Abstract
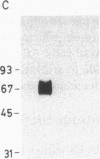
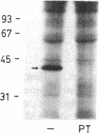
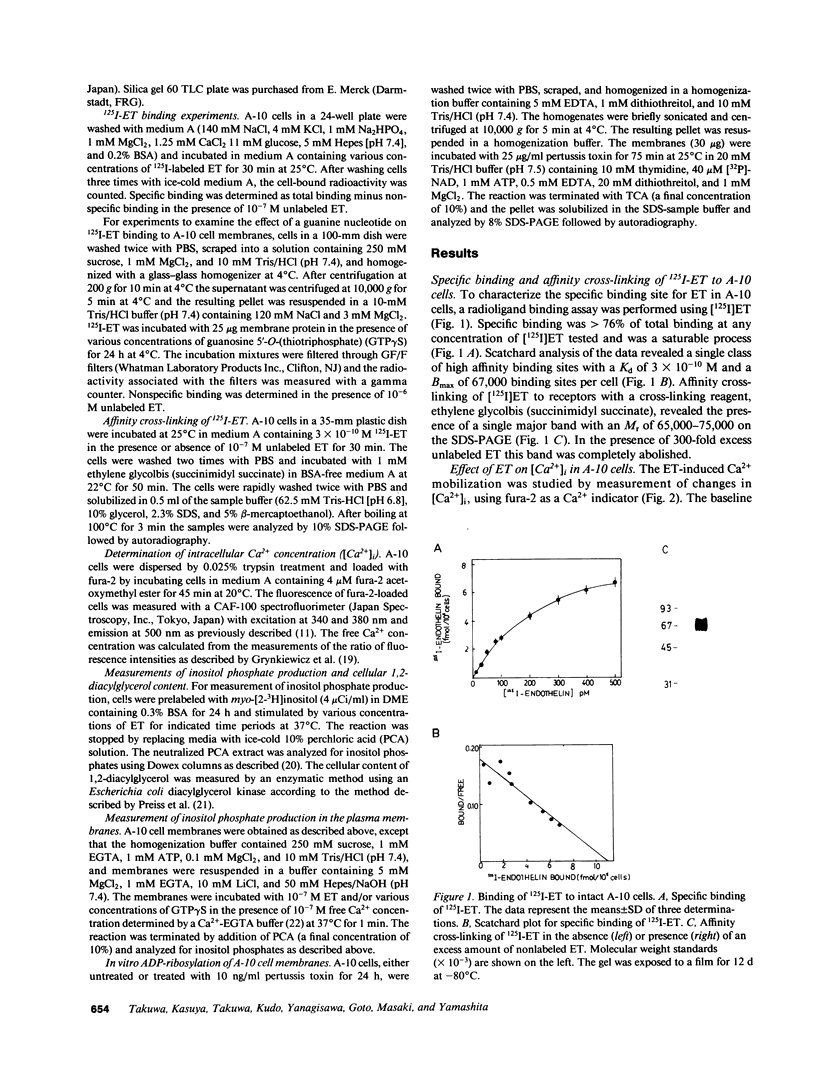
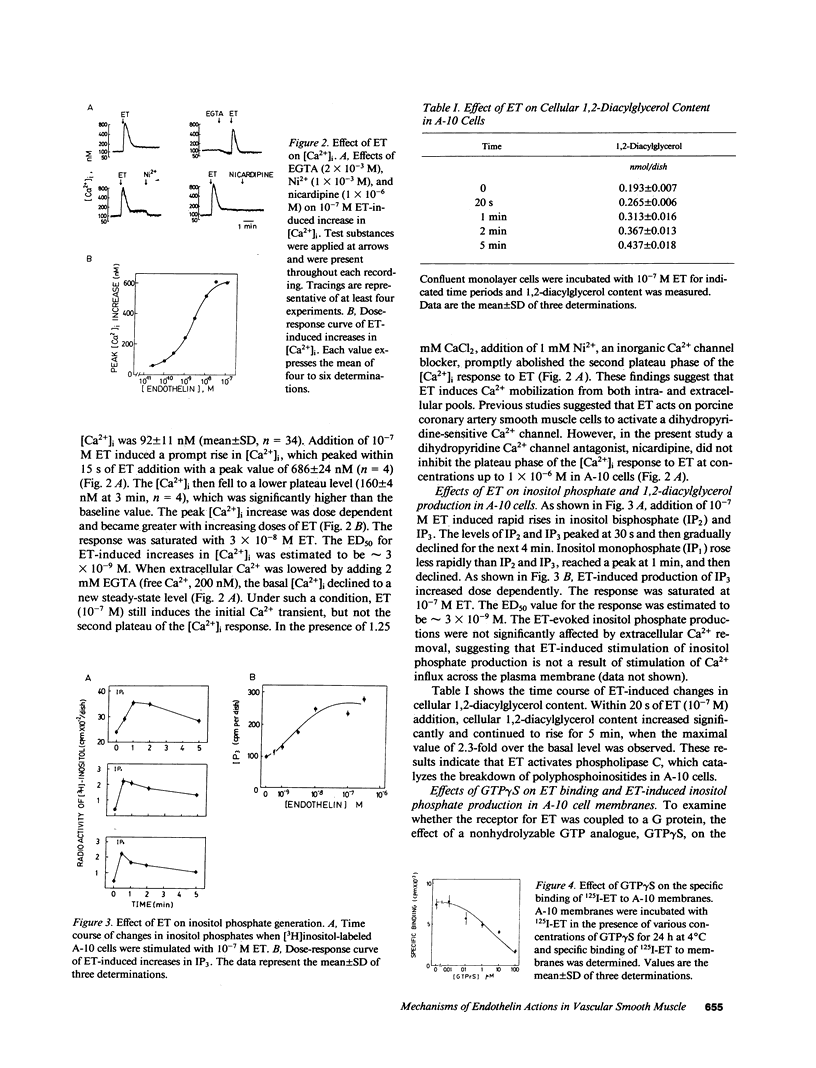
The mechanisms of endothelin-1 (ET) actions were investigated in cultured rat aortic vascular smooth muscle A-10 cells. The A-10 cells have a single class of high affinity binding sites for ET with an apparent Mr of 65,000-75,000 on SDS-PAGE. Stimulation of cells with ET induces mobilization of Ca2+ from both intra- and extracellular pools to produce a biphasic increase in cytoplasmic free Ca2+ concentration. ET increases cellular levels of inositol trisphosphate and 1,2-diacylglycerol, indicating activation of phospholipase C by ET. ET stimulates production of inositol phosphates in membranes prepared from A-10 cells in the presence of guanosine 5'-O-(thiotriphosphate) (GTP gamma S), but not in its absence. Further, specific binding of 125I-labeled ET to A-10 cell membranes is shown to be inhibited by GTP gamma S in a dose-dependent manner. Treatment of A-10 cells with pertussis toxin induces ADP-ribosylation of a 41,000-D membrane protein but fails to block the ET-induced increases in inositol phosphate production and Ca2+ mobilization. These results indicate that the receptor for ET is coupled to phospholipase C via a guanine nucleotide-binding regulatory protein which is distinct from the pertussis toxin substrate in A-10 cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badr K. F., Murray J. J., Breyer M. D., Takahashi K., Inagami T., Harris R. C. Mesangial cell, glomerular and renal vascular responses to endothelin in the rat kidney. Elucidation of signal transduction pathways. J Clin Invest. 1989 Jan;83(1):336–342. doi: 10.1172/JCI113880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Biden T. J., Peter-Riesch B., Schlegel W., Wollheim C. B. Ca2+-mediated generation of inositol 1,4,5-triphosphate and inositol 1,3,4,5-tetrakisphosphate in pancreatic islets. Studies with K+, glucose, and carbamylcholine. J Biol Chem. 1987 Mar 15;262(8):3567–3571. [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., Bolanowski M. A., Bennett C. D., Rands E., Diehl R. E. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986 May 1;321(6065):75–79. