Graphical abstract
Research highlights
▸ Context of infant trauma predicts outcome. ▸ Predictable infant trauma induces depressive-like behaviors. ▸ Unpredictable infant trauma induces anxiety-like behaviors. ▸ Predictable infant trauma can induce learning of cues of positive hedonic value. ▸ These infant trauma characteristics dramatically diverge from adult trauma effects.
Abbreviations: aPC, anterior piriform cortex; BLA, basolateral amygdala; CoA, cortical nucleus of the amygdala; CS, conditioned stimulus; FST, Forced Swim Test; pPC, posterior piriform cortex; PN, postnatal day
Keywords: Amygdala, Infant experiences, Depression, Odor–shock pairing, Plasticity, Safety signal
Abstract
Maltreatment from the caregiver induces vulnerability to later life psychopathologies, yet attraction and comfort is sometimes provided by cues associated with early life maltreatment. We used a rat model of early life maltreatment with odor–0.5 mA shock conditioning to produce depressive-like behaviors and questioned whether stimuli associated with maltreatment would restore emotional neurobehavioral function to control levels. Pups received daily novel odor–0.5 mA shock conditioning from postnatal day 8 to 12. This procedure produces a new maternal odor that controls pups’ attachment behaviors. In adulthood, either with or without the infant odor, animals received a Forced Swim Test, Sucrose Preference Test or assessment of amygdala and olfactory system functioning using field potential signal evoked by olfactory bulb paired-pulse electrical stimulation. Following neonatal odor–shock pairings, but not unpaired controls, adults without the odor present showed increased depression-like behavior in the Forced Swim Test and Sucrose Preference Test and a deficit in paired-pulse inhibition in amygdala and piriform (olfactory) cortex. All effects were brought to control levels when the infant conditioned odor was presented during behavioral and neural tests. The ability of cues associated with early life maltreatment to normalize behavior and amygdala activity suggests these cues provide adaptive value in adulthood.
1. Introduction
Childhood maltreatment is associated with later life psychiatric disorders and adverse brain development (Cicchetti and Toth, 2005, Connor et al., 2003, Gunnar, 2003, Heim et al., 2009, Pollak, 2003, Rutter et al., 2006, Stovall-McClough and Cloitre, 2006, Teicher et al., 2003, Zeanah et al., 2003) and has been replicated in animal models (Cameron et al., 2005, Cirulli et al., 2009, McEwen, 2008, Sanchez et al., 2001). While this relationship is not entirely clear, maltreatment from the caregiver appears to impart particularly strong vulnerability to later life psychiatric disorders, yet a strong attraction and comfort can sometimes be elicited by the cues associated with the early life maltreatment (Freud, 1997, Haynes-Seman, 1987). To explore the enduring effects of early life maltreatment, we used an olfactory fear conditioning paradigm in infant rats and questioned whether the odor associated with shock is attractive in later life. We also questioned whether the presence of this specific odor could restore depression-like behavior and neurobiological functions in adulthood to control levels.
Previous work has shown that, while shock is painful to pups (Barr, 1995, Collier and Bolles, 1980, Emerich et al., 1985, Fitzgerald, 2005), early life odor–shock learning produces an odor that has similar qualities to maternal odor. Indeed, this learned odor (i) induces approach responses in pups, (ii) can support nipple attachment and (iii) attenuates both amygdala activity and fear (Barr, 1995, Camp and Rudy, 1988, Haroutunian and Campbell, 1979, Moriceau and Sullivan, 2006, Raineki et al., 2010, Roth and Sullivan, 2005, Sullivan et al., 1990, Sullivan et al., 2000). The ecological relevance of this odor pain learning has been demonstrated within the nest. Specifically, rearing pups with a mother treated with a novel peppermint odor results in pups responding to peppermint as a new maternal odor, and peppermint activates olfactory structures normally responsive to natural maternal odor (Sullivan et al., 1990). Furthermore, the natural maternal odor loses its behavioral effect on pups and no longer enhances olfactory bulb activity. This natural learning paradigm is capable of producing a new maternal odor and its neural correlates even when the mother handles her pups roughly and fails to nurse (Raineki et al., 2010, Roth and Sullivan, 2005). This attachment learning is observed throughout the vertebrate kingdom when infants are totally dependent on their parents to survive, including chicks, infant dogs, nonhuman primates and humans (Harlow and Harlow, 1965, Helfer et al., 1997, Rajecki et al., 1978, Salzen, 1979, Sanchez et al., 2001). We suggest that this attachment system permits altricial animals to easily form a repertoire of proximity-seeking behaviors to the primary caregiver, regardless of the quality of the care they receive (Hofer and Sullivan, 2008). This early life maternal odor retains its value in pups as they mature. Specifically, the odor enhances sexual (Fillion and Blass, 1986, Mainardi et al., 1965, Marr and Gardner, 1965) and maternal behavior (Shah et al., 2002), influences mate choice (Moore et al., 1996) and can attenuate fear learning and amygdala activity (Sevelinges et al., 2007, Sevelinges et al., 2008). Thus, the odor appears to retain value into adulthood, although the behaviors it controls change from mother–infant interactions to behaviors important in adulthood.
While this early life shock conditioning appears beneficial by producing an odor that enhances interaction with the mother, other work suggests that early life stressors and shock have detrimental effects later in life and impact many brain areas, including the amygdala (Anisman et al., 1998, McEwen, 2003, Ressler and Mayberg, 2007). Specifically, a myriad of early life stress paradigms produce depressive-like behaviors as measured by increased time spent immobile in the Forced Swim Test (FST; Porsolt et al., 1977) and decreased sucrose consumption (Papp et al., 1991). Therefore, we next questioned whether early life odor–shock pairing, similar to other early life stressors (Cirulli et al., 2009, Ladd et al., 2000, Pryce et al., 2005), would induce depressive-like behaviors and disrupt amygdala function.
Furthermore, recent work has shown that depressive-like behaviors and related abnormal amygdala activity could be normalized with the presentation of a safety signal at levels comparable to the administration of the antidepressant fluoxetine (Muigg et al., 2007, Pollak et al., 2008, Roche et al., 2007). Interestingly, safety signals have also been shown to attenuate amygdala activity (Rogan et al., 2005). Here, we capitalized on these recent findings and questioned whether the odor paired with shock in infancy, which took on characteristics of the maternal odor (Raineki et al., 2010), might later function as a safety signal to normalize depressive-like behavior and amygdala activity in adulthood. In support of this hypothesis, we recently reported that this infant odor paired with shock retains its value with maturation and can attenuate fear learning and its related amygdala activity (Sevelinges et al., 2007, Sevelinges et al., 2008).
In summary, we hypothesized that early life paired odor–shock results not only in later life depressive-like behaviors and amygdala dysfunction, but also supports positive associations to cues (i.e. odor) paired with adversity (i.e. the shock) because of the infant's unique learning attachment system. We test this hypothesis by pairing shock with a novel odor in infancy and assessing the ability of the odor to normalize adult depressive-like behavior, amygdala function and olfactory structures.
2. Material and methods
2.1. Subjects and husbandry
Male Long Evans rats (n = 166) were born in the respective institutions’ animal care facilities from dams housed in polypropylene cages (34 cm × 29 cm × 17 cm) lined with abundant pine shavings, ad libitum food and water, and kept in a temperature (23 °C) and light (from 7:00 am to 7:00 pm) controlled room. Mothers were either purchased pregnant (France) or bred (USA) in the facilities. The day of parturition was considered postnatal day (PN) 0 and culling of litters to 12 pups occurred on PN0–1. To prevent litter effects on statistical analysis, no more than one male from a litter was used in an experimental conditioning/testing condition. Institutional approval was received for all procedures, which followed the National Institute of Health (USA) and European guidelines (France). An overlap in personnel conditioning/testing both infant and adult rats in both France and the USA ensured consistency of conditioning and testing of infant and adult animals between labs.
2.2. Infant odor–shock conditioning with peppermint
PN8 pups were assigned to one of the following experimental groups: Paired odor–shock (n = 66), Unpaired odor–shock (n = 45), Odor-only (n = 30), Shock-only (n = 9) or Naïve (n = 16). Pups were trained daily for 5 consecutive days in order to induce strong learning that has long-term effects lasting into adulthood (Moriceau et al., 2009, Sevelinges et al., 2007, Sevelinges et al., 2008, Tyler et al., 2007). Our conditioning sessions were begun during the sensitive period (PN8) and continued past the end of the sensitive period (PN10) into the “transitional sensitive period”, which ends at PN15 (Moriceau and Sullivan, 2006, Upton and Sullivan, 2010). The sensitive period is characterized by rapid, robust odor learning, where the odor takes on qualities of maternal odor. Specifically, an odor paired with milk, grooming-like tactile stimulation (stroking) or mild pain (tailpinch, 0.5 mA tail or foot shock) produces an odor that functions as the maternal odor (Raineki et al., 2010, Sullivan et al., 1986, Sullivan et al., 2000). The transitional sensitive period is characterized by maternal presence reinstating sensitive period learning (Moriceau et al., 2009, Raineki et al., 2010). Thus, provided odor–shock conditioning is initiated during the sensitive period (prior to PN10) and ends before the end of the transitional sensitive period (PN15), the odor–shock conditioning continues to support the unique sensitive period odor learning. Importantly, pups exhibit this odor preference despite showing pain related responses to the shock (Barr, 1995, Collier and Bolles, 1980, Emerich et al., 1985, Fitzgerald, 2005, Shair et al., 1997, Stehouwer and Campbell, 1978).
