Abstract
The use of microneedles for transdermal drug delivery is limited due to the risk of infection associated with formation of channels through the stratum corneum layer of the epidermis. The risk of infection associated with use of microneedles may be reduced by imparting these devices with antimicrobial properties. In this study, a photopolymerization-micromolding technique was used to fabricate microneedle arrays from a photosensitive material containing polyethylene glycol 600 diacrylate, gentamicin sulfate, and a photoinitiator. Scanning electron microscopy indicated that the photopolymerization-micromolding process produced microneedle arrays that exhibited good microneedle-to-microneedle uniformity. An agar plating assay revealed that microneedles fabricated with polyethylene glycol 600 diacrylate containing 2 mg mL−1 gentamicin sulfate inhibited growth of Staphylococcus aureus bacteria. Scanning electron microscopy revealed no platelet aggregation on the surfaces of platelet rich plasma-exposed undoped polyethylene glycol 600 diacrylate microneedles and gentamicin-doped polyethylene glycol 600 diacrylate microneedles. These efforts will enable wider adoption of microneedles for transdermal delivery of pharmacologic agents.
One novel mechanism for transdermal delivery of pharmacologic agents involves the use of microneedles.[1] Microneedles are structures that are similar in shape to hypodermic needles or lancets; these devices exhibit at least one dimension that is less than 1 mm in length. Bal et al. recently demonstrated that hollow and solid metal microneedles were able to penetrate ventral forearm skin in human volunteers; these devices were shown to disrupt the stratum corneum layer of the epidermis with minimal irritation and absence of pain.[2] Bacteria such as Staphylococcus aureus reside on the skin surface; a pore in the stratum corneum created by a microneedle could enable microorganisms to enter the circulatory system and cause systemic infection.[3] Sato et al. demonstrated that pathogenic microorganisms may be transferred from the surface of the skin to deeper regions of the skin with conventional hypodermic needles.[4] Birchall described the risk of infection associated with penetration of the stratum corneum by a microneedle.[5] He suggested that damage to the stratum coreum is transient and is relatively minor in nature; however, he also noted that mechanisms of skin repair in response to microneedle insertion are not completely understood.
Antimicrobial microneedles may be fabricated by combining biocompatible polymers such as polyethylene glycol with conventional antimicrobial agents such as gentamicin sulfate. One common method for fabricating polyethylene glycol structures involves free-radical photopolymerization of polyethylene glycol diacrylate macromers using ultraviolet light or visible light photoinitiators.[6] Miyano et al. demonstrated that polyethylene glycol microneedles penetrated a living human skin model, which suggested that the needles exhibited sufficient mechanical strength to form conduits through the stratum corneum.[7] Takano et al. recently demonstrated insertion of polyethylene glycol microneedles into a wet cultured human skin model that was placed under biaxial tension; in their study, shortening of inserted microneedles due to hydrolysis was observed.[8]
Gentamicin sulfate is utilized as a broad spectrum antimicrobial agent for treatment of pyodermas and other skin infections due to its efficacy against Gram negative organisms as well as some Gram positive organisms. For example, Changez et al. demonstrated that gentamicin is active against Staphylococcus, a pathogenic organism that is commonly found on the skin.[9] They demonstrated that the minimum inhibitory concentration, the lowest concentration showing no visible growth after incubation at 37°C for 18 h, of gentamicin for S. aureus (ATCC 259523) was 0.4 mg ml−1; they also determined that the minimum bactericidal concentration (MBC) and the break point sensitivity of gentamicin for S. aureus were 0.6 and 1 mg ml−1, respectively. Several biomedical polymers, including poly (methyl methacrylate), polylactic acid, polyglycolic acid, and polyethylene glycol, have been combined with gentamicin sulfate.[10,11] Gentamicin is extremely hydrophilic, which enables aqueous suspensions containing polyethylene glycol and gentamicin to be readily produced.[12]
Gentamicin-doped polyethylene glycol was made by mixing SR 610 polyethylene glycol 600 diacrylate (Sartomer, Paris, France) with 2wt% Irgacure 369 photoinitiator (Ciba Specialty Chemicals, Basel, Switzerland). Aqueous 10 mg mL−1 gentamicin sulfate (MP Biomedicals, Solon, OH) was subsequently added to this mixture in order to obtain a 2 mg mL−1 concentration. A mixture containing only polyethylene glycol and 2wt% Irgacure 369 photoinitiator was also prepared for comparison purposes. A photopolymerization-micromolding process was used to fabricate microneedle arrays from undoped polyethylene glycol 600 diacrylate and gentamicin-doped polyethylene glycol 600 diacrylate.