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Fargin A., Raymond J. R., Lohse M. J., Kobilka B. K., Caron M. G., Lefkowitz R. J. The genomic clone G-21 which resembles a beta-adrenergic receptor sequence encodes the 5-HT1A receptor. Nature. 1988 Sep 22;335(6188):358–360. doi: 10.1038/335358a0. [DOI] [PubMed] [Google Scholar]
- Goodhardt M., Ferry N., Geynet P., Hanoune J. Hepatic alpha 1-adrenergic receptors show agonist-specific regulation by guanine nucleotides. Loss of nucleotide effect after adrenalectomy. J Biol Chem. 1982 Oct 10;257(19):11577–11583. [PubMed] [Google Scholar]
- Goto K., Kasuya Y., Matsuki N., Takuwa Y., Kurihara H., Ishikawa T., Kimura S., Yanagisawa M., Masaki T. Endothelin activates the dihydropyridine-sensitive, voltage-dependent Ca2+ channel in vascular smooth muscle. Proc Natl Acad Sci U S A. 1989 May;86(10):3915–3918. doi: 10.1073/pnas.86.10.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hirata Y., Yoshimi H., Takata S., Watanabe T. X., Kumagai S., Nakajima K., Sakakibara S. Cellular mechanism of action by a novel vasoconstrictor endothelin in cultured rat vascular smooth muscle cells. Biochem Biophys Res Commun. 1988 Aug 15;154(3):868–875. doi: 10.1016/0006-291x(88)90220-3. [DOI] [PubMed] [Google Scholar]
- Kasuya Y., Takuwa Y., Yanagisawa M., Kimura S., Goto K., Masaki T. Endothelin-1 induces vasoconstriction through two functionally distinct pathways in porcine coronary artery: contribution of phosphoinositide turnover. Biochem Biophys Res Commun. 1989 Jun 30;161(3):1049–1055. doi: 10.1016/0006-291x(89)91349-1. [DOI] [PubMed] [Google Scholar]
- Kendall D. A., Nahorski S. R. Dihydropyridine calcium channel activators and antagonists influence depolarization-evoked inositol phospholipid hydrolysis in brain. Eur J Pharmacol. 1985 Sep 10;115(1):31–36. doi: 10.1016/0014-2999(85)90580-1. [DOI] [PubMed] [Google Scholar]
- Kojima I., Shibata H., Ogata E. Pertussis toxin blocks angiotensin II-induced calcium influx but not inositol trisphosphate production in adrenal glomerulosa cell. FEBS Lett. 1986 Aug 18;204(2):347–351. doi: 10.1016/0014-5793(86)80841-9. [DOI] [PubMed] [Google Scholar]