The Paired group received 11 presentations of a 30 s conditioned stimulus (CS – McCormick Pure Peppermint, Hunt Valley, MD; 2 L/min 1:10 peppermint vapor to air) and a 1 s unconditioned stimulus (US – hind limb shock; 0.5 mA). Peppermint odor was delivered via an olfactometer controlled by a ChonTrol (ChonTrol Corporation, San Diego, CA) with an inter-trial interval of 4 min. The Unpaired group was shocked 1.5–2 min following the CS odor presentation. Odor-only and Shock-only groups received only the odor CS or the shock US respectively. Naïve pups did not receive either the odor or the shock and were always from a litter without any conditioned pups. Pups were removed from the nest immediately before conditioning and placed in individual 600 mL clear plastic beakers and given a 10 min adaptation period to recover from experimenter handling before conditioning began. Pups were returned immediately to the nest following the 45 min conditioning.
2.3. Pup behavioral study
2.3.1. Shock-induced behavioral activation and vocalization
To verify that shock induced pain, we recorded behavioral responses and audible vocalizations to shock during conditioning in PN8 Paired (n = 6) and Unpaired (n = 6) pups. Behavioral responses were observed at the same time points in control Odor-only pups (n = 6). Due to motor immaturity, a general assessment of behavioral activity was done using a rating score where 0 = no movement of the four limbs or head, and 5 indicated movement of all four limbs and the head (Hall, 1979, Roth and Sullivan, 2005, Sullivan and Leon, 1986). Vocalizations were expressed as the percentage of trials (out of 11) on which vocalizations occurred.
2.3.2. Infant acquisition curve during odor–shock conditioning
To verify associative learning, we recorded pups’ behavioral responses to CS odor presentation during the 29 s before the shock to construct acquisition curves. Due to motor immaturity, a general assessment of behavioral activity was used to assess learning on the behavioral rating scale (0–5).
2.3.3. Assessment of conditioned odor to function as a maternal odor: infant nipple attachment test
To verify that the conditioned odor acquired properties of the maternal odor, pups were tested using a nipple attachment test with an anesthetized mother (urethane, 1.5 g/kg, i.p., prevents milk letdown) the day after conditioning. Since the maternal odor is required for pups’ nipple attachment, the natural maternal odor was removed (chemical wash of the mother's ventrum – 5 min wash of acetone, 5 min wash of alcohol, 5 min wash with water, and dried) (Hofer et al., 1976, Teicher and Blass, 1977). The washed mother was then placed in the testing chamber (25 cm × 40 cm × 20 cm) on her side to provide pups access to nipples. An individual pup was placed in the testing apparatus, perpendicular to the mother's ventrum, and the learned odor (peppermint) was presented in an air stream 2 cm above the center of the mother's ventrum (2 L/min, 1:10 maternal odor:air). During the 3 min test, the duration of pups’ nipple attachment was recorded.
2.3.4. Assessment of conditioned odor to function as a maternal odor: approach responses in the Y-maze test
To further ensure that odor–shock conditioning resulted in an odor preference, 18 additional pups (Paired, n = 6; Unpaired, n = 6, Odor-only, n = 6) were given a Y-maze test the day after conditioning. This test required pups to choose between two arms of a Plexiglas Y maze (start box: 8.5 cm (width) × 10 cm (length) × 8 cm (height); choice arms: 8.5 cm × 24 cm × 8 cm): one containing the peppermint odor CS (20 μL of peppermint odor placed on a KimWipe), and the other containing the familiar odor of pine shavings (20 mL of clean shaving in a Petri dish). A pup was placed in the start box during the 5 s before the door to each alley was opened. Each pup was given 60 s to choose an arm. A response was considered a choice when a pup's entire body moved beyond the entrance to the alley. Pups received five trials with 5 s between trials, and the floor was wiped clean between each trial (Sullivan and Wilson, 1991). Testing was done blind to the conditioning groups. These pups were used for assessment of adult behavior and brain function.
2.4. Adult behavioral and neurobiological studies
2.4.1. Adult assessment of retention of learned infant CS odor: Y-maze test
Retention of infant learning was assessed with a Y-maze test in 18 additional rats in adulthood (Infant Unpaired, n = 8; Infant Paired, n = 10). The Y-maze consisted of a start box (13 cm width, 18 cm length, 18 cm height) and two arms (13 cm × 65 cm × 18 cm) separated by two doors. This test required rats to choose between two arms of a Plexiglas Y-maze, one containing the CS odor and the other containing the familiar wood odor (20 mL of clean shaving in a Petri dish). To initiate a trial, the adult rat was placed in the start box for 5 s, the doors to each arm were opened and the animal was given 60 s to choose an arm. Rats received three trials and were removed to a holding chamber for 5 s between trials while the Y-maze floor was wiped clean. Testing was done blind to the conditioning groups.
2.4.2. Adult assessment of depression-like behaviors with and without the infant odor
To evaluate depression-like behavior at adulthood, rats were tested either in the FST or in the Sucrose Preference Test.
For the FST, we used the original Porsolt test (Porsolt et al., 1977). 49 animals (Infant Paired, n = 10; Infant Unpaired, n = 10; Infant Odor-only, n = 10; Infant Shock-only, n = 9; Infant Naïve, n = 10) were placed in a plastic cylinder (d = 25 cm; h = 65 cm; water temperature 28 ± 1 °C) such that the animal or tail did not touch the cylinder's floor. The cylinder was always covered with a clear plastic film to contain the odor if presented. Time spent immobile, i.e. the rat floated in upright position with minor movements necessary to maintain the head above water, and latency to immobility were analyzed. On day 1, animals were given a 15 min pretest swim. On day 2, rats were tested in a 5 min swim, either with or without the infant CS odor. The odor was delivered continuously through a tube inserted in the cylinder, using the same concentration and flow rate as used in infancy. Following both sessions, animals were gently dried, placed in a 32 °C chamber and returned to the home cage. The water was changed between animals.
For the Sucrose Preference Test, 24 (Infant Paired, n = 8; Infant Unpaired, n = 8; Infant Odor-only, n = 8) rats were given a 72 h acclimation period and given access to a 1% (w/v) sucrose/water solution or tap water in the home cage, to avoid neophobia during testing. Bottle positions were switched 2–3 times a day to prevent the formation of location preference. Before the Sucrose Preference Test, animals were deprived of water for approximately 3 h. The test began with two bottles containing either tap water or the sucrose solution placed into individual cages either with or without the infant CS present as a contextual cue. The odor was delivered every 2 min using the same concentration and flow rate as used in infancy. After 60 min, fluid intake was measured and sucrose preference was calculated as the percentage of sucrose solution ingested relative to the total amount of liquid consumed.
2.4.3. Adult odor specificity test and depressive-like behaviors
To evaluate whether odor presentation nonspecifically returns the depressive-like behaviors to control levels, we repeated the FST and Sucrose Preference test with a novel citral odor. Adult rats from the Infant Paired group were tested either in the FST (n = 9; n = 4 with and n = 5 without the novel citral odor) or the Sucrose Preference Test (n = 10; n = 4 with and n = 6 without the novel citral odor).
2.4.4. Adult electrophysiology on amygdala and piriform cortex with and without the infant odor
Electrophysiological recordings were done in adulthood following infant Paired (n = 7), Unpaired (n = 7) or Naïve (n = 6) experience, in order to evaluate the level of inhibitory interneuron activity in the amygdala and olfactory cortex under two experimental conditions: either with or without the infant odor delivered during the recordings.
Animals were anesthetized with Equithesin (mixture of chloral hydrate and sodium pentobarbital; 3 mL/kg, i.p.). A bipolar stimulating electrode was lowered in the left olfactory bulb. Four monopolar recording electrodes were implanted ipsilaterally in the anterior piriform cortex (aPC, A/P +2.2 mm, L/M 4 mm relative to Bregma), the posterior piriform cortex (pPC, A/P −1.8 mm, L/M 5.5 mm to Bregma), the basolateral amygdala (BLA, A/P −2.8 mm, L/M 5.1 mm relative to Bregma) and the cortical nucleus of the amygdala (CoA, A/P −2.3 mm, L/M 3.3 mm relative to Bregma). Accurate positioning of recording electrodes depth was achieved using the field potential profile evoked in each structure in response to electrical stimulation of the bipolar olfactory bulb electrodes (Mouly and Di Scala, 2006).