Details on the photopolymerization-micromolding process have been previously reported in ref.[13,14] A microneedle array master structure was fabricated with SR 259 polyethylene glycol dimethacrylate (Sartomer, Paris, France) containing 2wt% Irgacure 369 photoinitiator on a silanized glass cover slip substrate using two photon polymerization. In two photon polymerization, temporal and spatial overlap of photons enables polymerization and material hardening within well-defined and highly localized volumes. Femtosecond laser pulses (60 fs, 94 MHz, <450mW, 780 nm) from a titanium: sapphire laser (Kapteyn-Murnane, Boulder, CO) were focused using a 5 × microscope objective. The position of the laser focus was moved using a galvanoscanner and three translational stages. A HurrySCAN galvo-scanner (Scanlab AG, Puchheim, Germany) was used to control the laser focus position in the X-Y plane and three C-843 linear translational stages (Physik Instrumente, Karlsruhe, Germany) were utilized to control the laser focus position in three dimensions. A computer-aided design program was used in order to create stereolithography (STL) files, which guided the two photon polymerization process. The master structure was a five-by-five array of solid microneedles, in which the microneedles were separated by a 500 μm needle center-to-needle center spacing. Each microneedle in the array was designed with a length of 500 μm, a base diameter of 150 μm, and a tip angle of 45°. Due to their larger surfaces, arrays of microneedles provide drug delivery in higher amounts and over larger regions than solitary needles.[15] Microneedle arrays may be designed with redundant structures in case some of the microneedles are fractured or obstructed during insertion into the skin. The microneedle array was sputtered with a gold coating (sputter time = 245 s, sputter current =10 mA) in order to enhance separation of the mold from the master structure. It was subsequently attached to a glass microscope slide with double-sided tape.
This master structure was subsequently used to fabricate a polydimethylsiloxane (PDMS) mold. Sylgard® 184 silicone elastomer and curing agent (Dow Corning, Midland, MI) were mixed in a 10:1 weight ratio. This mixture was degassed under vacuum. A 20 mm diameter aluminum seal (Sigma Aldrich, St. Louis, MO) was positioned over the master structure. The mixture was poured over the array until the seal was completely filled. 100mbar vacuum was applied to the glass slide containing the master structure, aluminum seal, and unpolymerized PDMS mixture. The glass slide was subsequently placed on a heat plate. Thermal curing of the PDMS mixture was obtained by increasing the heat plate temperature from room temperature to 100 °C over 30 min and then maintaining the heat plate temperature at 100 °C for 30 min. After the polymerized PDMS mold had cooled, the glass slide was clamped to a table using two metal restraining bars. The aluminum seal was attached to a vertical actuator using screws. The PDMS mold and aluminum seal were separated from the master structure by lifting the PDMS mold away from the table using the vertical actuator. The polymethylsiloxane mold was ultrasonically cleaned in ethanol for 15 min.
Microneedle arrays were fabricated from the PDMS mold using a micromolding process. ∼50 μL of either gentamicin-doped polyethylene glycol 600 diacrylate or undoped polyethylene glycol 600 diacrylate was placed on the surface of the PDMS mold. The PDMS mold containing the unpolymerized polymer mixture was placed under 100 mbar vacuum for ∼5 min. The mold containing the unpolymerized polymer mixture was degassed under vacuum in order to enable the mixture to completely fill the mold. This degassing step removed air from the unpolymerized polymer mixture in the PDMS mold; trapped air may result in the formation of incomplete microneedles, missing microneedle tips, and other microneedle defects. The PDMS mold containing the unpolymerized polymer mixture was then pressed against a glass cover slip, which in turn was attached to a microscope slide using double-sided tape. The mold and the slide were fitted into a vertical actuator. The unpolymerized polymer mixture was then exposed to an ultraviolet curing lamp, which provided visible and ultraviolet light emission (Thorlabs, Newton, NJ). ATR-FTIR studies by Witte et al. have shown that complete photopolymerization of polyethylene glycol 600 diacrylate occurs with less than 3 min of ultraviolet light exposure.[16] After ultraviolet curing was complete, the microneedle array was separated from the PDMS mold using the vertical actuator.
