Abstract
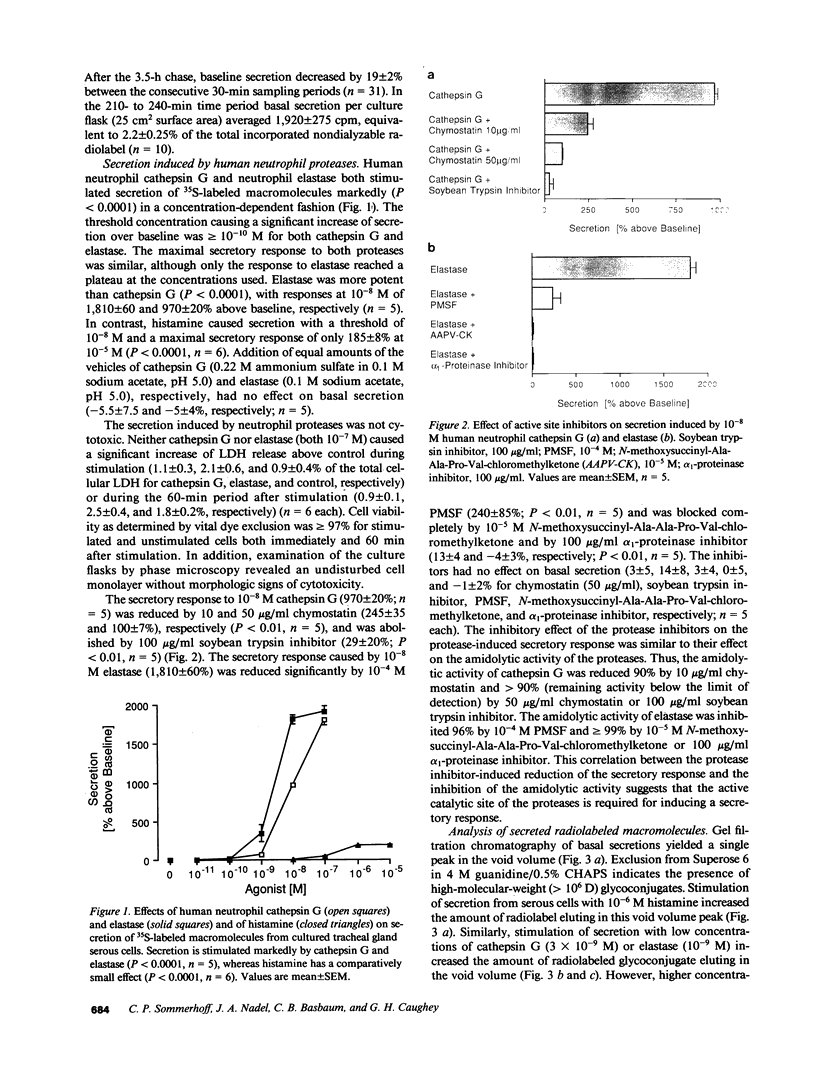
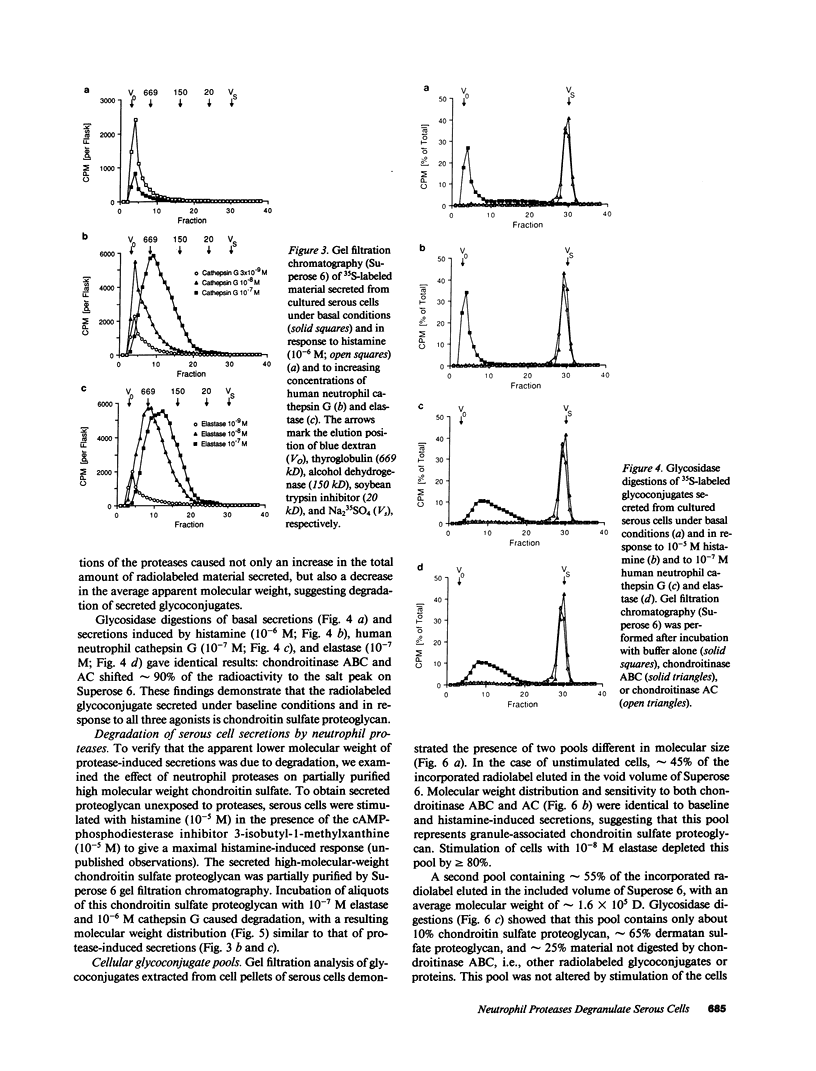
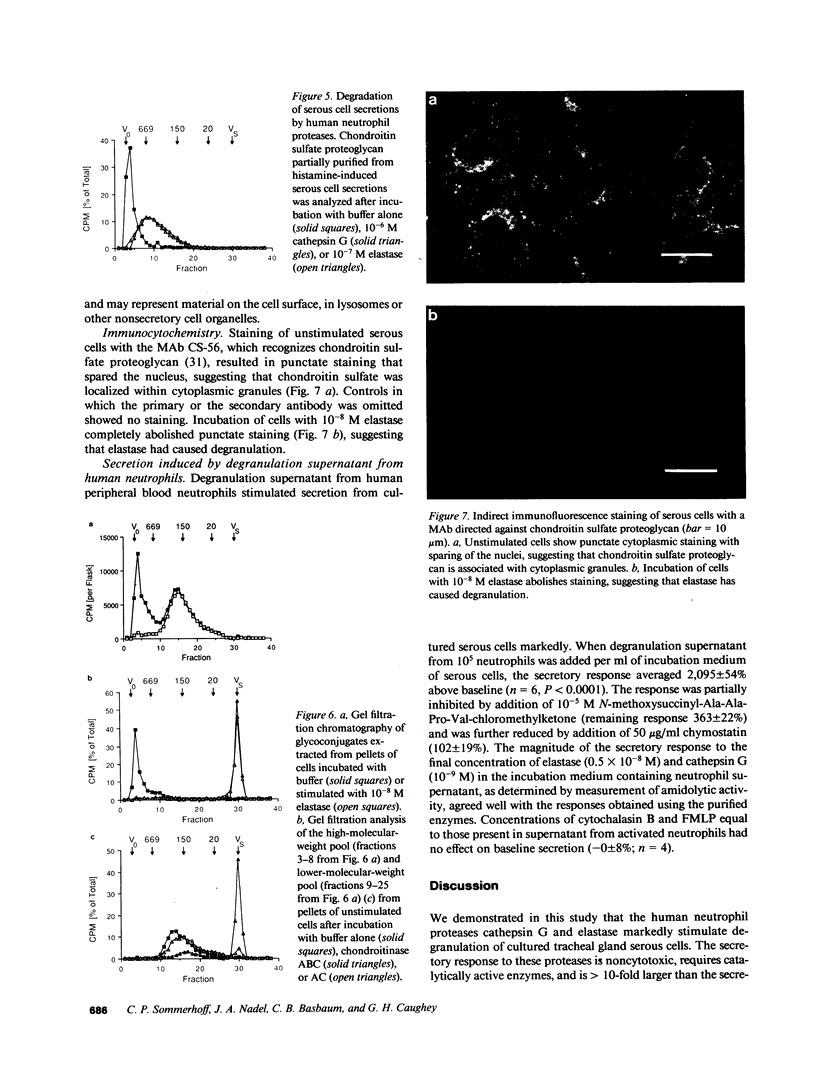
To investigate the hypothesis that neutrophil proteases stimulate airway gland secretion, we studied the effect of human cathepsin G and elastase on secretion of 35S-labeled macromolecules from cultured bovine airway gland serous cells. Both proteases stimulated secretion in a concentration-dependent fashion with a threshold of greater than or equal to 10(-10) M. Elastase was more potent than cathepsin G, causing a maximal secretory response of 1,810 +/- 60% over baseline at 10(-8) M. The maximal response to cathepsin G (1,810 +/- 70% over baseline at 10(-7) M) was similar to the maximal response to elastase. These responses were greater than 10-fold larger than the response to other agonists such as histamine. Protease-induced secretion was noncytotoxic and required catalytically active enzymes. The predominant sulfated macromolecule released by proteases was chondroitin sulfate proteoglycan. Immunocytochemical staining demonstrated chondroitin sulfate in cytoplasmic granules and decreased granular staining after stimulation of cells with elastase. The neutrophil proteases also degraded the proteoglycan released from serous cells. Cathepsin G and elastase in supernatant obtained by degranulation of human peripheral neutrophils also caused a secretory response. Thus, neutrophil proteases stimulate airway gland serous cell secretion of chondroitin sulfate proteoglycan and degrade the secreted product. These findings suggest a potential role for neutrophil proteases in the pathogenesis of increased and abnormal submucosal gland secretions in diseases associated with inflammation and neutrophil infiltration of the airways.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auberger P., Mary D., Breittmayer J. P., Aussel C., Fehlmann M. Chymotryptic-type protease inhibitors block the increase in Ca2+ and Il-2 production in activated Jurkat T cells. J Immunol. 1989 Feb 15;142(4):1253–1259. [PubMed] [Google Scholar]
- Avnur Z., Geiger B. Immunocytochemical localization of native chondroitin-sulfate in tissues and cultured cells using specific monoclonal antibody. Cell. 1984 Oct;38(3):811–822. doi: 10.1016/0092-8674(84)90276-9. [DOI] [PubMed] [Google Scholar]
- Basbaum C. B., Forsberg L. S., Paul A., Sommerhoff C., Finkbeiner W. E. Studies of tracheal secretion using serous cell cultures and monoclonal antibodies. Biorheology. 1987;24(6):585–588. doi: 10.3233/bir-1987-24610. [DOI] [PubMed] [Google Scholar]