Electrical stimulation was delivered in the olfactory bulb through a Master-8 stimulator (AMPI, Jerusalem, Israel). The electrical stimulus was a single monophasic square pulse, 0.1 ms duration, 0.2 Hz frequency, 300–500 μA intensity.
Paired-pulse stimulation of the olfactory bulb, with an interpulse interval of 20 ms, was used to assess the time course of short-term inhibition in the different recording sites. This interval allows the study of the functioning of local inhibitory feedback exerted by local GABAergic interneurons onto the main glutamatergic projection neurons (piriform cortex: Princivalle et al., 2000, Tseng and Haberly, 1988; amygdala: Muller et al., 2006, Sah et al., 2003). The effect of the conditioning (first) pulse was assessed by measuring changes in the response to the test (second) pulse. A ratio Test/Conditioning below 1 is observed when the response to the test pulse is smaller than the response to the conditioning pulse and characterizes paired-pulse inhibition. Evoked field potentials were recorded both with and without CS odor presentation (20 μL of peppermint odor placed on a KimWipe in a syringe and delivered manually during the recording sessions). Paired-pulse data obtained without odor have been included in a previous study (Sevelinges et al., 2008).
2.5. Data analyses
Intergroup comparisons were evaluated using Student's unpaired t-test or one-factor (groups) analysis of variance (ANOVA), followed by post hoc Fisher tests for between group comparisons. Intra-group comparisons during the 11 trials of the learning-curve were made using a repeated measure ANOVA (number of trial as a dependant factor and group as an independent factor). For all the statistical comparisons performed, the significance level was set at 0.05.
3. Results
3.1. Pup behavior
3.1.1. Shocks induce behavioral activation and vocalizations
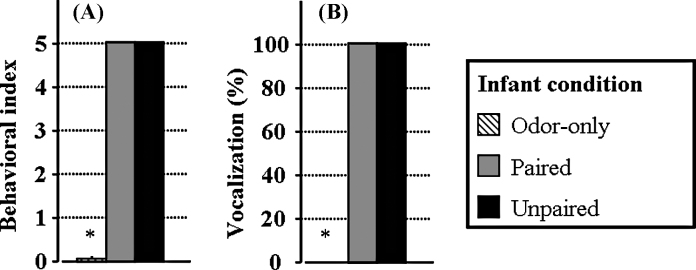
A one-way ANOVA revealed a significant effect of groups (F(2,15) > 100, p < 0.001) for shock-induced behaviors. The results showed that shocks induced a vigorous generalized movement of all limbs and head, i.e. a score of 5, in Paired and Unpaired groups, whereas at the same time points, Odor-only pups did not move, i.e. they exhibited a score of 0 (Fig. 1A). Post hoc analyses revealed that Paired and Unpaired groups exhibited a level of behavioral activation significantly higher than Odor-only pups (p < 0.001) but were not different from each other (p = 1.0).
Fig. 1.
Effects of shocks on behavioral activation and vocalizations in pups. The presentation of a 0.5 mA shock induces a similar level of behavioral activation (A) and vocalizations (B) in Paired and Unpaired odor–shock animals during the conditioning session. During this session, Odor-only animals exhibited none of these two parameters. *: significant difference from all other groups (p < 0.05).
For the shock-induced vocalizations, a one-way ANOVA revealed a significant effect between groups (F(2,15) > 100, p < 0.001). The results showed that Paired and Unpaired groups vocalized for each of the 1 s shocks whereas at the same time points, Odor-only pups did not vocalize (Fig. 1B). Post hoc analyses revealed that Paired and Unpaired groups exhibited a vocalization score significantly higher than Odor-only pups (p < 0.001) but were not different from each other (p = 1).
3.1.2. During conditioning, paired pups showed acquisition curves
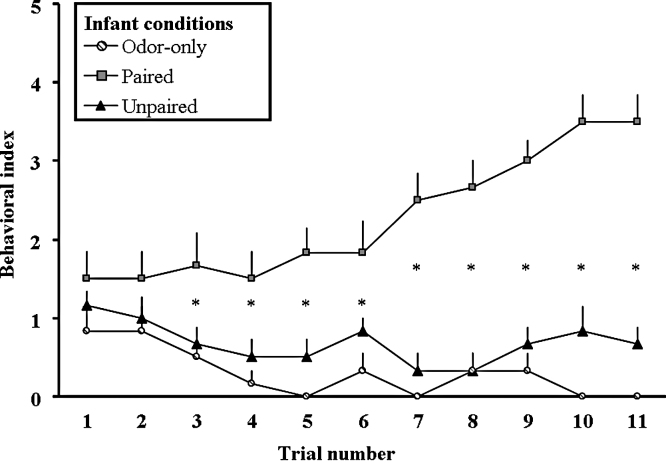
A repeated measure ANOVA carried out on the behavioral activation scores measured throughout the conditioning session (Fig. 2) showed a significant group effect (F(2,15) = 31.4, p < 0.001), trial effect (F(10,150) = 4.6, p < 0.001) and interaction between group × trial (F(20,150) = 8.6, p < 0.001). A repeated measure ANOVA for each group revealed that only Paired animals exhibited a significant odor-induced behavioral activation during the conditioning session (F(10,50) = 13.2, p < 0.0001). Finally, a one-way ANOVA for each trial revealed a significant effect of group from Trials 3 to 11. Post hoc analyses showed that the Paired animals exhibited an odor-induced behavioral activation significantly higher than Unpaired and Odor-only pups from Trials 3 to 11 (Trial 3: p < 0.05; Trials 4–11: p < 0.01). These two latter groups were not significantly different, except for Trial 10 (p = 0.04).
Fig. 2.
Acquisition curve to CS odor presentation during conditioning in infancy. During odor–shock conditioning, Paired animals exhibited a progressive increase of odor-induced behavioral activation. Animals from control groups (Unpaired and Odor-only) showed no behavioral activation in response to odor presentation. *: significant difference from all other groups (p < 0.05).
3.1.3. Despite painful shock, paired pups exhibited an attraction for the CS odor and the odor supported nipple attachment
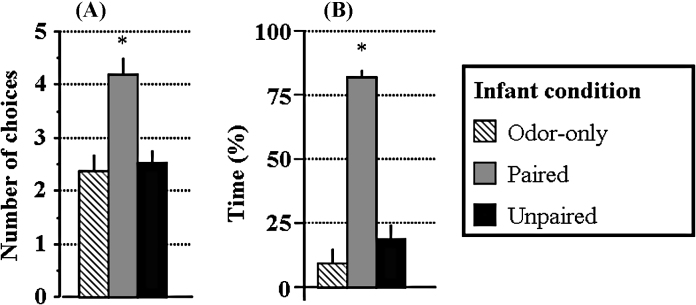
In the Y-maze test (Fig. 3A), PN8 Paired pups preferentially chose the arm containing the odor previously paired with the shock, whereas control pups (Unpaired or Odor-only) did not exhibit such an attraction. ANOVA confirmed a main effect of condition (F(2,15) = 76.1, p < 0.001). Post hoc Fisher tests showed that the Paired group was significantly different from the two control groups (p < 0.01) which were not different from each other (p = 0.69).
Fig. 3.
Odor–shock pairing produced an odor similar in quality to maternal odor since it produced an odor preference/approach to the odor, as well as supporting nipple attachment. Paired animals learned the odor that was expressed as (A) an odor preference as indicated by an approach to the odor in a Y-maze and (B) support of nipple attachment on a mother with the natural maternal odor removed. Unpaired and Odor-only control animals exhibited no learning in either test. *: significant difference from all other groups (p < 0.05).
For the nipple attachment test (Fig. 3B), a one-way ANOVA revealed a significant effect of group (F(2,15) = 76,1, p < 0.001). Post hoc Fisher's test showed that Paired animals spent significantly more time attached to the nipples in the presence of the infant odor CS than Unpaired and Odor-only animals (p < 0.001), which were not different from each other (p = 0.16).
3.2. Adult behavioral and neurobiological studies
3.2.1. Attraction for the CS odor was maintained into adulthood
Student's unpaired t-test revealed that in adulthood, Infant Paired animals preferentially chose the arm containing the infant peppermint odor while the Infant Unpaired animals performed close to chance (t = −3.3, p < 0.01; Fig. 4). This result showed that the odor attraction acquired during infancy is maintained into adulthood.
Fig. 4.
