Abstract
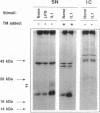
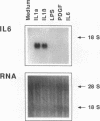
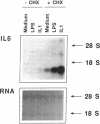
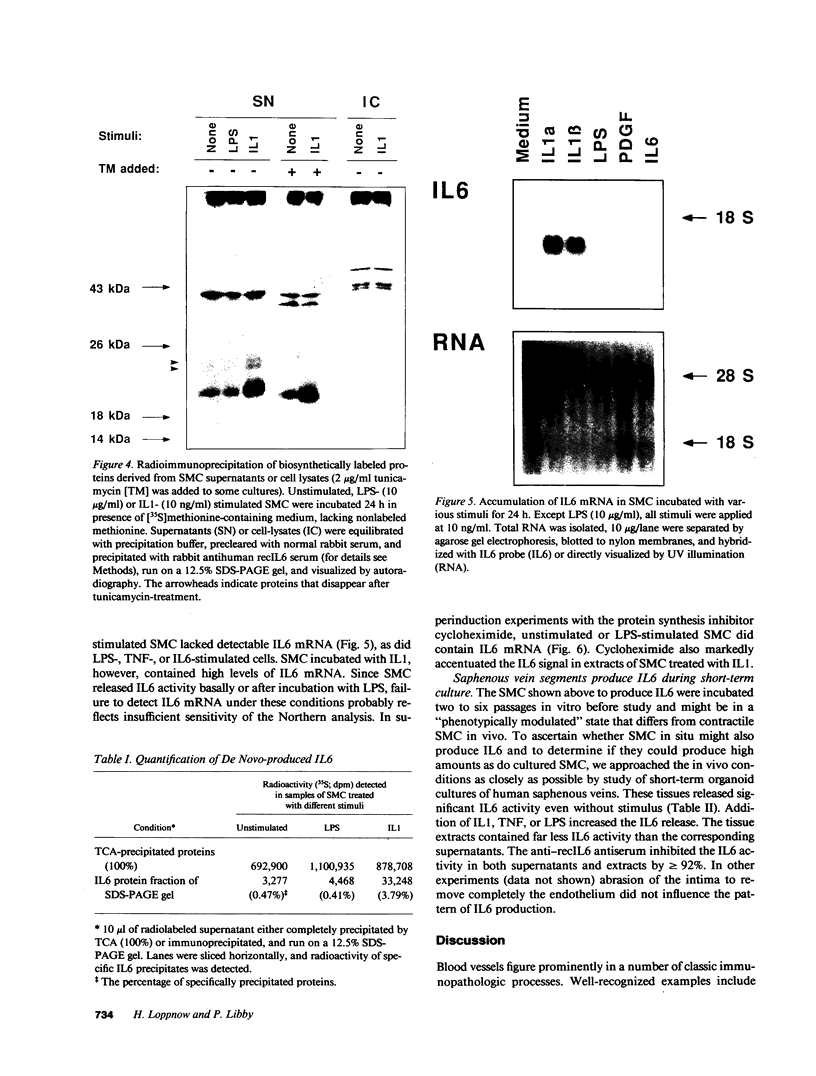
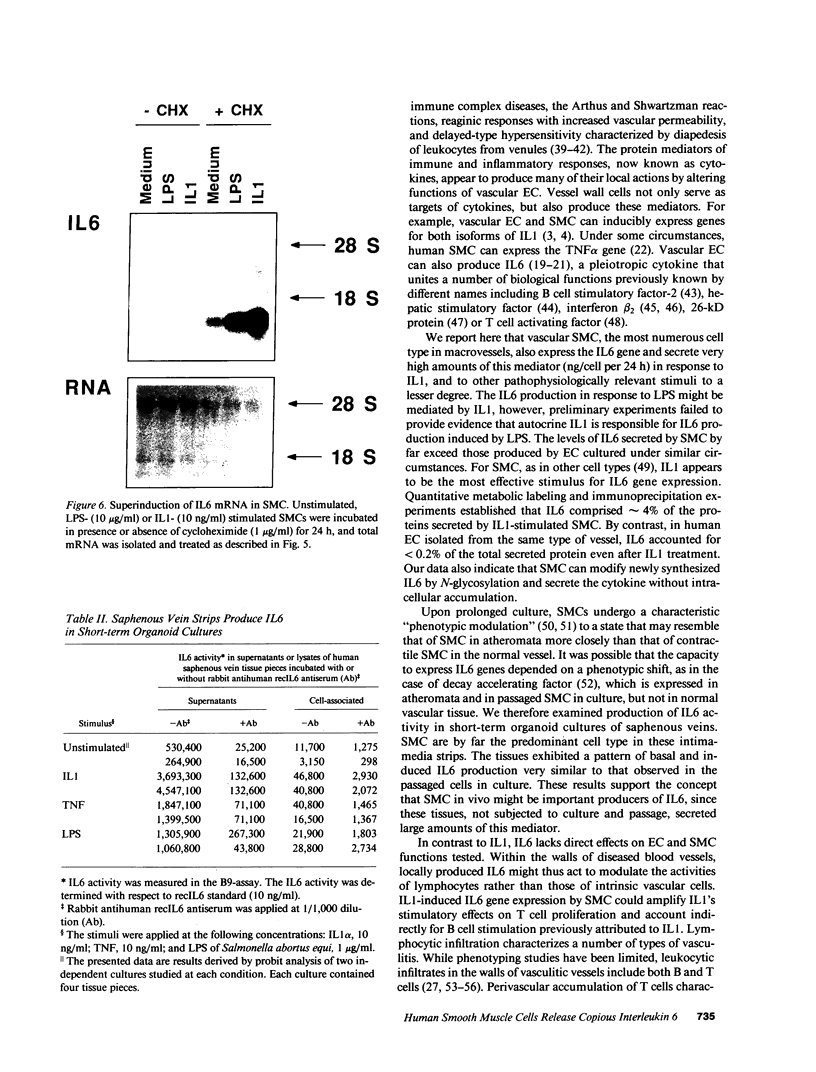
The cells that make up blood vessel walls appear to participate actively in local immune and inflammatory responses, as well as in certain vascular diseases. We tested here whether smooth muscle cells (SMC) can produce the important inflammatory mediator IL6. Unstimulated SMC in vitro elaborated 5 X 10(3) pg recIL6/24h (i.e., biological activity equivalent to 5 X 10(3) pg recombinant IL6 (recIL6), as determined in B9-assay with a recIL6 standard). Several pathophysiologically relevant factors augmented IL6 release from SMC including 10 micrograms LPS/ml (10(4) pg recIL6), 10 ng tumor necrosis factor/ml (4 X 10(4) pg recIL6), and most notably 10 ng IL1/ml (greater than or equal to 3.2 X 10(5) pg recIL6). Production of IL6 activity corresponded to IL6 mRNA accumulation and de novo synthesis. SMC released newly synthesized IL6 rapidly, as little metabolically labeled material remained cell-associated. In supernatants of IL1-stimulated SMC, IL6 accounted for as much as 4% of the secreted proteins. In normal vessels SMC seldom divide, but SMC proliferation can occur in hypertension or during atherogenesis. We therefore tested the relationship between IL6 production and SMC proliferation in response to platelet-derived growth factor (PDGF) in vitro. Quiescent SMC released scant IL6 activity, whereas PDGF (1-100 ng/ml) produced concentration-dependent and coordinate enhancement of SMC proliferation and IL6 release (linear regression of growth vs. IL6 release yielded r greater than 0.9). IL6 itself neither stimulated nor inhibited SMC growth or IL6 production. Intact medial strips studied in short-term organoid culture produced large quantities of IL6, similar to the results obtained with cultured SMC. These findings illustrate a new function of vascular SMC by which these cells might participate in local immunoregulation and in the pathogenesis of various important vascular diseases as well as in inflammatory responses generally.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Albrightson C. R., Baenziger N. L., Needleman P. Exaggerated human vascular cell prostaglandin biosynthesis mediated by monocytes: role of monokines and interleukin 1. J Immunol. 1985 Sep;135(3):1872–1877. [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984 Aug 1;160(2):618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolino F., Breviario F., Tetta C., Aglietta M., Mantovani A., Dejana E. Interleukin 1 stimulates platelet-activating factor production in cultured human endothelial cells. J Clin Invest. 