Summary
The mitotic checkpoint, also known as the spindle assembly checkpoint, delays anaphase onset until all chromosomes have reached bipolar tension on the mitotic spindle [1–3]. Once this is achieved, the protease separase is activated to cleave the chromosomal cohesin complex, thereby triggering anaphase. Cohesin cleavage releases tension between sister chromatids, but why the mitotic checkpoint now remains silent is poorly understood. Here, using budding yeast as a model, we show that loss of sister chromatid cohesion at anaphase onset would engage the mitotic checkpoint if this was not prevented by concomitant separase-dependent activation of the Cdc14 phosphatase. Cdc14, in turn, inactivates the mitotic checkpoint by dephosphorylating Sli15INCENP, a subunit of the conserved Aurora B kinase complex that forms part of the proposed chromosomal tension sensor. Dephosphorylation-dependent relocation of Sli15INCENP from centromeres to the central spindle during anaphase is seen in organisms from yeast to human [4–8]. Our results suggest that Sli15INCENP dephosphorylation is part of an evolutionarily conserved mechanism that prevents the mitotic checkpoint from reengaging when tension between sister chromatids is lost at anaphase onset.
Keywords: CELLBIO
Highlights
► Loss of cohesion at anaphase onset can reengage the mitotic checkpoint ► This is prevented by activation of the Cdc14 phosphatase at the same time ► Cdc14 inactivates the mitotic checkpoint by dephosphorylating Sli15INCENP ► A conserved mechanism inactivates the mitotic checkpoint in anaphase
Result and Discussion
Reengagement of the Mitotic Checkpoint Due to Loss of Tension in Anaphase
During chromosome alignment on the mitotic spindle, a single chromosome that has not yet come under bipolar tension is sufficient to delay mitotic progression [1–3]. Only when all chromosomes are bioriented, the mitotic checkpoint is silenced, leading to activation of the anaphase promoting complex (APC), a ubiquitin ligase complex. The APC now ubiquitinates, and thereby primes for degradation, the anaphase inhibitor securin, as well as mitotic cyclins. Securin destruction liberates the protease separase to trigger sister chromatid separation by cleaving the chromosomal cohesin complex while cyclin destruction downregulates mitotic cyclin-dependent kinase (Cdk) activity to promote mitotic exit. As sister chromatids split, the cohesive counterforce required for the build-up of tension on the metaphase plate is lost from all chromosomes. Reengagement of the mitotic checkpoint at this stage would inhibit the APC, stabilize securin and cyclins again, and thus impede further mitotic progression [9]. Why the ubiquitous loss of tension at anaphase onset goes undetected by the checkpoint remains poorly understood. One possibility is that the viscous drag of chromosomes on their way to the spindle poles substitutes for tension between sister chromatids, but this has not been experimentally addressed.
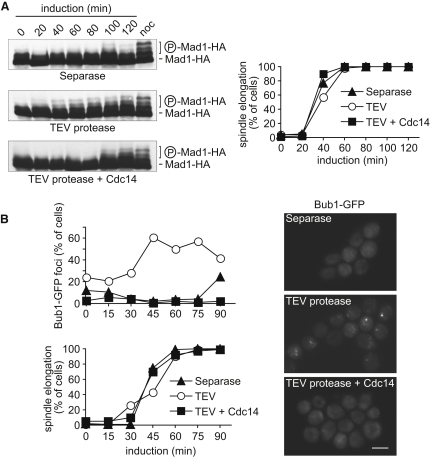
We set out to investigate whether loss of cohesion at anaphase onset would, in principle, reengage the mitotic checkpoint, and if so, how this is normally prevented. We studied budding yeast cells arrested in metaphase by depletion of the APC activator Cdc20. In these cells, we initiated anaphase onset by ectopic expression of either separase or the foreign TEV protease that also triggered loss of cohesion by cleaving accordingly engineered cohesin [10]. Mitotic checkpoint signaling was monitored by the phosphorylation status and kinetochore recruitment of the checkpoint components Mad1 and Bub1, respectively [11, 12]. Mad1 phosphorylation, accompanied by retarded electrophoretic mobility, a sign for checkpoint engagement, was not detectable during separase-triggered anaphase (Figure 1A), consistent with the notion that the mitotic checkpoint remains silent. Only at later time points, some Mad1 phosphorylation became apparent, which was probably the consequence of progression into the next cell cycle after separase expression [13]. In contrast, when anaphase onset was triggered by TEV protease expression, Mad1 became phosphorylated concomitant with anaphase onset. Similarly, recruitment of Bub1 into distinct nuclear foci, a marker for recognition of tensionless kinetochores by the checkpoint, was observed at the time of anaphase onset in response to TEV protease expression, but not after separase expression (Figure 1B). This suggests that loss of cohesion at anaphase onset results in a loss of tension that is, in principle, detected by the mitotic checkpoint, but an activity of separase, different from cohesin cleavage, prevents this. These observations are consistent with a recent report that the checkpoint protein BubR1 associates with anaphase chromosomes after TEV protease-induced cohesin cleavage in mitotically arrested Drosophila embryos [14].
