Abstract
To date, studies suggest that biological signaling by nitric oxide (NO) is primarily mediated by cGMP, which is synthesized by NO-activated guanylyl cyclases and broken down by cyclic nucleotide phosphodiesterases (PDEs). Effects of cGMP occur through three main groups of cellular targets: cGMP-dependent protein kinases (PKGs), cGMP-gated cation channels, and PDEs. cGMP binding activates PKG, which phosphorylates serines and threonines on many cellular proteins, frequently resulting in changes in activity or function, subcellular localization, or regulatory features. The proteins that are so modified by PKG commonly regulate calcium homeostasis, calcium sensitivity of cellular proteins, platelet activation and adhesion, smooth muscle contraction, cardiac function, gene expression, feedback of the NO-signaling pathway, and other processes. Current therapies that have successfully targeted the NO-signaling pathway include nitrovasodilators (nitroglycerin), PDE5 inhibitors [sildenafil (Viagra and Revatio), vardenafil (Levitra), and tadalafil (Cialis and Adcirca)] for treatment of a number of vascular diseases including angina pectoris, erectile dysfunction, and pulmonary hypertension; the PDE3 inhibitors [cilostazol (Pletal) and milrinone (Primacor)] are used for treatment of intermittent claudication and acute heart failure, respectively. Potential for use of these medications in the treatment of other maladies continues to emerge.
I. Introduction
The identification of nitric oxide (NO1), a small gaseous molecule, as a key biological signal was a landmark event in understanding regulation of many physiological functions. NO is composed of one nitrogen atom and one oxygen atom and has a half-life of several seconds (Ignarro, 2005). In 1992, it was named molecule of the year, and in 1998, three scientists, Robert Furchgott, Louis Ignarro, and Ferid Murad, were awarded the Nobel Prize in Physiology or Medicine “for their discoveries concerning nitric oxide as a signaling molecule in the cardiovascular system.” Thus, in contrast to many other molecules whose signaling mechanisms and biological effects have been studied for many years, our understanding of NO-signaling processes is still in its infancy. Despite its molecular simplicity, NO acts as a biological signal in a number of ways (Ignarro et al., 2002; Hofmann, 2005; Ignarro, 2005; Bryan et al., 2009; Foster et al., 2009; Groneberg et al., 2010). NO is the active component released from a number of nitrovasodilators, such as glyceryl trinitrate (nitroglycerin), that are widely used in the clinic for therapeutic relief of chest pain known as angina pectoris; nitroglycerin had been used clinically for many decades before the realization that NO is a natural signaling molecule in the cardiovascular system (Marsh and Marsh, 2000). Nitric oxide produced naturally and that derived from nitrovasodilators, such as nitroglycerin, act via the same molecular mechanisms to regulate functions of smooth muscle cells encircling blood vessels, but effects of NO vary significantly among different types of blood vessels. Moreover, the NO derived from glyceryl trinitrate, unlike that derived from endothelial cells and nerves, is released through a bioactivation process so that the effectiveness of the medication can be affected by the availability of enzymes and other factors required for this step. Effects of NO derived from nitroglycerin are modest in platelets and some other tissues, and tolerance to nitroglycerin is a significant clinical problem; both factors drive the need for alternate drugs that have similar overall actions.
A. Nitric Oxide as a Signaling Molecule
NO is synthesized by the catalytic action of a family of NO synthases (NOS) that convert the precursor amino acid, l-arginine, to NO and l-citrulline (Fig. 1) (Ignarro et al., 1999; Ignarro, 2005; Madhusoodanan and Murad, 2007; Bryan et al., 2009). The three families of NO synthases include the inducible NOS, which is commonly expressed in response to inflammatory stimuli and produces NO that is an important defense against pathogens; neuronal NOS (nNOS), which was first found in neuronal tissues and produces NO that is an important neurotransmitter particularly in nonadrenergic noncholinergic nerves; and endothelial NOS (eNOS), which was first found in endothelial cells and produces NO that acts as a paracrine signal in a number of systems, including the vasculature. NO produced by endothelial cells was initially designated by Robert Furchgott as the “endothelial-derived relaxation factor” (EDRF), which was extensively studied and characterized (Furchgott, 1995, 1998, 1999). Even though it was known that nitrates could produce relaxation of vascular smooth muscle similar to that observed with EDRF, it took many years to determine that EDRF was actually NO (Furchgott et al., 1987; Ignarro et al., 1987, 1988; Palmer et al., 1987; Buga et al., 1989; Furchgott, 1998; Ignarro, 1999, 2005).
