Abstract
Background
Neuropathic pain is reported to be common based on studies from specialty centers and survey studies. However, few prevalence estimates have been completed in a community population using clinical evaluation.
Objective
To develop an estimate of the prevalence of neuropathic pain in community dwelling adults.
Methods
Data from a mailed survey (n=3575 community respondents), telephone interview (n=905), and a clinical examination (n=205) were linked to estimate the population prevalence of neuropathic pain. Using the clinical examination as the “gold” standard, estimates from several screening tools were developed and adjusted to the Olmsted County, Minnesota adult population.
Results
The estimated community prevalence of neuropathic pain from the clinical examination (gold standard) was 9.8%. Most other estimates were lower, including a 3.0% population prevalence using the Berger criteria and 8.8% using the S-LANSS. Only the prevalence rate based on self-report of nerve pain was higher (12.4%). Overlap among the groups each tool identified as having “neuropathic predominant pain” was only modest and the groups had significantly different rates of depressive symptoms, anxiety, limited functional ability and use of complementary and alternative medicine (CAM).
Conclusions
The estimated rates and personal characteristics of community residents with “neuropathic pain” varies widely depending on the tools used to identify neuropathic pain. None of the screening tools compared well to clinical evaluation. The differences in the groups identified by alternative screening methods become of major importance when reporting neuropathic pain epidemiology, studying therapies for neuropathic pain or attempting to translate neuropathic pain research into clinical practice.
Keywords: neuropathic pain, pain, prevalence, population based, screening tools, study populations, clinical practice
Introduction
Chronic pain is a common and often frustrating problem for patients and physicians1 affecting 28% to 65% of U.S. adults depending on the frequency, intensity and duration of pain used to define “chronic”.2, 3 The World Health Organization has estimated that 22% of the world’s primary care patients have chronic debilitating pain making chronic pain a problem to be addressed by all physicians and health professionals.4
Chronic pain can be broadly divided into neuropathic or nociceptive categories. Neuropathic pain is defined as a chronic pain condition that occurs and persists in a heterogeneous group of etiologically different diseases characterized by primary lesions or dysfunction of the peripheral or central nervous system.5 Because diagnosis and treatment of neuropathic pain is complex requiring clinical evaluation and treatment is often incompletely effective, 5–8 people with neuropathic pain may require special attention compared to people with nociceptive pain.9–14
Information on the prevalence and characteristics of those with neuropathic pain in the community is limited. Much of the published information comes from studies in specialized cohorts such as people with low back pain,8, 10, 15 diabetes,16 multiple sclerosis, nerve entrapment syndrome8 or people who attend specialized health care sites such as neurology pain centers.17 The prevalence rates and clinical information reported from these sites are not likely to be generalizable to the community population.
Some recent community studies assess the prevalence of neuropathic pain using surveys tools such as the Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS)18 or self-report or administrative data tools such as the Berger criteria.9 Previous work by our group suggested that these tools have a range of sensitivities and specificities for identifying people with neuropathic pain. 19 We therefore wanted to develop a community population prevalence estimate based on clinical examination.