Figure 1.
Cdc14 Prevents Mitotic Checkpoint Engagement Due to Loss of Sister Chromatid Cohesion at Anaphase Onset
(A) Cells were arrested in metaphase by Cdc20 depletion, and expression of separase, TEV protease, or TEV protease together with Cdc14 was induced. Activation of the mitotic checkpoint was monitored by the phosphorylation-induced electrophoretic mobility shift of Mad1, fused to a HA-epitope tag to facilitate western detection. The same cells treated with the spindle poison nocodazole (5 μg/ml; noc), but uninduced, served as a positive control for mitotic checkpoint activation.
(B) As in (A), but checkpoint activation was visualized by the appearance of Bub1-GFP nuclear foci. Images are of cells 45 min after induction; scale bar represents 5 μm. Anaphase spindles of 4 μm or longer were scored as elongated. See also Figure S1.
Cdc14 Prevents Reengagement of the Mitotic Checkpoint during Anaphase
In addition to splitting sister chromatids, separase promotes activation of the Cdc14 phosphatase, a key Cdk opponent during budding yeast mitotic exit [13, 15]. To address whether Cdc14 makes cells insensitive to loss of tension at anaphase onset, we ectopically coexpressed Cdc14 with TEV protease in metaphase-arrested cells. This prevented both Mad1 phosphorylation and Bub1 foci formation in response to sister chromatid splitting (Figures 1A and 1B), indicating that Cdc14 can inactivate the responsiveness of the mitotic checkpoint to loss of tension. Ectopic Cdc14 expression also overcame a mitotic arrest induced by the spindle depolymerizing drug nocodazole (see Figure S1 available online), further emphasizing its capacity to inactivate the mitotic checkpoint.
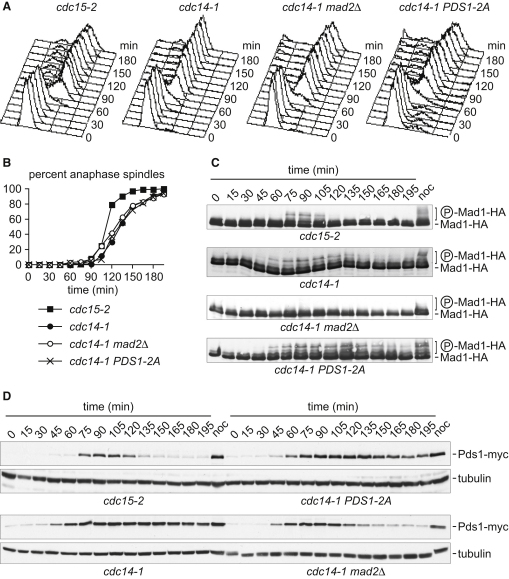
To confirm that Cdc14 is responsible for restraining the checkpoint in anaphase, we examined a cdc14-1 temperature-sensitive strain. As a control, we used cdc15-2 mutant cells that, like cdc14-1 cells, arrest in telophase at restrictive temperature but activate Cdc14 in early anaphase [15]. After synchronization in G1 using α-factor, both strains progressed through the early stages of the cell cycle with similar kinetics (Figure 2A). Anaphase spindle elongation started at the same time but took longer to complete in the case of cdc14-1 cells, most likely because of the Cdc14 requirement for stable spindle midzone formation, as described previously [5, 16–18] (Figure 2B). In cdc15-2 control cells, Mad1 phosphorylation became detectable at the time of S phase and disappeared again at the metaphase-to-anaphase transition (Figure 2C). In contrast, Mad1 phosphorylation persisted long into anaphase in cdc14-1 cells, indicating a failure to inactivate the mitotic checkpoint. Checkpoint engagement during anaphase is expected to inhibit the APC and consequently stabilize securin. Consistently, we observed high levels of securin in cdc14-1, but not cdc15-2, anaphase cells (Figure 2D). The persistence of securin was due to the mitotic checkpoint in cdc14-1 cells, because it was no longer observed after deletion of the gene encoding the checkpoint component Mad2. Anaphase spindle elongation was not advanced in cdc14-1 cells lacking Mad2, confirming that the rate of spindle elongation was affected by Cdc14 independently of mitotic checkpoint regulation.