A S3200 scanning electron microscope (Hitachi, Tokyo, Japan) equipped with a Robinson back scattered electron detector was used to examine microneedle arrays fabricated with gentamicin-doped polyethylene glycol 600 diacrylate and undoped polyethylene glycol 600 diacrylate. Fourier transform infrared spectroscopy (FTIR) was performed on cylindrical wafers of gentamicin-doped polyethylene glycol 600 diacrylate or undoped polyethylene glycol 600 diacrylate using a Nexus 470 system equipped with an OMNI sampler and a continuum microscope (Thermo Fisher, Waltham, MA). Analysis of the absorption spectra was performed using OMNIC™ software (Thermo Fisher, Waltham, MA). Platelet adhesion testing is an in vitro technique that is commonly used for examining adsorption of platelets, proteins, and other blood components to the surface of a biomaterial.[17] Fresh whole blood was obtained from a healthy adult volunteer. The blood was evaluated for the presence of anticoagulants and other pharmacologic agents that could affect protein-biomaterial or platelet-biomaterial interaction using platelet function, prothrombin time, and partial thromboplastic time studies. Sodium citrate was added to the fresh whole blood in order to prevent coagulation. The blood was centrifuged using a Plasma Saver system (Hemonetics, Braintree, MA) at 25 °C for 10 min, 25 °C for 10 min, and then at 4 °C for 1 h (operating speed = 3500 rpm). In this multiple-step centrifugation process, the first spin process served to separate low-platelet concentrated plasma from platelet rich plasma and red blood cells.[18] The mixture of platelet rich plasma and red blood cells was separated, and the platelet rich plasma was subsequently collected at the bottom of the test tube because of its high specific gravity. The platelet rich plasma was incubated at 37°C for 10 min and then frozen until testing. Microneedle arrays fabricated with gentamicin-doped polyethylene glycol 600 diacrylate or undoped polyethylene glycol 600 diacrylate were immersed in the platelet rich plasma solution and incubated at 37°C for 10 min. Weakly adherent platelets were removed by rinsing the microneedle arrays with 0.9% saline solution. The platelet rich plasma-exposed microneedle arrays were subsequently fixed in 4% glutaraldehyde and critical point dried. Scanning electron microscopy and energy dispersive X-ray spectroscopy were performed to examine protein and platelet adhesion on the platelet rich plasma-exposed microneedle arrays.
The antimicrobial properties of microneedle arrays fabricated with gentamicin-doped polyethylene glycol 600 diacrylate or undoped polyethylene glycol 600 diacrylate were evaluated using an agar plating method. The agar plating method is similar to a method that was outlined by the National Committee for Clinical Laboratory Standards and is used in clinical laboratories across the world, in which an antimicrobial agent diffuses outward from a disk over 18 h and the zone of inhibition is subsequently determined.[19] The agar plating method is appropriate for most pathogenic bacteria and provides qualitative data regarding susceptibility of microorganisms to a given antimicrobial agent. Overnight cultures of S. aureus ATCC 25923 (American Type Culture Collection, Manassas, VA) in tryptic soy broth (TSB) (VWR International, West Chester, PA) were centrifuged at 4500 rpm for 10 min. The resulting pellets were resuspended in 1X phosphate-buffered saline (PBS) in order to obtain a final cell density of ∼108 cells ml−1. 1× phosphate-buffered saline solution was prepared by diluting 10× phosphate-buffered saline (VWR International, West Chester, PA) using deionized water. A sterile swab was used in order to inoculate a lawn of the bacterium on tryptic soy agar plates (VWR International, West Chester, PA). Microneedle arrays fabricated with gentamicin-doped polyethylene glycol 600 diacrylate or undoped polyethylene glycol 600 diacrylate were then placed on the inoculated plates, in which the needles were projected into the agar. The plates were inverted and incubated for 24 h at 37°C. The microneedle arrays were subsequently removed from the agar. Inhibition of microbial growth was determined using optical microscopy.