This study assesses the community prevalence rate of neuropathic pain from clinical examination and compares that estimate to those calculated using the S-LANSS,18 the Berger Criteria9 and self-report in the same population. This information provides one of the first estimates of the population prevalence of neuropathic pain using the gold standard of clinical assessment, as well as determining the potential over- or under-identification of neuropathic pain assessed using screening methods for identification of neuropathic predominant pain.2, 12
Methods
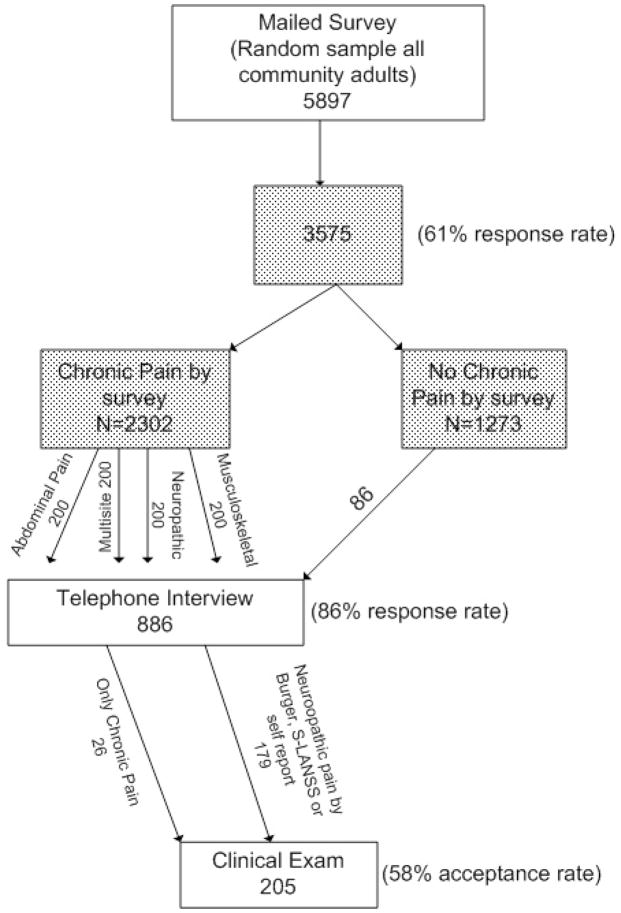
Four methods were linked to obtain the information used in this study; a mailed survey, medical record review, an in-depth telephone interview and clinical examination by a pain expert. (Figure) The mailed survey has been described previously.3 Briefly, 3575 adult residents (61% response rate) of Olmsted County, MN aged 30 years and older completed a four-page postal survey inquiring about age, gender, ethnicity, race, education, marital status and employment status, as well as two general questions on health status and quality of life. Subjects were then asked if they had any pain in the last 3 months or taken any pain medications in the last 3 months. Subjects responding positively to either of these questions were asked to continue with more detailed questions regarding pain symptoms, duration, frequency, sites of pain, and severity of pain. The survey also included the S-LANSS questionnaire18 as well as a specific query about whether the respondent believed they had “nerve pain”.
Figure 1.
Steps in the Olmsted County Community Pain Study
The medical records of all survey respondents were reviewed to identify all medical diagnoses within the past 2 years during any visit to any Olmsted County, MN health care facility. These diagnostic data were used to identify respondents whose ICD-9-CM codes met the Berger criteria9 suggesting they have condition that might be associated with neuropathic pain. Access to all medical record data is provided by the Rochester Epidemiology Project that links all Olmsted County residents to all sites of health care they visit.20
Next, an in-depth telephone interview was completed with a stratified random sample of 907 survey respondents who reported they had experienced pain for 3 months or longer (chronic pain). The candidates for the telephone interview were selected at random from the mail survey respondents who reported pain of 3 month or more duration until the enrollment goal of 900 was reached. Of the people invited, 76% responded positively and completed the interview. The telephone interview included screening questions for depression (PHQ-9),21 anxiety (Beck Anxiety Index),22 as well as sites of pain, types of therapy and information related to abdominal and joint pain not used in these analyses.
The final source of data was the clinical assessment completed by one of three pain specialists and has been described in a previous publication.19 The sample (n=179) who completed the clinical examination were selected from the 331 subjects who had an S-LANSS scores of ≥12 or reported having nerve pain on the mailed survey or who completed the mailed survey and met the Berger criteria on medical record review (acceptance rate of 54%). An additional 26 “control” subjects (32 invited, 81% acceptance) without any indication of neuropathic predominant pain (negative S-LANSS, Berger and nerve pain question) but with frequent, moderate to severe chronic pain also completed the clinical examination.
The clinical evaluation was based on the a standardized set of questions and physical examination procedures adapted from a protocol developed by PJ Dyck (personal communication, (Dyck.Peter@mayo.edu) based on IASP criteria for diagnosis of neuropathic pain23 and has been described previously. The essential elements of the evaluation included: the location and extent of the pain site, the described quality of the pain, reaction to pin prick and light touch, medical history of injury or chronic disease known to be associated with pain and the presence of allodynia and hypergesia.1,6,7,10,17,24,25 The examiners were given the discretion to ask additional questions and perform a more extensive examination if indicated but were blinded to survey responses, written medical records or results of previous laboratory or imaging studies. Each person was assigned a binary diagnosis of neuropathic pain or no neuropathic pain by the examiner. In people with multiple areas of pain, the subject was considered to have neuropathic pain if any of their painful areas was neuropathic in origin, even if the painful area was not the primary area of pain. The pain specialists were unable to classify the character of the subject’s pain in 3 cases, and these subjects were excluded from further analysis. Four subjects were examined by all three pain specialists and in each case the classifications were consistent among the three examiners.