Figure 2.
Persistent Mitotic Checkpoint Signaling in cdc14-1 Mutant Anaphase Cells
(A) Cells of the indicated genotypes were released from α-factor block in G1 into synchronous cell cycle progression at nonpermissive temperature (37°C) for the cdc14-1 and cdc15-2 alleles. Cell cycle progression was monitored by fluorescence-activated cell sorting (FACS) analysis of DNA content.
(B) Spindles of 4 μm or longer were scored as elongated.
(C) The Mad1 phosphorylation status in cells from the above experiment was analyzed by western blotting.
(D) Levels of securin (Pds1), fused to a myc epitope tag to facilitate detection, were analyzed by western blotting. Tubulin served as a loading control. See also Figure S2.
The above results suggest that the mitotic checkpoint is engaged in cdc14-1 anaphase cells. However, checkpoint silencing and securin destruction are thought to be a prerequisite for anaphase onset. Persistent Mad1 phosphorylation and securin in cdc14-1 cells might therefore be the consequence of checkpoint reengagement after it had initially been satisfied. A transient decrease in Mad1 phosphorylation and securin levels might have been obstructed by the limited mitotic synchrony of our cell population after release from α-factor block. When we performed a similar experiment with cells synchronized at the metaphase-to-anaphase transition by depletion and reinduction of Cdc20, transient securin destruction and checkpoint-dependent reaccumulation became obvious in cdc14-1, but not cdc15-2, cells (Figure S2). These observations suggest that Cdc14 is required to prevent mitotic checkpoint reengagement in anaphase.
It has been suggested that Cdc14 promotes securin destruction during anaphase by direct securin dephosphorylation. However, introduction of a nonphosphorylatable securin allele, PDS1-2A, that is no longer protected from degradation by Cdk phosphorylation [19] did not avert securin stabilization in cdc14-1 anaphase cells (Figure 2D). This is in contrast to the marked dependence of securin stabilization on Mad2, suggesting that securin accumulation in cdc14-1 mutant anaphase is primarily the consequence of the checkpoint.
Cdc14 Overcomes a Mitotic Checkpoint-Dependent Cell Cycle Delay
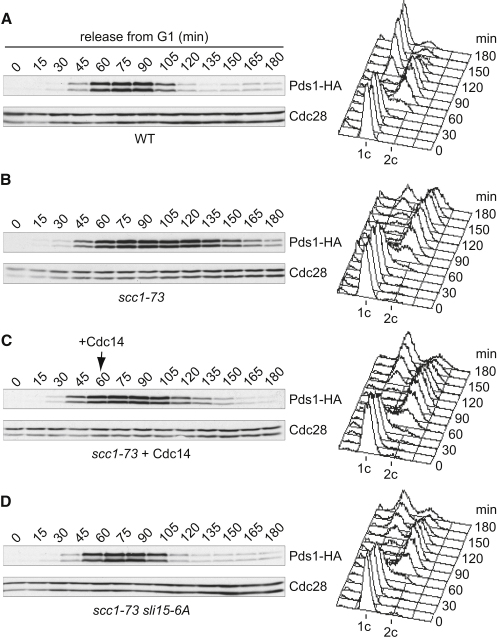
The above experiments have analyzed markers of the checkpoint and have suggested that Cdc14 is required to prevent checkpoint reengagement due to loss of tension at anaphase onset. The important physiological consequence of checkpoint signaling is a mitotic delay. We were unable to analyze a checkpoint-mediated delay to mitotic progression in cdc14-1 anaphase cells because of the essential requirement of Cdc14 for mitotic exit independently of checkpoint inactivation. To explore the potential of Cdc14 as a checkpoint regulator, we therefore analyzed its impact in a setting where mitosis is delayed in cells that fail to establish tension between sister chromatids as a result of defective sister chromatid cohesion. As described [20], securin destruction and progression through mitosis was delayed in cells carrying the temperature-sensitive cohesin subunit scc1-73 (Figures 3A and 3B). Ectopic Cdc14 expression in scc1-73 cells largely overcame the delay to both securin destruction and mitotic progression (Figure 3C). This demonstrates that Cdc14 can override a mitotic checkpoint delay due to absence of tension between sister chromatids. Whereas in this experiment Cdc14 overcame the checkpoint response to lack of tension in prometaphase, Cdc14 would normally disable the response to loss of tension in early anaphase, its normal time of activation.