- Basbaum C. B., Mann J. K., Chow A. W., Finkbeiner W. E. Monoclonal antibodies as probes for unique antigens in secretory cells of mixed exocrine organs. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4419–4423. doi: 10.1073/pnas.81.14.4419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh R. J., Travis J. Human leukocyte granule elastase: rapid isolation and characterization. Biochemistry. 1976 Feb 24;15(4):836–841. doi: 10.1021/bi00649a017. [DOI] [PubMed] [Google Scholar]
- Boat T. F., Cheng P. I., Klinger J. D., Liedtke C. M., Tandler B. Proteinases release mucin from airways goblet cells. Ciba Found Symp. 1984;109:72–88. doi: 10.1002/9780470720905.ch6. [DOI] [PubMed] [Google Scholar]
- Breuer R., Christensen T. G., Lucey E. C., Stone P. J., Snider G. L. An ultrastructural morphometric analysis of elastase-treated hamster bronchi shows discharge followed by progressive accumulation of secretory granules. Am Rev Respir Dis. 1987 Sep;136(3):698–703. doi: 10.1164/ajrccm/136.3.698. [DOI] [PubMed] [Google Scholar]
- Breuer R., Christensen T. G., Niles R. M., Stone P. J., Snider G. L. Human neutrophil elastase causes glycoconjugate release from the epithelial cell surface of hamster trachea in organ culture. Am Rev Respir Dis. 1989 Mar;139(3):779–782. doi: 10.1164/ajrccm/139.3.779. [DOI] [PubMed] [Google Scholar]
- Campbell C. H., Cunningham D. D. Binding sites for elastase on cultured human fibroblasts that do not mediate internalization. J Cell Physiol. 1987 Jan;130(1):142–149. doi: 10.1002/jcp.1041300120. [DOI] [PubMed] [Google Scholar]
- Campbell E. J. Human leukocyte elastase, cathepsin G, and lactoferrin: family of neutrophil granule glycoproteins that bind to an alveolar macrophage receptor. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6941–6945. doi: 10.1073/pnas.79.22.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E. J., White R. R., Senior R. M., Rodriguez R. J., Kuhn C. Receptor-mediated binding and internalization of leukocyte elastase by alveolar macrophages in vitro. J Clin Invest. 1979 Sep;64(3):824–833. doi: 10.1172/JCI109530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman D. L., Tuet I. K., Widdicombe J. H. Electrical properties of dog tracheal epithelial cells grown in monolayer culture. Am J Physiol. 1984 Mar;246(3 Pt 1):C355–C359. doi: 10.1152/ajpcell.1984.246.3.C355. [DOI] [PubMed] [Google Scholar]
- De Water R., Willems L. N., Van Muijen G. N., Franken C., Fransen J. A., Dijkman J. H., Kramps J. A. Ultrastructural localization of bronchial antileukoprotease in central and peripheral human airways by a gold-labeling technique using monoclonal antibodies. Am Rev Respir Dis. 1986 May;133(5):882–890. [PubMed] [Google Scholar]
