SUMMARY
Dehydration, and consequent intracellular hyperosmolarity, is a major challenge to land organisms, as it is associated with extraction of water from cells and disturbance of global cellular function. Organisms have thus developed a highly conserved regulatory mechanism that transduces the hyperosmolarity signal from the cell surface to the cell nucleus and adjusts the expression of cellular osmolarity-regulating genes. We recently found that the Rho-type guanine nucleotide exchange factor Brx, or AKAP13, is essential for osmotic stress-stimulated expression of nuclear factor of activated T-cells 5 (NFAT5), a key transcription factor of intracellular osmolarity. It accomplishes this by first attracting cJun kinase (JNK)-interacting protein (JIP) 4 and then coupling activated Rho-type small G-proteins to cascade components of the p38 MAPK signaling pathway, ultimately activating NFAT5. We describe the potential implications of osmotic stress and Brx activation in organ physiology and pathophysiology and connect activation of this system to key human homeostatic states.
Keywords: glucocorticoids, glucocorticoid receptor (GR), guanine nucleotide exchange factor (GEF), hyperosmolarity, cJun kinase-interacting protein (JIP), mitogen-activated protein kinase, nuclear factor of activated T-cells 5, small GTP-binding protein (small G-protein)
EXPERT COMMENTARY
1. Introduction
Dehydration represents a major challenge to land organisms as they evolved from life in the sea, an environment where organisms maintain their body fluid and extracellular osmolarity against surrounding seawater [1]. In organisms that live mainly on land, however, such as amphibians and mammals, dehydration is associated with extraction of water from their bodies and resultant extracellular and consequently intracellular hyperosmolarity. Thus, land organisms developed several biologic mechanisms for counteracting environmental dehydration through their evolution process from sea organisms. Osmolarity, or osmotic pressure, is mainly determined by net concentrations of soluble molecular particles, such as sodium, chloride and potassium, in addition to some small organic molecules, including derivatives and metabolites of sugars, amino acids, nucleic acids, peptides and micelle-type molecules like lipid carrying lipoproteins [2]. Since these small molecules cannot pass through the plasma membrane, extracellular hyperosmolarity or increased concentration of these molecules causes imbalance of osmotic pressure between the extracellular and intracellular compartments, resulting in a shift of water from the intra- to the extracellular space, until osmotic pressure equilibrates [2,3]. Loss of intracellular water disrupts various cellular functions, such as DNA repair and protein translation, mitochondrial function, cell cycle progression and consequent inhibition of proliferation and induction of secondary oxidative stress and apoptosis mechanisms [3,4], by condensing and denaturing intracellular molecules and by altering subcellular architecture [2].
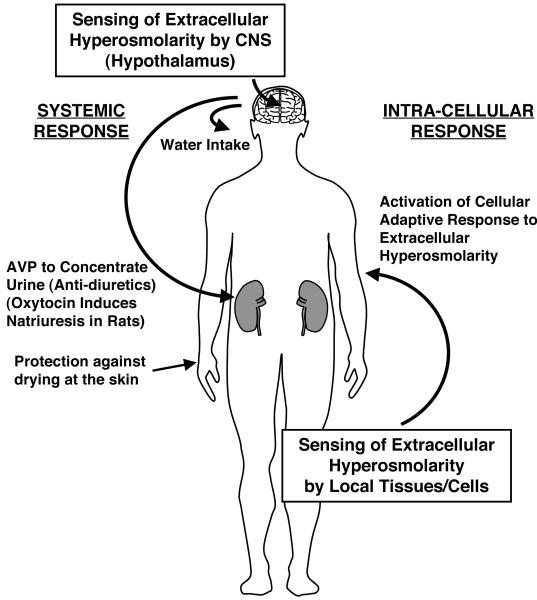
To avoid dehydration of the body, land organisms, including humans, have developed several systemic mechanisms for reducing water loss from their bodies (Figure 1). They thus decrease water evaporation from their body surface by a keratinized, lipid-containing outermost layer of the skin, the stratum corneum [5]. Once these organisms sense extracellular hyperosmolarity with their systemic osmosensor located in the hypothalamus of the central nervous system, they secrete arginine vasopressin (AVP) (and oxytocin in rats) from the posterior lobe of the pituitary gland, which in turn causes the kidney to concentrate urine and minimize water loss (anti-diuresis) [6]. Oxytocin also causes natriuresis in the kidney to reduce circulating levels of sodium in rats [6].
Figure 1.
Systemic and intra-cellular adaptive response to dehydration and subsequent extracellular/intracellular hyperosmolarity.
Humans have developed multi-layered protective mechanisms to counteract extracellular hyperosmolarity. For instance, they avoid water loss from the surface of the body is reduced by keratinized skin. Once extracellular hyperosmolarity is sensed by the central nervous system, the brain stimulates neuronal circuits activating water intake and secretion of the anti-diuretic hormone arginine vasopressin (AVP) from the posterior lobe of the pituitary gland to produce highly concentrated urine in the kidney. Oxytocin is also secreted in response to extracellular hyperosmolarity in rodents and induces natriuresis and hence excretion of electrolytes. Extracellular hyperosmolarity also stimulates/induces intracellular NFAT5 and activates a cellular adaptive response to osmotic stress, by inducing several osmotic stress-responsive genes and causing accumulation of organic osmolytes to equilibrate osmotic difference across the plasma membrane.
AVP: arginine vasopressin
In addition to these systemic protective mechanisms for dehydration, land organisms possess the intracellular machinery to protect their tissues and cells from extracellular hyperosmolarity, a universal cellular mechanism seen in virtually all organisms on earth [2-4]. This regulatory system -an adaptive response to osmotic stress-is very ancient and highly conserved throughout phylogeny from prokaryotic bacteria and eukaryotic yeast to humans [2]. This system, although not well elucidated as yet, senses elevation of extracellular osmolarity (osmotic stress) at the cellular surface level, transduces the signal through the cytoplasm to the nucleus, changes the transcription rates of osmotic stress-regulatory genes and accumulates organic molecules inside the cells to counterbalance the difference in osmotic pressure across the plasma membrane [2-4,7]. Although not entirely understood and still associated with controversy, recent intensive research has indicated that this regulatory system may include the guanine nucleotide exchange factor Brx and a downstream Rel-homology domain-containing transcription factor, the nuclear factor of activated T-cells 5 (NFAT5) [4,8-11]: Upon elevation of extracellular osmolarity, Brx activates Rho-type small guanine nucleotide triphosphate (GTP)-binding proteins (G-proteins) and p38 mitogen-activated protein kinase (MAPK), ultimately stimulating the transcriptional activity of NFAT5 on osmotic stress-regulatory genes, specifically aldose reductase, sodium/myoinositol cotransporter and sodium/chloride/betaine cotransporter to produce/uptake small organic osmolytes [8].
This intracellular adaptive system appears to be functional in virtually all human organs and tissues, in addition to renal medullary epithelial cells, which are continuously exposed to hyperosmotic intra-tubular fluid [9]. Specifically, studies have shown that T- and B-lymphocytes [8,12-14], macrophages [15], neurons [16], myoblasts [17] and embryonic fibroblasts [18] use this machinery to respond to extracellular hyperosmolarity. Gut, cornea, hepatocytes and epithelial cells lining the inter-vertebral discs/joints are all exposed to high osmolar fluids [9] (Table 1), and most likely possess this regulatory system to respond to cellular changes associated with extracellular hyperosmolarity, however this remains to be shown. In this review, we describe recent progress in the signaling cascade organizing the adaptive response to osmotic stress and its implications for organ physiology and pathophysiology in several human physiologic and pathologic states.