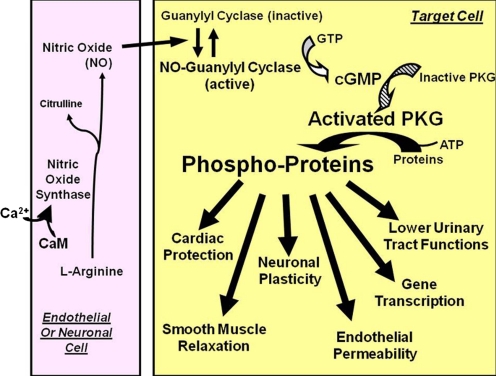
Fig. 1.
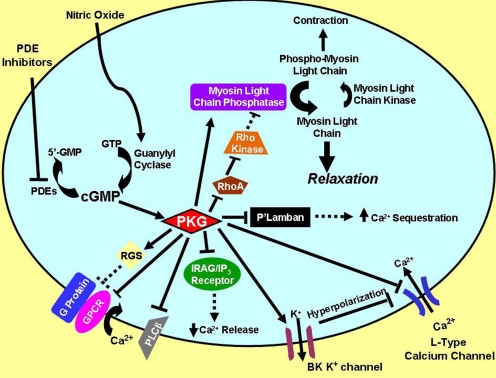
Nitric oxide signaling through cGMP. NO is synthesized from l-arginine by NO synthases located in neuronal, endothelial, or other cells. Calcium that enters the cell complexes with calmodulin and activates the synthases. The NO produced then diffuses through the intercellular space and traverses the cell membrane of a nearby target cell. Therein, NO binds to and activates NO-guanylyl cyclase, which increases synthesis of cGMP from GTP and results in activation of PKG phosphotransferase activity. These processes initiate a cascade of reactions that are amplified at each step as shown by increasingly larger arrows and text. CaM, calmodulin. [Adapted from Francis SH and Corbin JD (2005) Phosphodiesterase-5 inhibition: the molecular biology of erectile function and dysfunction. Urol Clin North Am 32:419–429. Copyright © 2005 Elsevier Ltd. Used with permission.]
The restricted monikers given to NOS isozymes are based on the tissues in which they were initially discovered, but they are expressed in many tissues. Both nNOS and eNOS are expressed constitutively, exhibit low basal activity, and are stimulated by calcium influx into the cell and calcium/calmodulin binding. Activities of these enzymes are regulated by several mechanisms, including phosphorylation, nitrosylation, interaction with other proteins, cofactor/substrate availability, and changes in transcription (Butt et al., 2000; Alderton et al., 2001; Fleming and Busse, 2003; Mitchell et al., 2005; Cary et al., 2006; Erwin et al., 2006; Fisslthaler and Fleming, 2009).
NO is produced and released from many cell types in the body, where it acts either as a neurotransmitter or as a paracrine agent. eNOS is highly expressed in endothelial cells, which is apparently the major source of plasma NO (Walter and Gambaryan, 2009). NO synthesis and release from endothelial cells is increased in response to mechanical shear stress of blood passing over the cell surface and to release of acetylcholine and perhaps other neurotransmitters and stimuli. NO is also released from neuronal cell terminals as a neurotransmitter in response to various depolarizing stimuli. NO extruded into the intercellular space from both origins then traverses the plasma membrane of nearby cells, where it acts as a signal to alter functions of target proteins and biological processes (Fig. 1). NO induces changes in target protein functions directly by binding covalently to tyrosines and cysteines (Foster et al., 2009) on those proteins or forming complexes with heme groups associated with those proteins (e.g., the NO-activated guanylyl cyclase (NO-GC) (Ignarro, 1999).
NO at nanomolar levels binds tightly to a prosthetic heme on the β-subunit of NO-GC, also known as the soluble guanylyl cyclase, and causes a 100- to 200-fold activation of the enzyme (Fig. 1) (Ignarro et al., 1982; Stone and Marletta, 1996; Friebe and Koesling, 2003; Russwurm and Koesling, 2004; Mullershausen et al., 2005; Cary et al., 2006; Derbyshire and Marletta, 2009). Activation of NO-GC increases conversion of GTP to cGMP, resulting in elevation of cGMP, which initiates the cGMP-signaling pathway and subsequent physiological changes (Waldman and Murad, 1988; Furchgott and Jothianandan, 1991; Bryan et al., 2009). Some reports demonstrate that a second molecule of NO may affect NO-GC functions by binding to an unknown site on the protein (Cary et al., 2006). Dissociation of NO from NO-GC or change in the redox status of the heme moiety rapidly reverses NO-GC activation. A number of compounds that activate NO-GC have been developed with hopes for clinical use. Activation of NO-GC by some of these (e.g., BAY 41-2272) is dependent on the heme moiety and synergizes with effects of NO. Activation by other compounds occurs via a NO-independent, heme-dependent action or a NO- and heme-independent process (e.g., BAY 58-2667) (Straub et al., 2001; Stasch et al., 2002; Schmidt et al., 2003; Egemnazarov et al., 2009; Stasch and Hobbs, 2009).