- Finkbeiner W. E., Nadel J. A., Basbaum C. B. Establishment and characterization of a cell line derived from bovine tracheal glands. In Vitro Cell Dev Biol. 1986 Oct;22(10):561–567. doi: 10.1007/BF02623514. [DOI] [PubMed] [Google Scholar]
- Goldstein W., Döring G. Lysosomal enzymes from polymorphonuclear leukocytes and proteinase inhibitors in patients with cystic fibrosis. Am Rev Respir Dis. 1986 Jul;134(1):49–56. doi: 10.1164/arrd.1986.134.1.49. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Physical properties and polydispersity of proteoglycan from bovine nasal cartilage. J Biol Chem. 1970 Oct 10;245(19):4920–4930. [PubMed] [Google Scholar]
- Heck L. W., Darby W. L., Hunter F. A., Bhown A., Miller E. J., Bennett J. C. Isolation, characterization, and amino-terminal amino acid sequence analysis of human neutrophil elastase from normal donors. Anal Biochem. 1985 Aug 15;149(1):153–162. doi: 10.1016/0003-2697(85)90488-9. [DOI] [PubMed] [Google Scholar]
- Heck L. W., Rostand K. S., Hunter F. A., Bhown A. Isolation, characterization, and amino-terminal amino acid sequence analysis of human neutrophil cathepsin G from normal donors. Anal Biochem. 1986 Oct;158(1):217–227. doi: 10.1016/0003-2697(86)90612-3. [DOI] [PubMed] [Google Scholar]
- Holtzman M. J., Fabbri L. M., O'Byrne P. M., Gold B. D., Aizawa H., Walters E. H., Alpert S. E., Nadel J. A. Importance of airway inflammation for hyperresponsiveness induced by ozone. Am Rev Respir Dis. 1983 Jun;127(6):686–690. doi: 10.1164/arrd.1983.127.6.686. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Janoff A., Raju L., Dearing R. Levels of elastase activity in bronchoalveolar lavage fluids of healthy smokers and nonsmokers. Am Rev Respir Dis. 1983 May;127(5):540–544. doi: 10.1164/arrd.1983.127.5.540. [DOI] [PubMed] [Google Scholar]
- Jeffery P. K. Morphologic features of airway surface epithelial cells and glands. Am Rev Respir Dis. 1983 Aug;128(2 Pt 2):S14–S20. doi: 10.1164/arrd.1983.128.2P2.S14. [DOI] [PubMed] [Google Scholar]
- Kido H., Fukusen N., Katunuma N. Antibody and inhibitor of chymase inhibit histamine release in immunoglobulin E-activated mast cells. Biochem Int. 1985 Jun;10(6):863–871. [PubMed] [Google Scholar]