Table 1. Tissues and body fluids known to demonstrate elevated osmolarity.
| Species | Tissues/body fluids | Associated physiologic/pathologic conditions | Osmolarity (mOsm/kg H2O) Mean ± S.E. (or distribution) |
References |
|---|---|---|---|---|
| Human | Serum | Diabetes mellitus (ketoacidosis) | 308 ± 4.9 | [69] |
| Uremia | 318 ± 6.3 | [70] | ||
| Dehydration after exercise | [71] | |||
| Heat stroke | 297 ± 1.0 | [72] | ||
| Fatal burn | 312 ± 22.1 | [73] | ||
| Inflammatory sites | Experimental inflammation induced by subcutaneous injection of BCG |
400-600 | [68] | |
| Tear | Keratoconjunctivitis sicca | 343 ± 32.3 | [100,101] | |
| Synovial (joint) fluid | Normal subjects | 404 ± 57 | [81] | |
| Faecal fluid | Under normal diet | 372 ± 11.4 | [102] | |
| Pig | Uterine fluids | Embryonic development in uteri | 320 ± 32 | [103] |
| Mouse | Spleen | Hyperosmotic microenvironment may be necessary for lymphocyte development and differentiation |
330-340 | [13] |
| Thymus | Hyperosmotic microenvironment may be necessary for lymphocyte development and differentiation |
320-330 | [13] | |
| Liver | 330-340 | [13] |
BCG: Bacillus Calmette-Guérin
2. The signaling system responsible for organizing adaptive response to osmotic stress
Yeast cells
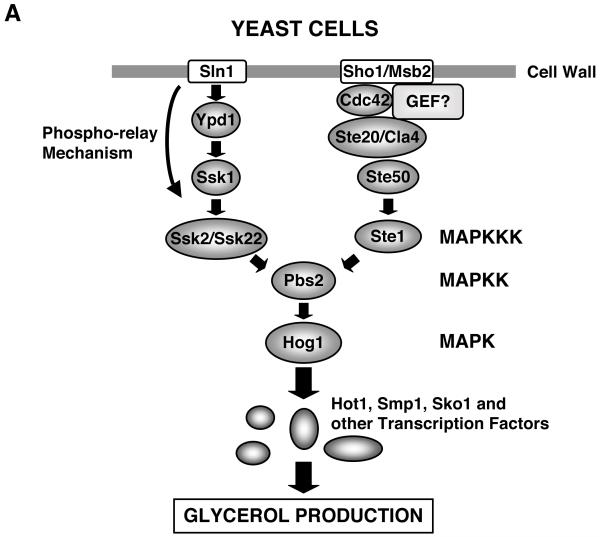
Since yeasts are immotile and frequently encounter hyperosmotic environments, as for instance in ripening fruits, these organisms have a highly active and sophisticated regulatory system for counteracting hyperosmolarity [19,20]. In response to osmotic stress, the budding yeast Saccharomyces cerevisiae produces hyperosmotic glycerol through activation of a signaling complex called the high osmolarity glycerol (Hog) pathway (Figure 2 A) [20-23]. This system has two signaling branches initiated respectively by structurally distinct and functionally independent cell surface osmosensing receptors, Sln1 (Sln1 branch) and Sho1/Hkr1-Msb2 (Sho1 branch), and subsequently activates the Hog mitogen-activated protein kinase (MAPK), a yeast homolog of the mammalian p38 MAPK [19,21,23,24], to stimulate further downstream transcription factors, such as Hot, Smp1, Sko1 and others [22]. The Sln1 branch employs a phospho-relay mechanism to activate Ssk2/Ssk22 MAPK kinase kinases (MAPKKKs) using several intermediate molecules, including Ypd1 and Ssk1 [25], while the Sho1 branch uses the Rho-type small G-protein Cdc42 and downstream kinases Ste20/Cla4 and Ste50 to stimulate Ste1 MAPKKK [20,26]. Independent signals of Ssk2/Ssk22 and Ste1 MAPKKK converge into activation of downstream MAPKK Pbs2 to finally stimulate Hog MPAK [20]. In the Sho1 branch, Cdc42 co-operates with Ste50 and Sho1 and acts as an adaptor for mediating osmotic stimuli-induced signal from Ste20/Cla4 to Ste11 and Pbs2 [23].
Figure 2.
Signal transduction systems for the adaptive response to osmotic stress in yeast and mammalian cells.
A: Intracellular signaling system for sensing and responding to extracellular hyperosmolarity in yeast.
Yeast cells have two branches (Sln1 and Sho1 branches) for transducing extracellular osmotic stress signal into the nucleus. Extracellular hyperosmolarity is sensed by the cell surface receptors Sln1 and Sho1/Hkr1-Msb2, respectively, and the evoked signal is transduced by multiple intermediate molecules, stimulating Pbs2 and its downstream Hog1 kinase. Hog1 kinase further activates transcription factors Hot, Smp1, Sko1, finally accumulating the osmolyte glycerol inside the cells. Note that the small G-protein Cdc42 plays a role in the Sho-1 branch-mediated signal transduction. Hog1 is a yeast ortholog of the mammalian p38 MAPK.
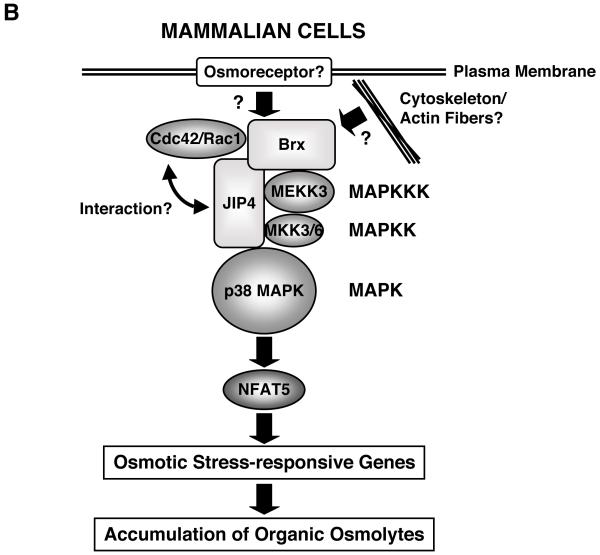
B: Intracellular signaling system for sensing and responding to extracellular hyperosmolarity in mammalian cells.
Upon exposure to extracellular hyperosmolarity, mammalian cells activate a signaling cascade similar to that of yeast, which consists of small G-proteins, p38 MPAK and NFAT5, to induce osmostress-responsive genes and accumulation of several organic osmolytes.
Brx plays a key role in mediating the signal of extracellular hyperosmolarity, sensed by yet undiscovered osmoreceptors and/or alterations in the cytoskeleton, to a downstream signaling cascade by forming a complex with small G-proteins, JIP4, p38 MAPK and its upstream kinases to finally activate NFAT5 and through it to stimulate the transcription rates of osmotic stress-responsive genes.
GEF: guanine nucleotide-exchange factor, HOG1: high osmolarity glycerol 1, JIP4: JNK-interacting protein 4, MAPK: mitogen-activated protein kinase, MAPKK: MAPK kinase, MAPKKK, MAPK kinase kinase, MEKK3: MAPK/ERK kinase kinase 3, NFAT5: nuclear factor of activated T-cells 5
Mammalian cells
In mammalian cells, the intracellular signaling system that mediates the adaptive response to osmotic stress has not been as well elucidated as in yeast cells, but its organization may have some similarity to that of yeast, although admittedly there is a significant difference between mammalian and yeast cells, as the latter have rigid cell walls, while the former do not (Figure 2 B). In mammalian cells, the system appears to consist of a multi-protein osmosensing complex that includes Rho-type small G-proteins and some kinases, including p38 MAPK [2,27-29]. Exposure to extracellular hyperosmolarity causes phosphorylation of the transcription factor nuclear factor of activated T-cells 5 (NFAT5)/tonicity enhancer binding protein (TonEBP; hereafter referred to as NFAT5), which contains a Rel-homology domain and shares a common Rel-like ancestor with rel/Dorsal/ the nuclear factor-κB (NF-κB) family proteins and the other NFAT transcription factors [10,15,30-34]. Extracellular hyperosmolarity leads to translocation of NFAT5 from the cytoplasm into the nucleus, where it stimulates the transcriptional activity of its responsive genes [12]. In addition to such functional modification of NFAT5, extracellular hyperosmolarity also stimulates protein expression of this transcription factor, possibly via direct stimulation of its transcription [8,32]. Some reports indicate that activation of p38 MAPK in response to extracellular hyperosmolarity stimulates NFAT5, while detailed mechanisms of action by which p38 MAPK stimulates NFAT5, such as phosphorylation of NFAT5 and subsequent induction of its nuclear translocation, have not been clearly demonstrated as yet [32,35,36]. Thus, further intensive research is required for clarifying the involvement of p38 MAPK and its mechanisms of action on NFAT5 during the adaptive cell response to extracellular hyperosmolarity.
Accumulation of active NFAT5 in the nucleus increases the transcription rates of hyperosmolarity-responsive genes, such as those of aldose reductase, taurine transporter and sodium/myoinositol cotransporter, to produce/uptake small organic osmolytes [2,7,10]. The promoter region of these hyperosmolarity-responsive genes contains several NFAT/TonEBP-binding sites through which NFAT5 binds to promoter DNA and activates the transcription of these genes [2,7]. In mammalian cells, cell surface osmosensing receptors, analogous to yeast Sln1 and Sh1, have not been identified as yet. Elucidation of the signaling pathway components between Rho-type small G-proteins and p38 MAPK has been a target of current research. We recently found that Brx, a guanine nucleotide exchange factor for the activation of Rho-type small G-proteins, plays an essential role in the activation of small G-proteins in response to osmotic stress, helping them couple to the stimulation of p38 MAPK in lymphocytes [8]. In the following sections, we examine the role of Brx in the adaptive response to osmotic stress through a p38 MAPK cascade and NFAT5, acting as a cytoplasmic integrator of the osmosensing cellular regulatory mechanism.
