Abstract
Over the last two decades much progress has been made in identifying and characterizing many of the molecules involved in understanding normal lens biology and its pathology. Much of this has been made possible through the establishment and use of the lens epithelial explant system. This simplistic tissue culture model, comprised of a sheet of lens epithelium on its native substratum, has been used effectively to study many cellular processes, including lens epithelial cell proliferation, fiber cell differentiation, cell apoptosis as well as epithelial to mesenchymal transformation of cells. In doing so, a number of key growth factors and cytokines, including members of the FGF, Wnt and TGFβ family have been shown to play essential roles in many of these cellular events. This has led to further studies exploring the signaling pathways downstream of these molecules in the lens, paving the way for the development of a number of in situ models (primarily transgenic mouse lines) to further explore in more detail the nature of these molecular and cellular interactions. To reciprocate, the lens epithelial explant system is increasingly being used to further characterize the nature of many complex phenotypes and pathologies observed in these in situ models, allowing us to selectively isolate and examine the direct impact of an individual molecule on a specific cellular response in lens cells. There is no question that the lens epithelial explant system has served as a powerful tool to further our understanding of lens biology and pathology, and there is no doubt that it will continue to serve in such a capacity, as new developments are realized and putative treatments for aberrant lens cell behaviour are to be trialed.
1. Introduction
The culturing of explanted tissues has long been a popular ex vivo/in vitro tool used to understand the mechanism(s) of cell and tissue interactions that occur during development, as well as the commitment of cells to specific lineages during tissue differentiation. The primary purpose of this review is to describe how the establishment and use of the ‘lens epithelial explant system’, first established in the early 1970’s, have significantly advanced our understanding of ocular lens development and differentiation, as well as pathological disturbances of the lens, such as cataract. How this system may be used to further our understanding of lens and eye development and disease in the future, will also be discussed.
1.1. Lens development and differentiation
The vertebrate ocular lens develops from a series of cumulative inductive cell and tissue interactions between a number of tissues, including the embryonic surface ectoderm of the head, the neural plate, mesoderm, foregut endoderm, the neural crest-derived mesenchyme and later interactions with the optic vesicle (OV), the presumptive retina (Chow and Lang 2001; Lovicu and McAvoy 2005). The lens placode begins as a thickening of the embryonic head ectoderm immediately adjacent to the OV. This interaction between the lens placode and OV results in invagination of the placode to form the lens pit, which eventually separates from the surface ectoderm to form the lens vesicle. At this critical stage of development, the lens acquires its distinctive architecture and polarity as the posterior lens vesicle cells elongate to form a mass of regularly aligned primary fiber cells (Figure 1). These differentiating fiber cells will subsequently make contact with the anterior lens vesicle cells, that are differentiating into the lens epithelium, to ultimately give rise to the mature lens.
Figure 1.
Schematic diagram demonstrating how the gradient of FGF stimulation affects the anteroposterior patterns of differentiation in the lens. The lens epithelium is frequently divided into two zones, the central lens epithelium (CE) and the germinative zone (GZ) which extends to the equator of the lens (EQ). The transitional zone (TZ), just posterior to the equator is where lens epithelial cells differentiate into fiber cells. Modified from (Lang and McAvoy 2004).
In order to demonstrate the importance of tissue-tissue interactions and the inductive signals involved in eye development, early embryologists used transplant experiments (Spemann 1901) and explant cultures to reveal the key role of the optic vesicle in lens formation. For example, transplant experiments in amphibians demonstrated that removal of the optic vesicle resulted in a lack of lens formation. These findings were later challenged since in other species a lens-like structure had been shown to develop in the absence of an optic vesicle. Yet in the mouse the optic vesicle remains an essential component for lens induction. The more current work of Grainger and colleagues also utilized embryological manipulation to show that lens induction is complex and involves four distinct stages, including early lens competence and bias, followed by specification of the placode and lens differentiation (Henry and Grainger 1987; Henry and Grainger 1990; Fisher 2004). Research has now been focused on determining the growth factors and transcription factors regulating these developmental stages. While studies examining the signals have mainly used mutant and transgenic mouse models, lens epithelial explant models, which are the highlight of this review, were used in many pioneering studies and ongoing studies to identify growth factors and signaling events involved in the regulation of lens cell behavior, in particular fiber cell differentiation.
The lens is a relatively simple tissue with a distinct polarity. An anterior monolayer of cuboidal epithelium, exposed mostly to the anterior ocular chamber, faces the cornea, whereas the differentiated lens fiber cells that make up the bulk of the lens are located more posteriorly, exposed to the vitreous chamber (Figure 1). While all of the anterior lens epithelial cells retain their ability to proliferate, proliferation in the postnatal lens is typically confined to the epithelial cells located just anterior to the lens equator, referred to as the germinative zone (McAvoy 1978a; McAvoy 1978b). As these cells proliferate, adjacent progenitors are displaced towards the equator and it is in this location that they withdraw from the cell cycle, elongate and terminally differentiate into secondary lens fiber cells.
The ocular environment, namely the ocular media, the aqueous and vitreous humor, have been shown to be critical for maintaining lens polarity. For example, classical experiments performed by Coulombre and Coulombre (1963) inverted the embryonic chick lens such that the lens epithelium faced the presumptive retina and was bathed by the vitreous humor, with the fiber cell region oriented towards the developing cornea. In this new configuration and ocular environment, after 5 days, the lens epithelial cells elongated into fiber cells and an anterior epithelium formed over the primary lens fiber region which ceased to grow in its new environment (Coulombre and Coulombre 1963). This experiment was also later repeated and corroborated using mice (Yamamoto 1976). Together these data indicated that factor(s) in the aqueous and vitreous humors are important for the establishment and maintenance of the lens epithelium and fiber cells, as well as for the continual regulation of lens epithelial cell proliferation and fiber cell differentiation.
1.2. Establishment of the lens epithelial explant model
It was clear from earlier studies in situ that the ocular environment provided the inductive cues to regulate lens cell behavior. Identification of the exact nature of these cues and the mechanisms involved had yet to be addressed, and this was facilitated by the development and introduction of the lens epithelial explant culture system, which was initially established using embryonic chick (Philpott and Coulombre 1965). In this system, the lens epithelium is kept adherent to its native basement membrane, the lens capsule, and isolated from the lens fiber cells. The constraints and benefits with which the underlying capsule imposed on the epithelial cells would later go on to prove important for studies examining the induction and progression of events associated with lens fiber differentiation, including the exit from the cell cycle, cell elongation and the synthesis of specific β- and γ-crystallins (abundant soluble proteins restricted to lens fiber cells). This is in contrast to other lens cell systems, such as dissociated lens epithelial cells or immortalized cell lines that typically undergo spontaneous changes in either their morphology and/or gene expression when placed in cell culture without their native basement membrane (Creighton, Mousa et al. 1976; Courtois, Barritault et al. 1980; Wang-Su, McCormack et al. 2003). Although some of these cultures do show features typical of fiber cell differentiation, for example, during lentoid body formation (Okada, Eguchi et al. 1971; Okada, Ito et al. 1975; De Pomerai, Pritchard et al. 1977; Russell, Fukui et al. 1977), the spontaneous nature of the changes makes the cultures difficult for studying the regulation of differentiation by external factors such as those that are operating in situ.