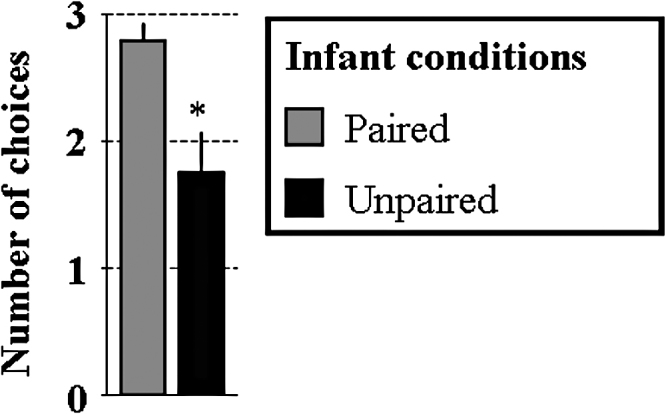
Infant odor attraction is maintained at adulthood. During the adult Y-maze test, Infant Paired, but not Infant Unpaired animals chose preferentially the arm containing the odor paired with shock during infancy. *: significant intergroup difference (p < 0.05).
3.2.2. Infant odor–shock pairing induced depression-like behaviors in adulthood, which was specifically restored by the infant odor
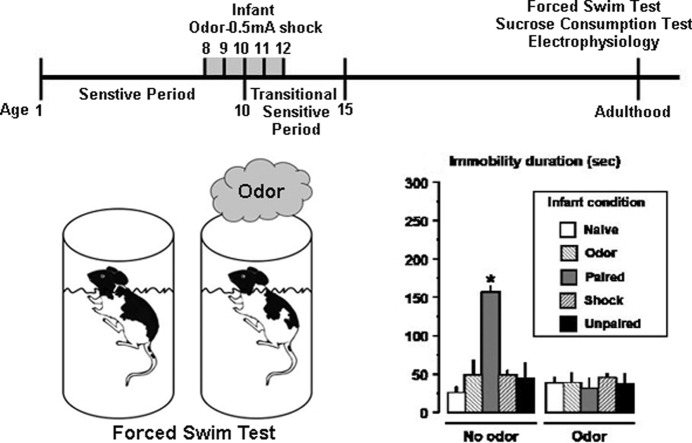
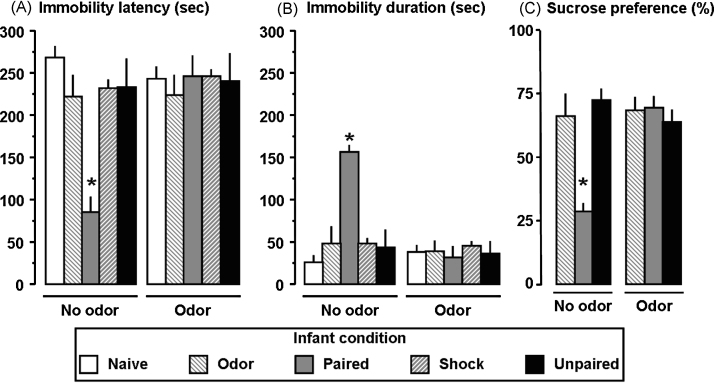
In adulthood, two-way ANOVA for depressive-like behavior showed a significant interaction of the Infant condition × Peppermint Odor for immobility in the FST (immobility latency, F(4,39) = 5.1, p < 0.01, Fig. 5A; immobility time, F(4,39) = 8.8, p < 0.001, Fig. 5B) and sucrose consumption (F(2,18) = 11.1, p < 0.001, Fig. 5C). Post hoc tests revealed that Infant Paired odor–shock animals exhibited a shorter latency and more immobility time in the FST, as well as lower levels of sucrose consumption compared to all other groups (p < 0.05). However, presentation of the infant peppermint odor paired with shock reversed these effects: Infant Paired animals behaved similarly to controls. Post hoc tests showed that Infant Paired odor–shock adult rats were significantly different from each control group as well as from the Paired group with infant peppermint odor for both the FST and Sucrose Test (p < 0.05).
Fig. 5.
Effects of infant odor–shock experience on adult depression-like behavior as assessed in the Forced Swim Test and Sucrose Preference Test. Adult animals with Infant Paired experience exhibited a lower latency to immobility (A), spent more time immobile (B) and exhibited an anhedonia in the Sucrose Preference Test (C). However, they behaved similarly to control animals when the infant learned peppermint odor was presented. *: significant intergroup differences (p < 0.001).
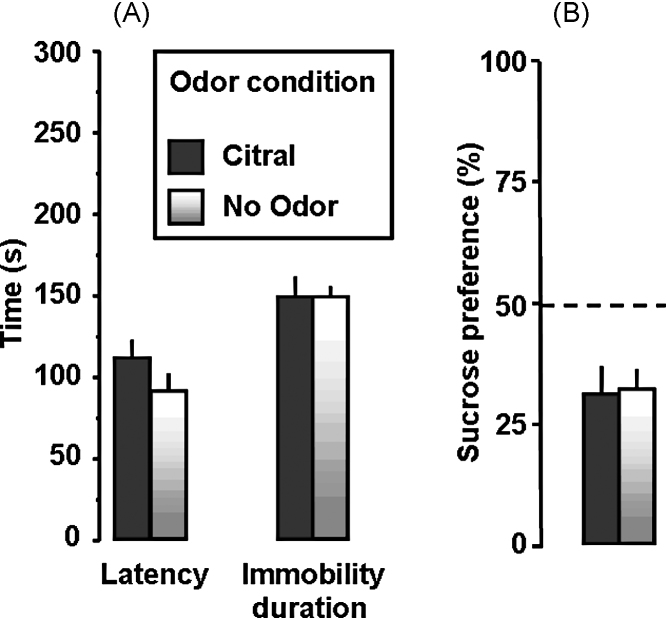
Moreover, we assessed the effect of the presentation of the novel citral odor in Infant Paired (peppermint) odor–shock animals on depressive-like behavior. Student's unpaired t-test showed that this novel citral odor did not attenuate depression-like behavior in the FST (immobility latency, t = 0.575, p = 0.58; FST immobility time, t < 1) nor in the Sucrose Preference Test (t < 1; Fig. 6A and B). The novelty-reaction cannot explain our results because the presence of the novel odor neither increased nor decreased swim levels in either our control or experimental groups. The results of our sucrose consumption test are consistent with this notion since the novel odor also did not change experimental or control levels over the course of the 1-h test, when the novelty effect would be eliminated.
Fig. 6.
Effects of the novel citral odor on adult depression-like behavior as assessed in the Forced Swim Test and Sucrose Preference Test. Adult animals with Infant Paired experience exhibited a lower latency to immobility, spent more time immobile (A), and exhibited an anhedonia in the Sucrose Preference Test (B). However, contrary to the infant odor paired with shock, the novel citral odor failed to restore the behavior to control level.
3.2.3. Infant odor–shock pairing induced a deficit in paired-pulse inhibition in amygdala and piriform cortex that was restored by the infant odor
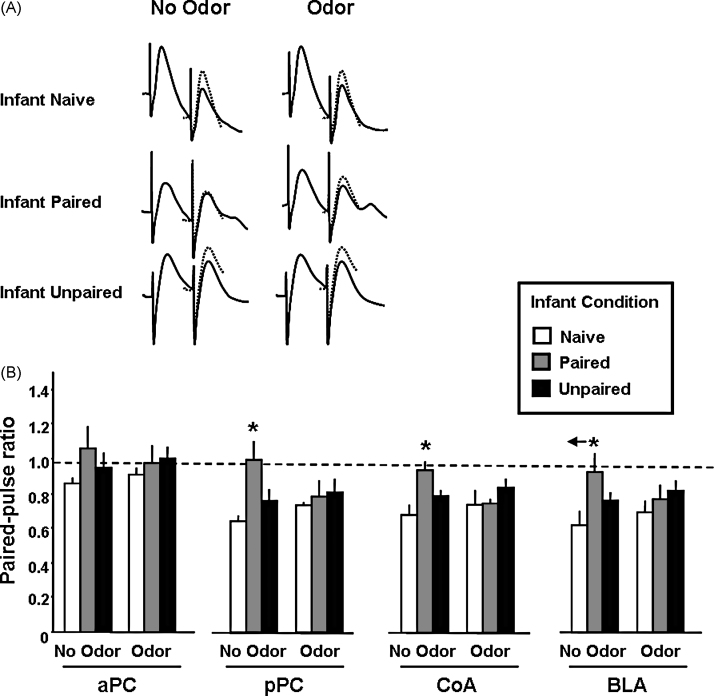
In adulthood, animals were also used for evoked field potential recordings in the piriform cortex and amygdala in response to paired-pulse stimulation of the olfactory bulb (Fig. 7). A three-way ANOVA revealed a significant interaction of the Infant conditioning group × adult odor presence × structure (F(2,68) = 59.46, p < 0.001). Further post hoc tests showed that Infant Paired odor–shock adult animals exhibited no paired-pulse inhibition in the posterior piriform (olfactory) cortex, cortical nucleus of the amygdala and basolateral amygdala in contrast to control Infant Naïve and Infant Unpaired animals (p < 0.05). Interestingly, presentation of the infant peppermint odor brought the Infant Paired animals’ paired-pulse ratios to control levels. However, Infant Paired odor–shock experience did not induce any detectable changes in the anterior piriform cortex paired-pulse in adult animals.