3. Molecular structure and known functions of Brx
Brx is one of the guanine nucleotide exchange factors (GEFs) for Rho-type small G-proteins, which consists of ~60 members in humans [37,38]. Most of the GEFs, including the Brx-subfamily, have GEF domains with specific protein motifs characterized by the Dbl-homology (DH) domain followed by the pleckstrin-homology (PH) domain, and catalyze the conversion of small G-proteins from the GDP-bound form to the GTP-bound form [37,38]. Small G-proteins bound with GDP are inactive, while their GTP-bound form is active [39,40]. Active small G-proteins control target molecules by switching them on or off via physical interaction [39]. After regulating the activity of their target molecules, GTP-bound small G-proteins are immediately returned to their GDP-associated form via intrinsic GTPase activity (GAP); thus, GEFs are essential molecules in controlling the biologic actions of small G-proteins. Small G-proteins are also stored in an inactive form in association with guanine nucleotide dissociation inhibitors (GDIs) [39,41], which add further complexity to the regulation of small G-proteins.
The small G-proteins have been classified into five subgroups, the Ras, Ran, Rab, Sar/Arf and Rho families, depending on functional and/or structural similarities [39]. The Rho family proteins, which include RhoA, Cdc42, Rac1 and their subfamily molecules, play important roles in the organization of the cytoskeleton, embryonic development and regulation of gene expression after activation by numerous extracellular stimuli [39,40,42].
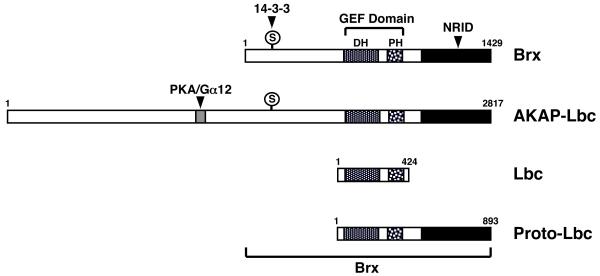
Among a Brx-type subgroup of GEFs, the Lbc molecule, a 424-residue oncogenic protein with unregulated exchange factor activity transforming NIH-3T3 cells, was discovered in 1995 [43] (Figure 3). In 1998, we first identified a 1429-residue protein, called Brx, by using the ligand-binding domain (LBD) of the retinoic X receptor β as bait in an expression cloning scheme [44]. Brx acts not only as a Rho family small G-protein-specific GEF, but also enhances the transcriptional activity of the estrogen receptors α and β and the glucocorticoid receptor through their nuclear receptor-interacting domain (NRID) [44-46]. Later, the proto-oncogenic form of Lbc, proto-Lbc, was isolated containing an additional C-terminal region that attenuated its transforming activity [47]. AKAP-Lbc or AKAP13 was subsequently reported as an even larger splicing variant of Brx, containing an additional N-terminal, and consisting of 1389 amino acid residues [48]. This longer form of Brx contains a protein kinase A (PKA)-docking domain for interaction with the classic G-protein Gα12 in its N-terminal portion and a full Brx sequence-containing a GEF domain, as well as a NRID at its C-terminal half [48]. Brx and AKAP-Lbc also have one 14-3-3-binding site with a serine at amino acid 1565 of AKAP-Lbc located N-terminally to their GEF domains; anchoring of Gα12 to this PKA-docking domain induces phosphorylation of this serine residue to allow binding to 14-3-3 [49]. Binding of 14-3-3 to these GEFs in turn regulates the exchange activity of Rho-small G-proteins. Inclusion of the NRID, the 14-3-3-binding site and/or a PKA-docking domain in Brx and AKAP-Lbc indicates that this family of GEFs acts as an integrator of signal transduction pathways to orchestrate independent signals toward final biologic actions by controlling the activity of Rho-type small G-proteins.
Figure 3.
Linearized models of Brx-related guanine nucleotide exchange factors (GEFs) and their functional domains.
All Brx-related GEFs contain the GEF domain harboring the DH and PH domains in the mid region of the molecule. Brx, AKAP-Lbc and proto-Lbc contain NRID in their C-terminal end, while AKAP-Lbc has a PKA/Gα12 docking domain in its N-terminal portion. Brx and AKAP-Lbc also contain one 14-3-3-binding site.
DH: Dbl-homology, GEF: guanine nucleotide-exchange factor, NRID: nuclear receptor-interacting domain, PH: pleckstrin-homology, PKA: protein kinase A
Since Brx is a GEF for Rho-type small G-proteins, it is expected that it may play a regulatory role in several biologic actions catalyzed by this class of small G-proteins. Brx is highly expressed in several organs and tissues, such as the spleen, thymus, peripheral leukocytes, skeletal muscles and testis, while its is moderately expressed in the ovary, placenta and lung [44]. Brx, however, is not or minimally expressed in the liver, brain, small intestine, colon and prostate [44]. This characteristic tissue distribution of Brx may indicate that this GEF plays important roles in regulation of the immune, reproductive and the musculoskeletal systems. We recently found that mice with a genetic ablation of the akap-lbc (AKAP13 gene) developed a severe heart defect during fetal organogenesis; the hearts had very thin ventricular walls with deficient sarcomere formation in the cardiomyocytes, and these fetuses died at embryonic day 10.5 to 11.0 [50]. Since Brx appears to be activated by alteration of cellular cytoskeletal architecture [51], it is likely that Brx is an interface between cell morphology and various cellular functions, including steroid hormone actions in the cells.
4. Brx as a cytoplasmic integrator mediating osmotic stress-induced signaling into the nucleus through p38 MAPK and NFAT5
During phenotypic analysis of brx haploinsufficient mice, we noticed that spleens were smaller than those of the wild type animals [8]. At the histologic level, these mice also had smaller splenic follicles [8]. We therefore performed a microarray-mediated transcriptome analysis on the spleen of brx haploinsufficient mice to look for the causative genes located downstream of Brx responsible for the observed phenotype [8]. We unexpectedly found that nfat5 was the most down-regulated gene among ~300 differentially expressed genes [8], while brx haploinsufficient mice also demonstrated reduced mRNA levels of several NFAT5-responsive genes [8]. In further analyses on the signaling cascade downstream of Brx in lymphocytes, we found that osmotic stress-mediated induction of NFAT5 required the Brx GEF domain and p38 mitogen-activated kinase (MAPK) [8]. Thus, Brx is a key component of the intracellular signaling cascade transmitting the extracellular hyperosmolarity-induced signal to the nucleus of cells [8] (Figure 2 B). Since mRNA levels of NFAT5 were also down-regulated in the medulla of the kidney from brx haploinsufficient mice [8], Brx may also play a role in the adaptive response to osmotic stress in the renal medullary epithelium.
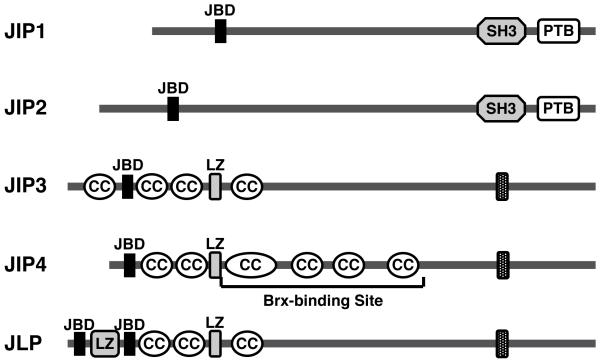
In agreement with these observations, a yeast two-hybrid screening assay revealed that Brx attracted the cJun kinase (JNK)-interacting protein (JIP) 4 through its C-terminal domain in response to osmotic stress [8]. JIP4 is a recently identified member of the JIP family of proteins [52,53]. This family consists of 5 members, JIP1, 2, 3, 4 and JLP (JNK-associated leucine zipper proteins), along with several splicing variants [52,53] (Figure 4). All JIP family members form homo-oligomers, while JIP2 also hetero-oligomerizes with JIP1 and 3 [52-54]. JIP proteins function as scaffolds for the MAPK family proteins, and positively or negatively regulate their activities by forming a complex of the kinases with their upstream and downstream factors, including MKKs, MKKKs, kinesin light chain (KLC), the insulin receptor substrates and several GEFs, such as p190RhoGEF, Tiam1 and Ras-GRF1 [52,53,55-57]. Thus, JIP proteins organize signaling specificity and facilitate the activation of pathway components by appropriately localizing them to specific subcellular sites or target molecules. JIP proteins also act as integration points for different signals to regulate the activity of MAPK proteins [53].
Figure 4.
Linearized models of JIP family proteins and their functional/structural domains.