The chick explant system was initially adopted to study the effects of serum on lens epithelial cell elongation (Piatigorsky and Rothschild 1971, with later studies identifying insulin and subsequently insulin-like growth factor, as inducers of this elongation (Piatigorsky and Rothschild 1971; Beebe, Silver et al. 1987). During this same period, a mammalian lens epithelial explant system was established using rat tissue, the focus of this review, to examine similar events regulating lens cellular processes in the presence of different stimuli, in particular retinal extracts (McAvoy 1980; McAvoy and Fernon 1984). The key feature of this explant model was that lens epithelial cells could survive for prolonged periods in culture without the need for serum or addition of exogenous proteins (Campbell and McAvoy 1984; McAvoy and Fernon 1984). Similar to the chick lens explants, the rat lens epithelial cells when cultured on their native capsule remained viable and maintained their in situ phenotype. Based on this, the explant system could now be used to test a range of soluble factors on lens epithelial cell behaviour that would not be influenced by other additives (such as those found in serum). More importantly this system would go on to become a reliable and accurate model for studying lens fiber differentiation, with cells exhibiting many characteristics of this process in vitro, including both molecular and morphological changes (outlined in detail below), characteristic of fiber cells in situ. Moreover, these differentiation events are shown to occur in an orderly regulatory fashion, as in vivo, and could be recapitulated under the control of external stimuli, rather than arising as spontaneous or random events. In agreement with earlier developmental studies that demonstrated that the OV was a regulator of lens development and differentiation, co-culture experiments using the rat lens epithelial explant system showed that in the presence of the neural retina (NR, a derivative of the OV) the explant cells underwent proliferation and differentiation (McAvoy and Fernon 1984). Further work showed that retina-conditioned media (RCM) could induce the same effects as the NR co-culture. For example, under the influence of RCM, explanted lens epithelial cells become enlarged and elongated, acquiring the capacity to synthesise β– and γ–crystallins. Furthermore, the capacity of explants to synthesize β-crystallin at significant rates appeared to precede that for γ-crystallin by several days. All of these changes paralleled the known events that occur in vivo as epithelial cells in the equatorial region of the lens differentiate into fiber cells (McAvoy 1978a).
In order to identify the inducing factor in the retina, the RCM was fractionated to reveal the prototypic fibroblast growth factors (FGFs), acidic and basic FGF, now more commonly known as FGF1 and FGF2, respectively (Campbell and McAvoy 1986). FGF2 was shown to be five times more potent than FGF1 in inducing lens fiber differentiation in rat lens epithelial explants, as assessed by the degree of cell multilayering and elongation and their concomitant accumulation of β-crystallin (Chamberlain and McAvoy 1987; Chamberlain and McAvoy 1989; McAvoy and Chamberlain 1989). More detailed morphological studies to follow, examining this FGF-induced differentiation process at the ultrastructural level (Lovicu and McAvoy 1989), demonstrated many of the differentiation features found in situ, including ordered cell elongation and packing, a reduction and subsequent loss in cytoplasmic organelles, the formation of specialized cell-cell junctions including finger like processes, flaps and imprints, gap junctions and ball and socket joints, as well as distinctive phases of nuclear modifications associated with cell denucleation. Lens explants not treated with FGF retained their monolayer of cuboidal epithelial cells. This process, whereby fiber cells elongated, lost their organelles and nuclei, a feature critical for lens transparency in situ, was now able to be replicated in FGF-treated lens explants.
2. Preparation of Rat Lens Epithelial Explants
An extensive and detailed account of the techniques and considerations involved in the preparation of mammalian lens epithelial explants has been described previously by Lovicu and McAvoy, 2008. For the last 25 years, from when this technique was first established (McAvoy and Fernon 1984), little has changed in its mode of preparation. In essence, the explant procedure aims to isolate the intact lens epithelium which is facilitated by the fact that it remains continually associated with its thick native basement membrane, the lens capsule. The strong adherence of the lens epithelium to the lens capsule ensures successful production of a lens epithelial explant by simply manipulating and securing a flat mount preparation of the lens capsule to the base of a tissue culture dish (Figure 2). Although any suitably sized lens can be used for the explanting procedure, owing primarily to its larger size, the lens of the postnatal rat has been routinely adopted in preparation of mammalian lens epithelial explants. The simplicity of the explanting procedure lends itself to not requiring much in the way of specialist equipment. Under aseptic conditions, using a dissecting microscope, a fiber-optic light source and watchmaker’s forceps, the eye and lens can be readily manipulated in order to isolate the lens epithelium.
Figure 2.
Representative figures of a rat lens epithelial explant. A. Bright field image demonstrating how an explant is pinned to the base of a tissue culture dish using fine forceps. B. Scanning electron micrograph of a rat lens epithelial explant pinned to the base of a tissue culture dish. C. Higher magnification view of explant (taken from box in B), demonstrating tightly packed sheet of lens epithelial cells which cover the surface of the lens epithelial explant. Modified from Lovicu and McAvoy, 2008.
Following enucleation of the ocular tissue from the animal model of choice, when isolating the lens, care should to be taken to avoid prematurely rupturing the lens capsule. Using fine forceps to pinch and tear the sclera where it meets the cornea, the lens should readily expel and cleanly separate from its surrounding ocular tissues. Once the lens has been isolated, and any adherent extra-lenticular tissues removed, the lenses are ready for explantation. Although there are many variations to the type of explant that can be prepared (see Lovicu and McAvoy, 2008), the ‘standard’ lens epithelial explant, once secured to the base of the tissue culture dish, has the lens epithelial cells facing and exposed directly to the media, with their underlying lens capsule in direct contact with the base of the tissue culture dish. The exposed surface of the lens epithelial cells would normally have been in contact with the opposing lens fiber mass in situ. To obtain this type of explant it is important that before dissecting the intact lens that it is orientated with the anterior epithelium facing the base of the dish and the posterior pole uppermost. It is at this posterior pole that the posterior (thinner) lens capsule is gently torn, with tears not extending beyond the lens equator. Insufficient tearing of the lens capsule, failing to expose all the posterior tips of the fiber cells, will impair any subsequent procedures. At this point the fiber cell mass will readily dissociate from the epithelial cells. By simply securing one point of the lens capsule to the base of the tissue culture dish with forceps, the other pair of forceps is used to grab and gently roll off the fiber cell mass, exposing the lens epithelial cells. To gently secure the lens epithelial explant to the base of the tissue culture dish, while one edge of the lens capsule is continually held in place to the base of the tissue culture dish with forceps, the other pair of forceps is used to pin the capsule around its circumference to the base of the dish, simultaneously flattening the lens capsule/lens epithelial explant in the process (Figure 2). The ‘pinning’ with forceps of the lens explant to the dish entails applying gentle pressure, firm enough to physically allow the capsule to lightly embed itself into the base of the tissue culture dish. Only a few points of adherence are required to secure the explant; however, the introduction of additional points of contact will permit a stronger adherence, allowing more rigorous processing of the tissue for subsequent assays (given that many of these assays are carried out in the original culture dish).