- Kubo T., Fukuda K., Mikami A., Maeda A., Takahashi H., Mishina M., Haga T., Haga K., Ichiyama A., Kangawa K. Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature. 1986 Oct 2;323(6087):411–416. doi: 10.1038/323411a0. [DOI] [PubMed] [Google Scholar]
- Marsden P. A., Danthuluri N. R., Brenner B. M., Ballermann B. J., Brock T. A. Endothelin action on vascular smooth muscle involves inositol trisphosphate and calcium mobilization. Biochem Biophys Res Commun. 1989 Jan 16;158(1):86–93. doi: 10.1016/s0006-291x(89)80180-9. [DOI] [PubMed] [Google Scholar]
- Miasiro N., Yamamoto H., Kanaide H., Nakamura M. Does endothelin mobilize calcium from intracellular store sites in rat aortic vascular smooth muscle cells in primary culture? Biochem Biophys Res Commun. 1988 Oct 14;156(1):312–317. doi: 10.1016/s0006-291x(88)80841-6. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi T., Morris R. C., Jr, Ives H. E. Endothelin-induced increases in vascular smooth muscle Ca2+ do not depend on dihydropyridine-sensitive Ca2+ channels. J Clin Invest. 1989 Aug;84(2):635–639. doi: 10.1172/JCI114209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Okajima F., Katada T., Ui M. Coupling of the guanine nucleotide regulatory protein to chemotactic peptide receptors in neutrophil membranes and its uncoupling by islet-activating protein, pertussis toxin. A possible role of the toxin substrate in Ca2+-mobilizing receptor-mediated signal transduction. J Biol Chem. 1985 Jun 10;260(11):6761–6768. [PubMed] [Google Scholar]
- Pang D. C., Johns A., Patterson K., Botelho L. H., Rubanyi G. M. Endothelin-1 stimulates phosphatidylinositol hydrolysis and calcium uptake in isolated canine coronary arteries. J Cardiovasc Pharmacol. 1989;13 (Suppl 5):S75–S84. doi: 10.1097/00005344-198900135-00018. [DOI] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Rasmussen H., Takuwa Y., Park S. Protein kinase C in the regulation of smooth muscle contraction. FASEB J. 1987 Sep;1(3):177–185. [PubMed] [Google Scholar]
- Resink T. J., Scott-Burden T., Bühler F. R. Endothelin stimulates phospholipase C in cultured vascular smooth muscle cells. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1360–1368. doi: 10.1016/s0006-291x(88)81025-8. [DOI] [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Silberberg S. D., Poder T. C., Lacerda A. E. Endothelin increases single-channel calcium currents in coronary arterial smooth muscle cells. FEBS Lett. 1989 Apr 10;247(1):68–72. doi: 10.1016/0014-5793(89)81242-6. [DOI] [PubMed] [Google Scholar]