Figure 3.
Cdc14 Relieves the Mitotic Checkpoint Delay Due to Absence of Tension
(A and B) Wild-type (A) and scc1-73 (B) cells were grown in YP medium containing raffinose as carbon source, arrested in G1 using α-factor, and released into synchronous cell cycle progression at restrictive temperature (35°C). α-factor was added back at 75 min for rearrest in the following G1. Cell cycle progression was monitored by FACS analysis of DNA content and western blotting against securin (Pds1) fused to a HA-epitope tag. Cdc28 served as a loading control.
(C) In a second scc1-73 culture, Cdc14 expression from the GAL1 promoter was induced by galactose addition at 60 min.
(D) A third scc1-73 culture carried the sli15-6A allele. See also Figure S3.
Sli15INCENP Dephosphorylation Inactivates the Mitotic Checkpoint
How does Cdc14 inactivate the mitotic checkpoint? It has been suggested that APC-dependent degradation of the Mps1 kinase disables the checkpoint in anaphase [21]. Mps1 degradation is in part mediated by the APC activator Cdh1, whose binding to the APC requires dephosphorylation by Cdc14. However, Cdh1 activation is a late event during mitotic exit, and, consistently, we found that Mps1 levels declined only late and gradually in anaphase (Figure S3). Mps1 degradation may therefore not act fast enough to render the mitotic checkpoint insensitive to loss of tension at anaphase onset. Furthermore, Mps1 remained stable, whereas the mitotic checkpoint was efficiently inactivated in response to separase expression in mitotically arrested cells (Figure S3). These observations suggest that Cdc14 inactivates the mitotic checkpoint by a different or additional mechanism.
A candidate Cdc14 substrate for checkpoint inactivation is Sli15INCENP. It forms part of the conserved Aurora B kinase complex at centromeres, required for conveying lack of tension to the mitotic checkpoint [20]. Its Cdc14-dependent dephosphorylation at anaphase onset mediates Sli15INCENP relocation from centromeres to the spindle midzone [5, 22]. To investigate the consequences of Sli15INCENP dephosphorylation, we employed cells carrying the sli15-6A allele in which six Cdk phosphorylation sites have been mutated, mimicking a dephosphorylated state independently of Cdc14 [5, 16]. sli15-6A cells were unable to delay mitosis in response to defective sister chromatid cohesion in scc1-73 cells (Figure 3D). This suggests that Sli15 phosphorylation is a prerequisite for its mitotic checkpoint function and that its dephosphorylation, which normally occurs in anaphase, inactivates the checkpoint.
The Sli15-6A protein was proficient in its essential function in chromosome biorientation on the mitotic spindle [23] (Figure S4), as well as in the mitotic checkpoint response to spindle depolymerization by nocodazole (Figure S1). Sli15-6A therefore appears to separate the functions of the Aurora B kinase complex in (1) the mitotic checkpoint response to loss of tension and (2) the error correction of kinetochore microtubule attachments and the checkpoint response to nocodazole treatment. This separation of function could be quantitative in nature, relating to reduced sli15-6A kinetochore levels as a result of its premature relocation to the mitotic spindle [5]. Error correction may require lower levels of the Aurora B kinase compared to generation of the mitotic checkpoint signal in response to loss of tension. A mitotic checkpoint function of the Aurora B kinase complex, independently of generating unattached kinetochores, has previously been documented in vertebrates and fission yeast [24–27].