Relaxation of vascular and gastrointestinal smooth muscle, inhibition of platelet aggregation, blunting of cardiac hypertrophy, protection against ischemia/reperfusion damage of the heart, and improvement in cognitive functions are among the myriad physiological processes that are apparently regulated by NO-induced elevation of cellular cGMP. Evidence suggests that these effects are largely mediated through activation of cGMP-dependent protein kinase I (PKGI) isozymes (Hofmann, 2005; Lohmann and Walter, 2005; Lincoln et al., 2006; Mullershausen et al., 2006; Kass et al., 2007a; Hofmann et al., 2009). cGMP-gated channels, cyclic nucleotide (cN) phosphodiesterases (PDEs), and PKGII are also important targets for cGMP actions.
NO as the “first messenger” in the NO/cGMP/PKG signaling pathway initiates a cascade of phosphorylation reactions in which the magnitude of each step is enzymatically amplified, a process that is critical for the resulting physiological effects (Fig. 1). Even if sufficient NO is generated by endothelial and/or neuronal cells, an imbalance in the rates of cGMP synthesis and degradation, dysfunction or reduced levels of proteins mediating steps in the cGMP-signaling pathway, and other processes can impair the physiological response. In many forms of vascular disease, there is an imbalance among steps in the pathway. Low levels of NO production caused by endothelial dysfunction is a widespread medical problem that occurs in patients with metabolic syndrome, hypertension, hypercholesterolemia, diabetes, and other maladies (Celermajer et al., 1993; Musicki and Burnett, 2007; Gratzke et al., 2010). In other instances (e.g., restenosis of blood vessels or penile priapism), levels and functions of target proteins such as PKG or PDEs are altered (Celermajer et al., 1993; Kugiyama et al., 1996; Lincoln et al., 2001; Champion et al., 2005). A thorough understanding of the NO/cGMP/PKG signaling pathway and the characteristics and functions of proteins involved in this pathway is required for maximizing potential for innovative pharmacological interventions that could ameliorate these maladies.
B. Biological Importance of Nitric Oxide/cGMP Signaling through cGMP-dependent Protein Kinases
The cGMP/PKG signaling pathway was initially thought to be restricted in tissue distribution and physiological actions. That concept has been challenged by the many reports that document the role of this signaling pathway in diverse tissues, even in tissues in which overall levels of the signaling components are very low (Hofmann, 2005; Hofmann et al., 2009). The biological importance of NO/cGMP/PKG signaling was first appreciated for promoting vascular smooth muscle relaxation and platelet disaggregation (Walter, 1989; Murad et al., 1992; Warner et al., 1994; Murad, 1996; Feletou et al., 2008; Walter and Gambaryan, 2009; Gratzke et al., 2010). Effects of NO/cGMP/PKG signaling on differentiation/proliferation of vascular smooth muscle in response to growth factors, vasoactive peptides, physical damage and other stimuli have also been clearly demonstrated. However, these effects are still poorly understood and seem to vary depending on the vessels from which the cells originate, conditions under which studies are conducted (primary cultures versus passaged cells), stage of cell differentiation, and the challenge/stimulus used (Dumitrascu et al., 2006; Lincoln et al., 2006; Bouallegue et al., 2007; Lukowski et al., 2008; Weinmeister et al., 2008; Hofmann et al., 2009).
Creation of eNOS-null mice (Friebe et al., 2007), nNOS-null-mice (Huang et al., 1993), and PKGI- and PKGII-null mice (both global and tissue-specific knockouts) (Pfeifer et al., 1998; Hofmann, 2005; Hofmann et al., 2009) has provided a powerful set of tools for dissecting PKG functions. Results from studies with these animals in conjunction with the use of traditional pharmacological tools continue to provide insights into NO/cGMP actions. The roles of PKGI isozymes have been documented in many processes including gastrointestinal motility, blood flow, neuronal plasticity, erectile function, lower urinary tract functions, endothelial permeability, and cardiac protection (Lincoln et al., 1995; Sausbier et al., 2000; Rybalkin et al., 2002; Shimizu-Albergine et al., 2003; Qin et al., 2004; Tegeder et al., 2004; Hofmann, 2005; Takimoto et al., 2005b; Fiedler et al., 2006; Agostino et al., 2007; Costa et al., 2008; Hofmann et al., 2009; Kleppisch and Feil, 2009; Salloum et al., 2009). Appreciation of the importance and complexity of the actions of NO/cGMP/PKGI signaling in diverse vascular tissues is driving the search for improved new therapies for systemic hypertension, cardiac failure, cardiac reperfusion injury, vascular smooth muscle proliferation, atherogenesis, endothelial dysfunction, and Raynaud's disease. Renewed efforts are also afoot to identify drugs and treatment strategies that influence this signaling pathway because of the potential for pharmacological relief of malfunctions in nonvascular cell types as well (Sandner et al., 2007; Bryan et al., 2009; Egemnazarov et al., 2009; Hofmann et al., 2009; Kleppisch and Feil, 2009; Krieg et al., 2009; Lapp et al., 2009; Reaume and Sokolowski, 2009). Effective medications that foster NO/cGMP/PKGI signaling in penile and pulmonary vascular beds have already provided the lead to major advances and hold promise for treatment of other maladies.