3. Research Utilizing Rat Lens Epithelial Explants
3.1. FGF-induced cell proliferation and fiber differentiation
The pioneering discovery of FGF as a potent inducer of lens fiber differentiation, using the rat lens epithelial explant system, led to further revelations of its unique activity, none more compelling than its ability to induce a differential response in lens epithelial cells depending on its dose (McAvoy and Chamberlain 1989). For example, exposure to a low dose of FGF induced lens epithelial cells in explants to undergo cell proliferation. As the concentration of FGF is increased, cells acquire the ability to migrate and at even higher concentrations, lens cells in explants were shown to elongate and accumulate β-crystallin, indicative of fiber cell differentiation. Based on this dose-dependent response of lens cells to FGF, and taken together with other studies examining the ocular distribution and levels of FGF and FGF receptors in the eye, it was proposed that the distinctive polarity of the lens and the tightly regulated spatial processes of lens cell proliferation and fiber differentiation are controlled by a FGF gradient in vivo. Indeed, many subsequent experimental in situ models and lines of evidence have supported this hypothesis. These include the use of transgenic mouse models with altered FGF and FGFR patterns of expression in the eye/lens (Robinson, MacMillan-Crow et al. 1995; Robinson, Overbeek et al. 1995; Lovicu, de Iongh et al. 1997; Lovicu and Overbeek 1998; Lovicu and McAvoy 2005) leading to altered patterns of lens cell differentiation, and the compelling demonstration that conditionally ablating FGF receptor signaling in the lens (in mice with lenses deficient for fgfr1, fgfr2 and fgfr3), led to a block in lens differentiation (Zhao, Yang et al. 2008).
More rigorous analysis of FGF-induced lens fiber differentiation demonstrated spatial and age-related differences in responsiveness of lens cells in explants to FGF (Richardson and McAvoy 1988; Lovicu and McAvoy 1992). For example, as the age of the donor rat increased, lens epithelial cells in explants underwent the process of lens fiber differentiation at a reduced rate, with a consistent decline in the expression of γ–crystallin (Richardson and McAvoy 1988; Lovicu and McAvoy 1992). This later result was of specific interest as γ–crystallin levels decline as the human lens ages. It was further shown that the central cells of explants from adult rats responded differently to FGF when compared to more peripheral cells in lens explants, with central cells failing to accumulate β-crystallin in response to FGF at concentrations that had been shown to stimulate this response in comparable cells in neonatal rat lens explants. Overall, these findings demonstrated a decrease in responsiveness to FGF with age, being more pronounced in central lens epithelial cells. This is consistent with the decreasing growth rate of the lens with age, which may be attributed, at least in part, to the reduction in FGF receptor expression in the lens, leading to a decline in responsiveness of cells to FGF (de Iongh and McAvoy 1993; de Iongh, Lovicu et al. 1997).
3.2. FGF Signaling and Differentiation of the Lens
As outlined previously, creation of the rat lens epithelial explant system was central to the identification of FGFs as key molecules involved in regulating lens cell behavior, specifically lens epithelial cell proliferation and fiber cell differentiation. Three distinct dose-dependent responses to FGF were identified using the explant system, including proliferation, migration and differentiation, and these were found to occur in a sequential fashion with the lowest dose inducing proliferation and the highest differentiation. More recent work has focused on the relevant signaling pathways that underlie these FGF responses (Iyengar, Wang et al. 2007). FGF is known to activate a cascade of intracellular regulatory molecules through binding to receptor tyrosine kinases including mitogen activated protein kinase (MAPK/ERK1/2) and phosphatidylinositol 3-kinase (PI3-K) Akt signaling pathways. Using the rat lens explant system, these pathways have been shown to be important in FGF-induced cell elongation and fiber cell specific expression of β– and γ-crystallins (Lovicu and McAvoy 2001; Wang, Stump et al. 2009). For example, distinct phosphorylation profiles of ERK1/2 and Akt accompanied FGF-induced fiber cell differentiation in the explant system and the use of selective inhibitors to these pathways were shown to be required for different aspects of fiber cell differentiation (Wang, Stump et al. 2009). Blocking ERK1/2 resulted in inhibition in fiber cell elongation but not β-crystallin or γ-crystallin expression (Lovicu and McAvoy 2001; Wang, Stump et al. 2009); however, Akt inhibition was shown to block both FGF-induced cell elongation, as well as β and γ-crystallin expression (Wang, Stump et al. 2009). When vitreous humor was used to stimulate fiber cell differentiation, the Akt inhibitor could not abolish these features of differentiation, demonstrating that FGF cannot completely replace the effects of vitreous and that other growth factors are likely to activate PI3-K/Akt signaling (Wang, Stump et al. 2009).
Aqueous-induced lens cell proliferation is also regulated by similar signaling pathways including ERK1/2 and PI3K/Akt. In a recent study by Iyengar and colleagues, the effects of aqueous on explant proliferation was compared to a variety of growth factors, including IGF-1, PDGF-A, EGF and FGF-2. It was shown that FGF was similar to aqueous in that it induced a sustained ERK-1/2 signaling profile (up to 6h) whereas the other growth factors only produced a shorter, more transient increase in ERK1/2 phosphorylation (Iyengar, Patkunanathan et al. 2006). A selective FGFR inhibitor perturbed the sustained aqueous-induced ERK1/2 signaling to a shorter duration, comparable to that seen with the other growth factors. When other selective receptor tyrosine kinase (RTK) inhibitors were employed, the duration of ERK1/2 signaling induced by aqueous was maintained, but the levels of ERK1/2 phosphorylation stimulated at the early phases of this cycle were reduced. Interestingly, although the use of the various RTK inhibitors in the explant system impaired ERK1/2 phosphorylation levels or duration, not one of these inhibitors alone could block the mitogenic effects of aqueous, yet combinations of the inhibitors were effective in completely blocking the mitogenic effect of aqueous, providing that FGFR signaling was blocked (Iyengar, Patkunanathan et al. 2006). Although this suggested that FGF is a key regulator of aqueous-induced cell proliferation in lens explants, other ocular mitogens are likely to play a role in this process which is the subject of current and future research.