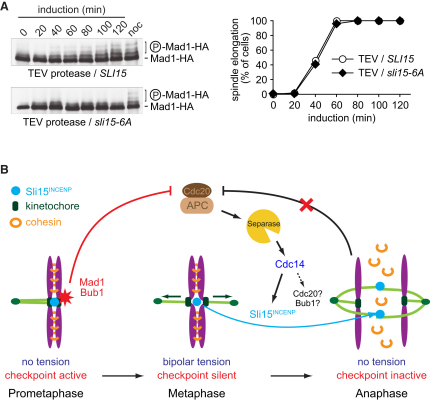
We finally tested whether Sli15INCENP dephosphorylation is indeed sufficient to prevent mitotic checkpoint engagement when tension between sister chromatids is lost at anaphase onset. We induced sister chromatid separation in metaphase-arrested sli15-6A cells by TEV protease expression. Unlike in SLI15 control cells, in which Mad1 became phosphorylated at the time of anaphase onset, this response was no longer observed in sli15-6A cells (Figure 4A). This suggests that Sli15 dephosphorylation turns off the ability of cells to respond to loss of tension between sister chromatids at anaphase onset. We note that despite compromised anaphase A movement, kinetochores do not obviously lose microtubule attachment during TEV protease-triggered anaphase [16]. This is consistent with the possibility that centromere retention of the Aurora B kinase complex reengages the mitotic checkpoint in anaphase without activating error correction, possibly because of the kinetochore microtubule geometry at this time.
Figure 4.
Nonphosphorylatable Sli15-6A Prevents Mitotic Checkpoint Reengagement in Anaphase
(A) Budding yeast cells harboring wild-type SLI15 or the sli15-6A allele were arrested in metaphase by Cdc20 depletion. Loss of sister chromatid cohesion was triggered by TEV protease expression. Mad1 phosphorylation and anaphase spindle elongation were monitored as in Figure 1.
(B) Model for mitotic checkpoint inactivation in anaphase. During chromosome alignment on the mitotic spindle in prometaphase, a mitotic checkpoint signal, including the Mad1 and Bub1 proteins, emanates from kinetochores that have not yet come under tension. This prevents APC activation by Cdc20. Generation of the checkpoint signal depends on the physical proximity between the Aurora B kinase complex and its targets on tensionless kinetochores. Once bipolar tension is established in metaphase, the checkpoint is silenced and the APC degrades securin to activate separase. Cohesin cleavage now triggers anaphase, and tension is lost again from kinetochores. This would reactivate the checkpoint, but this is prevented by Sli15INCENP dephosphorylation and consequent relocation of the Aurora B kinase complex to the spindle midzone. Dephosphorylation of additional Cdk targets might contribute to maintain an inactive checkpoint. See also Figure S4.
A Conserved Mechanism to Inactivate the Mitotic Checkpoint in Anaphase
We show here that loss of tension between sister chromatids at anaphase onset, caused by cleavage of cohesin, would in principle reengage the mitotic checkpoint. This is prevented in budding yeast by concomitant activation of the Cdc14 phosphatase, which, helped by cyclin proteolysis and Cdk downregulation, leads to dephosphorylation of Sli15INCENP. The Aurora B kinase complex is an integral part of the mitotic checkpoint also in vertebrates [24–26]. Its sudden relocation from the inner centromere to the spindle midzone, promoted by INCENP dephosphorylation, is a hallmark feature of this “chromosomal passenger” complex [4, 6–8]. In an accompanying study, Vázquez-Novelle and Petronczki ([28], this issue of Current Biology) show that relocation of the Aurora B kinase complex is required to prevent untimely checkpoint protein recruitment to human kinetochores in anaphase, suggesting that a conserved mechanism prevents the mitotic checkpoint from reengaging in anaphase.