- Kim K. C., Wasano K., Niles R. M., Schuster J. E., Stone P. J., Brody J. S. Human neutrophil elastase releases cell surface mucins from primary cultures of hamster tracheal epithelial cells. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9304–9308. doi: 10.1073/pnas.84.24.9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger J. D., Tandler B., Liedtke C. M., Boat T. F. Proteinases of Pseudomonas aeruginosa evoke mucin release by tracheal epithelium. J Clin Invest. 1984 Nov;74(5):1669–1678. doi: 10.1172/JCI111583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagunoff D., Chi E. Y., Wan H. Effects of chymotrypsin and trypsin on rat peritoneal mast cells. Biochem Pharmacol. 1975 Sep 1;24(17):1573–1578. doi: 10.1016/0006-2952(75)90081-7. [DOI] [PubMed] [Google Scholar]
- Mullen J. B., Wright J. L., Wiggs B. R., Paré P. D., Hogg J. C. Structure of central airways in current smokers and ex-smokers with and without mucus hypersecretion: relationship to lung function. Thorax. 1987 Nov;42(11):843–848. doi: 10.1136/thx.42.11.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy D. I., Strittmatter W. J. Requirement for metalloendoprotease in exocytosis: evidence in mast cells and adrenal chromaffin cells. Cell. 1985 Mar;40(3):645–656. doi: 10.1016/0092-8674(85)90213-2. [DOI] [PubMed] [Google Scholar]
- Niles R. M., Christensen T. G., Breuer R., Stone P. J., Snider G. L. Serine proteases stimulate mucous glycoprotein release from hamster tracheal ring organ culture. J Lab Clin Med. 1986 Nov;108(5):489–497. [PubMed] [Google Scholar]
- Ohlsson K., Olsson I. The extracellular release of granulocyte collagenase and elastase during phagocytosis and inflammatory processes. Scand J Haematol. 1977 Aug;19(2):145–152. doi: 10.1111/j.1600-0609.1977.tb02339.x. [DOI] [PubMed] [Google Scholar]
- Ohlsson K., Tegner H. Granulocyte collagenase, elastase and plasma protease inhibitors in purulent sputum. Eur J Clin Invest. 1975 Jun 12;5(3):221–227. doi: 10.1111/j.1365-2362.1975.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Paul A., Picard J., Mergey M., Veissiere D., Finkbeiner W. E., Basbaum C. B. Glycoconjugates secreted by bovine tracheal serous cells in culture. Arch Biochem Biophys. 1988 Jan;260(1):75–84. doi: 10.1016/0003-9861(88)90426-2. [DOI] [PubMed] [Google Scholar]
- Peterson M. W., Stone P., Shasby D. M. Cationic neutrophil proteins increase transendothelial albumin movement. J Appl Physiol (1985) 1987 Apr;62(4):1521–1530. doi: 10.1152/jappl.1987.62.4.1521. [DOI] [PubMed] [Google Scholar]
- Phipps R. J., Denas S. M., Sielczak M. W., Wanner A. Effects of 0.5 ppm ozone on glycoprotein secretion, ion and water fluxes in sheep trachea. J Appl Physiol (1985) 1986 Mar;60(3):918–927. doi: 10.1152/jappl.1986.60.3.918. [DOI] [PubMed] [Google Scholar]