1986 Jun;77(6):2027–2033. doi: 10.1172/JCI112532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley-Campbell J. H., Campbell G. R., Ross R. Phenotype-dependent response of cultured aortic smooth muscle to serum mitogens. J Cell Biol. 1981 May;89(2):379–383. doi: 10.1083/jcb.89.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley-Campbell J. H., Campbell G. R. What controls smooth muscle phenotype? Atherosclerosis. 1981 Nov-Dec;40(3-4):347–357. doi: 10.1016/0021-9150(81)90145-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Content J., De Wit L., Pierard D., Derynck R., De Clercq E., Fiers W. Secretory proteins induced in human fibroblasts under conditions used for the production of interferon beta. Proc Natl Acad Sci U S A. 1982 May;79(9):2768–2772. doi: 10.1073/pnas.79.9.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran R. S., Gimbrone M. A., Jr, Bevilacqua M. P., Mendrick D. L., Pober J. S. Induction and detection of a human endothelial activation antigen in vivo. J Exp Med. 1986 Aug 1;164(2):661–666. doi: 10.1084/jem.164.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defilippi P., Poupart P., Tavernier J., Fiers W., Content J. Induction and regulation of mRNA encoding 26-kDa protein in human cell lines treated with recombinant human tumor necrosis factor. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4557–4561. doi: 10.1073/pnas.84.13.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin M. L., Rothlein R., Bhan A. K., Dinarello C. A., Springer T. A. Induction by IL 1 and interferon-gamma: tissue distribution, biochemistry, and function of a natural adherence molecule (ICAM-1). J Immunol. 1986 Jul 1;137(1):245–254. [PubMed] [Google Scholar]
- Emeson E. E., Robertson A. L., Jr T lymphocytes in aortic and coronary intimas. Their potential role in atherogenesis. Am J Pathol. 1988 Feb;130(2):369–376. [PMC free article] [PubMed] [Google Scholar]
- Garman R. D., Raulet D. H. Characterization of a novel murine T cell-activating factor. J Immunol. 1987 Feb 15;138(4):1121–1129. [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gephardt G. N., Ahmad M., Tubbs R. R. Pulmonary vasculitis (Wegener's granulomatosis). Immunohistochemical study of T and B cell markers. Am J Med. 1983 Apr;74(4):700–704. doi: 10.1016/0002-9343(83)91030-6. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Holm J., Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989 Jul;135(1):169–175. [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Issekutz T. B., Stoltz J. M., vd Meide P. Lymphocyte recruitment in delayed-type hypersensitivity. The role of IFN-gamma. J Immunol. 1988 May 1;140(9):2989–2993. [PubMed] [Google Scholar]