C. Cellular cGMP Production and Breakdown
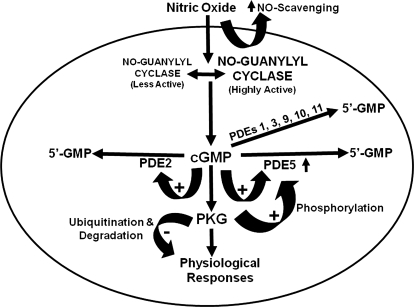
Synthesis of cGMP in tissues is catalyzed by two classes of GCs, the NO-GC and GCs that are linked to receptors activated by peptide agonists (Wedel and Garbers, 1998; Potter et al., 2009); only the NO-GC will be discussed herein. NO-GC is largely cytosolic, although a portion is associated with the particulate fraction in certain cells, and it is thought to primarily, but not exclusively, generate cytosolic cGMP pools (Russwurm et al., 2001; Castro et al., 2006; Piggott et al., 2006). cGMP level in the whole cell or in specific intracellular pools is primarily determined by the balance between activities of the GCs and cN PDEs that break down cGMP (Fig. 2). Cyclic nucleotides can be exported from cells by the action of some members of the ATP-dependent multidrug resistance transporter protein family (Jedlitschky et al., 2000, 2004), but where this has been studied, the quantitative contribution of cN export to lower cellular cN level compared with that of PDE action is small (Barber and Butcher, 1981). Herein, we focus on the roles of PKGI isozymes and cGMP-hydrolyzing PDEs in mediating effects of NO-induced cGMP signaling in mammalian tissues. The role of cGMP signaling initiated by activation of particulate GCs in many physiological processes is well established (Potter et al., 2009). It is also clear that, in some instances, physiological responses due to increased synthesis of cGMP by particulate and NO-GCs intersect and/or overlap and that downstream effects may be mediated/impacted by some of the same proteins (e.g., PKGs, PDEs, and cation channels). However, consideration of actions elicited by particulate GCs is beyond the scope of this review.
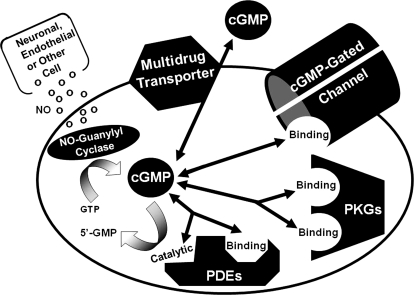
Fig. 2.
Cellular targets of cGMP. Multiple intracellular proteins can interact with cGMP, including the cGMP-gated cation channel, PKG, allosteric sites and catalytic sites on certain PDEs, and certain multidrug transporter proteins. The level of cellular cGMP is determined largely by the balance between its synthesis by guanylyl cyclase and breakdown by PDEs, although the transporters may play a role in some cells. The different shapes of the pockets on the respective proteins indicate that the catalytic sites of PDEs (shown as half-diamonds), the allosteric sites of PDEs (shown as half-octagons) and the allosteric sites on PKG and cation channels (shown as half-circles) are structurally and evolutionarily unrelated. The affinity of the multidrug transporter for cGMP is low, and its role in exporting cGMP from the cell is minor compared with the action of PDEs.