3.3. TGFβ and Lens Pathology
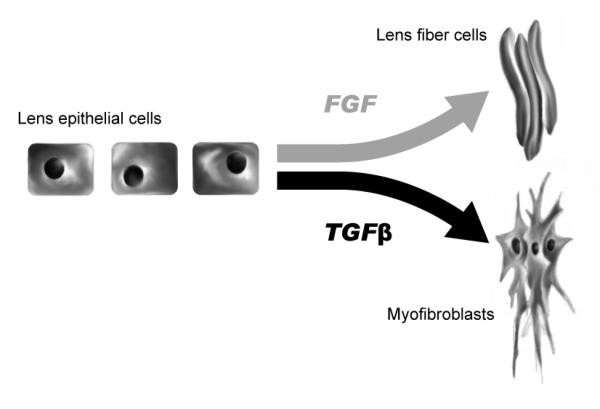
In the process of characterizing the important influence that FGF had on cells in rat lens epithelial explants, other growth factors that were thought to influence the behavior of lens cells in explants were examined. Transforming growth factor β (TGFβ) was a candidate molecule, since in other systems it had been shown to regulate growth and differentiation and to modulate FGF response/activity. Moreover, it was known that TGFβ was available to lens cells in situ and that its active forms were not only present but elevated in the ocular media from patients following cataract surgery and in other ocular insults (Cousins, McCabe et al. 1991). Using the rat lens epithelial explant system it was demonstrated that exposure to TGFβ, in the absence or presence of FGF, resulted in pronounced effects on lens epithelial cell morphology, very different to what was earlier seen in response to FGF (Figure 3, 4). These changes included a rapid elongation of cells, the aberrant accumulation of ECM, alpha smooth muscle actin (αSMA) reactivity, lens capsule wrinkling and cell death by apoptosis, all characteristic features of an epithelial-to-mesenchymal transformation (EMT) (Liu, Hales et al. 1994; Wallentin, Wickstrom et al. 1998). This response to TGFβ was found to be age-dependent with explants from 10 day old rats only responding to TGFβ in the presence of FGF, whereas cells in explants from older donors (weanling to adult rats) exhibiting morphological changes in response to TGFβ alone. Furthermore, pockets of ECM-like material were detected between cells following TGFβ treatment, suggesting that TGFβ may regulate capsule synthesis during postnatal life. The elongation of cells following TGFβ treatment was reminiscent of that seen in FGF-treated explants; however, these cells tapered away to very pointed ends and were more spindle shaped than lens fiber cells. These tapered elongate cells did not accumulate crystallins as seen in fiber cells. It was noted that these cells resembled those identified in anterior subcapsular cataract (ASC) as well as those associated with posterior capsular opacification (PCO), also known as secondary cataract (Font and SA 1974; Novotny and Pau 1984; Kappelhof and Vrensen 1992). In PCO, lens epithelial cells from the anterior and equatorial regions, left behind after surgery, have the capacity to survive, proliferate and transdifferentiate through an EMT into large spindle-shaped cells, referred to as myofibroblasts. The spindle-shaped cells in TGFβ-treated explants were also identified as myofibroblasts. Unlike lens epithelial cells (LECs), these myofibroblasts ceased to produce type IV collagen (a component of the lens capsule) and crystallin proteins, in place secreting an abnormal accumulation of ECM, including type I and III collagen, desmin, tenascin and fibronectin (Lovicu, Schulz et al. 2002).
Figure 3.
Schematic diagram illustrating FGF and TGFβ induction of lens epithelial cells into lens fiber cells or myofibroblasts cells, respectively. Modified from (Lang and McAvoy 2004)
Figure 4.
Lens epithelial explant culture of 21 day old rats, immunostained for α-SMA. Untreated explants do not exhibit αSMA immunoreactivity (A), whereas explants treated with 4ng/ml of TGFβ-2 for 4 days exhibit α-SMA accumulation (green) (B). The co-treatment of explants with TGFβ-2 (4ng/ml) and 25μM of MMPI-2/-9 (C), resulted in the suppression of α-SMA accumulation. Blue represents 4′,6-diamidino-2-phenylindole (DAPI) staining for nuclei.
The ability of TGFβ to initiate cataractous changes in LECs was first highlighted in rat lens explant studies (Liu, Hales et al. 1994); however, the effect of TGFβ has since been confirmed and extended using a variety of rat, mouse, rabbit, bovine, canine and human models (Mansfield, Cerra et al. 2004). In addition, whole rat lenses exposed to TGFβ were shown to develop opacities that resemble ASC plaques, which upon examination were shown to contain myofibroblasts and aberrant ECM deposition (Hales, Chamberlain et al. 1995). Many of these models were developed to further investigate the mechanism underlying PCO and have consisted of seeding of LECs onto structures such as Plexiglass, plastic or bovine capsules with or without intraocular replacement lenses (IOLs), to determine their effects on proliferation and migration (McDonnell, Rowen et al. 1985; Nishi, Nishi et al. 1992). A capsular bag model similar to that produced in vivo following cataract surgery, and similar to the rat lens epithelial explant model, was also developed to monitor LEC migration as it occurs during PCO from the anterior equatorial margin onto the posterior capsule (Gimbel and Neuhann 1990; Nagamoto and Bissen-Miyajima 1994; Nagamoto and Hara 1996). Other derivations of this capsular bag model have been undertaken by Wormstone and co-workers and Saxby and colleagues (Wormstone, Liu et al. 1997; Saxby, Rosen et al. 1998). Importantly, utilizing their capsular bag model, Wormstone and colleagues were able to demonstrate that addition of TGFβ accelerated LEC transformation and capsule wrinkling, both of which are thought to induce light scattering (Wormstone, Tamiya et al. 2002). Furthermore, co-culturing with an anti-TGFβ antibody (CAT-152) suppressed TGFβ-induced development of PCO, implicating TGFβ in its etiology (Wormstone, Tamiya et al. 2002)).
As mentioned earlier, treatment of lens cells in rat explants with TGFβ has also been shown to result in substantial cell loss through apoptosis. These findings were considered paradoxical since a major feature of PCO is the survival of the remnant LECs following cataract surgery. Thus it was hypothesized that PCO was likely dependent on the action of additional growth factors and not just on TGFβ alone (Mansfield, Cerra et al. 2004). Mansfield and colleagues used the rat lens explant system to demonstrate that exposure of the cells to FGF-2 counteracted the ability of TGFβ to induce cell loss. The co-culture with FGF-2 and TGFβ; however, still resulted in the additional PCO characteristics reported previously following TGFβ treatment, including the appearance of myofibroblasts and capsular wrinkling (Mansfield, Cerra et al. 2004).
3.4 Wnt Signalling
TGFβ has been shown to regulate expression of the Wnt growth factor family, and proteins that comprise the Wnt signalling pathway, which are expressed in the lens throughout development and in the adult (Stark, Biggs et al. 2000). Recent studies using rat lens epithelial explants have shown the importance of Wnt signalling in lens fiber cell differentiation. For example, treatment of the explants with Wnt alone, lead to the accumulation of β–crystallin, indicative of fiber cell differentiation. The increase in β-crystallin promoter activity appeared to be related to the Wnt/β-catenin pathway since transclocation of β-catenin to the nucleus was observed. Interestingly, while Wnt treatment caused accumulation of β-crystallin, this was found to occur in the absence of cell elongation, another aspect of fiber cell differentiation. However, after short exposure to FGF, explants were also able to show elongation when stimulated with Wnt suggesting that Wnts can also promote the morphological aspects of fiber cell differentiation in a process that requires FGF signalling. Unlike, the accumulation of β–crystallin, the elongation of cells in the explant in response to Wnt was found to be independent of β-catenin.