Scanning electron micrographs of microneedle arrays fabricated with undoped polyethylene glycol 600 diacrylate and gentamicin-doped polyethylene glycol 600 diacrylate are shown in Figure 1(a) and 1(b), respectively. The microneedles in both arrays exhibited good correspondence with the CAD microneedle designs; in addition, good microneedle-to-microneedle uniformity was observed in both arrays. The microneedle arrays fabricated by photopolymerization-micromolding also exhibited good correspondence with the master structure. The microneedles in both arrays demonstrated base diameters of 150 μm, lengths of 500 μm, tip angles of 45°, and >10 μm tip diameters. Voids within microneedles and truncated microneedle tips were observed in a small fraction of the microneedles within the microneedle arrays; these features were attributed to incomplete degassing of the unpolymerized polyethylene glycol solution. Energy-dispersive X-ray spectroscopy found that gentamicin-doped polyethylene glycol 600 diacrylate microneedle arrays and undoped polyethylene glycol 600 diacrylate microneedle arrays were comprised of carbon, oxygen, and hydrogen. This finding confirms that other elements were not introduced during the photopolymerization-micromolding process.
Fig. 1.
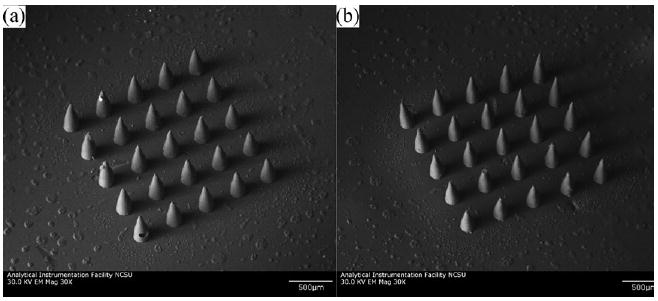
Scanning electron micrographs of microneedle arrays. (a) Microneedle array fabricated with undoped polyethylene glycol 600 diacrylate. (b) Microneedle array fabricated with gentamicin-doped polyethylene glycol 600 diacrylate.
The FTIR spectrum of photo-initiated polymerization of polyethylene glycol 600 diacrylate in the presence of Irgacure 369 photoinitiator (2-benzyl-2-dimethylamino-1-(4-morpholinophenyl)- butanone-1) contained large sharp peaks at 2870, 1732, and 1102 cm−1 as well as a broad peak at 3519 cm−1; this spectrum showed good correspondence with absorption spectra of polyethylene glycol 600 diacrylate prepared by other methods (e.g., redox initiation).[20–22] The addition of gentamicin sulfate to polyethylene glycol 600 diacrylate did not significantly alter the absorption spectrum (Fig. 2). Peaks at 1740 and 1102 cm−1 were attributed to stretching vibration of the aliphatic carbonyl groups and the ether groups, respectively.[22,23] Irgacure 369 photoinitiator forms radicals by means of a Norrish type I mechanism, which involves homolytic alpha cleavage between the carbonyl group and the α carbon atom upon photoexcitation.[24]
Fig. 2.
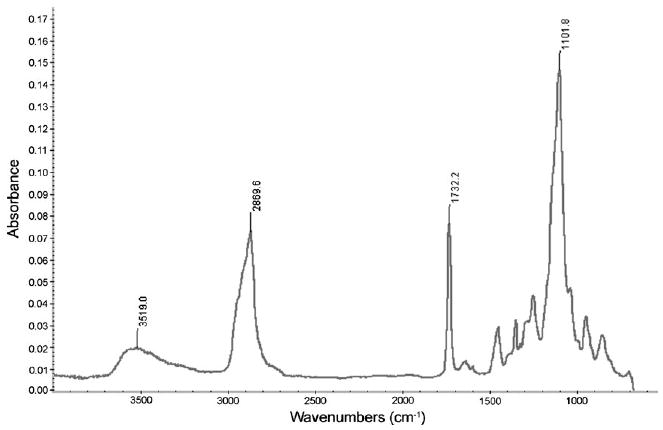
Fourier transform infrared spectrum of gentamicin-doped polyethylene glycol 600 diacrylate.