JIP family consists of 5 members and their splicing isoforms. All have a JNK-binding domain (JBD) in their N-terminal portion, while JIP1 and 2 contain SH3 and PTB domains in their C-terminal end. JIP3 and 4 and JLP have a putative transmembrane domain (TM) in their C-terminal end. JIP4 interacts with Brx through its middle portion enclosing multiple coiled-coil structures.
CC: coiled-coil, JIP: JNK-interacting protein, JLP: JNK-associated leucine zipper protein, JBD: JNK-binding domain, LZ: leucine zipper, PTB: phosphotyrosine-binding domain, SH3: Src homology-3 domain
Among the JIP family proteins, JIP4 that binds Brx functions as a scaffold in the p38 MAPK cascade activation by communicating with p38 MAPK and its upstream kinases MKK3, MKK6 and MEKK3 [52-54]. Mouse JIP4 consists of 1142 amino acids and contains the p38 MAPK-binding and leucine zipper domains in its N-terminal portion, multiple coiled-coil structures in its middle portion, and the putative trans-membrane (TM) domain in the C-terminal region (Figure 4). The C-terminal portion of Brx between amino acids 1042 and 1429 physically interacts with the part of mouse JIP4 enclosed by amino acids 251 and 750 located in its middle portion [8] (Figure 4). Since Brx specifically mediated osmotic stress-stimulated p38 MAPK activity to NFAT5 expression but showed no effect on this kinase activity induced by the other extracellular activators [8], Brx may function as a molecular rectifier by coupling small G-proteins activated by Brx in response to upstream osmostress-induced signal to the JIP4/p38 MAPK complex (Figure 2 B), which directs otherwise diverse actions of p38 MAPK into a specific cellular pathway for stimulating NFAT5 expression [58]. Taken together, our recent results revealed a new role for Brx as a signal integrator in the osmotic stress-activating signaling pathway through small G-proteins and p38 MAPK [8,9].
The signaling cascade(s) upstream of Brx and Rho-type small G-proteins remain(s) to be elucidated. Mammalian cells may contain cell surface osmoreceptors analogous to yeast Sln1 and Sho1, and Brx might interact with these osmosensors to activate small G-proteins. Alternatively, osmotic stimuli might provoke the cellular adaptive response by directly influencing the cytoskeleton, which in this case, may function as a sensor of cell volume [59-62]. Indeed, extracellular hyperosmolarity reduces cellular volume by shifting intracellular water to the outside of the cells, and a substantial number of reports indicate that Rho-type small G-proteins and their GEFs are central players in the formation/remodeling of cytoskeletal organization [62]. We recently found that Brx was significantly induced by mechanical stress in leiomyoma cells and was associated with actin fibers –pivotal components of the cytoskeleton [51]. Since mechanical stress dramatically alters the cytoskeleton similarly to osmotic stress [63,64], it is possible that Brx is activated by osmotic stress through modulation of the cytoskeleton (Figure 2 B), but this remains to be examined.
5. Implication of osmotic stress to inflammation and organ physiology and pathology
Immune system
Mammalian adaptive response to osmotic stress mediated through Brx is functional in lymphocytes and is required for B-lymphocyte differentiation and normal development of the spleen [8]. Immune organs, including the spleen and the thymus, have physiologically elevated osmotic pressure [13,14] (Table 1). Persistent osmotic stress-mediated activation of NFAT5 may confer normal development and proliferation of both T-and B-cells, possibly by inducing TNFα family cytokines, such as TNFα, lymphotoxin-β and B-cell activating factor (BAFF), which have putative NFAT response elements in their promoter regions [8,12-15,65]. In addition to lymphocytes, macrophages, important immune accessory cells responsible for antigen processing and presentation [66], also respond to osmotic stress and can activate NFAT5 [15]. These pieces of evidence indicate that osmotic stress may be a component of the physiologic regulation of the immune system, in addition to its well-known protective effect in renal medullary epithelial cells [2]. Of course, these components of the immune system respond variably to osmotic stress with respect to specific thresholds of osmotic pressure [15]. More research is required to determine whether mildly hypertonic extracellular fluid in these immune organs is sufficient to elicit unambiguous functional properties. Indeed, most of the reported experiments were performed in much higher levels of osmolarity than those observed in vivo [8,12,36,67], and thus, its is intriguing that mildly elevated extracellular hyperosmolarity observed in animals could activate NFAT5-mediated adaptive response in immune cells.
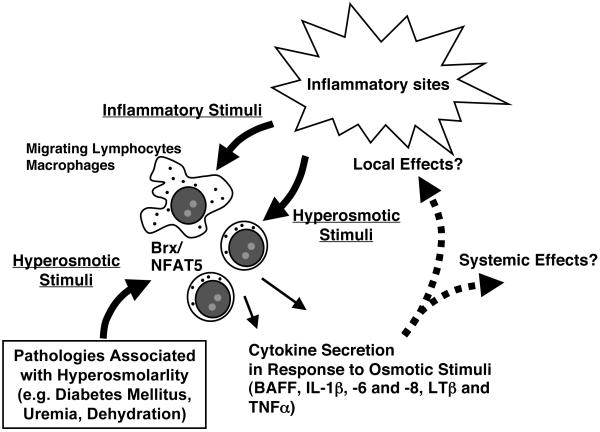
Regulation of the immune system by osmotic stress may not be restricted to the immune organs spleen and thymus, but local inflammatory sites also feature elevated osmolarity in general, with electrolytes, bioactive molecules and cell debris discharged from leukocytes, resident cells, blood vessels and even exogenous pathogens [68] (Table 1). The cellular machinery for responding to hyperosmolarity would protect migrating immune cells from the elevated osmolarity on one hand, while it may stimulate production of various cytokines and other bioactive compounds to further modulate the local as well as systemic immune reaction on the other (Figure 5).
Figure 5.
Brx/NFAT5-mediated adaptive response to osmotic stress in a putative inflammatory site and in pathologic conditions associated with extracellular hyperosmolarity.
Inflammation activates lymphocytes and macrophages accumulated in an inflammatory site not only through injurious agents and cytokines but also through osmotic stress. The Brx/NFAT5-mediated intracellular signaling system stimulates further expression of cytokines to modulate local and systemic inflammatory reactions, while it induces hyperosmolarity-responsive genes to protect immune and immune accessory cells from the local hyperosmolar environment. Pathologic conditions associated with extracellular hyperosmolarity may alter the various functions of white cells through activation of Brx/NFAT5-mediated signaling system.
BAFF: B-cell activating factor, IL: interleukin, LTβ: lymphotoxin β, NFAT5: nuclear factor of activated T-cells 5, TNFα: tumor necrosis factor α
Osmotic stress-mediated alteration in immune function might also play a role in the pathogenesis of several disorders associated with extracellular hyperosmolarity. For example, diabetes mellitus, uremia, diabetes insipidus, dehydration, heat stroke and tissue burns feature extracellular hyperosmolarity (Table 1), and are associated with an altered immune response and dysregulation in the secretion of cytokines, such as the interleukin (IL)-1β, −6 and −8 [69-79]. Hyperosmolarity-mediated NFAT5 activation in macrophages also plays a role in the vascular homeostasis under high-salt diet [80]. Extracellular hyperosmolarity in the skin of animals on a high-salt diet stimulates NFAT5 in residential macrophages, which in turn facilitates secretion of the vascular endothelial growth factor-C that counteracts the elevated extracellular fluid through expanding the skin vascular bed by increasing expression of endothelial nitric oxide synthase and production of the vasodilatory nitric oxide [80]. Inflamed joints, the intestine and the cornea are also sites of altered osmolarity [81-84]. Hyperosmolarity-induced secretion of various cytokines may not only cause dysregulation of the immune system itself, but also affect proper function of distant organs, such as the liver, brain, bone, fat and heart, and may lead to the systemic sickness syndrome and other physiologic or pathologic conditions [85-90].
Endocrine and other organs/tissues
The NFAT5-mediated adaptive response to extracellular hyperosmolarity may be active virtually in all human organs and tissues, however, there could be different potencies and thresholds in their responsiveness, as they are equally exposed to the hyperosmolar extracellular fluid once dehydration becomes apparent. This adaptive system may be of particular importance in some tissues and cells, such as epithelial cells lining the gastro-intestinal tract, uterus and inter-vertebral disc and joints, because these cells encounter hyperosmolar fluids continuously or periodically [9] (Table 1). As seen in the immune organs, the machinery for responding to hyperosmolarity might cause organ-, tissue- and/or cell type-specific responses against elevated osmolarity, as they would need to adjust their own functions in the hyperosmolar environment, in addition to protecting themselves. For example, the osmolarities of the fallopian, oviduct and uterine fluids are physiologically elevated [91,92], and levels of hyperosmolarity in these fluids are critical for embryos to develop to the blastocyst stage [92] (Table 1). These pieces of evidence indicate that hyperosmolarity influences embryonic development, possibly regulating their proliferation/differentiation and apoptosis functions.