- Jirik F. R., Podor T. J., Hirano T., Kishimoto T., Loskutoff D. J., Carson D. A., Lotz M. Bacterial lipopolysaccharide and inflammatory mediators augment IL-6 secretion by human endothelial cells. J Immunol. 1989 Jan 1;142(1):144–147. [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Gabbiani G., Hansson G. K. Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J Clin Invest. 1985 Jul;76(1):125–131. doi: 10.1172/JCI111934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T., Hirano T. Molecular regulation of B lymphocyte response. Annu Rev Immunol. 1988;6:485–512. doi: 10.1146/annurev.iy.06.040188.002413. [DOI] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Libby P., O'Brien K. V. Culture of quiescent arterial smooth muscle cells in a defined serum-free medium. J Cell Physiol. 1983 May;115(2):217–223. doi: 10.1002/jcp.1041150217. [DOI] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Auger K. R., Robbins A. H., Birinyi L. K., Dinarello C. A. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am J Pathol. 1986 Aug;124(2):179–185. [PMC free article] [PubMed] [Google Scholar]
- Libby P., Ordovas J. M., Birinyi L. K., Auger K. R., Dinarello C. A. Inducible interleukin-1 gene expression in human vascular smooth muscle cells. J Clin Invest. 1986 Dec;78(6):1432–1438. doi: 10.1172/JCI112732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipford E. H., Jr, Margolick J. B., Longo D. L., Fauci A. S., Jaffe E. S. Angiocentric immunoproliferative lesions: a clinicopathologic spectrum of post-thymic T-cell proliferations. Blood. 1988 Nov;72(5):1674–1681. [PubMed] [Google Scholar]
- Loppnow H., Libby P. Adult human vascular endothelial cells express the IL6 gene differentially in response to LPS or IL1. Cell Immunol. 1989 Sep;122(2):493–503. doi: 10.1016/0008-8749(89)90095-6. [DOI] [PubMed] [Google Scholar]
- Lovett D. H., Larsen A. Cell cycle-dependent interleukin 1 gene expression by cultured glomerular mesangial cells. J Clin Invest. 1988 Jul;82(1):115–122. doi: 10.1172/JCI113558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Castle C. K., Goodman L. V., Weisgraber K. H., Mahley R. W., Shooter E. M., Gebicke-Haerter P. J. Expression of apolipoprotein E by cultured vascular smooth muscle cells is controlled by growth state. J Cell Biol. 1988 Sep;107(3):1207–1213. doi: 10.1083/jcb.107.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May L. T., Helfgott D. C., Sehgal P. B. Anti-beta-interferon antibodies inhibit the increased expression of HLA-B7 mRNA in tumor necrosis factor-treated human fibroblasts: structural studies of the beta 2 interferon involved. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8957–8961. doi: 10.1073/pnas.83.23.8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movat H. Z., Burrowes C. E., Cybulsky M. I., Dinarello C. A. Acute inflammation and a Shwartzman-like reaction induced by interleukin-1 and tumor necrosis factor. Synergistic action of the cytokines in the induction of inflammation and microvascular injury. Am J Pathol. 1987 Dec;129(3):463–476. [PMC free article] [PubMed] [Google Scholar]