- Smith C. D., Lane B. C., Kusaka I., Verghese M. W., Snyderman R. Chemoattractant receptor-induced hydrolysis of phosphatidylinositol 4,5-bisphosphate in human polymorphonuclear leukocyte membranes. Requirement for a guanine nucleotide regulatory protein. J Biol Chem. 1985 May 25;260(10):5875–5878. [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Yanagisawa M., Yamashita K., Masaki T. A novel vasoactive peptide endothelin stimulates mitogenesis through inositol lipid turnover in Swiss 3T3 fibroblasts. J Biol Chem. 1989 May 15;264(14):7856–7861. [PubMed] [Google Scholar]
- Takuwa Y., Kelley G., Takuwa N., Rasmussen H. Protein phosphorylation changes in bovine carotid artery smooth muscle during contraction and relaxation. Mol Cell Endocrinol. 1988 Nov;60(1):71–86. doi: 10.1016/0303-7207(88)90121-9. [DOI] [PubMed] [Google Scholar]
- Takuwa Y., Rasmussen H. Measurement of cytoplasmic free Ca2+ concentration in rabbit aorta using the photoprotein, aequorin. Effect of atrial natriuretic peptide on agonist-induced Ca2+ signal generation. J Clin Invest. 1987 Jul;80(1):248–257. doi: 10.1172/JCI113055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa Y., Takuwa N., Rasmussen H. Carbachol induces a rapid and sustained hydrolysis of polyphosphoinositide in bovine tracheal smooth muscle measurements of the mass of polyphosphoinositides, 1,2-diacylglycerol, and phosphatidic acid. J Biol Chem. 1986 Nov 5;261(31):14670–14675. [PubMed] [Google Scholar]
- Wahl M. I., Daniel T. O., Carpenter G. Antiphosphotyrosine recovery of phospholipase C activity after EGF treatment of A-431 cells. Science. 1988 Aug 19;241(4868):968–970. doi: 10.1126/science.2457254. [DOI] [PubMed] [Google Scholar]
- Waisman D. M., Gimble J. M., Goodman D. B., Rasmussen H. Studies of the Ca2+ transport mechanism of human erythrocyte inside-out plasma membrane vesicles. I. Regulation of the Ca2+ pump by calmodulin. J Biol Chem. 1981 Jan 10;256(1):409–414. [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]