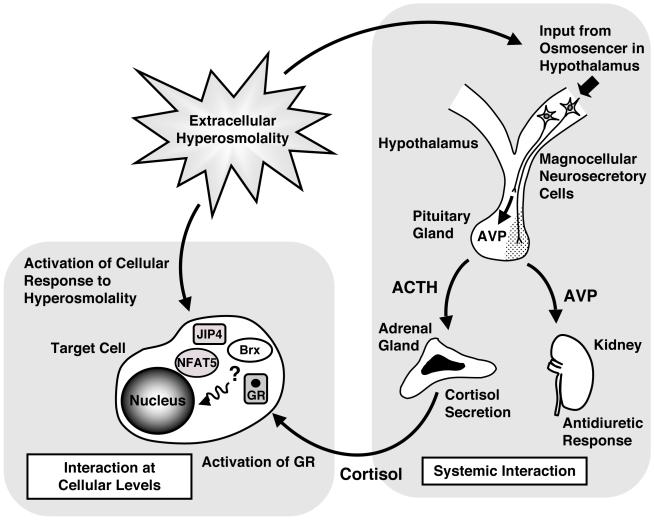
Further, the extracellular hyperosmolarity-induced adaptive response might alter various physiologic functions and influence pathologic courses. We previously demonstrated that Brx bound the glucocorticoid receptor (GR) and enhanced its transcriptional activity by physically interacting with this receptor through its C-terminal NRID [46]. This steroid receptor transduces diverse action of glucocorticoid hormones, which are secreted from the adrenal gland in response to the adrenocorticotropic hormone (ACTH) and act as end-effector products of the hypothalamic-pituitary-adrenal axis, an important system for regulating the adaptive response to external and internal stressors [93-95]. Indeed, extracellular hyperosmolarity is a strong external stressor, stimulating secretion of arginine vasopressin (AVP) from the hypothalamic paraventricular and suprachiasmatic nucleus [96]. Secreted AVP further stimulates ACTH release from the anterior lobe of the pituitary gland and activates the HPA axis in synergy with corticotropin releasing hormone [96], in addition to inducing an anti-diuretic response in the kidney [97]. Although Brx is little expressed in the brain, it is still possible that this molecule contributes also to the function of the osmoreceptor located in the hypothalamus [98]. It is likely that organisms activate their HPA axis-mediated stress system in addition to their NFAT5-mediated cellular adaptive response to osmotic stress; the former glucocorticoid/GR-mediated stress response system and the latter osmo-responsive system may interact with each other at the cellular level and ultimately influence the various cellular activities regulated by cortisol (Figure 6). This interaction might occur at the level of Brx. That is, GR and JIP4 may share a binding site or bind closely on Brx, thus, these molecules could influence each other’s functions via Brx. Since GR interacts with numerous transcription factors, including NF-κB and other NFAT transcription factors, and mutually regulates their activities [94,99], NFAT5 might also interact with the GR and regulate GR-induced transcriptional activity of glucocorticoid-responsive genes, further altering cellular activities linked to glucocorticoid actions. Brx and its longer form AKAP-Lbc also physically and functionally interact with the estrogen receptor and components of the PKA signal pathway, which has strong activity in cell growth, differentiation and even cell death [44,45,48]. Thus, the adaptive response to dehydration-linked osmotic stress or extracellular hyperosmolarity appears to be an integral component of cellular regulation, which determines the functional specificity of the cells as well as their fate, such as proliferation, differentiation and death, ultimately changing the functions of organs and tissues.
Figure 6.
Mutual interaction between the adaptive response system to osmotic stress and the HPA axis-mediated stress response system at the systemic and cellular levels. Extracellular hyperosmolarity activates the HPA axis/cortisol secretion through hypothalamic AVP secreted into the pituitary portal system in addition to the stimulation of anti-diuretic response in the kidney through systemically secreted AVP. At a cellular level, GR activated by cortisol and osmotic stress signal components such as Brx, JIP4 and NFAT5 may influence each other’s activity.
ACTH: adrenocorticotropic hormone, AVP: arginine vasopressin, GR: glucocorticoid receptor, JIP4: JNK-interacting protein 4, NFAT5: nuclear factor of activated T-cells 5
FIVE-YEAR VIEW
The molecules located upstream of intracellular Brx have not yet been elucidated. Using recent results obtained in yeast as a model system, such upstream molecules, for example mammalian cell surface osmoreceptors analogous to yeast Sln1 and Sho1/Mbs2, could be identified by searching for molecules that associate with Brx. Brx also associates with actin filaments, which are important components of the cell cytoskeleton; substantial numbers of reports indicate them to be a “receptor” for extracellular hyperosmolarity, by sensing alterations of cell morphology following absorption of water from the cells. Identification of the molecules, which physically interact with both Brx and actin filaments, could provide a clue for elucidating “mechanical” receptors for osmotic stress. The involvement of p38 MAPK activation in the adaptive response to extracellular hyperosmolarity should be clearly addressed. The mechanism responsible for the activation of NFAT5 was described, however, the signaling cascade responsible for phosphorylation of NFAT5, its cytoplasmic to nuclear translocation and mRNA induction have not been well identified as yet. We hope these phenomena will be elucidated during the next 5 years. The pathologic importance of hyperosmolarity, particularly the mild to moderate elevations encountered in several pathologic conditions, in the activation of the immune system at inflammatory sites also remains to be clarified. Using mice with genetic defects in components of the signaling pathways involved in osmotic stress, such as Brx, NFAT5 and JIP4, and induction of inflammation in these animals, will be particularly interesting.
KEY ISSUES
Extracellular hyperosmolarity causes a shift of water from the intracellular to the extracellular compartment towards a state of equilibration between intra- and extracellular osmotic pressures. Since molecules producing osmotic pressure cannot pass freely through the plasma membrane, cell dehydration and shrinkage occur.
Dehydration of the intracellular compartment causes cellular dysfunction in part by condensation and denaturation of intracellular molecules and by altering the subcellular architecture.
To counteract extracellular hyperosmolarity, organisms have developed a regulatory mechanism in which the Rho-type small G-proteins, kinases, including p38 MAPK, and the NFAT5 transcription factor play significant roles.
Brx may act as a cytoplasmic integrator of signal transduction pathways, orchestrating independent signals towards coordinated biologic actions possibly by coupling activated Rho-type small G-proteins and their signaling pathway components.
NFAT5 contains the Rel-homology domain and shares a common Rel-like ancestor with rel/Dorsal/NF-κB family proteins and the other NFAT transcription factors. NFAT5 increases the transcription rates of hyperosmolarity-responsive genes in response to cellular changes associated with extracellular osmolarity.
JIP4 functions as a scaffold in the p38 MAPK cascade activation by communicating with p38 MAPK and its upstream kinases MKK3, MKK6 and MEKK3. JIP4 physically interacts with Brx in response to osmotic stimuli and helps couple activated small G-proteins to the p38 MAPK signal components.
Extracellular hyperosmolarity appears to be a component of the physiologic regulation of the immune organs, such as the spleen and the thymus. It may also be a component of inflammation, as inflammatory sites are associated with elevated extracellular osmolarity, while the cellular machinery for responding to extracellular hyperosmolarity appears to be beneficial for protecting immune cells from ambient hyperosmolarity and to stimulate production of various cytokines and other bioactive compounds to further modulate the local as well as the systemic immune reaction.
The cellular machinery responding to extracellular hyperosmolarity may be active in virtually all organs and tissues, however, there may be tissue-specific potencies and thresholds. It may also alter specific functions of organs and tissues by communicating with other signaling pathways, in addition to protecting them from hyperosmolarity.
More research is needed to determine unambiguously an adaptive osmotic response of immune tissues, especially at the mild to moderate levels of extracellular hyperosmolarity observed in the spleen and the thymus or associated with various physiologic or pathologic conditions. Osmotic stress-dependent and -independent functions of Brx, NFAT5 and other components of the osmotic stress-signaling pathway should be also distinguished.
FINANCIAL DISCLOSURE/ACKNOWLEDGEMENTS
This work is supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD, and the University of Athens, Athens, Greece.
REFERENCES
- 1.Griffith RW. Composition of blood serum of deep-sea fishes. Biol. Bull. 1981;160:250–264. [Google Scholar]
- 2.Burg MB, Kwon ED, Kultz D. Regulation of gene expression by hypertonicity. Annu Rev Physiol. 1997;59:437–455. doi: 10.1146/annurev.physiol.59.1.437. [DOI] [PubMed] [Google Scholar]
- 3.Burg MB, Ferraris JD, Dmitrieva NI. Cellular response to hyperosmotic stresses. Physiol Rev. 2007;87(4):1441–1474. doi: 10.1152/physrev.00056.2006. ** This review article comprehensively explains adaptive response to osmotic stress.