- REID L. Measurement of the bronchial mucous gland layer: a diagnostic yardstick in chronic bronchitis. Thorax. 1960 Jun;15:132–141. doi: 10.1136/thx.15.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y., Merrill W. W. Airway changes in young smokers that may antedate chronic obstructive lung disease. Med Clin North Am. 1981 May;65(3):667–689. doi: 10.1016/s0025-7125(16)31518-8. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- Schalkwijk J., Joosten L. A., van den Berg W. B., van de Putte L. B. Degradation of cartilage proteoglycans by elastase is dependent on charge-mediated interactions. Rheumatol Int. 1988;8(1):27–33. doi: 10.1007/BF00541347. [DOI] [PubMed] [Google Scholar]
- Schick B., Austen K. F. Rat serosal mast cell degranulation mediated by chymase, an endogenous secretory granule protease: active site-dependent initiation at 1 degree C. J Immunol. 1986 May 15;136(10):3812–3818. [PubMed] [Google Scholar]
- Schick B., Austen K. F., Schwartz L. B. Activation of rat serosal mast cells by chymase, an endogenous secretory granule protease. J Immunol. 1984 May;132(5):2571–2577. [PubMed] [Google Scholar]
- Selak M. A., Chignard M., Smith J. B. Cathepsin G is a strong platelet agonist released by neutrophils. Biochem J. 1988 Apr 1;251(1):293–299. doi: 10.1042/bj2510293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer J., Bigby B. G., Stulbarg M., Holtzman M. J., Nadel J. A., Ueki I. F., Leikauf G. D., Goetzl E. J., Boushey H. A. O3-induced change in bronchial reactivity to methacholine and airway inflammation in humans. J Appl Physiol (1985) 1986 Apr;60(4):1321–1326. doi: 10.1152/jappl.1986.60.4.1321. [DOI] [PubMed] [Google Scholar]
- Seltzer J., Scanlon P. D., Drazen J. M., Ingram R. H., Jr, Reid L. Morphologic correlation of physiologic changes caused by SO2-induced bronchitis in dogs. The role of inflammation. Am Rev Respir Dis. 1984 May;129(5):790–797. doi: 10.1164/arrd.1984.129.5.790. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Campbell E. J. Cathepsin G in human mononuclear phagocytes: comparisons between monocytes and U937 monocyte-like cells. J Immunol. 1984 May;132(5):2547–2551. [PubMed] [Google Scholar]
- Shore S. A., Kariya S. T., Anderson K., Skornik W., Feldman H. A., Pennington J., Godleski J., Drazen J. M. Sulfur-dioxide-induced bronchitis in dogs. Effects on airway responsiveness to inhaled and intravenously administered methacholine. Am Rev Respir Dis. 1987 Apr;135(4):840–847. doi: 10.1164/arrd.1987.135.4.840. [DOI] [PubMed] [Google Scholar]
- Smedly L. A., Tonnesen M. G., Sandhaus R. A., Haslett C., Guthrie L. A., Johnston R. B., Jr, Henson P. M., Worthen G. S. Neutrophil-mediated injury to endothelial cells. Enhancement by endotoxin and essential role of neutrophil elastase. J Clin Invest. 1986 Apr;77(4):1233–1243. doi: 10.1172/JCI112426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobonya R. E., Taussig L. M. Quantitative aspects of lung pathology in cystic fibrosis. Am Rev Respir Dis. 1986 Aug;134(2):290–295. doi: 10.1164/arrd.1986.134.2.290. [DOI] [PubMed] [Google Scholar]
