemus M 2004 Puberty, ovarian steroids, and stress. Ann NY Acad Sci 1021:124–133 [DOI] [PubMed] [Google Scholar]
- Jessop DS, Dallman MF, Fleming D, Lightman SL 2001 Resistance to glucocorticoid feedback in obesity. J Clin Endocrinol Metab 86:4109–4114 [DOI] [PubMed] [Google Scholar]