- 4.Ho SN. Intracellular water homeostasis and the mammalian cellular osmotic stress response. J Cell Physiol. 2006;206(1):9–15. doi: 10.1002/jcp.20445. [DOI] [PubMed] [Google Scholar]
- 5.Jungersted JM, Hellgren LI, Jemec GB, Agner T. Lipids and skin barrier function--a clinical perspective. Contact Dermatitis. 2008;58(5):255–262. doi: 10.1111/j.1600-0536.2008.01320.x. [DOI] [PubMed] [Google Scholar]
- 6.Bourque CW, Oliet SH. Osmoreceptors in the central nervous system. Annu Rev Physiol. 1997;59:601–619. doi: 10.1146/annurev.physiol.59.1.601. [DOI] [PubMed] [Google Scholar]
- 7.Ho SN. The role of NFAT5/TonEBP in establishing an optimal intracellular environment. Arch Biochem Biophys. 2003;413(2):151–157. doi: 10.1016/s0003-9861(03)00130-9. * This review explains the roles and actions of NFAT5 in the adaptive response to osmotic stress.
- 8.Kino T, Takatori H, Manoli I, et al. Brx mediates the response of lymphocytes to osmotic stress through the activation of NFAT5. Sci Signal. 2009;2(57):ra5. doi: 10.1126/scisignal.2000081. ** This original article demonstrates for the first time the involvement of Brx GEF in the signal transduction of the adaptive response to osmotic stress.
- 9.Aramburu J, Lopez-Rodriguez C. Brx shines a light on the route from hyperosmolarity to NFAT5. Sci Signal. 2009;2(65):pe20. doi: 10.1126/scisignal.265pe20. [DOI] [PubMed] [Google Scholar]
- 10.Aramburu J, Drews-Elger K, Estrada-Gelonch A, et al. Regulation of the hypertonic stress response and other cellular functions by the Rel-like transcription factor NFAT5. Biochem Pharmacol. 2006;72(11):1597–1604. doi: 10.1016/j.bcp.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Haussinger D. The role of cellular hydration in the regulation of cell function. Biochem J. 1996;313:697–710. doi: 10.1042/bj3130697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Rodriguez C, Aramburu J, Jin L, Rakeman AS, Michino M, Rao A. Bridging the NFAT and NF-κB families: NFAT5 dimerization regulates cytokine gene transcription in response to osmotic stress. Immunity. 2001;15(1):47–58. doi: 10.1016/s1074-7613(01)00165-0. * This original article demonstrates the mechanisms of NFAT5 activation and NFAT-mediated stimulation of cytokine gene expression.
- 13.Go WY, Liu X, Roti MA, Liu F, Ho SN. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc Natl Acad Sci U S A. 2004;101(29):10673–10678. doi: 10.1073/pnas.0403139101. ** This original article demonstrates the importance of NFAT5 and osmotic stress in the development of splenic lymphocytes in nfat5 haploinsufficient mice
- 14.Trama J, Go WY, Ho SN. The osmoprotective function of the NFAT5 transcription factor in T cell development and activation. J Immunol. 2002;169(10):5477–5488. doi: 10.4049/jimmunol.169.10.5477. [DOI] [PubMed] [Google Scholar]
- 15.Morancho B, Minguillon J, Molkentin JD, Lopez-Rodriguez C, Aramburu J. Analysis of the transcriptional activity of endogenous NFAT5 in primary cells using transgenic NFAT-luciferase reporter mice. BMC Mol Biol. 2008;9:13. doi: 10.1186/1471-2199-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam AK, Ko BC, Tam S, et al. Osmotic response element-binding protein (OREBP) is an essential regulator of the urine concentrating mechanism. J Biol Chem. 2004;279(46):48048–48054. doi: 10.1074/jbc.M407224200. [DOI] [PubMed] [Google Scholar]
- 17.O’Connor RS, Mills ST, Jones KA, Ho SN, Pavlath GK. A combinatorial role for NFAT5 in both myoblast migration and differentiation during skeletal muscle myogenesis. J Cell Sci. 2007;120(Pt 1):149–159. doi: 10.1242/jcs.03307. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Rodriguez C, Antos CL, Shelton JM, et al. Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc Natl Acad Sci U S A. 2004;101(8):2392–2397. doi: 10.1073/pnas.0308703100. ** This original article confirms the roles of NFAT5 in the protection of renal epithelial cells from their hyperosmotic environment using nfat5 knockout mice.
- 19.Hohmann S. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol Rev. 2002;66(2):300–372. doi: 10.1128/MMBR.66.2.300-372.2002. * This excellent review article explains the signaling components of the adaptive response to osmotic stress in the yeast.
- 20.Saito H, Tatebayashi K. Regulation of the osmoregulatory HOG MAPK cascade in yeast. J Biochem. 2004;136(3):267–272. doi: 10.1093/jb/mvh135. [DOI] [PubMed] [Google Scholar]
- 21.Raitt DC, Posas F, Saito H. Yeast Cdc42 GTPase and Ste20 PAK-like kinase regulate Sho1-dependent activation of the Hog1 MAPK pathway. EMBO J. 2000;19(17):4623–4631. doi: 10.1093/emboj/19.17.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tatebayashi K, Tanaka K, Yang HY, et al. Transmembrane mucins Hkr1 and Msb2 are putative osmosensors in the SHO1 branch of yeast HOG pathway. EMBO J. 2007;26(15):3521–3333. doi: 10.1038/sj.emboj.7601796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tatebayashi K, Yamamoto K, Tanaka K, et al. Adaptor functions of Cdc42, Ste50, and Sho1 in the yeast osmoregulatory HOG MAPK pathway. EMBO J. 2006;25(13):3033–3044. doi: 10.1038/sj.emboj.7601192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Widmann C, Gibson S, Jarpe MB, Johnson GL. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiol Rev. 1999;79(1):143–180. doi: 10.1152/physrev.1999.79.1.143. [DOI] [PubMed] [Google Scholar]
- 25.Saito H. Histidine phosphorylation and two-component signaling in eukaryotic cells. Chem Rev. 2001;101(8):2497–2509. doi: 10.1021/cr000243+. [DOI] [PubMed] [Google Scholar]
- 26.Maeda T, Takekawa M, Saito H. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science. 1995;269(5223):554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- 27.Lunn JA, Rozengurt E. Hyperosmotic stress induces rapid focal adhesion kinase phosphorylation at tyrosines 397 and 577. Role of Src family kinases and Rho family GTPases. J Biol Chem. 2004;279(43):45266–45278. doi: 10.1074/jbc.M314132200. [DOI] [PubMed] [Google Scholar]
- 28.Uhlik MT, Abell AN, Johnson NL, et al. Rac-MEKK3-MKK3 scaffolding for p38 MAPK activation during hyperosmotic shock. Nat Cell Biol. 2003;5(12):1104–1110. doi: 10.1038/ncb1071. [DOI] [PubMed] [Google Scholar]
- 29.Sheikh-Hamad D, Di Mari J, Suki WN, Safirstein R, Watts BA, 3rd, Rouse D. p38 kinase activity is essential for osmotic induction of mRNAs for HSP70 and transporter for organic solute betaine in Madin-Darby canine kidney cells. J Biol Chem. 1998;273(3):1832–1837. doi: 10.1074/jbc.273.3.1832. [DOI] [PubMed] [Google Scholar]
- 30.Macian F, Lopez-Rodriguez C, Rao A. Partners in transcription: NFAT and AP-1. Oncogene. 2001;20(19):2476–2489. doi: 10.1038/sj.onc.1204386. [DOI] [PubMed] [Google Scholar]
- 31.Tsai TT, Guttapalli A, Agrawal A, Albert TJ, Shapiro IM, Risbud MV. MEK/ERK signaling controls osmoregulation of nucleus pulposus cells of the intervertebral disc by transactivation of TonEBP/OREBP. J Bone Miner Res. 2007;22(7):965–974. doi: 10.1359/jbmr.070322. [DOI] [PubMed] [Google Scholar]
- 32.Lee JH, Kim M, Im YS, Choi W, Byeon SH, Lee HK. NFAT5 induction and its role in hyperosmolar stressed human limbal epithelial cells. Invest Ophthalmol Vis Sci. 2008;49(5):1827–1835. doi: 10.1167/iovs.07-1142. [DOI] [PubMed] [Google Scholar]
- 33.Graef IA, Gastier JM, Francke U, Crabtree GR. Evolutionary relationships among Rel domains indicate functional diversification by recombination. Proc Natl Acad Sci U S A. 2001;98(10):5740–5745. doi: 10.1073/pnas.101602398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keyser P, Borge-Renberg K, Hultmark D. The Drosophila NFAT homolog is involved in salt stress tolerance. Insect Biochem Mol Biol. 2007;37(4):356–362. doi: 10.1016/j.ibmb.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Dahl SC, Handler JS, Kwon HM. Hypertonicity-induced phosphorylation and nuclear localization of the transcription factor TonEBP. Am J Physiol Cell Physiol. 2001;280(2):C248–253. doi: 10.1152/ajpcell.2001.280.2.C248. [DOI] [PubMed] [Google Scholar]
- 36.Ko BC, Lam AK, Kapus A, Fan L, Chung SK, Chung SS. Fyn and p38 signaling are both required for maximal hypertonic activation of the osmotic response element-binding protein/tonicity-responsive enhancer-binding protein (OREBP/TonEBP) J Biol Chem. 2002;277(48):46085–46092. doi: 10.1074/jbc.M208138200. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt A, Hall A. Guanine nucleotide exchange factors for Rho GTPases: turning on the switch. Genes Dev. 2002;16(13):1587–1609. doi: 10.1101/gad.1003302. [DOI] [PubMed] [Google Scholar]
- 38.Cherfils J, Chardin P. GEFs: structural basis for their activation of small GTP-binding proteins. Trends Biochem Sci. 1999;24(8):306–311. doi: 10.1016/s0968-0004(99)01429-2. [DOI] [PubMed] [Google Scholar]
- 39.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81(1):153–208. doi: 10.1152/physrev.2001.81.1.153. ** This review article covers the known actions, molecular structures and mechanisms of action of the small G-proteins.