- Sommerhoff C. P., Caughey G. H., Finkbeiner W. E., Lazarus S. C., Basbaum C. B., Nadel J. A. Mast cell chymase. A potent secretagogue for airway gland serous cells. J Immunol. 1989 Apr 1;142(7):2450–2456. [PubMed] [Google Scholar]
- Stockley R. A., Burnett D. Alpha,-antitrypsin and leukocyte elastase in infected and noninfected sputum. Am Rev Respir Dis. 1979 Nov;120(5):1081–1086. doi: 10.1164/arrd.1979.120.5.1081. [DOI] [PubMed] [Google Scholar]
- Suter S., Schaad U. B., Tegner H., Ohlsson K., Desgrandchamps D., Waldvogel F. A. Levels of free granulocyte elastase in bronchial secretions from patients with cystic fibrosis: effect of antimicrobial treatment against Pseudomonas aeruginosa. J Infect Dis. 1986 May;153(5):902–909. doi: 10.1093/infdis/153.5.902. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Imajoh S., Emori Y., Kawasaki H., Minami Y., Ohno S. Calcium-activated neutral protease and its endogenous inhibitor. Activation at the cell membrane and biological function. FEBS Lett. 1987 Aug 17;220(2):271–277. doi: 10.1016/0014-5793(87)80828-1. [DOI] [PubMed] [Google Scholar]
- Tetley T. D., Smith S. F., Burton G. H., Winning A. J., Cooke N. T., Guz A. Effects of cigarette smoking and drugs on respiratory tract proteases and antiproteases. Eur J Respir Dis Suppl. 1987;153:93–102. [PubMed] [Google Scholar]
- UVNAES B., ANTONSSON J. TRIGGERING ACTION OF PHOSPHATIDASE A AND CHYMOTRYPSINS ON DEGRANULATION OF RAT MESENTERY MAST CELLS. Biochem Pharmacol. 1963 Aug;12:867–873. doi: 10.1016/0006-2952(63)90117-5. [DOI] [PubMed] [Google Scholar]
- Varsano S., Basbaum C. B., Forsberg L. S., Borson D. B., Caughey G., Nadel J. A. Dog tracheal epithelial cells in culture synthesize sulfated macromolecular glycoconjugates and release them from the cell surface upon exposure to extracellular proteinases. Exp Lung Res. 1987;13(2):157–184. doi: 10.3109/01902148709064316. [DOI] [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Hoffstein S. Leukocytic proteases and the immunologic release of lysosomal enzymes. Am J Pathol. 1972 Sep;68(3):539–564. [PMC free article] [PubMed] [Google Scholar]
- Weitz J. I., Crowley K. A., Landman S. L., Lipman B. I., Yu J. Increased neutrophil elastase activity in cigarette smokers. Ann Intern Med. 1987 Nov;107(5):680–682. doi: 10.7326/0003-4819-107-5-680. [DOI] [PubMed] [Google Scholar]