Scanning electron micrographs of microneedle arrays fabricated using undoped polyethylene glycol 600 diacrylate and gentamicin-doped polyethylene glycol 600 diacrylate after exposure to platelet rich plasma are provided in Figure 3(a) and 3(b), respectively. No protein aggregation was observed on the gentamicin-doped polyethylene glycol 600 diacrylate microneedle array and the undoped polyethylene glycol 600 diacrylate microneedle array. In addition, no platelet aggregation was observed on the gentamicin-doped polyethylene glycol 600 diacrylate microneedle array and the undoped polyethylene glycol 600 diacrylate microneedle array. Metal-containing materials exposed to platelet rich plasma under similar conditions demonstrated a dense fibrin network with aggregated platelets.[17] Small, widely scattered crystals were observed on the surfaces of the gentamicin-doped polyethylene glycol 600 diacrylate microneedle array and the undoped polyethylene glycol 600 diacrylate microneedle array. The presence of sodium, chlorine, and phosphorus in the energy dispersive X-ray spectra suggests that sodium chloride crystals precipitated from the platelet rich plasma. Precipitation of sodium chloride crystals from platelet rich plasma appears to be autonomous from platelet and protein adsorption processes. Previous studies have shown that polyethylene glycol is resistant to adhesion of cells and adsorption of proteins; for example, Moon et al., Delong et al., and Nagaoka et al. demonstrated that polyethylene glycol diacrylate-based materials exhibit nonspecific resistance to adhesion of cells, adsorption of proteins, and formation of thrombi.[26–28] The ability of polyethylene glycol surfaces to resist proteins and other biological molecules is attributed to formation of hydrogen bonds with water molecules and is enhanced by steric stabilization. Steric stabilization is a repulsive force that results from: (i) the loss in conformational freedom of polyethylene glycol chains when proteins move closer to polyethylene glycol surfaces and (ii) osmotic interactions between proteins and polyethylene glycol surfaces.[29] These properties are appealing for microneedle-based devices, since protein fouling and cell adhesion can impede transport of pharmacologic agents from the microneedle to the surrounding tissues.
Fig. 3.

Scanning electron micrographs of microneedle arrays after exposure to platelet rich plasma. (a) Microneedle array fabricated with undoped polyethylene glycol 600 diacrylate. (b) Microneedle array fabricated with gentamicin-doped polyethylene glycol 600 diacrylate.
The agar plating assay results for microneedle arrays fabricated using undoped polyethylene glycol 600 diacrylate and gentamicin-doped polyethylene glycol 600 diacrylate are shown in Figure 4(a) and 4(b), respectively. The microneedle arrays were removed from the agar plates in order to facilitate imaging of microbial growth. No zone of inhibition was observed for the microneedle array fabricated with undoped polyethylene glycol 600 diacrylate; S. aureus growth was observed over the entire agar plate, including underneath the microneedle array. The microneedle array fabricated with gentamicin-doped polyethylene glycol 600 diacrylate had a circular zone of inhibition (diameter = 26.8 mm). The zone of inhibition indicated that gentamicin sulfate was released from polyethylene glycol 600 diacrylate during the agar plating assay. Acellular bubble-like structures were observed on the agar surface, which were attributed to hydrogel degradation. Previous work by Matsuda et al. demonstrated that PEG-based microneedles and other microstructures undergo degradation at the surface and in the bulk due to absorption of water.[30] According to Miyano et al., there are minimal concerns regarding fracture of polyethylene glycol microneedles during insertion since hydrolytic degradation of polyethylene glycol microneedles may occur on the skin surface.[7] Takano et al. describe retention of polyethylene glycol microneedles on the skin surface for extended periods of time; for example, hydrolysis of polyethylene glycol microneedles on the skin surface may allow for gradual release of antimicrobial agents as well as other pharmacologic agents.[8] Disposal of polyethylene glycol microneedles may be performed by means of boiling or other low cost procedures.[7]
Fig. 4.