To assess the representativeness of each sample, the responders were compared to the non-responders for number of diagnoses of chronic diseases, age, gender and number of visits to any Olmsted County, MN health care facility during 20004 and 2005. These results have been described previously. (Watkins paper) Those attending the clinical examination were an average of 5 years younger and more likely to be women than those refusing participation in the clinical examination.
Data analysis
Summary statistics are presented for each group. Population-level prevalence rates and descriptive measures were estimated using data not only from the sample of 205 people examined but from the stratum of survey respondees with possible neuropathic pain and the stratum with chronic, frequent, moderate or severe pain but without suggestions of neuropathic pain origin. Since the group examined (N=205) was not a random sample of the entire 3557 survey sample, population estimates required weighting the sample estimates according to the proportion each stratum represented in the stratified sampling scheme. Specifically, the population estimate for the rate of neuropathic pain was computed as the proportion of people with positive S-LANSS, meeting Berger criteria or self-reporting nerve pain in the survey population times the proportion of neuropathic pain confirmed in the group with these characteristics who attended the clinical examination plus the proportion of “control subjects” (those with frequent, severe chronic pain) in the survey population times the proportion who had a diagnosis of neuropathic pain on clinical examination from the control group examined. Variances of these weighted estimates were computed using the usual formulas for the variance of products and sums of random variables. Confidence intervals were computed using Gaussian approximation methods.
Each stage of the sampling was examined for possible bias due to non-response. For the mailed survey, with its large sample size, there were some statistically significant differences between responders and non-responders, but no clinically significant effects on rates of chronic pain.13 In the telephone survey, no statistically significant demographic differences were found between responders and non-responders.
Comparisons between subgroups were made using Wilcoxon rank-sum tests or Chi-squared tests. Since comparing the screening criteria was not one of the a priori goals of the study, p-values from these comparisons must be considered exploratory and suggestive, rather than confirmatory.
Results
Of all survey respondents (n=3575), 2302 (64.4%) reported chronic pain of at least 3 months duration. Of those reporting chronic pain, 315 subjects (13.7%) scored ≥ 12 on the S-LANSS, and 443 (19.2%) self-reported nerve pain. One hundred seven subjects (4.6%) met Berger criterion from the medical record review. This translates to 8.8% of all survey respondents having a score on the S-LANSS considered indicative of neuropathic predominant pain (≥12), 12.4% having self-reported nerve pain and 3.0% meeting the Berger criteria (Table 1).
Table 1.
Prevalence rates estimated by different tools
| Method of defining neuropathic pain | Numbers of cases and population size used to make estimate | % of community adult population % (95% CI) |
% of community adult population with self-reported chronic pain % (95% CI) |
|---|---|---|---|
| Clinical examination | 75/205 | 9.8 (6.2–13.4) | 15.2 (11.6 – 8.9) |
| S-LANSS18 | 315/3575 | 8.8 (7.9 – 9.8) | 13.7 (12.3 – 15.2) |
| Berger criteria9 | 107/3575 | 3.0 (2.5 – 3.6) | 4.6 (3.8 – 5.6) |
| Self-reported “nerve pain” | 443/3575 | 12.4 (11.4 – 13.6) | 19.3 (17.7 – 21.0) |
Of the 179 people examined who had a screening assessment suggestive of neuropathic pain (positive Berger, S-LANSS or self reported “nerve pain”, 71 were clinically confirmed to have neuropathic pain (40.3%). An additional 3 were indeterminant (the examiners could not decided) and the rest (105, 59%) were considered to not have neuropathic pain.