Fig. 7.
Effects of infant odor–shock experience on field potential responses recorded in the anterior (aPC) and posterior (pPC) piriform cortex, cortical (CoA) and basolateral (BLA) amygdala in response to paired-pulse electrical stimulation of the olfactory bulb, using a 20 ms inter-pulse interval. (A) Representative individual mean signals (n = 12 sweeps) recorded in the BLA in response to the conditioning (first) and test (second) pulses delivered to the olfactory bulb with or without the infant learned odor present. The signal induced by the conditioning pulse (dotted line) is superimposed on the signal resulting from the test pulse. (B) Mean paired-pulse ratios (mean test signal amplitude/mean conditioning signal amplitude ± SEM) obtained in the different experimental groups, in the four recording sites. A ratio <1 characterizes paired-pulse inhibition, whereas a ratio >1 corresponds to paired-pulse facilitation. Infant Paired animals exhibited a deficit in paired-pulse inhibition compared to control animals in the pPC, CoA and BLA. However, presentation of the infant learned peppermint odor brought paired-pulse ratios to control values. *: significant intergroup differences (p < 0.05).
4. Discussion
4.1. Infant odor–shock pairing induces depression-like behavior
The present data show that infant odor–0.5 mA shock pairings result in a learned odor preference and produce an odor that controls pups’ nipple attachment, despite the aversiveness of the shocks. These results support previous work indicating that this aversive conditioning with slight to moderate pain produces an odor preference in young infant rats (Camp and Rudy, 1988, Haroutunian and Campbell, 1979, Sullivan et al., 1986) due to the lack of amygdala participation in this infant learning (Moriceau et al., 2006, Sullivan et al., 2000). Following this conditioning, the odor becomes a new maternal odor that gains control over pups’ interactions with the mother and attachment (Moriceau and Sullivan, 2006, Raineki et al., 2010, Roth and Sullivan, 2005, Sullivan et al., 1986, Sullivan et al., 2000). However, the adult consequence of this early life adverse experience results in higher levels of depression-like behavior, as assessed through the FST and the Sucrose Preference Test. These results support previous clinical studies (Cicchetti and Toth, 2005, Connor et al., 2003, Gunnar, 2003, Heim et al., 2009, Heim and Nemeroff, 2001, McEwen, 2003, Nemeroff, 2004, Pollak, 2003, Rutter et al., 2006, Stovall-McClough and Cloitre, 2006, Teicher et al., 2003, Zeanah et al., 2003) and animal models (Card et al., 2005, Cameron et al., 2005, Cirulli et al., 2009, Kosten et al., 2006, McEwen, 2008, Pryce et al., 2002, Sanchez et al., 2001, Sevelinges et al., 2008) indicating that exposure to early life adverse experiences increases vulnerability to emotional and cognitive dysfunctions, including depressive-like behaviors in adulthood.
It is important to note that shock unpaired with the odor does not result in infant learning (Kucharski and Spear, 1984, Sullivan et al., 2000), nor does it result in detectable behavioral or neural effects in adulthood (Sevelinges et al., 2007, Sevelinges et al., 2008), suggesting that the contingency of adversity in infancy has a significant impact on the enduring effects of adversity. Indeed, in the present document, we reported that the neonatal predictability of the shock (via odor) appears critical in determining both the behavioral and neurobiological effects since only the Paired (predictable) shock animals, but not the Unpaired and Shock-only animals, showed deficits in the FST and the Sucrose Preference Test.
The clinical literature and early life experience animal literature indicate that the type, patterning and age of early life trauma produce different outcomes, although the mechanisms for this divergence are not well understood. Indeed, early life maternal separation, exposure to a stressed mother or exposure to adverse events (i.e. shock) produce diverse effects in adulthood using both cognitive and emotional assessments (Cameron et al., 2005, Cirulli et al., 2009, McEwen, 2008, Sanchez et al., 2001). Based on the present results of only paired odor–shock inducing later life depressive-like behavior, we suggest that the predictability of the adverse effect may be one such variable. Indeed, our previous data indicates the unpredictable unpaired infant shock is associated with anxiety in adulthood as assessed by latency to leave a dark box and enter a lighted alley (Tyler et al., 2007) and supports previous work showing that the predictability of shock was associated with later life impairment (Bell and Denenberg, 1962, Levine, 2005). Therefore, we propose that early life shock produces adverse outcomes in early life, unpredictable shock is associated with an animal model of anxiety, while predictable shock appears to be associated with an animal model of depression. These influences of early life shock and its predictability may be limited to infancy, since the adult literature suggests that unpredictable adverse events are associated with depression-like behavior and related to the “learned helplessness” (Seligman and Maier, 1967, Sherman et al., 1979). One hypothesis to explain this discrepancy is the “predictive adaptation hypothesis” (for review see Oitzl et al., 2010) postulating that infant aversive events “program” the individual to the world it will live in.
4.2. Neurobiological basis of depressive-like behavior induced by infant odor–shock pairings
Clinical studies have indicated that the amygdala is associated with depression (Dreverts et al., 2008, Monk et al., 2008, Nestler et al., 2002a, Nestler et al., 2002b, Ressler and Mayberg, 2007, Sheline et al., 1998, Whalen et al., 2002). Animal models have also supported the relationship between the amygdala and depressive-like behaviors (Nestler et al., 2002a, Nestler et al., 2002b, Ressler and Mayberg, 2007). Importantly, adverse effects of early life trauma are seen in neural processing within the olfactory pathway and the amygdala. Specifically, paired odor–shock experience in infancy result in adult lower levels of paired-pulse inhibition in a neural network involving the posterior piriform cortex, cortical nucleus of the amygdala, and basolateral amygdala (present results; Sevelinges et al., 2007, Sevelinges et al., 2008). This effect appears to reflect mainly a decrease in GABAergic interneuron function in the inhibitory feedback exerted by local GABAergic interneurons onto the main glutamatergic projection neurons (piriform cortex: Princivalle et al., 2000, Tseng and Haberly, 1988; amygdala: Muller et al., 2006, Sah et al., 2003). Interestingly, it has been proposed that decreased GABA functioning may be associated with anxiety and depression in animal models (Brambilla et al., 2003).
4.3. The infant odor may function as a safety signal
Since the infant conditioned odor functioned as a maternal odor and supported attachment to the mother, we speculated that the odor may have acquired the value of a “paradoxical safety signal”, which is known to reduce conditioned fear responses, normalize FST (decrease immobility) and normalize amygdala activity (Pollak et al., 2008, Rogan et al., 2005, Schiller et al., 2008). A “safety signal” is typically formed when it predicts the non-occurrence of the aversive event and/or produces a reduction in fear and/or anxiety (Bolles and Robert, 1970, Candido et al., 2004, Chan et al., 2001, Josselyn et al., 2005, Ostroff et al., 2010, Pollak et al., 2008, Rogan et al., 2005, Schiller et al., 2008). Paradoxically, as is reviewed above for infant conditioning, a safety signal appears to be produced from the odor even when it is paired with pain. In infancy, this odor blocks fear conditioning and prevents amygdala plasticity in older pups by blocking the release of the stress hormone corticosterone (Moriceau and Sullivan, 2006, Shionoya et al., 2007, Stanton and Levine, 1990, Wiedenmayer et al., 2003), which prevents infant amygdala plasticity (Moriceau and Sullivan, 2006, Moriceau et al., 2006). Furthermore, this safety signal appears to be retained into adulthood as indicated by the natural and learned maternal odors’ abilities to enhance sexual (Fillion and Blass, 1986, Mainardi et al., 1965, Marr and Gardner, 1965, Moore et al., 1996) and maternal behaviors (Shah et al., 2002), improve olfactory discrimination learning (Blais et al., 2006), reduce conditioned odor aversion (Sevelinges et al., 2009a, Sevelinges et al., 2009b) and reduce fear learning and amygdala activity (Sevelinges et al., 2007, Sevelinges et al., 2008). The present data suggest this odor also reduces depressive-like behavior, which is also consistent with the notion of a safety signal (Pollak et al., 2008, Rogan et al., 2005). Together, these results suggest that both learned and natural maternal odors have characteristics consistent with a safety signal in both infancy and adulthood.