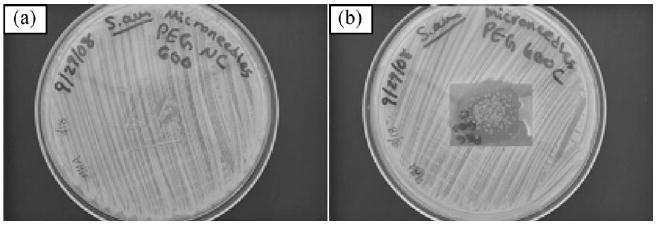
Agar plating assay results. (a) Microneedle array fabricated with undoped polyethylene glycol 600 diacrylate against Staphylococcus aureus. (b) Microneedle array fabricated with gentamicin-doped polyethylene glycol 600 diacrylate against Staphylococcus aureus. The zone of inhibition for the microneedle array fabricated with gentamicin-doped polyethylene glycol 600 diacrylate was 26.8 mm in diameter. The bubble-like structures were acellular and were attributed to hydrogel degradation.
In this study, microneedle arrays with antimicrobial properties were fabricated using a photosensitive material containing polyethylene glycol 600 diacrylate, gentamicin sulfate, and a photoinitiator. The photopolymerization-micromolding process was used to produce microneedle arrays that exhibited good microneedle-to-microneedle uniformity. An agar plating assay revealed that the microneedle array fabricated using gentamicin-doped polyethylene glycol 600 diacrylate inhibited growth of S. aureus bacteria. A 26.8 mm diameter zone of inhibition was observed, which indicated that gentamicin sulfate was released from the microneedle array. In addition, no platelet aggregation was observed on the surface of the platelet rich plasma-exposed undoped polyethylene glycol 600 diacrylate microneedles and gentamicin-doped polyethylene glycol 600 diacrylate microneedles. The results of this study suggest that gentamicin-doped polyethylene glycol 600 diacrylate may be used to fabricate microneedles and other medical devices that exhibit antimicrobial functionality.
Contributor Information
Shaun D Gittard, Joint Department of Biomedical Engineering, University of North Carolina, and North Carolina State University, Chapel Hill, Raleigh, NC 27599-7115, USA.
Aleksandr Ovsianikov, Department of Nanotechnology, Laser Zentrum Hannover, Hollerithalle 8, 30419 Hannover, Germany.
Hasan Akar, Department of Nanotechnology, Laser Zentrum Hannover, Hollerithalle 8, 30419 Hannover, Germany.
Boris Chichkov, Department of Nanotechnology, Laser Zentrum Hannover, Hollerithalle 8, 30419 Hannover, Germany.
Nancy A Monteiro-Riviere, Joint Department of Biomedical Engineering, University of North Carolina, and North Carolina State University, Chapel Hill, Raleigh, NC 27599-7115, USA; Center for Chemical Toxicology Research and Pharmacokinetics, North Carolina State University, Raleigh, NC 27695, USA.
Shane Stafslien, Center for Nanoscale Science and Engineering, North Dakota, State University, 1805 Research Park Drive, Fargo, ND, 58102, USA.
Bret Chisholm, Center for Nanoscale Science and Engineering, North Dakota, State University, 1805 Research Park Drive, Fargo, ND, 58102, USA.
Chun-Che Shin, Institute of Clinical Medicine, School of Medicine, National Yang-Ming University, Taipei 112, Taiwan; Division of Cardiovascular Surgery, Taipei Veterans General Hospital, Taipei 112, Taiwan; Cardiovascular Research Center, National Yang-Ming University, Taipei 112, Taiwan.
Chun-Ming Shih, Graduate Institute of Medical Sciences, School of Medicine, Taipei Medical University, Taipei 110, Taiwan.
Shing-Jong Lin, Institute of Clinical Medicine, School of Medicine, National Yang-Ming University, Taipei 112, Taiwan; Cardiovascular Research Center, National Yang-Ming University, Taipei 112, Taiwan; Division of Cardiology, Taipei Veterans General Hospital, Taipei 112, Taiwan.