Of the 26 people examined who had chronic frequent and severe pain (control subjects), 6 (15.4%) were judged by clinical examination to have neuropathic pain. The other 20 were judged to have no element of neuropathic pain. Using these percentages to move to the to the community population level, the prevalence of chronic neuropathic pain based on clinical examination is 9.8% (Table 1).
To more fully explore the differences among people identified as potential or predominantly neuropathic pain by each of the methods of assessment (Berger, S-LANSS, self-report and clinical examination), Table 2 presents age, gender, education and rates of failure to seek care for the pain for indivduals from each tool. For example, of those identified as possible neuropathic pain by the S-LANNS (n=315) only 138 self-reported nerve pain and 24 met the Berger criteria. For the 179 people with potential neuropathic pain the agreement was as follows: S-LANSS and clinical examination---52% positive agreement, Berger and clinical examination—18% positive agreement and self report and clinical examination—43% positive agreement.
Table 2.
Comparison of individuals identified by screening methods and confirmed by clinical examination.
| All subjects with chronic pain N=2302 |
|||
|---|---|---|---|
| S-LANNS positive N = 315 |
Self-report of nerve pain N = 443 |
Berger criteria met N = 107 |
|
| S-LANNS positive | 315 | 138 | 24 |
| Self-report of nerve pain | 443 | 43 | |
| Berger criteria met | 107 | ||
Tables 3 and 4 demonstrate that not only are different groups of individuals identified by each of the assessment methods, but that the individuals identified have differing characteristics. Those identified on clinical examination are older, more likely to be women and less likely to have a college education and to withhold concerns about their pain from their physicians compared to the characteristics of individuals identified by the S-LANSS, or self-report (p all <0.05). Rates of elevated depression scores show a tendency to vary among the groups (p = 0.07) with the anxiety scores being statistically significantly different (p < 0.04). The highest anxiety rate is among those positive on clinical examination but the highest on depression scales is among those who are S-LANSS positive. Physical function scores vary little among the groups.
Table 3.
Demographic characteristics of those with neuropathic pain assessed by different methods
| Characteristic | Clinical examination positive N=71 |
Clinical examination extrapolated to Olmsted County, MN population | S-LANSS18 positive N= 315 |
Berger criteria9 met N = 107 |
Self- reported nerve pain N = 443 |
|---|---|---|---|---|---|
| Age Mean (s.d.) |
58.7 (12.4) | 60.4 (4.6) | 53.6 (13.7) | 59.9 (14.4) | 55.1 (13.6) |
| Gender % female |
71% | 72% | 66% | 62% | 56% |
| More than high school education % | 34% | 27% | 33% | 38% | 35% |
| Silent sufferer *% | 8.0% | 6.3% | 10.4% | 3.7% | 13.0% |
Those reporting they had not discussed their pain with any physician or nurse
Table 4.
Depression, anxiety, and physical functioning among people with neuropathic pain assessed by different methods.
| Co-morbidity | Clinical examination positive N = 71 |
S-LANSS positive N = 315 |
Berger criteria met N = 107 |
Self-reported nerve pain N = 443 |
|---|---|---|---|---|
| Depression PHQ9 Mean score (s.d.) | 4.32 (4.86) | 4.72 (5.05) | 3.13 (4.35) | 3.72 (4.83) |
| (PHQ-9 ≥10) % | 12.2% | 16.4% | 5.1% | 10.8% |
| Anxiety (Beck anxiety index BAI) Mean score |
2.24(2.75) | 2.24(2.99) | 0.85(1.44) | 1.77(2.71) |
| BAI ≥4 % | 27.0% | 23.0% | 5.1% | 18.3% |
| Physical functioning scale Mean score (s.d.) |
22.5(5.4) | 23.3(5.1) | 23.18(5.3) | 23.98(5.3) |
Use of complementary and alternative care (CAC) shows a trend toward differences among groups identified by different tools. (p = .09). Use of all types of CAC were common, highlighting the importance of asking about this type of resource use. (Table 5)
Table 5.