- Moyer C. F., Strandberg J. D., Reinisch C. L. Systemic mononuclear-cell vasculitis in MRL/Mp-lpr/lpr mice. A histologic and immunocytochemical analysis. Am J Pathol. 1987 May;127(2):229–242. [PMC free article] [PubMed] [Google Scholar]
- Munro J. M., Cotran R. S. The pathogenesis of atherosclerosis: atherogenesis and inflammation. Lab Invest. 1988 Mar;58(3):249–261. [PubMed] [Google Scholar]
- Nachman R. L., Hajjar K. A., Silverstein R. L., Dinarello C. A. Interleukin 1 induces endothelial cell synthesis of plasminogen activator inhibitor. J Exp Med. 1986 Jun 1;163(6):1595–1600. doi: 10.1084/jem.163.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawroth P. P., Bank I., Handley D., Cassimeris J., Chess L., Stern D. Tumor necrosis factor/cachectin interacts with endothelial cell receptors to induce release of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1363–1375. doi: 10.1084/jem.163.6.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines E. W., Dower S. K., Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989 Jan 20;243(4889):393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- Ritchie D. G., Fuller G. M. Hepatocyte-stimulating factor: a monocyte-derived acute-phase regulatory protein. Ann N Y Acad Sci. 1983 Jun 27;408:490–502. doi: 10.1111/j.1749-6632.1983.tb23268.x. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis--an update. N Engl J Med. 1986 Feb 20;314(8):488–500. doi: 10.1056/NEJM198602203140806. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., May L. T., Tamm I., Vilcek J. Human beta 2 interferon and B-cell differentiation factor BSF-2 are identical. Science. 1987 Feb 13;235(4790):731–732. doi: 10.1126/science.3492764. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Sagar A. D. Heterogeneity of poly(I) x poly(C)-induced human fibroblast interferon mRNA species. Nature. 1980 Nov 6;288(5786):95–97. doi: 10.1038/288095a0. [DOI] [PubMed] [Google Scholar]
- Seifert P. S., Hansson G. K. Decay-accelerating factor is expressed on vascular smooth muscle cells in human atherosclerotic lesions. J Clin Invest. 1989 Aug;84(2):597–604. doi: 10.1172/JCI114204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi M., Breviario F., Proserpio P., Biondi A., Vecchi A., Van Damme J., Dejana E., Mantovani A. IL-1 stimulates IL-6 production in endothelial cells. J Immunol. 1989 Jan 15;142(2):549–553. [PubMed] [Google Scholar]