Yea-Yang Su, Institute of Clinical Medicine, School of Medicine, National Yang-Ming University, Taipei 112, Taiwan.
Roger J Narayan, Email: roger_narayan@msn.com, Joint Department of Biomedical Engineering, University of North Carolina, and North Carolina State University, Chapel Hill, Raleigh, NC 27599-7115, USA.
References
- 1.Arora A. Int J Pharm. 2008;364:227. doi: 10.1016/j.ijpharm.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bal SM. Eur J Pharm Sci. 2008;35:193. doi: 10.1016/j.ejps.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 3.Kalia YN. J Invest Dermtol. 1998;111:320. doi: 10.1046/j.1523-1747.1998.00289.x. [DOI] [PubMed] [Google Scholar]
- 4.Sato S. Anesthesiology. 1996;85:1276. doi: 10.1097/00000542-199612000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Birchall JC. Exp Rev Med Dev. 2006;3:1. doi: 10.1586/17434440.3.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Gabler S. Int J Mater Eng Innov. 2009;1:1. [Google Scholar]
- 7.Miyano T. Solid-State Sensors, Actuators and Microsystems Conference, IEEE; Los Alamitos, CA. 2007. [Google Scholar]
- 8.Takano N. J Solid Mech Mater Eng. 2009;3:604. [Google Scholar]
- 9.Changez M. Biomaterials. 2004;25:139. doi: 10.1016/s0142-9612(03)00466-6. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn KD. J Pharm Biomed Anal. 2008;48:612. doi: 10.1016/j.jpba.2008.05.041. [DOI] [PubMed] [Google Scholar]
- 11.Brin YS. J Biomater Sci. 2009;20:1081. doi: 10.1163/156856209X444439. [DOI] [PubMed] [Google Scholar]
- 12.Naraharisetti PK. J Control Rel. 2005;102:345. doi: 10.1016/j.jconrel.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 13.Doraiswamy A. Acta Biomater. 2006;2:267. doi: 10.1016/j.actbio.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Gittard SD. J Diabetes Sci Tech. 2009;3:304. doi: 10.1177/193229680900300211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.J H, Gardeniers GE. J Microelectromech Syst. 2003;12:855. [Google Scholar]
- 16.Witte RP. J Biomed Mater Res. 2004;71A:508. doi: 10.1002/jbm.a.30179. [DOI] [PubMed] [Google Scholar]
- 17.Andara M. Diamond Relat Mater. 2006;15:1941. [Google Scholar]
- 18.Lozada JL. J Oral Implantol. 2001;27:38. doi: 10.1563/1548-1336(2001)027<0038:PPAISG>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 19.Performance standards for antimicrobial disk susceptibility tests (M2-A6) National Committee for Clinical Laboratory Standards; Wayne, PA: 1997. [Google Scholar]
- 20.Tan GX. Polymer Bull. 2008;61:91. [Google Scholar]
- 21.Imani M. Iranian Polymer J. 2007;16:13. [Google Scholar]
- 22.Zhang X. Int J Biol Macromol. 2008;43:456. doi: 10.1016/j.ijbiomac.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Dyer JR. Applications of absorption spectroscopy of organic compounds. Vol. 1 Prentice Hall; Englewood Cliffs, NJ: 1965. [Google Scholar]
- 24.Houbertz R. Appl Surf Sci. 2005;247:504. [Google Scholar]
- 25.Andrade JD. Adv Chem Ser. 1996;248:51. [Google Scholar]
- 26.DeLong SA. Biomaterials. 2005;26:3227. doi: 10.1016/j.biomaterials.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 27.Moon JJ. Tissue Eng A. 2009;15:579. doi: 10.1089/ten.tea.2008.0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagaoka S. Biomaterials. 1990;11:119. doi: 10.1016/0142-9612(90)90126-b. [DOI] [PubMed] [Google Scholar]
- 29.Atha DH. J Biol Chem. 1981;256:2108. [PubMed] [Google Scholar]
- 30.Matsuda T. J Biomed Mater Res. 2002;62:395. doi: 10.1002/jbm.10295. [DOI] [PubMed] [Google Scholar]