Wnt signalling has also been shown to be important in TGFβ induced EMT and cataract formation. Several of the Wnts and their Frizzled receptors were shown to be upregulated in association with TGFβ induced cataract development (Chong, Stump et al. 2009). This was also found to be the case for the TGFβ treated explants. In addition, TGFβ was shown to promote translocation of β-catenin from the cell margins to the nucleus in the explants suggesting that TGFβ may promote the canonical Wnt signalling function of β–catenin. However, as suggested by the investigators of these studies Wnt and Frizzled upregulation may also be related to promotion of non-canonical Wnt signalling. For example, the Wnt/Planar Cell Polarity (PCP) pathway involves a different domain than the canonical β-catenin signalling pathway and is involved in reorganization of the cell cytoskeleton, which is a key feature of EMT. Further studies in this area will be required to determine the specific requirement for Wnt in TGFβ-induced pathological changes in the lens and how this differs from Wnt stimulation of epithelial to fiber cell transition in the normal lens.
3.5 Matrix Metalloproteinases, EMT and Cataract
The Matrix Metalloproteinases (MMPs) are a family of zinc-dependent matrix degrading enzymes, involved in multiple diseases, including fibrosis and have recently been shown to be involved in a cataracts associated with elevated TGFβ, such as ASC and PCO (West-Mays and Pino 2007). In particular, the gelatinases, MMP-2 and MMP-9 have been shown to be induced by TGFβ (human) in lens capsular bags (Wormstone, Tamiya et al. 2002; Wong, Daniels et al. 2004). Studies that directly test whether MMPs promote PCO, include that in which a broad-spectrum MMP inhibitor, GM6001, was shown to significantly inhibit the migration of LECs on human donor lens capsules (Wong, Daniels et al. 2004). A significant reduction in capsular contraction was also observed in the GM6001-treated capsular bags. The ability of MMPIs to perturb ASC formation has also been demonstrated (Dwivedi, Pino et al. 2006). These studies were carried out by using the excised rat lens model described previously in which whole lenses were cultured with TGFβ. The treated lenses developed distinct ASC plaques within six days that closely mimic human ASC (Hales, Chamberlain et al. 1995). It was then shown that co-treatment of the excised lenses with TGFβ and either GM6001, the broad MMPI, or a MMP2/9 specific inhibitor, significantly suppressed ASC formation (Dwivedi, Pino et al. 2006). This was confirmed at the histological level since cross-sections of the lenses treated with TGFβ revealed the presence of numerous plaques, exhibiting strong immunoreactivity to αSMA, yet all of the lenses co-cultured with TGFβ and GM6001 or the MMP2/9 inhibitor, did not. Since no αSMA reactivity was observed in the inhibitor treated lenses, these data further suggested the requirement for MMPs in the EMT of LECs. The loss of the cell-cell adhesion molecule, E-cadherin, and F-actin stress fiber rearrangement are phenotypic changes associated with TGFβ-mediated EMT. This was demonstrated using the rat lens epithelial explants in which TGFβ treatment was shown to induce the loss of E-cadherin expression its delocalization from the cell junctions (Banh, Deschamps et al. 2007). TGFβ/FGF co-treated explants showed an even greater loss of E-cadherin expression. Indeed, using the whole lens model, it was further shown that TGFβ causes E-cadherin disruption resulting in E-cadherin shedding, whereby extracellular E-cadherin fragments were detected in the conditioned media (Dwivedi, Pino et al. 2006). Interestingly, co-treatment with MMPIs reduced the appearance of the E-cadherin fragments suggesting that this may be the mechanism by which MMPIs suppress ASC formation. Importantly, investigators in the West-Mays’ laboratory have recently extended these findings to the rat lens explant system. Using the explant system we have found that the TGFβ induced changes such as the induction in αSMA expression and loss of E-cadherin localization at the membrane are inhibited by the co-treatment with MMPIs (Figure 4). Further work will determine which MMPs are critical in mediating this disruption and how E-cadherin disruption contributes to EMT in the lens.
3.6 Lens Epithelial Cell Survival and Cell Cycle
The ocular lens is an encapsulated organ, consisting of only two cell types. It is also avasular and is devoid of innervation. Because of these features it has been postulated that if any cell type could survive without signals or secretory factors from other cell types it would be the LECs. Indeed, the early demonstration that rat lens epithelial cells on their capsules, as explants, could survive for long periods of time in culture supported this notion. Ishizaki and colleagues (Ishizaki, Voyvodic et al. 1993) further investigated this phenomenon and provided evidence to indicate that LECs secrete factor(s) which promote their own survival. A number of lines of evidence were given to support this hypothesis. For example, the rat LECs could survive long periods of time in culture as dissociated cells, without their capsule, as long as they were cultured at high density. Direct cell contact; however, was not required since they could survive in high density agarose cultures where direct cell contact did not occur. Conditioned media from the high density cultures was also shown to promote survival of the LECs cultured at low density. Finally, when explant cultures were treated with the protein kinase inhibitor, staurosporine, almost all of the cells underwent apoptosis, suggesting that cell signaling is important for survival. This work pointed to the fact that the rat lens explant cultures secrete survival signals. Multiple survival factors and signals for LECs have since been identified and shown to promote survival of LECs and/or protect LECs from apoptosis. These include FGF, lens epithelium derived growth factor, IGF-1, transferrin and Nitric Oxide. The effect of NO on the rat lens explants was recently examined by Chamberlain and colleagues (Chamberlain, Mansfield et al. 2008). NO is a diffusible molecule that is generated by iNOS and is normally present in low concentrations in a number of ocular tissues, such as the lens, as well as in the aqueous humor. It can either induce cell death or promote cell survival depending on the cell type and the environmental context. Using the rat lens explant system, Chamberlain and colleagues demonstrated that NO was able to act as a survival factor for LECs and protect them against TGFβ-induced pathological changes. Further understanding of the factors and signals that promote LEC survival or induce LEC apoptosis will undoubtedly be useful in targeting these cells following cataract surgery and help in the prevention of PCO.
Investigators have also used the rat lens explant system to examine the role that the ubiquitin-proteosome pathway (UPP) plays in LEC proliferation and differentiation (Guo, Shang et al. 2004; Guo, Shang et al. 2006). The UPP pathway is a major cytosolic proteolytic pathway in most eukatyotic cells involved in the regulation of many processes including cell cycle regulation, transcription, protein quality control, DNA repair and immune responses. The explant system was used to specifically test the hypothesis that the UPP plays an important role in regulating FGF-induced lens cell proliferation and differentiation. Indeed it was revealed that inhibition of UPP activity delayed and diminished the early FGF-induced proliferation as well as subsequent differentiation of cells in lens explants (Guo, Shang et al. 2004; Guo, Shang et al. 2006). They further showed that degradation of the cell cycle regulators, p21 and p27 is regulated by UPP and that Cul1 is involved in the ubiquination of the cyclin dependent kinases.