The resulting model of Aurora B kinase regulation as part of the mitotic checkpoint is illustrated in Figure 4B. The spatial proximity between inner centromeric Aurora B kinase and not-yet-identified phosphorylation targets at the outer kinetochore, probably including Ndc80, is thought to initiate checkpoint signaling [29, 30]. Once biorientation is achieved, the kinetochore undergoes a conformational change in response to the exerted physical tension [31, 32]. This increases the distance between Aurora B kinase and the outer kinetochore and brings its phosphorylation targets out of reach [23, 29]. Protein phosphatase 1, resident at the outer kinetochore, now removes the phosphoepitopes and thereby silences the checkpoint [27, 33, 34]. At anaphase onset, however, kinetochores revert to their tensionless conformation [31]. This would bring the outer kinetochore back into proximity of Aurora B kinase, leading to reengagement of the mitotic checkpoint. We propose that Sli15INCENP dephosphorylation and consequent dissociation of the Aurora B kinase complex from centromeres prevents unscheduled checkpoint reactivation at this time.
In addition to Sli15INCENP, a number of other mitotic checkpoint components undergo cell cycle regulation. Cdk-dependent phosphorylation of fission yeast Bub1 and vertebrate Cdc20 are required for a functional mitotic checkpoint [35–37]. The dephosphorylation timing of these proteins during mitotic exit and the phosphatases responsible remains to be characterized. Although Sli15INCENP dephosphorylation inactivates the mitotic checkpoint at the very source of the checkpoint signal, we speculate that dephosphorylation of these additional targets, as well as Mps1 degradation [21], contributes to inactivate the mitotic checkpoint at anaphase onset and to keep it inactive until well into the next cell cycle. This ensures that loss of tension, which causes a robust block to mitotic progression in prometaphase, will not impede mitotic exit and return to G1 once the signal to the separation of sister chromatids has been given.
Experimental Procedures
Yeast Strains and Techniques
Details of the yeast strains used in this study can be found in Table S1. Genes were fused at their endogenous gene loci with affinity epitope tags for western blot detection or a 3xGFP cassette for detection by fluorescent microscopy using polymerase chain reaction products. Arrest of cells in metaphase by depletion of Cdc20 under control of the MET3 promoter and expression of separase, TEV protease, or Cdc14 under inducible control of the GAL1 promoter were as described previously [10, 16]. Analysis of Mad1 phosphorylation was performed by electrophoresis of whole cell extracts and prepared using an alkaline extraction method [38] on low crosslinking SDS-polyacrylamide gels (8%; acrylamide to bisacrylamide ratio 33.5:0.3), followed by western blotting. Antibodies used for western detection were α-HA clone 12CA5, α-myc clone 9E10, α-Clb2 serum (sc-9071), α-PSTAIRE serum recognizing Cdc28 (sc-53, both Santa Cruz Biotechnology), α-tubulin antibody clone YOL1/34 (AbD Serotec), and α-actin serum (ab8227, Abcam).
Microscopy
Cells expressing Bub1-3xGFP were fixed in 100% ethanol and mounted on 2% agarose pads for examination. Recruitment to kinetochores was confirmed by its colocalization with Ndc80-RFP (data not shown). Spindle elongation was analyzed in formaldehyde-fixed cells by indirect immunofluorescence using α-tubulin antibody clone YOL1/34. Fluorescent images were acquired using an Axioplan 2 imaging microscope (Zeiss) equipped with a 100× (NA = 1.45) Plan-Neofluar objective and an ORCA-ER camera (Hamamatsu).
Acknowledgments
We thank D. Morgan for providing the PDS1-2A allele, T. Higuchi for constructing the sli15-6A strain, M.D. Vázquez-Novelle and M. Petronczki for sharing unpublished results, and T. Toda and members of our laboratory for discussion and critical reading of the manuscript.
Published online: July 8, 2010
Footnotes
Supplemental Information includes four figures and one table and can be found with this article online at doi:10.1016/j.cub.2010.06.023.