4.4. Infant odor normalizes amygdala and piriform cortex activity
The ability of the learned odor to restore amygdala activity, a brain area associated with fear and depression (Balu et al., 2009, Castro et al., 2010, Fanselow and Gale, 2003, Matsuzawa-Yanagida et al., 2008, Muigg et al., 2007, Roche et al., 2007, Shishkina et al., 2007, Varea et al., 2007), is also consistent with the notion of the maternal odor as a safety signal (Pollak et al., 2008, Rogan et al., 2005, Schiller et al., 2008). With respect to the present results, the amygdala appears to be targeted by infant paired odor–shock. However, unexpectedly, presentation of the odor paired with shock in infancy was capable of restoring control levels of the neural measurements. The odor signal, which arrives in the amygdala either directly from the olfactory bulb or indirectly through the piriform cortex (Haberly and Price, 1978a, Haberly and Price, 1978b, Luskin and Price, 1982, Luskin and Price, 1983, McDonald, 1998, Price, 1973, Savander et al., 1996, Swanson and Petrovich, 1998), restored neural functioning at various points along the olfactory pathway to the amygdala.
4.5. Implications
These data complement the existing literature on the enduring effects of early life aversive experiences indicating lasting effects on emotional dysregulation and amygdala. However, the present studies also suggest that other enduring effects of early life trauma can also be dependent upon learning and expressed in unexpected ways due to unique learning characteristics during infancy. Specifically, the odor associated with shock in infancy has acquired value to attenuate the enduring effects of trauma on both behavior and brain.
Acknowledgments
This work was supported by NIH-DC009910, NIH-MH091451, NSF-IOB0850527/0544406 to RMS, Eurodoc grant to YS, and financial support of the Agence Nationale de la Recherche (ANR), under the Programme National de Recherche en Alimentation et Nutrition Humaine project ANR-05-PNRA-1.E7 AROMALIM to AMM. The authors would like to thank Lisa Salstein for proofreading the manuscript.
References
- Anisman H., Zaharia M.D., Meaney M.J., Merali Z. Do early-life events permanently alter behavioral and hormonal responses to stressors? Int. J. Dev. Neurosci. 1998;16:149–164. doi: 10.1016/s0736-5748(98)00025-2. [DOI] [PubMed] [Google Scholar]
- Balu D.T., Hodes G.E., Anderson B.T., Lucki I. Enhanced sensitivity of the MRL/MpJ mouse to the neuroplastic and behavioral effects of chronic antidepressant treatments. Neuropsychopharmacology. 2009;34:1764–1773. doi: 10.1038/npp.2008.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr G.A. Ontogeny of nociception and antinociception. NIDA Res. Monogr. 1995;158:172–201. [PubMed] [Google Scholar]
- Bell R.W., Denenberg V.H. The interrelationships of shock and critical periods in infancy as they affect adult learning and activity. Anim. Behav. 1962;11:21–27. [Google Scholar]
- Blais I., Terkel J., Goldblatt A. Long-term impact of early olfactory experience on later olfactory conditioning. Dev. Psychobiol. 2006;48:501–507. doi: 10.1002/dev.20176. [DOI] [PubMed] [Google Scholar]
- Bolles R.C., Robert C. Species-specific defense reactions and avoidance learning. Psychol. Rev. 1970;77:32–48. [Google Scholar]
- Brambilla P., Perez J., Barale F., Schettini G., Soares J.C. GABAergic dysfunction in mood disorders. Mol. Psychiatry. 2003;8:721–737. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- Cameron N.M., Champagne F.A., Parent C., Fish E.W., Ozaki-Kuroda K., Meaney M.J. The programming of individual differences in defensive responses and reproductive strategies in the rat through variations in maternal care. Neurosci. Biobehav. Rev. 2005;29:843–865. doi: 10.1016/j.neubiorev.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Camp L.L., Rudy J.W. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Dev. Psychobiol. 1988;21:25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Candido A., Gonzalez F., de Brugada I. Safety signals from avoidance learning but not from yoked classical conditioning training pass both summation and retardation tests for inhibition. Behav. Processes. 2004;66:153–160. doi: 10.1016/j.beproc.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Card J.P., Levitt P., Gluhovsky M., Rinaman L. Early experience modifies the postnatal assembly of autonomic emotional motor circuits in rats. J. Neurosci. 2005;25:9102–9111. doi: 10.1523/JNEUROSCI.2345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro J.E., Varea E., Marquez C., Cordero M.I., Poirier G., Sandi C. Role of the amygdala in antidepressant effects on hippocampal cell proliferation and survival and on depression-like behavior in the rat. PLoS One. 2010;5:e8618. doi: 10.1371/journal.pone.0008618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.H., Morell J.R., Jarrard L.E., Davidson T.L. Reconsideration of the role of the hippocampus in learned inhibition. Behav. Brain Res. 2001;119:111–130. doi: 10.1016/s0166-4328(00)00363-6. [DOI] [PubMed] [Google Scholar]
- Cicchetti D., Toth S.L. Child maltreatment. Annu. Rev. Clin. Psychol. 2005;1:409–438. doi: 10.1146/annurev.clinpsy.1.102803.144029. [DOI] [PubMed] [Google Scholar]
- Cirulli F., Francia N., Berry A., Aloe L., Alleva E., Suomi S.J. Early life stress as a risk factor for mental health: role of neurotrophins from rodents to non-humans primates. Neurosci. Biobehav. Rev. 2009;33:573–585. doi: 10.1016/j.neubiorev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A.C., Bolles R.C. The ontogenesis of defensive reactions to shock in preweanling rats. Dev. Psychobiol. 1980;13:141–150. doi: 10.1002/dev.420130206. [DOI] [PubMed] [Google Scholar]
- Connor D.F., Doerfler L.A., Volungis A.M., Steingard R.J., Melloni R.H., Jr. Aggressive behavior in abused children. Ann. N. Y. Acad. Sci. 2003;1008:79–90. doi: 10.1196/annals.1301.009. [DOI] [PubMed] [Google Scholar]
- Dreverts W.C., Price J.L., Furey M.L. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct. Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich D.F., Scalzo F.M., Enters E.K., Spear N.E., Spear L.P. Effects of 6-hydroxydopamine-induced catecholamine depletion on shock-precipitated wall climbing of infant rat pups. Dev. Psychobiol. 1985;18:215–227. doi: 10.1002/dev.420180303. [DOI] [PubMed] [Google Scholar]
- Fanselow M.S., Gale G.D. The amygdala, fear, and memory. Ann. N. Y. Acad. Sci. 2003;985:125–134. doi: 10.1111/j.1749-6632.2003.tb07077.x. [DOI] [PubMed] [Google Scholar]
- Fillion T.J., Blass E.M. Infantile experience with suckling odors determines adult sexual behavior in male rats. Science. 1986;231:729–731. doi: 10.1126/science.3945807. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. The development of nociceptive circuits. Nat. Rev. Neurosci. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- Freud, S.S., 1997. A child is being beaten. Yale University Press. Collected writings compiled by the International Psycho-Analytic Association.