Use of Complementary and Alternative Medicine (CAM) Therapies for Pain
| CAM type | Clinical examination positive N = 71 |
S-LANSS positive N = 315 |
Berger criteria met N = 107 |
Self-reported nerve pain N = 443 |
|---|---|---|---|---|
| Acupuncture | 10.8% | 15.1% | 12.8% | 13.8% |
| Chiropractic Care | 50.0% | 44.3% | 51.3% | 52.1% |
| Herbal supplements | 25.7% | 24.3% | 10.3% | 19.6% |
Discussion
In Olmsted County neuropathic pain is common occurring in about 1 in 10 community adult residents age 30 and older as confirmed by clinical examination. This is similar to the estimate using the S-LANSS score of ≥12 from written surveys (8.8%). Self-report resulted in the highest estimated prevalence at 12.4% and the Berger criteria provided much lower prevalence estimates of 3.0%. Even though the S-LANNS and clinical examination provided similar estimates, the agreement between the two methods as to who had neruopathic pain was only 52%.
Torrance et al. found that 8.0% of 6000 primary care patients from practices in the UK had a positive S-LANSS score in response to a mailed survey.12 This represented 17.0% of all patients who reported pain of 3 months or greater duration. Our 9.8% community prevalence rate from the clinical examination represents 15.2% of those with self-reported chronic pain of greater than 3 months duration. This 15.2% of the Olmsted County population with chronic neuropathic pain corresponds to the 17.0% reported n the UK population. The differences appear to be based on the method used to identify neuropathic pain, clinical examination in our patients versus the S-LANSS in the U.K. population.
Since we included people with neuropathic pain of all intensities, we anticipated that our prevalence rates would be higher than those reported by other studies that included only people reporting moderate or severe pain.14 Assessment of pain severity is entirely subjective, and in our population, even those who chose to label their pain as “mild” reported pain interference with physical functioning and sleep. Therefore, the exclusion of people with mild chronic neuropathic pain does not appear to be justified if estimates are to help identify clinically meaningful pain conditions.3 In addition, we did not require that the neuropathic pain be the only pain experienced by the patient or even the primary pain as described by the subject. This may serve to increase our prevalence rates, however, few of the other studies of pain prevalence report whether they included only primary pain site or all types of pain when estimating rates of chronic neuropathic pain.1,2,6,8,9,11–16
As anticipated studies that evaluated the frequency of neuropathic pain in specialized populations had much higher rates of prevalence.8, 10, 15–17 While these studies have relevance to clinical care of people with diabetes or low back pain, the information taken from specialty populations has limited relevance to a generalist’s population of patients. Therefore, this study provides additional support to the survey studies that used proxy measures but did include the community population.2
Each of the four different methods we used to estimate prevalence identified different individuals. Our exploratory analysis of the individuals’ characteristics across screening tools shows several differences, some statistically significant and others suggesting trends. These differences in characteristics include some that could be considered important functional status outcomes such as sleep or activity limitations and pain severity and are monitored or used as outcomes in pain-related studies. Therefore, using different tools to select the study population may affect outcomes. Even if clinical examination is used as the final entry criterion, the population included in a study that used self-report versus S-LANNS as an entry screening criteria would likely have subjects with different baseline levels of pain severity, age, and co-morbid conditions of depression and anxiety, all factors that affect the ability of any intervention to modify pain, satisfaction with care and adherence to an intervention treatment.