4. Future Directions
The development of the rat lens epithelial explant system has provided a unique opportunity to study and identify the growth factors required for the maintenance and differentiation of the mammalian lens. For example, this system was integral in identifying FGF as the major regulator of lens fiber cell differentiation. Current and future studies will continue to employ this system to further identify the signaling pathways activated by FGF, as well as other growth factors in the ocular media. The ability of FGF and vitreous to regulate the three-dimensional organization and functional properties of the lens, namely the ability to transmit light, has recently been explored using improved explant systems (O’Connor, Wederell et al. 2008). By developing inverted and paired explant systems, O’Connor and colleagues have shown that the two distinct lens cell types, epithelial and fiber cells can be maintained in the same explant culture with a cellular composition, ultrastructure and organization similar to that observed in the lens in vivo (O’Connor, Wederell et al. 2008). Interestingly, it was observed that better development and maintenance of transparency of the explant was achieved in the presence of vitreous rather than FGF alone. This 3-dimensional system will therefore be important for further identifying the additional vitreous-derived factors important for lens differentiation, growth and transparency.
Studies using the explant system have also contributed to our understanding of pathological disturbances in the lens such as that which occurs during ASC formation and PCO. As outlined earlier, it was with the rat lens explant system that TGFβ was first identified as a key inducer of the EMT and fibrosis observed in these cataracts. This system, along with the human capsular bag model that stemmed from it will continue to be a useful model for characterizing the pathological changes in the lens. These culture systems will also be important for determining and testing potential inhibitors of PCO. For example, a number of pharmacological antagonists have been explored including agents that effect cell growth such as ethylenediaminetetraacetic acid (EDTA), and thapsigargin, as well as those that inhibit multiple aspects of PCO including LEC proliferation, migration, EMT and capsular contraction, such as the MMPIs, and the proteosome inhibitor, MG132 (Duncan, Wormstone et al. 1997; Wong, Daniels et al. 2004; Awasthi and Wagner 2006). However, to date, agents that inhibit PCO have not gone beyond Phase I clinical trials. The delivery of these agents is likely to be a key factor in their success in the clinic. The use of variety of gene delivery techniques, such as viral vectors, could be explored in the explant system using candidate inhibitors. Another promising delivery method that could be investigated in the explants is that from an intraocular lens (IOL). Delivery from an IOL offers the advantage of controlled release of the modulating agent and perhaps reduced toxicity to surrounding ocular tissues. Indeed recent studies from the West-Mays’ laboratory in collaboration with Dr. Sheardown and colleagues have shown that MMPIs can be released from PDMS, a model IOL material, and effectively inhibit MMP activity in a lens epithelial cell line, FHL124 (Morarescu, submitted). These studies further showed that the MMPIs can prevent TGFβ induced migration in these cells. Future studies will employ these materials with the rat lens explant model to further determine the ability of the released MMPIs to suppress TGFβ-induced EMT.
Acknowledgements
We would like to acknowledge the work of Meg Covert and Glen Oomen for the design and creation of the schematic diagrams shown in Figures 1 and 3.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: Pending patent application - West-Mays, JA. Matrix Metalloproteinase Inhibitors of TGFβ-induced Subcapsular Cataract Formation. NO. H310923US
References
- Awasthi N, Wagner BJ. Suppression of human lens epithelial cell proliferation by proteasome inhibition, a potential defense against posterior capsular opacification. Invest Ophthalmol Vis Sci. 2006;47(10):4482–9. doi: 10.1167/iovs.06-0139. [DOI] [PubMed] [Google Scholar]
- Banh A, Deschamps PA, et al. The role of Hsp70 and Hsp90 in TGF-beta-induced epithelial-to-mesenchymal transition in rat lens epithelial explants. Mol Vis. 2007;13:2248–62. [PubMed] [Google Scholar]
- Beebe DC, Silver MH, et al. Lentropin, a protein that controls lens fiber formation, is related functionally and immunologically to the insulin-like growth factors. Proc Natl Acad Sci U S A. 1987;84(8):2327–30. doi: 10.1073/pnas.84.8.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MT, McAvoy JW. Onset of fibre differentiation in cultured rat lens epithelium under the influence of neural retina-conditioned medium. Exp Eye Res. 1984;39(1):83–94. doi: 10.1016/0014-4835(84)90117-9. [DOI] [PubMed] [Google Scholar]
- Campbell MT, McAvoy JW. A lens fibre differentiation factor from calf neural retina. Exp Cell Res. 1986;163(2):453–66. doi: 10.1016/0014-4827(86)90076-5. [DOI] [PubMed] [Google Scholar]
- Chamberlain CG, Mansfield KJ, et al. Nitric oxide, a survival factor for lens epithelial cells. Mol Vis. 2008;14:983–91. [PMC free article] [PubMed] [Google Scholar]
- Chamberlain CG, McAvoy JW. Evidence that fibroblast growth factor promotes lens fibre differentiation. Curr Eye Res. 1987;6(9):1165–9. doi: 10.3109/02713688709034890. [DOI] [PubMed] [Google Scholar]
- Chamberlain CG, McAvoy JW. Induction of lens fibre differentiation by acidic and basic fibroblast growth factor (FGF) Growth Factors. 1989;1(2):125–34. doi: 10.3109/08977198909029122. [DOI] [PubMed] [Google Scholar]
- Chong CC, Stump RJ, et al. TGFbeta promotes Wnt expression during cataract development. Exp Eye Res. 2009;88(2):307–13. doi: 10.1016/j.exer.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RL, Lang RA. Early eye development in vertebrates. Annu Rev Cell Dev Biol. 2001;17:255–96. doi: 10.1146/annurev.cellbio.17.1.255. [DOI] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ. Lens Development: Fiber Elongation and Lens Orientation. Science. 1963;142:1489–90. doi: 10.1126/science.142.3598.1489. [DOI] [PubMed] [Google Scholar]
- Courtois Y, Barritault D, et al. Growth factors and cell cultures: a new approach for research in ophthalmology. C R Seances Soc Biol Fil. 1980;174(4):676–85. [PubMed] [Google Scholar]