II. Intracellular Mediators of Nitric Oxide/cGMP Action
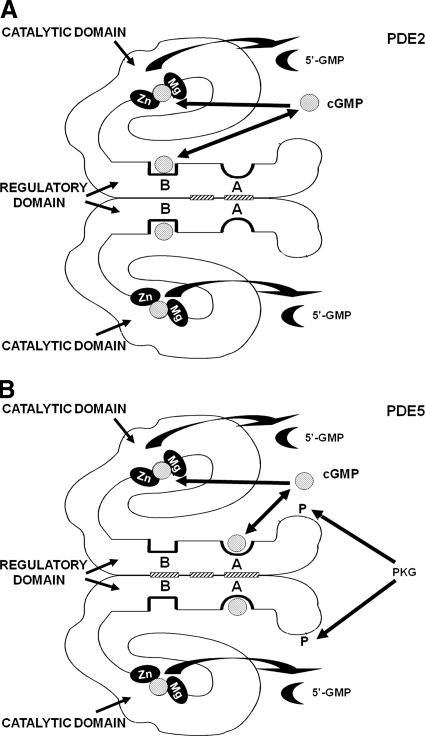
Cellular proteins that are directly targeted by cGMP and participate in cGMP-signaling include PKGs, cGMP-gated cation channels, cGMP-hydrolyzing PDEs, and PDEs that contain allosteric cGMP-binding sites (Figs. 2 and 3); the affinities of these sites for cGMP vary and, in some instances, are modulated by phosphorylation or other modifications (Francis et al., 2005). The cGMP-binding sites on PKGs and cGMP-gated cation channels are homologous to the cAMP-binding site in the catabolite gene-activator protein (CAP) and those in the cAMP-dependent protein kinases (PKA) and exchange proteins activated by cAMP (EPACs), whereas the catalytic and allosteric cGMP-binding sites on PDEs are evolutionarily unrelated to either the CAP-related sites or each other (Fig. 3) (Francis et al., 2005). Each type of site has distinct structural topography and novel interactions with cGMP that have fostered development of selective activators or inhibitors (Sekhar et al., 1992; Thomas et al., 1992; Butt et al., 1994b; Beltman et al., 1995; Wu et al., 2004; Ke and Wang, 2007; Zoraghi et al., 2007; Heikaus et al., 2008; Martinez et al., 2008; Poppe et al., 2008). Differences in the sites have been determined from 1) amino acid sequences, 2) X-ray crystal and NMR structures, 3) site-directed mutagenesis, 4) cGMP analog specificity, and 5) kinetic characteristics, such as cGMP dissociation rates.
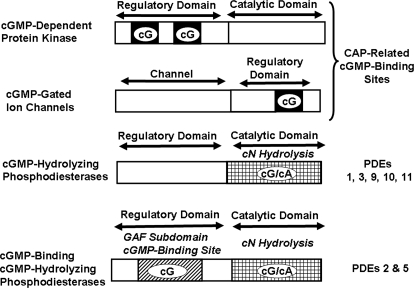
Fig. 3.
Comparison of structures of major intracellular cGMP receptors. Images demonstrate that PKGs have two allosteric cGMP-binding sites compared with one cGMP-binding site on the subunits of the cGMP-gated channels; the sites on the PKG and the channels belong to the CAP family of cN-binding sites. cGMP-hydrolyzing PDEs contain a catalytic site at which cGMP is converted to 5′-GMP, and some of these PDEs contain an allosteric cGMP-binding site that is located in one of the GAF subdomains found in these proteins. The term GAF is the acronym for a protein domain conserved in cGMP-binding PDEs/Anabaena adenylyl cyclases, and Escherichia coli FhlA.
A. Cross-Talk among Cyclic Nucleotide Targets
Unless selectivity for cGMP versus cAMP is extremely high, there is typically some interaction of cGMP with sites that prefer cAMP and interaction of cAMP with sites that prefer cGMP (Jiang et al., 1992; Lincoln et al., 1995; Wu et al., 2004). This heterologous binding can significantly affect physiological events that occur after elevation of either cN and should always be a consideration in interpreting results. Knowledge of the types and levels of PKAs, PKGs, and cN PDEs in a cell is critical when considering these possibilities, because NO-induced increases in cGMP may act through PKGs, PKAs, or dual-specificity PDEs (i.e., PDEs that break down both cNs). Use of a collection of pharmacological agents such as selective inhibitors or activators for these proteins is helpful in developing a more accurate picture of the pathway involved, but use of such reagents has inherent limitations (Burkhardt et al., 2000; Beavo et al., 2006; Taniguchi et al., 2006; Vandeput et al., 2009). In addition, variations in the NO signal, the persistence of NO-induced changes in the phosphorylation or expression of certain proteins, and the sensitivity of cells to subsequent NO challenges may vary depending on the composite of stimuli that the cell experiences as well as the particular cell type being studied. The cGMP concentration in the basal state or after NO exposure undoubtedly varies among cell types, and the pattern, time, and quantitative exposure to endogenously generated NO for a given cell also varies. As a result, cGMP in some cells may readily reach levels that would activate cAMP-signaling pathways. Vascular smooth muscle cells and platelets undergo sustained exposure to endothelially derived NO with localized bursts of NO; the degree of NO exposure can vary depending on sheer stress, oxidative exposure, and other factors. Vascular smooth muscle cells in the penile corpus cavernosum also receive extended exposure to a low level of endothelially derived NO, but the entire vasculature of the corpus cavernosum experiences sporadic tissue-wide surges of neuronally derived NO during sexual arousal (Burnett, 2006; Gratzke et al., 2010).