The Berger criteria were never suggested as a screening tool to be used to identify cases if neuropathic pain9 and therefore the very different prevalence rate may not be surprising. The Berger criteria are considered quite specific and poorly sensitive, identifying people with known etiologies of their neuropathic pain such as diabetic neuropathy or radiculopathy. Some might suggest that this group would represent those who are the most ill. However, our information on co-morbidities assessed by depression and anxiety screening tools as well as functional status measures, suggest that those who meet the Berger criteria are not more depressed, more anxious or have lower physical functioning scores than those identified by other methods. Moreover, they do report similar rates of use of complementary and alternative care such as acupuncture and chiropractic care for their pain. Those identified by the Berger criteria are less likely to be “silent sufferers”—people who fail to report their pain to their physicians3 than those identified by other tools, with only about 3% stating they had not discussed their pain with their physicians. This may be due to the receipt of medical care related to the pain etiology: To meet the Berger criteria, the person must have been diagnosed with one or more conditions commonly associated with neuropathic pain. The presence of the diagnosis is likely to trigger discussion about the presence of neuropathic pain.9
Morley-Forster recently expressed doubt about the validity of the most recent U.S. estimate of neuropathic pain prevalence.26 That estimate states that only two million adults in the United States (about 2%) have neuropathic pain.25 Using the data from the clinical examination from this study data and assuming that rates of pain prevalence are similar across racial groups, we estimate that almost 16 million Americans suffer from chronic neuropathic pain. We also agree with Morley-Forster that there appears to be an unmet need for effective medical treatment for neuropathic pain. 26 Among the group of people with neuropathic pain problems on clinical examination, 8.0% stated that they had not discussed the pain with any physician. In addition, the significant portion of the people with chronic neuropathic pain who use acupuncture (more than 1 in 10), herbal remedies (about 1 in 5), and chiropractic care (about 1 in 2) suggest that traditional medical care alone is not solving their pain problem or that they choose not to use the traditional approaches currently available. The proportion of neuropathic pain sufferers who are using alternative types of care is larger than the recent reported (2002) proportion of the U.S. adult population who use acupuncture (<5%) or chiropractic care (7.4%) for any reason.27 The proportion of chronic neuropathic pain sufferers who use herbal supplements is the same as reported for the U.S. population generally (18.6%).27
People in our community sample are over 90% White Americans and our findings may not be generalizable to the entire US population. Our study results are also limited by our modest sample size for those attending the clinical examination. However, coupled with other data from a previously published study regarding the specificity and sensitivity of the S-LANSS,19 they suggest that the estimated prevalence of neuropathic pain is somewhat higher than the survey studies using the S-LANSS report but in the same range of 8 to 10%. The people identified by the S-LANSS have only about 52% overlap and show a trend toward different demographic and clinical characteristics.
Conclusions
Neuropathic pain occurs in about 1 in every 10 adults over age 30. The prevalence rate and people identified varied depending on the method of identification of neuropathic pain. This may have significant implications for researchers deciding how to select subjects for studies of neuropathic pain.
Acknowledgments
Supported by a grant from AstraZeneca and NIH (AR30582)
References
- 1.Verhaak PFM, Kerssens JJ, Dekker J, et al. Prevalence of chronic benign pain disorder among adults: a review of the literature. Pain. 1998;77:231–239. doi: 10.1016/S0304-3959(98)00117-1. [DOI] [PubMed] [Google Scholar]
- 2.Portenoy RK, Ugarte C, Fuller I, et al. Population-based survey of pain in the United States: differences among White, African-American and Hispanic subjects. Journal of Pain. 2004;5(6):317–328. doi: 10.1016/j.jpain.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Watkins E, Wollan P, Melton LJ, et al. Silent Pain Sufferers. Mayo Clin Proc. 2006;81(2):167–171. doi: 10.4065/81.2.167. [DOI] [PubMed] [Google Scholar]