Supplemental Information
References
- 1.Rieder C.L., Schultz A., Cole R., Sluder G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J. Cell Biol. 1994;127:1301–1310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li X., Nicklas R.B. Mitotic forces control a cell-cycle checkpoint. Nature. 1995;373:630–632. doi: 10.1038/373630a0. [DOI] [PubMed] [Google Scholar]
- 3.Santaguida S., Musacchio A. The life and miracles of kinetochores. EMBO J. 2009;28:2511–2531. doi: 10.1038/emboj.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Earnshaw W.C., Bernat R.L. Chromosomal passengers: Toward an integrated view of mitosis. Chromosoma. 1991;100:139–146. doi: 10.1007/BF00337241. [DOI] [PubMed] [Google Scholar]
- 5.Pereira G., Schiebel E. Separase regulates INCENP-Aurora B anaphase spindle function through Cdc14. Science. 2003;302:2120–2124. doi: 10.1126/science.1091936. [DOI] [PubMed] [Google Scholar]
- 6.Parry D.H., Hickson G.R.X., O'Farrell P.H. Cyclin B destruction triggers changes in kinetochore behavior essential for successful anaphase. Curr. Biol. 2003;13:647–653. doi: 10.1016/s0960-9822(03)00242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruneberg U., Neef R., Honda R., Nigg E.A., Barr F.A. Relocation of Aurora B from centromeres to the central spindle at the metaphase to anaphase transition requires MKlp2. J. Cell Biol. 2004;166:167–172. doi: 10.1083/jcb.200403084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hümmer S., Mayer T.U. Cdk1 negatively regulates midzone localization of the mitotic kinesin Mklp2 and the chromosomal passenger complex. Curr. Biol. 2009;19:607–612. doi: 10.1016/j.cub.2009.02.046. [DOI] [PubMed] [Google Scholar]
- 9.Tinker-Kulberg R.L., Morgan D.O. Pds1 and Esp1 control both anaphase and mitotic exit in normal cells and after DNA damage. Genes Dev. 1999;13:1936–1949. doi: 10.1101/gad.13.15.1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uhlmann F., Wernic D., Poupart M.-A., Koonin E.V., Nasmyth K. Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell. 2000;103:375–386. doi: 10.1016/s0092-8674(00)00130-6. [DOI] [PubMed] [Google Scholar]
- 11.Hardwick K.G., Murray A.W. Mad1p, a phosphoprotein component of the spindle assembly checkpoint in budding yeast. J. Cell Biol. 1995;131:709–720. doi: 10.1083/jcb.131.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillett E.S., Espelin C.W., Sorger P.K. Spindle checkpoint proteins and chromosome-microtubule attachment in budding yeast. J. Cell Biol. 2004;164:535–546. doi: 10.1083/jcb.200308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sullivan M., Uhlmann F. A non-proteolytic function of separase links the onset of anaphase to mitotic exit. Nat. Cell Biol. 2003;5:249–254. doi: 10.1038/ncb940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliveira R.A., Hamilton R.S., Pauli A., Davis I., Nasmyth K. Cohesin cleavage and Cdk inhibition trigger formation of daughter nuclei. Nat. Cell Biol. 2010;12:185–192. doi: 10.1038/ncb2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stegmeier F., Visintin R., Amon A. Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell. 2002;108:207–220. doi: 10.1016/s0092-8674(02)00618-9. [DOI] [PubMed] [Google Scholar]
- 16.Higuchi T., Uhlmann F. Stabilization of microtubule dynamics at anaphase onset promotes chromosome segregation. Nature. 2005;433:171–176. doi: 10.1038/nature03240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodbury E.L., Morgan D.O. Cdk and APC activities limit the spindle-stabilizing function of Fin1 to anaphase. Nat. Cell Biol. 2007;9:106–112. doi: 10.1038/ncb1523. [DOI] [PubMed] [Google Scholar]
- 18.Khmelinskii A., Roostalu J., Roque H., Antony C., Schiebel E. Phosphorylation-dependent protein interactions at the spindle midzone mediate cell cycle regulation of spindle elongation. Dev. Cell. 2009;17:244–256. doi: 10.1016/j.devcel.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Holt L.J., Krutchinsky A.N., Morgan D.O. Positive feedback sharpens the anaphase switch. Nature. 2008;454:353–357. doi: 10.1038/nature07050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biggins S., Murray A.W. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 2001;15:3118–3129. doi: 10.1101/gad.934801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palframan W.J., Meehl J.B., Jaspersen S.L., Winey M., Murray A.W. Anaphase inactivation of the spindle checkpoint. Science. 