- 40.Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- 41.Olofsson B. Rho guanine dissociation inhibitors: pivotal molecules in cellular signalling. Cell Signal. 1999;11(8):545–554. doi: 10.1016/s0898-6568(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 42.Aznar S, Lacal JC. Rho signals to cell growth and apoptosis. Cancer Lett. 2001;165(1):1–10. doi: 10.1016/s0304-3835(01)00412-8. [DOI] [PubMed] [Google Scholar]
- 43.Zheng Y, Olson MF, Hall A, Cerione RA, Toksoz D. Direct involvement of the small GTP-binding protein Rho in lbc oncogene function. J Biol Chem. 1995;270(16):9031–9034. doi: 10.1074/jbc.270.16.9031. [DOI] [PubMed] [Google Scholar]
- 44.Rubino D, Driggers P, Arbit D, et al. Characterization of Brx, a novel Dbl family member that modulates estrogen receptor action. Oncogene. 1998;16(19):2513–2526. doi: 10.1038/sj.onc.1201783. * This original article describes the cloning of the human Brx gene and shows that Brx is a Rho-type GEF.
- 45.Driggers PH, Segars JH, Rubino DM. The proto-oncoprotein Brx activates estrogen receptor β by a p38 mitogen-activated protein kinase pathway. J Biol Chem. 2001;276(50):46792–476797. doi: 10.1074/jbc.M106927200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kino T, Souvatzoglou E, Charmandari E, et al. Rho family Guanine nucleotide exchange factor Brx couples extracellular signals to the glucocorticoid signaling system. J Biol Chem. 2006;281(14):9118–9126. doi: 10.1074/jbc.M509339200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sterpetti P, Hack AA, Bashar MP, et al. Activation of the Lbc Rho exchange factor proto-oncogene by truncation of an extended C terminus that regulates transformation and targeting. Mol Cell Biol. 1999;19(2):1334–1345. doi: 10.1128/mcb.19.2.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diviani D, Soderling J, Scott JD. AKAP-Lbc anchors protein kinase A and nucleates Gα12-selective Rho-mediated stress fiber formation. J Biol Chem. 2001;276(47):44247–44257. doi: 10.1074/jbc.M106629200. * This original article describes the cloning of the larger human AKAP transcript and demonstrates that its product acts as a GEF and has a PKA-docking domain.
- 49.Diviani D, Abuin L, Cotecchia S, Pansier L. Anchoring of both PKA and 14-3-3 inhibits the Rho-GEF activity of the AKAP-Lbc signaling complex. Embo J. 2004;23(14):2811–2820. doi: 10.1038/sj.emboj.7600287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDaniel K, Mayers C, Venere M, et al. Brx, a cytoplasmic protein capable of augmenting estrogen action, is essential for murine development. J Soc Gynecol Invest. 2004;11(2 (Suppl):199A. [Google Scholar]
- 51.Rogers R, Norian J, Malik M, et al. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol. 2008;198(4) doi: 10.1016/j.ajog.2007.11.057. 474 e471-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- 53.Whitmarsh AJ. The JIP family of MAPK scaffold proteins. Biochem Soc Trans. 2006;34(Pt 5):828–832. doi: 10.1042/BST0340828. [DOI] [PubMed] [Google Scholar]
- 54.Kelkar N, Standen CL, Davis RJ. Role of the JIP4 scaffold protein in the regulation of mitogen-activated protein kinase signaling pathways. Mol Cell Biol. 2005;25(7):2733–2743. doi: 10.1128/MCB.25.7.2733-2743.2005. * This original article demonstrates the biologic function of JIP4.
- 55.Standen CL, Kennedy NJ, Flavell RA, Davis RJ. Signal Transduction Cross-talk Mediated by JIP and IRS Scaffold Protein Complexes. Mol Cell Biol. 2009 doi: 10.1128/MCB.00155-09. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ge W, Wu J, Zhai J, et al. Binding of p190RhoGEF to a destabilizing element on the light neurofilament mRNA is competed by BC1 RNA. J Biol Chem. 2002;277(45):42701–42705. doi: 10.1074/jbc.M206635200. [DOI] [PubMed] [Google Scholar]
- 57.Buchsbaum RJ, Connolly BA, Feig LA. Interaction of Rac exchange factors Tiam1 and Ras-GRF1 with a scaffold for the p38 mitogen-activated protein kinase cascade. Mol Cell Biol. 2002;22(12):4073–4085. doi: 10.1128/MCB.22.12.4073-4085.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi Y, Gaestel M. In the cellular garden of forking paths: how p38 MAPKs signal for downstream assistance. Biol Chem. 2002;383(10):1519–1536. doi: 10.1515/BC.2002.173. [DOI] [PubMed] [Google Scholar]
- 59.Pedersen SF, Hoffmann EK, Mills JW. The cytoskeleton and cell volume regulation. Comp Biochem Physiol A Mol Integr Physiol. 2001;130(3):385–399. doi: 10.1016/s1095-6433(01)00429-9. [DOI] [PubMed] [Google Scholar]
- 60.Henson JH. Relationships between the actin cytoskeleton and cell volume regulation. Microsc Res Tech. 1999;47(2):155–162. doi: 10.1002/(SICI)1097-0029(19991015)47:2<155::AID-JEMT7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 61.Hoffmann EK, Dunham PB. Membrane mechanisms and intracellular signalling in cell volume regulation. Int Rev Cytol. 1995;161:173–262. doi: 10.1016/s0074-7696(08)62498-5. [DOI] [PubMed] [Google Scholar]
- 62.Di Ciano-Oliveira C, Thirone AC, Szaszi K, Kapus A. Osmotic stress and the cytoskeleton: the R(h)ole of Rho GTPases. Acta Physiol (Oxf) 2006;187(1-2):257–272. doi: 10.1111/j.1748-1716.2006.01535.x. [DOI] [PubMed] [Google Scholar]
- 63.Li C, Xu Q. Mechanical stress-initiated signal transduction in vascular smooth muscle cells in vitro and in vivo. Cell Signal. 2007;19(5):881–891. doi: 10.1016/j.cellsig.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 64.Wang JH, Thampatty BP, Lin JS, Im HJ. Mechanoregulation of gene expression in fibroblasts. Gene. 2007;391(1-2):1–15. doi: 10.1016/j.gene.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Drews-Elger K, Ortells MC, Rao A, Lopez-Rodriguez C, Aramburu J. The transcription factor NFAT5 is required for cyclin expression and cell cycle progression in cells exposed to hypertonic stress. PLoS One. 2009;4(4):e5245. doi: 10.1371/journal.pone.0005245. * This original article demonstrates that NFAT5 regulates cell cycle activity upon stimulation with osmotic stress.