- 4.Lepine JP, Briley M. The epidemiology of pain in depression. Hum Psychopharmacol. 2004;19(Suppl 1):S3–7. doi: 10.1002/hup.618. [DOI] [PubMed] [Google Scholar]
- 5.Colombo B, Annovazzi PO, Comi G. Medications for neuropathic pain: current trends. Neurol Sci. 2006;27(Suppl 2):S183–9. doi: 10.1007/s10072-006-0598-7. [DOI] [PubMed] [Google Scholar]
- 6.Schwartzman RJ, Grothusen J, Kiefer TR, et al. Neuropathic central pain: epidemiology, etiology, and treatment options. Arch Neurol. 2001;58(10):1547–50. doi: 10.1001/archneur.58.10.1547. [DOI] [PubMed] [Google Scholar]
- 7.Nicholson BD. Diagnosis and management of neuropathic pain: a balanced approach to treatment. J Am Acad Nurse Pract. 2003;15(12 Suppl):3–9. [PubMed] [Google Scholar]
- 8.Galvez R, Rejas J, Perez M, et al. Prevalence of neuropathic pain in Spain: clinical, working and health care implications. Med Clin (Barc) 2005;125(6):221–9. doi: 10.1157/13077380. [DOI] [PubMed] [Google Scholar]
- 9.Berger A, Dukes EM, Oster G. Clinical characteristics and economic costs of patients with painful neuropathic disorders. J Pain. 2004;5(3):143–9. doi: 10.1016/j.jpain.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Freynhagen R, Baron R, Tolle T, et al. Screening of neuropathic pain components in patients with chronic back pain associated with nerve root compression: a prospective observational pilot study (MIPORT) Curr Med Res Opin. 2006;22(3):529–37. doi: 10.1185/030079906X89874. [DOI] [PubMed] [Google Scholar]
- 11.Maguire MF, Ravenscroft A, Beggs D, et al. A questionnaire study investigating the prevalence of the neuropathic component of chronic pain after thoracic surgery. Eur J Cardiothorac Surg. 2006;29(5):800–5. doi: 10.1016/j.ejcts.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Torrance N, Smith BH, Bennett MI, et al. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain. 2006;7(4):281–9. doi: 10.1016/j.jpain.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Watkins E, Wollan P, Melton L, III, et al. A population in pain. Report from the Olmsted County Health Study. Pain Med Jrnl. 2007 doi: 10.1111/j.1526-4637.2007.00280.x. In Press. [DOI] [PubMed] [Google Scholar]
- 14.Portenoy R. Development and testing of a neuropathic pain screening questionnaire: ID Pain. Curr Med Res Opin. 2006;22(8):1555–65. doi: 10.1185/030079906X115702. [DOI] [PubMed] [Google Scholar]
- 15.Kaki AM, El-Yaski AZ, Youseif E. Identifying neuropathic pain among patients with chronic low-back pain: use of the Leeds Assessment of Neuropathic Symptoms and Signs pain scale. Reg Anesth Pain Med. 2005;30(5):422–8. doi: 10.1016/j.rapm.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Argoff CE, Cole BE, Fishbain DA, et al. Diabetic peripheral neuropathic pain: clinical and quality-of-life issues. Mayo Clin Proc. 2006;81(4 Suppl):S3–11. doi: 10.1016/s0025-6196(11)61474-2. [DOI] [PubMed] [Google Scholar]
- 17.Montero Homs J, Gutierrez-Rivas E, Pardo Fernandez J, et al. Epidemiological study of prevalence, incident and neuropathic pain characterization in neurology units. PREVADOL study. Neurologia. 2005;20(8):385–9. [PubMed] [Google Scholar]
- 18.Bennett DL. Neurotrophic factors: important regulators of nociceptive function. Neuroscientist. 2001;7(1):13–7. doi: 10.1177/107385840100700105. [DOI] [PubMed] [Google Scholar]
- 19.Weingarten TN, Watson JC, Hooten WM, et al. Validation of the S-LANSS in the Community Setting. Pain. 2006 doi: 10.1016/j.pain.2007.07.030. Submitted. [DOI] [PubMed] [Google Scholar]
- 20.Melton LJI. History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 21.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steer RA, Rissmiller DJ, Ranieri WF, et al. Structure of the computer-assisted Beck Anxiety Inventory with psychiatric patients. J Pers Assess. 1993;60(3):532–42. doi: 10.1207/s15327752jpa6003_10. [DOI] [PubMed] [Google Scholar]
- 23.Anonymous. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain - Supplement. 1986;3:S1–226. [Journal Article] [PubMed] [Google Scholar]
- 24.Dworkin RH, Backonja MM, Rowbotham MC, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60(11):1524–34. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 25.Backonja MM, Galer BS. Pain assessment and evaluation of patients who have neuropathic pain. Neurol Clin. 1998;16(4):775–90. doi: 10.1016/s0733-8619(05)70097-9. [DOI] [PubMed] [Google Scholar]
- 26.Morley-Foster P. Prevalence of neuropathic pain and the need for treatment. Pain Res Manag. 2006;11(Suppl A):5A–10A. [Google Scholar]
- 27.Tindle HA, Davis RB, Phillips RS, DM, et al. Trends in use of complementary and alternative medicine by US adults: 1997–2002. Altern Ther Health Med. 2005;11(1):42–9. [PubMed] [Google Scholar]