2006;313:680–684. doi: 10.1126/science.1127205. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan M., Hornig N.C.D., Porstmann T., Uhlmann F. Studies on substrate recognition by the budding yeast separase. J. Biol. Chem. 2004;279:1191–1196. doi: 10.1074/jbc.M309761200. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka T.U., Rachidi N., Janke C., Pereira G., Galova M., Schiebel E., Stark M.J.R., Nasmyth K. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108:317–329. doi: 10.1016/s0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- 24.Kallio M.J., McCleland M.L., Stukenberg P.T., Gorbsky G.J. Inhibition of aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Curr. Biol. 2002;12:900–905. doi: 10.1016/s0960-9822(02)00887-4. [DOI] [PubMed] [Google Scholar]
- 25.Hauf S., Cole R.W., LaTerra S., Zimmer C., Schnapp G., Walter R., Heckel A., van Meel J., Rieder C.L., Peters J.-M. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vigneron S., Prieto S., Bernis C., Labbé J.-C., Castro A., Lorca T. Kinetochore localization of spindle checkpoint proteins: Who controls whom? Mol. Biol. Cell. 2004;15:4584–4596. doi: 10.1091/mbc.E04-01-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanoosthuyse V., Hardwick K.G. A novel protein phosphatase 1-dependent spindle checkpoint silencing mechanism. Curr. Biol. 2009;19:1176–1181. doi: 10.1016/j.cub.2009.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vázquez-Novelle M.D., Petronczki M. Relocation of the chromosomal passenger complex prevents mitotic checkpoint engagement at anaphase in human cells. Curr. Biol. 2010;20:1402–1407. doi: 10.1016/j.cub.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 29.Liu D., Vader G., Vromans M.J.M., Lampson M.A., Lens S.M.A. Sensing chromosome bi-orientation by spatial separation of aurora B kinase from kinetochore substrates. Science. 2009;323:1350–1353. doi: 10.1126/science.1167000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kemmler S., Stach M., Knapp M., Ortiz J., Pfannstiel J., Ruppert T., Lechner J. Mimicking Ndc80 phosphorylation triggers spindle assembly checkpoint signalling. EMBO J. 2009;28:1099–1110. doi: 10.1038/emboj.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joglekar A.P., Bloom K., Salmon E.D. In vivo protein architecture of the eukaryotic kinetochore with nanometer scale accuracy. Curr. Biol. 2009;19:694–699. doi: 10.1016/j.cub.2009.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan X., O'Quinn R.P., Pierce H.L., Joglekar A.P., Gall W.E., DeLuca J.G., Carroll C.W., Liu S.-T., Yen T.J., McEwen B.F. Protein architecture of the human kinetochore microtubule attachment site. Cell. 2009;137:672–684. doi: 10.1016/j.cell.2009.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinsky B.A., Nelson C.R., Biggins S. Protein phosphatase 1 regulates exit from the spindle checkpoint in budding yeast. Curr. Biol. 2009;19:1182–1187. doi: 10.1016/j.cub.2009.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu D., Vleugel M., Backer C.B., Hori T., Fukagawa T., Cheeseman I.M., Lampson M.A. Regulated targeting of protein phosphatase 1 to the outer kinetochore by KNL1 opposes Aurora B kinase. J. Cell Biol. 2010;188:809–820. doi: 10.1083/jcb.201001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaguchi S., Decottignies A., Nurse P. Function of Cdc2p-dependent Bub1p phosphorylation and Bub1p kinase activity in the mitotic and meiotic spindle checkpoint. EMBO J. 2003;22:1075–1087. doi: 10.1093/emboj/cdg100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chung E., Chen R.-H. Phosphorylation of Cdc20 is required for its inhibition by the spindle checkpoint. Nat. Cell Biol. 2003;5:748–753. doi: 10.1038/ncb1022. [DOI] [PubMed] [Google Scholar]
- 37.D'Angiolella V., Mari C., Nocera D., Rametti L., Grieco D. The spindle checkpoint requires cyclin-dependent kinase activity. Genes Dev. 2003;17:2520–2525. doi: 10.1101/gad.267603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kushnirov V.V. Rapid and reliable protein extraction from yeast. Yeast. 2000;16:857–860. doi: 10.1002/1097-0061(20000630)16:9<857::AID-YEA561>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.