1. Cross-Talk among Cyclic Nucleotide-Dependent Protein Kinases.
PKG and PKA are homologous proteins and despite exhibiting 50- to 200-fold selectivity for cGMP and cAMP, respectively, each can also be activated by the other cN within physiological ranges of that nucleotide (Francis et al., 1988; Butt et al., 1992; Jiang et al., 1992; Lincoln et al., 1995; Francis and Corbin, 1999; Hofmann et al., 2009). In all instances, phosphotransferase activity of the respective kinase is increased, and in a few instances, the biological action results at least in part from cross-activation of the “other” kinase. There are many well documented examples of this “cross-activation” in biological processes (Jiang et al., 1992; Kurjak et al., 1999; White et al., 2000; Sellak et al., 2002; Barman et al., 2003; Browner et al., 2004; Burnette and White, 2006; Wörner et al., 2007). However, cross-activation does not seem to occur in some cells; its incidence may reflect effects of selective subcellular microdomains and compartmentation of proteins involved in these pathways (Massberg et al., 1999; Weber et al., 2007).
2. Cross-Talk among Cyclic Nucleotide Phosphodiesterases.
cGMP and cAMP compete for catalytic sites of PDEs that hydrolyze both cNs (Bender and Beavo, 2006a; Conti and Beavo, 2007). Several reports support cross-talk between cGMP and cAMP at PDE catalytic sites (e.g., PDE3 isozymes) that have modest cN selectivity (Maurice and Haslam, 1990; Jang et al., 1993; Aizawa et al., 2003; Surapisitchat et al., 2007). In almost all cells, basal cAMP level significantly exceeds that of cGMP. Consequently, an increase in cGMP could more effectively compete with cAMP for interaction with these sites. This would decrease breakdown of cAMP by dual-specificity PDEs, such as PDE1, PDE2, PDE3, PDE10, or PDE11, resulting in cAMP elevation and increased signaling through cAMP/PKA/EPAC-signaling pathways (Han et al., 1999; Maurice, 2005). Several reports suggest that this occurs after modest elevation of cGMP in endothelial cells or platelets (Maurice and Haslam, 1990; Maurice, 2005; Surapisitchat et al., 2007). In endothelial cells that contain significant amounts of PDE2 and PDE3, a modest elevation of cAMP blunts thrombin-induced permeability, and low concentrations of NO or atrial natriuretic peptide that elevate cGMP slightly potentiate this effect (Surapisitchat et al., 2007); cGMP seems to enhance cAMP level and cAMP signaling by competing for the PDE3 active site. However, treatments that produce a large increase in cGMP in these cells or over-expression of PDE2 counters the cAMP-mediated decrease in permeability; results suggest that the higher level of cGMP acts by binding to an allosteric site on PDE2 that activates PDE2-mediated breakdown of cAMP in these cells. Elevation of cAMP is unlikely to affect catalytic function in such cGMP-specific PDEs as PDE5, PDE6, and PDE9, which have much higher affinity for cGMP than for cAMP. cGMP also binds to an allosteric site in PDE5 and activates PDE5 catalytic function; this site in PDE5 is highly selective for cGMP, so it is unlikely that changes in cAMP would significantly affect its interaction with cGMP. However, the allosteric cGMP-binding site in PDE2 is less selective and may bind cAMP in some circumstances (Thomas et al., 1990a; Wu et al., 2004; Francis et al., 2006).
B. cGMP-Dependent Protein Kinase I
The two PKG families (PKGI and PKGII) are derived from separate genes (prkg1 and prkg2). Only the PKGI family is discussed herein because its members are more commonly involved when cGMP signaling is mediated by NO. In some instances, however, PKGI isoenzymes can mediate the effects of cGMP elevation produced by particulate GCs. PKGI isozymes (PKGIα and PKGIβ) are products of alternative splicing and differ only in the N-terminal ∼100 amino acids (Wernet et al., 1989; Francis and Corbin, 1999; Francis et al., 2005; Hofmann, 2005). Both bind two cGMP molecules per monomer and have similar preferences (kcat and Km) for phosphorylation of synthetic peptide substrates, although there are important differences with certain protein substrates as described below (Surks et al., 1999; Tang et al., 2003; Francis et al., 2005; Schlossmann and Desch, 2009). PKGIα and PKGIβ are commonly coexpressed in varying proportions (Eigenthaler et al., 1992; Sekhar et al., 1992; Geiselhöringer et al., 2004; Hofmann, 2005). In hypotonic lysates of many cells, both isozymes are abundant in the cytosol. However, in platelets, PKGIβ predominates and is almost entirely membrane-bound (Eigenthaler et al., 1992); it is now clear that PKGI isozymes can segregate into specific compartments in numerous cell types, but the mechanisms providing for these localizations are not fully understood. The sequence of the divergent ∼100 N-terminal amino acids of PKGIα and PKGIβ affect 1) cGMP affinity, 2) cN analog selectivity, 3) protein-substrate specificity, 4) state of activation, and 5) subcellular localization (Wolfe et al., 1989b; Sekhar et al., 1992; Ruth et al., 1997; Surks et al., 1999; Ammendola et al., 2001; Richie-Jannetta et al., 2003; Tang et al., 2003; Francis et al., 2005).