- Cousins SW, McCabe MM, et al. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 1991;32(8):2201–11. [PubMed] [Google Scholar]
- Creighton MO, Mousa GY, et al. Differentiation of rat lens epithelial cells in tissue culture. (I) Effects of cell density, medium and embryonic age of initial culture. Differentiation. 1976;6(3):155–67. doi: 10.1111/j.1432-0436.1976.tb01482.x. [DOI] [PubMed] [Google Scholar]
- de Iongh R, McAvoy JW. Spatio-temporal distribution of acidic and basic FGF indicates a role for FGF in rat lens morphogenesis. Dev Dyn. 1993;198(3):190–202. doi: 10.1002/aja.1001980305. [DOI] [PubMed] [Google Scholar]
- de Iongh RU, Lovicu FJ, et al. Differential expression of fibroblast growth factor receptors during rat lens morphogenesis and growth. Invest Ophthalmol Vis Sci. 1997;38(9):1688–99. [PubMed] [Google Scholar]
- De Pomerai DI, Pritchard DJ, et al. Biochemical and immunological studies of lentoid formation in cultures of embryonic chick neural retina and day-old chick lens epithelium. Dev Biol. 1977;60(2):416–27. doi: 10.1016/0012-1606(77)90139-7. [DOI] [PubMed] [Google Scholar]
- Duncan G, Wormstone IM, et al. Thapsigargin-coated intraocular lenses inhibit human lens cell growth. Nat Med. 1997;3(9):1026–8. doi: 10.1038/nm0997-1026. [DOI] [PubMed] [Google Scholar]
- Dwivedi DJ, Pino G, et al. Matrix metalloproteinase inhibitors suppress transforming growth factor-beta-induced subcapsular cataract formation. Am J Pathol. 2006;168(1):69–79. doi: 10.2353/ajpath.2006.041089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. a. R. G., Lovicu F. J. a. R., L. M. Development of the Ocular Lens. Cambridge University Press; New York: 2004. Lens Induction and Determination; pp. 27–47. [Google Scholar]
- Font R, SA B. A light and electron microscopic study of anterior subcapsular cataracts. American Journal of Ophthalmology. 1974;78:972–984. doi: 10.1016/0002-9394(74)90811-3. [DOI] [PubMed] [Google Scholar]
- Gimbel HV, Neuhann T. Development, advantages, and methods of the continuous circular capsulorhexis technique. J Cataract Refract Surg. 1990;16(1):31–7. doi: 10.1016/s0886-3350(13)80870-x. [DOI] [PubMed] [Google Scholar]
- Guo W, Shang F, et al. Differential regulation of components of the ubiquitin-proteasome pathway during lens cell differentiation. Invest Ophthalmol Vis Sci. 2004;45(4):1194–201. doi: 10.1167/iovs.03-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Shang F, et al. Ubiquitin-proteasome pathway function is required for lens cell proliferation and differentiation. Invest Ophthalmol Vis Sci. 2006;47(6):2569–75. doi: 10.1167/iovs.05-0261. [DOI] [PubMed] [Google Scholar]
- Hales AM, Chamberlain CG, et al. Cataract induction in lenses cultured with transforming growth factor-beta. Invest Ophthalmol Vis Sci. 1995;36(8):1709–13. [PubMed] [Google Scholar]
- Henry JJ, Grainger RM. Inductive interactions in the spatial and temporal restriction of lens-forming potential in embryonic ectoderm of Xenopus laevis. Dev Biol. 1987;124(1):200–14. doi: 10.1016/0012-1606(87)90472-6. [DOI] [PubMed] [Google Scholar]
- Henry JJ, Grainger RM. Early tissue interactions leading to embryonic lens formation in Xenopus laevis. Dev Biol. 1990;141(1):149–63. doi: 10.1016/0012-1606(90)90110-5. [DOI] [PubMed] [Google Scholar]
- Ishizaki Y, Voyvodic JT, et al. Control of lens epithelial cell survival. J Cell Biol. 1993;121(4):899–908. doi: 10.1083/jcb.121.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar L, Patkunanathan B, et al. Aqueous humour- and growth factor-induced lens cell proliferation is dependent on MAPK/ERK1/2 and Akt/PI3-K signalling. Exp Eye Res. 2006;83(3):667–78. doi: 10.1016/j.exer.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Iyengar L, Wang Q, et al. Duration of ERK1/2 phosphorylation induced by FGF or ocular media determines lens cell fate. Differentiation. 2007;75(7):662–8. doi: 10.1111/j.1432-0436.2007.00167.x. [DOI] [PubMed] [Google Scholar]
- Kappelhof JP, Vrensen GF. The pathology of after-cataract. A minireview. Acta Ophthalmol Suppl. 1992;205:13–24. doi: 10.1111/j.1755-3768.1992.tb02176.x. [DOI] [PubMed] [Google Scholar]
- Lang RA, McAvoy JW, Lovicu FJ, Robinson ML. Development of the Ocular Lens. Cambridge University Press; New York: 2004. Growth Factors in Lens Development; pp. 261–289. [Google Scholar]
- Liu J, Hales AM, et al. Induction of cataract-like changes in rat lens epithelial explants by transforming growth factor beta. Invest Ophthalmol Vis Sci. 1994;35(2):388–401. [PubMed] [Google Scholar]
- Lovicu FJ, de Iongh RU, et al. Expression of FGF-1 and FGF-2 mRNA during lens morphogenesis, differentiation and growth. Curr Eye Res. 1997;16(3):222–30. doi: 10.1076/ceyr.16.3.222.15408. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Structural analysis of lens epithelial explants induced to differentiate into fibres by fibroblast growth factor (FGF) Exp Eye Res. 1989;49(3):479–94. doi: 10.1016/0014-4835(89)90056-0. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. The age of rats affects the response of lens epithelial explants to fibroblast growth factor. An ultrastructural analysis. Invest Ophthalmol Vis Sci. 1992;33(7):2269–78. [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. FGF-induced lens cell proliferation and differentiation is dependent on MAPK (ERK1/2) signalling. Development. 2001;128(24):5075–84. doi: 10.1242/dev.128.24.5075. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, McAvoy JW. Growth factor regulation of lens development. Dev Biol. 2005;280(1):1–14. doi: 10.1016/j.ydbio.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Overbeek PA. Overlapping effects of different members of the FGF family on lens fiber differentiation in transgenic mice. Development. 1998;125(17):3365–77. doi: 10.1242/dev.125.17.3365. [DOI] [PubMed] [Google Scholar]
- Lovicu FJ, Schulz MW, et al. TGFbeta induces morphological and molecular changes similar to human anterior subcapsular cataract. Br J Ophthalmol. 2002;86(2):220–6. doi: 10.1136/bjo.86.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield KJ, Cerra A, et al. FGF-2 counteracts loss of TGFbeta affected cells from rat lens explants: implications for PCO (after cataract) Mol Vis. 2004;10:521–32. [PubMed] [Google Scholar]
- McAvoy JW. Cell division, cell elongation and distribution of alpha-, beta- and gamma- crystallins in the rat lens. J Embryol Exp Morphol. 1978a;44:149–65. [PubMed] [Google Scholar]