- Gunnar M.R. Integrating neuroscience and psychological approaches in the study of early experiences. Ann. N. Y. Acad. Sci. 2003;1008:238–247. doi: 10.1196/annals.1301.024. [DOI] [PubMed] [Google Scholar]
- Haberly L.B., Price J.L. Association and commissural fiber systems of the olfactory cortex of the rat. I. Systems originating in the piriform cortex and adjacent areas. J. Comp. Neurol. 1978;181:781–807. doi: 10.1002/cne.901810407. [DOI] [PubMed] [Google Scholar]
- Haberly L.B., Price J.L. Association and commissural fiber systems of the olfactory cortex of the rat. II. Systems originating in the olfactory peduncle. J. Comp. Neurol. 1978;181:781–807. doi: 10.1002/cne.901810407. [DOI] [PubMed] [Google Scholar]
- Hall W.G. Feeding and behavioral activation in infant rats. Science. 1979;205:206–209. doi: 10.1126/science.451591. [DOI] [PubMed] [Google Scholar]
- Harlow H., Harlow M. The affectional system. In: Schrier A., Harlow H., Stollnitz F., editors. vol. 2. Academic Press; New York: 1965. (Behavior of Nonhuman Primates). [Google Scholar]
- Haroutunian V., Campbell B.A. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205:927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- Haynes-Seman C. Developmental origins of moral masochism: a failure-to-thrive toddler's interactions with mother. Child Abuse Negl. 1987;11:319–330. doi: 10.1016/0145-2134(87)90005-6. [DOI] [PubMed] [Google Scholar]
- Heim C., Nemeroff C.B. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C., Bradley B., Mletzko T.C., Deveau T.C., Musselman D.L., Nemeroff C.B., Ressler K.J., Binder E.B. Effect of childhood trauma on adult depression and neuroendocrine function: sex-specific moderation by CRH receptor 1 gene. Front. Behav. Neurosci. 2009;3:41. doi: 10.3389/neuro.08.041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer M.E., Kempe R.S., Krugman R.D. University of Chicago Press; Chicago: 1997. The Battered Child. [Google Scholar]
- Hofer M.A., Shair H., Singh P. Evidence that maternal ventral skin substances promote suckling in infant rats. Physiol. Behav. 1976;17:131–136. doi: 10.1016/0031-9384(76)90279-1. [DOI] [PubMed] [Google Scholar]
- Hofer M.A., Sullivan R.M. Towards a neurobiology of attachment. In: Nelson C.A., Luciana M., editors. Handbook of Developmental Cognitive Neuroscience. 2nd ed. MIT Press; 2008. pp. 787–806. [Google Scholar]
- Josselyn S.A., Falls W.A., Gewirtz J.C., Pistell P., Davis M. The nucleus accumbens is not critically involved in mediating the effects of a safety signal on behavior. Neuropsychopharmacology. 2005;30:17–26. doi: 10.1038/sj.npp.1300530. [DOI] [PubMed] [Google Scholar]
- Kosten T.A., Lee H.J., Kim J.J. Early life stress impairs fear conditioning in adult male and female rats. Brain Res. 2006;1087:142–150. doi: 10.1016/j.brainres.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Kucharski D., Spear N.E. Conditioning of aversion to an odor paired with peripheral shock in the developing rat. Dev. Psychobiol. 1984;17:465–479. doi: 10.1002/dev.420170505. [DOI] [PubMed] [Google Scholar]
- Ladd C.O., Huot R.L., Thrivikraman K.V., Nemeroff C.B., Meaney M.J., Plotsky P.M. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog. Brain Res. 2000;122:81–103. doi: 10.1016/s0079-6123(08)62132-9. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30:939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Luskin M.B., Price J.L. The distribution of axon collaterals from the olfactory bulb and the nucleus of the horizontal limb of the diagonal band to the olfactory cortex, demonstrated by double retrograde labeling techniques. J. Comp. Neurol. 1982;209:249–263. doi: 10.1002/cne.902090304. [DOI] [PubMed] [Google Scholar]
- Luskin M.B., Price J.L. The topographic organization of associational fibers of the olfactory system in the rat, including centrifugal fibers to the olfactory bulb. J. Comp. Neurol. 1983;216:264–291. doi: 10.1002/cne.902160305. [DOI] [PubMed] [Google Scholar]
- Mainardi D., Marsan M., Pasquali A. Causation of sexual preference in the house mouse. The behavior of mice reared by parents whose odor was artificially altered. Atti. Soc. Ital. Sci. Nat. 1965;104:325–338. [Google Scholar]
- Marr J.N., Gardner L.E. Early olfactory experience and later social behavior in the rat preference, sexual responsiveness, and care of young. J. Genet. Psychol. 1965;107:167–174. doi: 10.1080/00221325.1965.10532774. [DOI] [PubMed] [Google Scholar]
- Matsuzawa-Yanagida K., Narita M., Nakajima M., Kuzumaki N., Niikura K., Nozaki H. Usefulness of antidepressants for improving the neuropathic pain-like state and pain-induced anxiety through actions at different brain sites. Neuropsychopharmacology. 2008;33:1952–1965. doi: 10.1038/sj.npp.1301590. [DOI] [PubMed] [Google Scholar]
- McDonald A.J. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Early life influences on life-long patterns of behavior and health. Ment. Retard. Dev. Disabil. Res. Rev. 2003;9:149–154. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Understanding the potency of stressful early life experiences on brain and body function. Metabolism. 2008;57:S11–S15. doi: 10.1016/j.metabol.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C.S., Klein R.G., Telzer E.H., Schroth E.A., Mannuzza S., Moulton J.L., III, Guardino M., Masten C.L., McClure-Tone E.B., Fromm S., Blair R.J., Pine D.S., Ernst M. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am. J. Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Moore C.L., Jordan L., Wong L. Early olfactory experience, novelty, and choice of sexual partner by male rats. Physiol. Behav. 1996;60:1361–1367. doi: 10.1016/s0031-9384(96)00249-1. [DOI] [PubMed] [Google Scholar]
- Moriceau S., Raineki C., Holman J.D., Holman J.G., Sullivan R.M. Enduring neurobehavioral effects of early life trauma mediated through learning and corticosterone suppression. Front. Behav. Neurosci. 2009;3:22. doi: 10.3389/neuro.08.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Sullivan R.M. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat. Neurosci. 2006;9:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Wilson D.A., Levine S., Sullivan R.M. Dual circuitry for odor–shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J. Neurosci. 2006;26:6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouly A.M., Di Scala G. Entorhinal cortex stimulation modulates amygdala and piriform cortex responses to olfactory bulb inputs in the rat. Neuroscience. 2006;137:1131–1141. doi: 10.1016/j.neuroscience.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Muigg P., Hoelzl U., Palfrader K., Neumann I., Wigger A., Landgraf R. Altered brain activation pattern associated with drug-induced attenuation of enhanced depression-like behavior in rats bred for high anxiety. Biol. Psychiatry. 2007;61:782–796. doi: 10.1016/j.biopsych.2006.08.035. [DOI] [PubMed] [Google Scholar]
- Muller J.F., Mascagni F., McDonald A.J. Pyramidal cells of the rat basolateral amygdala: synaptology and innervation by parvalbumin-immunoreactive interneurons. J. Comp. Neurol. 2006;494:635–650. doi: 10.1002/cne.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff C.B. Neurobiological consequences of childhood trauma. J. Clin. Psychiatry. 2004;65(Suppl. 1):18–28. [PubMed] [Google Scholar]
- Nestler E.J., Barrot M., DiLeone R.J., Eisch A.J., Gold S.J., Monteggia L.M. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Nestler E.J., Gould E., Manji H., Buncan M., Duman R.S., Greshenfeld H.K., Hen R., Koester S., Lederhendler I., Meaney M., Robbins T., Winsky L., Zalcman S. Preclinical models: status of basic research in depression. Biol. Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- Oitzl M.S., Champagne S.L., van der Veen R., de Kloet E.R. Brain development under stress: hypotheses of glucocorticoid actions revisited. Neurosci. Biobehav. Rev. 2010;34:853–866. doi: 10.1016/j.neubiorev.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Ostroff L.E., Cain C.K., Bedont J., Monfils M.H., Ledoux J.E. Fear and safety learning differentially affect synapse size and dendritic translation in the lateral amygdala. Proc. Natl. Acad. Sci. U.S.A. 2010;107:9418–9423. doi: 10.1073/pnas.0913384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp M., Willner P., Muscat R. An animal model of anhedonia: attenuation of sucrose consumption and place preference conditioning by chronic unpredictable mild stress. Psychopharmacology (Berl.) 1991;104:255–259. doi: 10.1007/BF02244188. [DOI] [PubMed] [Google Scholar]
- Pollak S.D. Experience-dependent affective learning and risk for psychopathology in children. Ann. N. Y. Acad. Sci. 2003;1008:102–111. doi: 10.1196/annals.1301.011. [DOI] [PubMed] [Google Scholar]
- Pollak D.D., Monje F.J., Zuckerman L., Denny C.A., Drew M.R., Kandel E.R. An animal model of a behavioral intervention for depression. Neuron. 2008;60:149–161. doi: 10.1016/j.neuron.2008.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt R.D., Le Pichon M., Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Price J.L. An autoradiographic study of complementary laminar patterns of termination of afferent fibers to the olfactory cortex. J. Comp. Neurol. 1973;150:87–108. doi: 10.1002/cne.901500105. [DOI] [PubMed] [Google Scholar]
- Princivalle A., Spreafico R., Bowery N., De Curtis M. Layer-specific immunocytochemical localization of GABABR1a and GABABR1b receptors in the rat piriform cortex. Eur. J. Neurosci. 2000;12:1516–1520. doi: 10.1046/j.1460-9568.2000.01060.x. [DOI] [PubMed] [Google Scholar]