1. Interaction with cGMP.
cGMP binds to allosteric sites in the PKG regulatory domain and increases phosphotransferase activity 3- to 10-fold (Lincoln et al., 1977; Wolfe et al., 1989a; Francis and Corbin, 1999; Hofmann et al., 2009; Schlossmann and Desch, 2009). Unlike PKA isozymes, the more N-terminal cN-binding site in PKGI isozymes has higher affinity for cGMP than does the more C-terminal site (Reed et al., 1996, 1997). PKGI isozymes are found in particular subcellular membrane fractions, in complex with certain cytosolic proteins, and as free cytosolic proteins; they are also reported to reversibly translocate among cellular compartments after changes in cGMP level (Pryzwansky et al., 1990, 1995; Cornwell et al., 1991; Wyatt et al., 1991; Surks et al., 1999; Yuasa et al., 1999, 2000b; Geiselhöringer et al., 2004; Fiedler et al., 2006; Antl et al., 2007; Stout et al., 2007; Zhang et al., 2007a; Casteel et al., 2008; Sharma et al., 2008; Wilson et al., 2008; Takimoto et al., 2009). The current consensus is that cGMP concentration may vary substantially in different cellular compartments as a result of a variety of factors (e.g., selective localization of the proteins that provide for cGMP synthesis, breakdown, or extrusion) that confine certain pools of cGMP to particular areas of the cell. Activation of the respective PKGs located within those microdomains is predicted to vary accordingly (Castro et al., 2006; Fischmeister et al., 2006; Piggott et al., 2006; Takimoto et al., 2007). Moreover, the extent of cGMP elevation or PKGI activation that is required to elicit a cellular response is still not known.
Despite having allosteric cGMP-binding sites that are identical in amino acid sequence, PKGIα affinity for cGMP (Kd ∼0.1 μM) is ∼10-fold greater than that of PKGIβ (Kd∼ 0.5–1.0 μM), and cN analog specificities differ substantially (Wolfe et al., 1989a; Sekhar et al., 1992; Poppe et al., 2008). Some cN analogs (e.g., 1,N2-phenyletheno-cGMP) are more potent PKGI activators than cGMP, and others, (e.g., 8-bromo-β-phenyl-1,N2-ethenoguanosine-3′,5′-cyclic monophosphorothioate, Rp-isomer) bind to the allosteric site but only partially activate catalysis; the latter compound is commonly used as a PKGI inhibitor because it blocks access of the more effective activator, cGMP, to the binding sites (Sekhar et al., 1992; Butt et al., 1994b; Taylor et al., 2004; Poppe et al., 2008; Valtcheva et al., 2009). Cyclic nucleotide analogs have proved to be highly useful tools in investigating biological effects mediated by PKAs, EPACs, or PKGs; the analogs freely traverse the cell membrane to directly act on the target proteins, thereby circumventing involvement of proteins that generate the signaling molecule, factors involved in delivery of the signal to the target cell, specific membrane receptors, or the adenylyl or guanylyl cyclases that produce the cNs (Beebe et al., 1988a,b; Francis et al., 1988; Christensen et al., 2003; Dao et al., 2006; Poppe et al., 2008). The pattern of potencies with which a collection of cN analogs elicits a biological response is a powerful indicator of the intracellular receptor that mediates the effect. Several analogs that strongly select for PKGIα over PKGIβ have been reported, but analogs that are adequately selective for PKGIβ have not been found (Sekhar et al., 1992). Some cN analogs that potently activate PKGs are resistant to hydrolysis by PDEs and, in many cases, act as potent inhibitors of particular PDEs. The potential for development of drugs based on the dual action of these types of analogs is well worth consideration, because they would act at two steps in the pathway to synergistically increase signaling.