- 66.Mahnke K, Ring S, Bedke T, Karakhanova S, Enk AH. Interaction of regulatory T cells with antigen-presenting cells in health and disease. Chem Immunol Allergy. 2008;94:29–39. doi: 10.1159/000154854. [DOI] [PubMed] [Google Scholar]
- 67.Li Y, Sassano A, Majchrzak B, et al. Role of p38α Map kinase in Type I interferon signaling. J Biol Chem. 2004;279(2):970–979. doi: 10.1074/jbc.M309927200. [DOI] [PubMed] [Google Scholar]
- 68.Schwartz L, Guais A, Pooya M, Abolhassani M. Is inflammation a consequence of extracellular hyperosmolarity? J Inflamm (Lond) 2009;6:21. doi: 10.1186/1476-9255-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoffman WH, Helman SW, Passmore G. Acute activation of peripheral lymphocytes during treatment of diabetic ketoacidosis. J Diabetes Complications. 2001;15(3):144–149. doi: 10.1016/s1056-8727(00)00142-2. [DOI] [PubMed] [Google Scholar]
- 70.Wu SG, Jeng FR, Wei SY, et al. Red blood cell osmotic fragility in chronically hemodialyzed patients. Nephron. 1998;78(1):28–32. doi: 10.1159/000044878. [DOI] [PubMed] [Google Scholar]
- 71.Mitono H, Endoh H, Okazaki K, et al. Acute hypoosmolality attenuates the suppression of cutaneous vasodilation with increased exercise intensity. J Appl Physiol. 2005;99(3):902–908. doi: 10.1152/japplphysiol.00156.2005. [DOI] [PubMed] [Google Scholar]
- 72.Ito T, Itoh T, Hayano T, Yamauchi K, Takamata A. Plasma hyperosmolality augments peripheral vascular response to baroreceptor unloading during heat stress. Am J Physiol Regul Integr Comp Physiol. 2005;289(2):R432–R440. doi: 10.1152/ajpregu.00027.2004. [DOI] [PubMed] [Google Scholar]
- 73.Holtfreter B, Bandt C, Kuhn SO, et al. Serum osmolality and outcome in intensive care unit patients. Acta Anaesthesiol Scand. 2006;50(8):970–977. doi: 10.1111/j.1399-6576.2006.01096.x. [DOI] [PubMed] [Google Scholar]
- 74.Wong CK, Szeto CC, Chan MH, Leung CB, Li PK, Lam CW. Elevation of pro-inflammatory cytokines, C-reactive protein and cardiac troponin T in chronic renal failure patients on dialysis. Immunol Invest. 2007;36(1):47–57. doi: 10.1080/08820130600745505. [DOI] [PubMed] [Google Scholar]
- 75.Yano A, Nakao K, Sarai A, et al. Elevated serum interleukin-18 levels might reflect the high risk of hospitalization in patients on peritoneal dialysis. Nephrology (Carlton) 2005;10(6):576–582. doi: 10.1111/j.1440-1797.2005.00497.x. [DOI] [PubMed] [Google Scholar]
- 76.Nakanishi I, Moutabarrik A, Okada N, et al. Interleukin-8 in chronic renal failure and dialysis patients. Nephrol Dial Transplant. 1994;9(10):1435–1442. [PubMed] [Google Scholar]
- 77.Descamps-Latscha B, Herbelin A, Nguyen AT, et al. Balance between IL-1β, TNF-α, and their specific inhibitors in chronic renal failure and maintenance dialysis. Relationships with activation markers of T cells, B cells, and monocytes. J Immunol. 1995;154(2):882–892. [PubMed] [Google Scholar]
- 78.Ostrowski K, Schjerling P, Pedersen BK. Physical activity and plasma interleukin-6 in humans--effect of intensity of exercise. Eur J Appl Physiol. 2000;83(6):512–515. doi: 10.1007/s004210000312. [DOI] [PubMed] [Google Scholar]
- 79.Bird MD, Kovacs EJ. Organ-specific inflammation following acute ethanol and burn injury. J Leukoc Biol. 2008;84(3):607–613. doi: 10.1189/jlb.1107766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Machnik A, Neuhofer W, Jantsch J, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med. 2009;15(5):545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]
- 81.Shanfield S, Campbell P, Baumgarten M, Bloebaum R, Sarmiento A. Synovial fluid osmolality in osteoarthritis and rheumatoid arthritis. Clin Orthop Relat Res. 1988;(235):289–295. [PubMed] [Google Scholar]
- 82.Nemeth ZH, Deitch EA, Szabo C, Hasko G. Hyperosmotic stress induces nuclear factor-κB activation and interleukin-8 production in human intestinal epithelial cells. Am J Pathol. 2002;161(3):987–996. doi: 10.1016/s0002-9440(10)64259-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vernia P, Gnaedinger A, Hauck W, Breuer RI. Organic anions and the diarrhea of inflammatory bowel disease. Dig Dis Sci. 1988;33(11):1353–1358. doi: 10.1007/BF01536987. [DOI] [PubMed] [Google Scholar]
- 84.Foulks GN. The correlation between the tear film lipid layer and dry eye disease. Surv Ophthalmol. 2007;52(4):369–374. doi: 10.1016/j.survophthal.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 85.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12(5):255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 86.Goldstein BI, Kemp DE, Soczynska JK, McIntyre RS. Inflammation and the phenomenology, pathophysiology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J Clin Psychiatry. 2009 doi: 10.4088/JCP.08r04505. [DOI] [PubMed] [Google Scholar]
- 87.Papa S, Bubici C, Zazzeroni F, Franzoso G. Mechanisms of liver disease: the crosstalk between the NF-κB and JNK pathways. Biol Chem. 2009 doi: 10.1515/BC.2009.111. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu R, Kim CS, Kang JH. Inflammatory components of adipose tissue as target for treatment of metabolic syndrome. Forum Nutr. 2009;61:95–103. doi: 10.1159/000212742. [DOI] [PubMed] [Google Scholar]
- 89.Fildes JE, Shaw SM, Yonan N, Williams SG. The immune system and chronic heart failure: is the heart in control? J Am Coll Cardiol. 2009;53(12):1013–1020. doi: 10.1016/j.jacc.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 90.Kim JH, Bachmann RA, Chen J. Interleukin-6 and insulin resistance. Vitam Horm. 2009;80:613–633. doi: 10.1016/S0083-6729(08)00621-3. [DOI] [PubMed] [Google Scholar]
- 91.Gorski J, Hou Q. Embryonic estrogen receptors: do they have a physiological function? Environ Health Perspect. 1995;103(Suppl 7):69–72. doi: 10.1289/ehp.95103s769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li R, Whitworth K, Lai L, et al. Concentration and composition of free amino acids and osmolalities of porcine oviductal and uterine fluid and their effects on development of porcine IVF embryos. Mol Reprod Dev. 2007;74(9):1228–1235. doi: 10.1002/mrd.20682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kino T, De Martino MU, Charmandari E, Mirani M, Chrousos GP. Tissue glucocorticoid resistance/hypersensitivity syndromes. J Steroid Biochem Mol Biol. 2003;85(2-5):457–467. doi: 10.1016/s0960-0760(03)00218-8. [DOI] [PubMed] [Google Scholar]
- 94.Chrousos GP, Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Sci STKE. 2005;2005(304):pe48. doi: 10.1126/stke.3042005pe48. [DOI] [PubMed] [Google Scholar]
- 95.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med. 1995;332(20):1351–1362. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 96.Antoni FA. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol. 1993;14(2):76–122. doi: 10.1006/frne.1993.1004. [DOI] [PubMed] [Google Scholar]
- 97.Weitzman RE, Kleeman CR. The clinical physiology of water metabolism. Part I: The physiologic regulation of arginine vasopressin secretion and thirst. West J Med. 1979;131(5):373–400. [PMC free article] [PubMed] [Google Scholar]
- 98.Eddington DO, Baldwin EL, Segars JH, Wu TJ. Estrogen effects on the expression of Brx in the brain and pituitary of the mouse. Brain Res Bull. 2006;69(4):447–451. doi: 10.1016/j.brainresbull.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 99.Zhan Y, Gerondakis S, Coghill E, et al. Glucocorticoid-induced TNF receptor expression by T cells is reciprocally regulated by NF-κB and NFAT. J Immunol. 2008;181(8):5405–5413. doi: 10.4049/jimmunol.181.8.5405. [DOI] [PubMed] [Google Scholar]
- 100.Yildiz EH, Fan VC, Banday H, et al. Evaluation of a new tear osmometer for repeatability and accuracy, using 0.5-microL (500-Nanoliter) samples. Cornea. 2009;28(6):677–680. doi: 10.1097/ICO.0b013e318198396b. [DOI] [PubMed] [Google Scholar]
- 101.Gilbard JP, Farris RL, Santamaria J., 2nd Osmolarity of tear microvolumes in keratoconjunctivitis sicca. Arch Ophthalmol. 1978;96(4):677–681. doi: 10.1001/archopht.1978.03910050373015. [DOI] [PubMed] [Google Scholar]
- 102.Bohr J, Jarnerot G, Tysk C, Jones I, Eriksson S. Effect of fasting on diarrhoea in collagenous colitis. Digestion. 2002;65(1):30–34. doi: 10.1159/000051928. [DOI] [PubMed] [Google Scholar]
- 103.Collins JL, Baltz JM. Estimates of mouse oviductal fluid tonicity based on osmotic responses of embryos. Biol Reprod. 1999;60(5):1188–1193. doi: 10.1095/biolreprod60.5.1188. [DOI] [PubMed] [Google Scholar]