- McAvoy JW. Cell division, cell elongation and the co-ordination of crystallin gene expression during lens morphogenesis in the rat. J Embryol Exp Morphol. 1978b;45:271–81. [PubMed] [Google Scholar]
- McAvoy JW. Induction of the eye lens. Differentiation. 1980;17(3):137–49. doi: 10.1111/j.1432-0436.1980.tb01091.x. [DOI] [PubMed] [Google Scholar]
- McAvoy JW, Chamberlain CG. Fibroblast growth factor (FGF) induces different responses in lens epithelial cells depending on its concentration. Development. 1989;107(2):221–8. doi: 10.1242/dev.107.2.221. [DOI] [PubMed] [Google Scholar]
- McAvoy JW, Fernon VT. Neural retinas promote cell division and fibre differentiation in lens epithelial explants. Curr Eye Res. 1984;3(6):827–34. doi: 10.3109/02713688409000795. [DOI] [PubMed] [Google Scholar]
- McDonnell PJ, Rowen SL, et al. Posterior capsule opacification. An in vitro model. Arch Ophthalmol. 1985;103(9):1378–81. doi: 10.1001/archopht.1985.01050090130047. [DOI] [PubMed] [Google Scholar]
- Nagamoto T, Bissen-Miyajima H. A ring to support the capsular bag after continuous curvilinear capsulorhexis. J Cataract Refract Surg. 1994;20(4):417–20. doi: 10.1016/s0886-3350(13)80177-0. [DOI] [PubMed] [Google Scholar]
- Nagamoto T, Hara E. Lens epithelial cell migration onto the posterior capsule in vitro. J Cataract Refract Surg. 1996;22(Suppl 1):841–6. doi: 10.1016/s0886-3350(96)80172-6. [DOI] [PubMed] [Google Scholar]
- Nishi O, Nishi K, et al. Synthesis of interleukin-1 and prostaglandin E2 by lens epithelial cells of human cataracts. Br J Ophthalmol. 1992;76(6):338–41. doi: 10.1136/bjo.76.6.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny GE, Pau H. Myofibroblast-like cells in human anterior capsular cataract. Virchows Arch A Pathol Anat Histopathol. 1984;404(4):393–401. doi: 10.1007/BF00695223. [DOI] [PubMed] [Google Scholar]
- O’Connor MD, Wederell ED, et al. Generation of transparency and cellular organization in lens explants. Exp Eye Res. 2008;86(5):734–45. doi: 10.1016/j.exer.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Okada TS, Eguchi G, et al. The expression of differentiation by chicken lens epithelium in in vitro cell culture. Dev Growth Differ. 1971;13(4):323–36. doi: 10.1111/j.1440-169x.1971.00323.x. [DOI] [PubMed] [Google Scholar]
- Okada TS, Ito Y, et al. Differentiation of lens in cultures of neural retinal cells of chick embryos. Dev Biol. 1975;45(2):318–29. doi: 10.1016/0012-1606(75)90069-x. [DOI] [PubMed] [Google Scholar]
- Philpott GW, Coulombre AJ. Lens Development. Ii. The Differentiation of Embryonic Chick Lens Epithelial Cells in Vitro and in Vivo. Exp Cell Res. 1965;38:635–44. doi: 10.1016/0014-4827(65)90387-3. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J, Rothschild SS. Effect of serum on the synthesis of RNA and of DNA in the cultured lens epithelium of the chick embryo: initiation of lens fiber formation in vitro. Biochim Biophys Acta. 1971;238(1):86–98. doi: 10.1016/0005-2787(71)90013-x. [DOI] [PubMed] [Google Scholar]
- Richardson NA, McAvoy JW. Age-related changes in fibre differentiation of rat lens epithelial cells in vitro. Exp Eye Res. 1988;46(2):259–67. doi: 10.1016/s0014-4835(88)80083-6. [DOI] [PubMed] [Google Scholar]
- Robinson ML, MacMillan-Crow LA, et al. Expression of a truncated FGF receptor results in defective lens development in transgenic mice. Development. 1995;121(12):3959–67. doi: 10.1242/dev.121.12.3959. [DOI] [PubMed] [Google Scholar]
- Robinson ML, Overbeek PA, et al. Extracellular FGF-1 acts as a lens differentiation factor in transgenic mice. Development. 1995;121(2):505–14. doi: 10.1242/dev.121.2.505. [DOI] [PubMed] [Google Scholar]
- Russell P, Fukui HN, et al. Tissue culture of lens epithelial cells from normal and Nakano mice. Invest Ophthalmol Vis Sci. 1977;16(3):243–6. [PubMed] [Google Scholar]
- Saxby L, Rosen E, et al. Lens epithelial cell proliferation, migration, and metaplasia following capsulorhexis. Br J Ophthalmol. 1998;82(8):945–52. doi: 10.1136/bjo.82.8.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spemann H. Uber Korrelationene in der Entwicklung des Auges. Verh. Anat. Ges. 1901;15:61–69. (Vers Bonn.) [Google Scholar]
- Stark MR, Biggs JJ, et al. Characterization of avian frizzled genes in cranial placode development. Mech Dev. 2000;93(1-2):195–200. doi: 10.1016/s0925-4773(00)00263-x. [DOI] [PubMed] [Google Scholar]
- Wallentin N, Wickstrom K, et al. Effect of cataract surgery on aqueous TGF-beta and lens epithelial cell proliferation. Invest Ophthalmol Vis Sci. 1998;39(8):1410–8. [PubMed] [Google Scholar]
- Wang Q, Stump R, et al. MAPK/ERK1/2 and PI3-kinase signalling pathways are required for vitreous-induced lens fibre cell differentiation. Exp Eye Res. 2009;88(2):293–306. doi: 10.1016/j.exer.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang-Su ST, McCormack AL, et al. Proteome analysis of lens epithelia, fibers, and the HLE B-3 cell line. Invest Ophthalmol Vis Sci. 2003;44(11):4829–36. doi: 10.1167/iovs.03-0556. [DOI] [PubMed] [Google Scholar]
- West-Mays JA, Pino G. Matrix Metalloproteinases as Mediators of Primary and Secondary Cataracts. Expert Rev Ophthalmol. 2007;2(6):931–938. doi: 10.1586/17469899.2.6.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TT, Daniels JT, et al. MMP inhibition prevents human lens epithelial cell migration and contraction of the lens capsule. Br J Ophthalmol. 2004;88(7):868–72. doi: 10.1136/bjo.2003.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormstone IM, Liu CS, et al. Human lens epithelial cell proliferation in a protein-free medium. Invest Ophthalmol Vis Sci. 1997;38(2):396–404. [PubMed] [Google Scholar]
- Wormstone IM, Tamiya S, et al. TGF-beta2-induced matrix modification and cell transdifferentiation in the human lens capsular bag. Invest Ophthalmol Vis Sci. 2002;43(7):2301–8. [PubMed] [Google Scholar]
- Yamamoto Y. Growth of lens and ocular environment: role of neural retina in the growth of the mouse lens revealed by implantation experiment. Dev. Growth Differ. 1976;18:273–278. doi: 10.1111/j.1440-169X.1976.00273.x. [DOI] [PubMed] [Google Scholar]
- Zhao H, Yang T, et al. Fibroblast growth factor receptor signaling is essential for lens fiber cell differentiation. Dev Biol. 2008;318:276–88. doi: 10.1016/j.ydbio.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]