- Pryce C.R., Rüedi-Bettschen D., Dettling A.C., Feldon J. Early life stress: long-term physiological impact in rodents and primates. News Physiol. Sci. 2002;17:150–155. doi: 10.1152/nips.01367.2001. [DOI] [PubMed] [Google Scholar]
- Pryce C.R., Ruedi-Bettschen D., Dettling A.C., Weston A., Russig H., Ferger B., Feldon J. Long-term effects of early-life environmental manipulations in rodents and primates: potential animal models in depression research. Neurosci. Biobehav. Rev. 2005;29:649–674. doi: 10.1016/j.neubiorev.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Raineki C., Moriceau S., Sullivan R.M. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biol. Psychiatry. 2010;67:1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajecki D.W., Lamb M.E., Obmascher P. Towards a general theory of infantile attachment: a comparative review of aspects of the social bond. Behav. Brain Sci. 1978;3:417–464. [Google Scholar]
- Ressler K.J., Mayberg H.S. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat. Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche M., Harkin A., Kelly J.P. Chronic fluoxetine treatment attenuates stressor-induced changes in temperature, heart rate, and neuronal activation in the olfactory bulbectomized rat. Neuropsychopharmacology. 2007;32:1312–1320. doi: 10.1038/sj.npp.1301253. [DOI] [PubMed] [Google Scholar]
- Rogan M.T., Leon K.S., Perez D.L., Kandel E.R. Distinct neural signatures for safety and danger in the amygdala and striatum of the mouse. Neuron. 2005;46:309–320. doi: 10.1016/j.neuron.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Roth T.L., Sullivan R.M. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol. Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Rutter M., Moffitt T.E., Caspi A. Gene-environment interplay and psychopathology: multiple varieties but real effects. J. Child Psychol. Psychiatry. 2006;47:26–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Sah P., Faber E.S., Lopez De Armentia M., Power J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Salzen E.A. The ontogeny of fear in animals. In: Sluckin W., editor. Fear in Animals and Man. Van Nostrand Reinhold; NY: 1979. pp. 125–163. [Google Scholar]
- Sanchez M.M., Ladd C.O., Plotsky P.M. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev. Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Savander V., LeDoux J.E., Pitkanen A. Topographic projections from the periamygdaloid cortex to select subregions of the lateral nucleus of the amygdala in the rat. Neurosci. Lett. 1996;211:167–170. doi: 10.1016/0304-3940(96)12750-6. [DOI] [PubMed] [Google Scholar]
- Schiller D., Levy I., Niv Y., LeDoux J.E., Phelps E.A. From fear to safety and back: reversal of fear in the human brain. J. Neurosci. 2008;28:11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman M.E., Maier S.F. Failure to escape traumatic shock. J. Exp. Psychol. 1967;74:1–9. doi: 10.1037/h0024514. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y., Levy F., Mouly A.M., Ferreira G. Rearing with artificially scented mothers attenuates conditioned odor aversion in adulthood but not its amygdala dependency. Behav. Brain Res. 2009;198:313–320. doi: 10.1016/j.bbr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y., Moriceau S., Holman P., Miner C., Muzny K., Gervais R. Enduring effects of infant memories: infant odor–shock conditioning attenuates amygdala activity and adult fear conditioning. Biol. Psychiatry. 2007;62:1070–1079. doi: 10.1016/j.biopsych.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y., Mouly A.M., Levy F., Ferreira G. Long-term effects of infant learning on adult conditioned odor aversion are determined by the last preweaning experience. Dev. Psychobiol. 2009;51:389–398. doi: 10.1002/dev.20378. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y., Sullivan R.M., Messaoudi B., Mouly A.M. Neonatal odor–shock conditioning alters the neural network involved in odor fear learning at adulthood. Learn. Mem. 2008;15:649–656. doi: 10.1101/lm.998508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A., Oxley G., Lovic V., Fleming A.S. Effects of preweaning exposure to novel maternal odors on maternal responsiveness and selectivity in adulthood. Dev. Psychobiol. 2002;41:187–196. doi: 10.1002/dev.10064. [DOI] [PubMed] [Google Scholar]
- Shair H.N., Masmela J.R., Brunelli S.A., Hofer M.A. Potentiation and inhibition of ultrasonic vocalization of rat pups: regulation by social cues. Dev. Psychobiol. 1997;30:195–200. doi: 10.1002/(sici)1098-2302(199704)30:3<195::aid-dev2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Sheline Y.I., Gado M.H., Price J.L. Amygdala core nuclei volumes are decreased in recurrent major depression. NeuroReport. 1998;9:2023–2028. doi: 10.1097/00001756-199806220-00021. [DOI] [PubMed] [Google Scholar]
- Sherman A.D., Allers G.L., Petty F., Henn F.A. A neuropharmacologically relevant animal model of depression. Neuropharmacology. 1979;18:891–893. doi: 10.1016/0028-3908(79)90087-x. [DOI] [PubMed] [Google Scholar]
- Shionoya K., Moriceau S., Bradstock P., Sullivan R.M. Maternal attenuation of hypothalamic paraventricular nucleus norepinephrine switches avoidance learning to preference learning in preweanling rat pups. Horm. Behav. 2007;52:391–400. doi: 10.1016/j.yhbeh.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishkina G.T., Kalinina T.S., Dygalo N.N. Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neuroscience. 2007;150:404–412. doi: 10.1016/j.neuroscience.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Stanton M.E., Levine S. Inhibition of infant glucocorticoid stress response: specific role of maternal cues. Dev. Psychobiol. 1990;23:411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- Stehouwer D.J., Campbell B.A. Habituation of the forelimb-withdrawal response in neonatal rats. J. Exp. Psychol. Anim. Behav. Process. 1978;4:104–119. doi: 10.1037//0097-7403.4.2.104. [DOI] [PubMed] [Google Scholar]
- Stovall-McClough K.C., Cloitre M. Unresolved attachment, PTSD, and dissociation in women with childhood abuse histories. J. Consult. Clin. Psychol. 2006;74:219–228. doi: 10.1037/0022-006X.74.2.219. [DOI] [PubMed] [Google Scholar]
- Sullivan R.M., Hofer M.A., Brake S.C. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Dev. Psychobiol. 1986;19:615–623. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- Sullivan R.M., Landers M., Yeaman B., Wilson D.A. Good memories of bad events in infancy. Nature. 2000;407:38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R.M., Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Brain Res. 1986;392:278–282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- Sullivan R.M., Wilson D.A. Neural correlates of conditioned odor avoidance in infant rats. Behav. Neurosci. 1991;105:307–312. doi: 10.1037//0735-7044.105.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R.M., Wilson D.A., Wong R., Correa A., Leon M. Modified behavioral and olfactory bulb responses to maternal odors in preweanling rats. Brain Res. Dev. 1990;53:243–247. doi: 10.1016/0165-3806(90)90013-o. [DOI] [PubMed] [Google Scholar]
- Swanson L.W., Petrovich G.D. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Teicher M.N., Andersen S.L., Polcari A., Andersen C.M., Navalta C.P., Kim D.M. The neurobiological consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Blass E.M. First suckling response of the newborn albino rat: the roles of olfaction and amniotic fluid. Science. 1977;198:635–636. doi: 10.1126/science.918660. [DOI] [PubMed] [Google Scholar]
- Tseng G.F., Haberly L.B. Characterization of synaptically mediated fast and slow inhibitory processes in piriform cortex in an in vitro slice preparation. J. Neurophysiol. 1988;59:1352–1376. doi: 10.1152/jn.1988.59.5.1352. [DOI] [PubMed] [Google Scholar]
- Tyler K., Moriceau S., Sullivan R.M., Greenwood-van Meerveld B. Long-term colonic hypersensitivity in adult rats induced by neonatal unpredictable vs predictable shock. Neurogastroenterol. Motil. 2007;19:761–768. doi: 10.1111/j.1365-2982.2007.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton K.L., Sullivan R.M. Defining ages limits of the sensitive period for attachment learning in rat pups. Dev. Psychobiol. 2010;52:453–464. doi: 10.1002/dev.20448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varea E., Castillo-Gomez E., Gomez-Climent M.A., Blasco-Ibanez J.M., Crespo C., Martinez-Guijarro F.J. Chronic antidepressant treatment induces contrasting patterns of synaptophysin and PSA-NCAM expression in different regions of the adult rat telencephalon. Eur. Neuropsychopharmacol. 2007;17:546–557. doi: 10.1016/j.euroneuro.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Wiedenmayer C.P., Magarinos A.M., McEwen B.S., Barr G.A. Mother lowers glucocorticoid levels of preweanling rats after acute threat. Ann. N. Y. Acad. Sci. 2003;1008:304–307. doi: 10.1196/annals.1301.038. [DOI] [PubMed] [Google Scholar]
- Whalen P.J., Shin L.M., Somerville L.H., McLean A.A., Kim H. Functional neuroimaging studies of the amygdala in depression. Semin. Clin. Neuropsychiatry. 2002;7:234–242. doi: 10.1053/scnp.2002.35219. [DOI] [PubMed] [Google Scholar]
- Zeanah C.H., Keyes A., Settles L. Attachment relationships experiences and childhood psychopathology. Ann. N. Y. Acad. Sci. 2003;1008:22–30. doi: 10.1196/annals.1301.003. [DOI] [PubMed] [Google Scholar]