- Tarkowski A., Jonsson R., Sanchez R., Klareskog L., Koopman W. J. Features of renal vasculitis in autoimmune MRL lpr/lpr mice: phenotypes and functional properties of infiltrating cells. Clin Exp Immunol. 1988 Apr;72(1):91–97. [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., Tippens D., Gordon D., Ross R., Gown A. M. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987 Jan;126(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Wagner C. R., Vetto R. M., Burger D. R. Expression of I-region-associated antigen (Ia) and interleukin 1 by subcultured human endothelial cells. Cell Immunol. 1985 Jun;93(1):91–104. doi: 10.1016/0008-8749(85)90391-0. [DOI] [PubMed] [Google Scholar]
- Warner S. J., Auger K. R., Libby P. Human interleukin 1 induces interleukin 1 gene expression in human vascular smooth muscle cells. J Exp Med. 1987 May 1;165(5):1316–1331. doi: 10.1084/jem.165.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner S. J., Auger K. R., Libby P. Interleukin 1 induces interleukin 1. II. Recombinant human interleukin 1 induces interleukin 1 production by adult human vascular endothelial cells. J Immunol. 1987 Sep 15;139(6):1911–1917. [PubMed] [Google Scholar]
- Warner S. J., Friedman G. B., Libby P. Immune interferon inhibits proliferation and induces 2'-5'-oligoadenylate synthetase gene expression in human vascular smooth muscle cells. J Clin Invest. 1989 Apr;83(4):1174–1182. doi: 10.1172/JCI113998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner S. J., Friedman G. B., Libby P. Regulation of major histocompatibility gene expression in human vascular smooth muscle cells. Arteriosclerosis. 1989 May-Jun;9(3):279–288. doi: 10.1161/01.atv.9.3.279. [DOI] [PubMed] [Google Scholar]
- Warner S. J., Libby P. Human vascular smooth muscle cells. Target for and source of tumor necrosis factor. J Immunol. 1989 Jan 1;142(1):100–109. [PubMed] [Google Scholar]
- Weissenbach J., Chernajovsky Y., Zeevi M., Shulman L., Soreq H., Nir U., Wallach D., Perricaudet M., Tiollais P., Revel M. Two interferon mRNAs in human fibroblasts: in vitro translation and Escherichia coli cloning studies. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7152–7156. doi: 10.1073/pnas.77.12.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasukawa K., Hirano T., Watanabe Y., Muratani K., Matsuda T., Nakai S., Kishimoto T. Structure and expression of human B cell stimulatory factor-2 (BSF-2/IL-6) gene. EMBO J. 1987 Oct;6(10):2939–2945. doi: 10.1002/j.1460-2075.1987.tb02598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein A., Ruggieri R., Korn J. H., Revel M. Structure and expression of cDNA and genes for human interferon-beta-2, a distinct species inducible by growth-stimulatory cytokines. EMBO J. 1986 Oct;5(10):2529–2537. doi: 10.1002/j.1460-2075.1986.tb04531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zullo J. N., Cochran B. H., Huang A. S., Stiles C. D. Platelet-derived growth factor and double-stranded ribonucleic acids stimulate expression of the same genes in 3T3 cells. Cell. 1985 Dec;43(3 Pt 2):793–800. doi: 10.1016/0092-8674(85)90252-1. [DOI] [PubMed] [Google Scholar]