The striking difference in affinities of PKGIα and PKGIβ for cGMP may have implications for distinct physiological roles of these enzymes, but selective actions based on this difference have not been convincingly demonstrated (Weber et al., 2007). It cannot be ruled out that the difference in affinity for cGMP of the isozymes measured in vitro is due to a missing cellular factor that modulates the affinities in vivo. If this difference in affinity for cGMP exists in cells, this could provide for a progressive increase in PKGI catalytic activity over a broad range of cGMP concentration. Such a continuous increase in PKGI-mediated phosphotransferase activity could potentially target different substrates with PKGIα phosphorylating substrates at lower level of cGMP and PKGIβ phosphorylating others at higher level of cGMP. Under basal conditions in which cGMP is low or after a slight increase in cGMP synthesis, the greater affinity of PKGIα for cGMP could provide for sufficient activation of this isozyme to modulate certain functions without incurring significant activation of PKGIβ. Phosphorylation of some substrates is apparently restricted to PKGIβ action [e.g., the inositol 1,4,5-trisphosphate (IP3) receptor-associated PKG substrate (IRAG) (Ammendola et al., 2001; Schlossmann and Desch, 2009) and the multifunctional transcriptional regulator (TFII-I) (Casteel et al., 2005) (see section II.D)]. Assuming that the PKGI affinities determined in vitro reflect those in the intact cell, it would be predicted that in the absence of compartmentation, these PKGIβ-mediated phosphorylations would occur only at relatively high cGMP levels and after complete activation of PKGIα. A broad range of PKG sensitivity to cGMP is supported by studies of PKG function in cerebral arteries using DT-2, a specific PKG peptide inhibitor (Dostmann et al., 2000; Taylor et al., 2004). Even in the absence of agents to increase cGMP, DT-2 treatment increases basal tone consistent with blockage of a low level of PKG activity. At the cGMP level found in arteries under basal conditions (∼0.1 μM), only significant activation of PKGIα, not of PKGIβ, would be predicted (Francis et al., 1988; Wolfe et al., 1989a). Likewise, basal PKGIα activity has been suggested to blunt the rise in intracellular calcium in response to thrombin receptor stimulation (Christensen and Mendelsohn, 2006).
2. Structural Features.
PKGI monomers contain a regulatory domain that is located in the more N-terminal portion of the protein and a catalytic domain that is located in the more C-terminal portion; each contains multiple subdomains that provide for specific functions (Lincoln et al., 1977; Monken and Gill, 1980; Takio et al., 1984; Wernet et al., 1989; Wolfe et al., 1989a; Francis et al., 2005; Hofmann, 2005; Hofmann et al., 2009). In the regulatory domain, these include the following: 1) a dimerization and localization subdomain provided by an extended leucine zipper motif, 2) overlapping autoinhibitory and autophosphorylation subdomains, and 3) a cGMP-binding subdomain containing two homologous cGMP-binding sites arranged in tandem. In the catalytic domain, there are two major subdomains: 1) a subdomain that binds Mg2+/ATP and 2) a protein substrate-binding subdomain (Fig. 4).
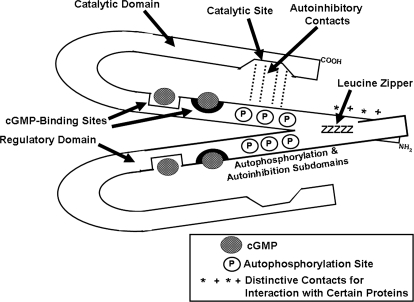
Fig. 4.
Working model of PKGI. PKGI isozymes are homodimers that are dimerized by an extended leucine zipper region (ZZZZZ). Unique faces of the zipper regions that provide for selective homodimerization of PKGIα and PKGIβ and their selective interaction with specific cellular proteins are indicated by the combined asterisks and plus symbols (* + * +). Multiple sites of autophosphorylation are indicated by encircled P and multiple autoinhibitory contacts are indicated by dashed lines (- - - - -). The cGMP-binding sites are homologous, but the more amino-terminal site has higher affinity for cGMP, indicated by the heavy dark semicircle.
The respective leucine/isoleucine zipper motif at the N terminus of each PKGI monomer provides for high-affinity homodimerization (Richie-Jannetta et al., 2003; Francis et al., 2005; Sharma et al., 2008), selective interaction with particular proteins, and subcellular localization in some instances (Gudi et al., 1997; Surks et al., 1999; Yuasa et al., 1999; Schröder et al., 2003; Fiedler et al., 2006; Antl et al., 2007; Zhang et al., 2007a; Casteel et al., 2008; Sharma et al., 2008; Wilson et al., 2008). Features provided by the leucine/isoleucine zipper and other residues in the N-terminal subdomain also improve affinity for cGMP binding and influence cGMP-analog specificity (Wolfe et al., 1989b; Ruth et al., 1991, 1997; Richie-Jannetta et al., 2003). In PKGIα, the leucine/isoleucine zipper involves five heptad repeats that are stabilized by hydrophobic residues and an extensive network of hydrogen bonds (Sharma et al., 2008). The leucine/isoleucine zipper in PKGIβ incudes eight heptad repeats; all eight repeats are involved in dimerization, but six repeats are adequate to mediate dimerization in vitro (Richie-Jannetta et al., 2003). Although the respective leucine/isoleucine zippers provide for homodimerization and affect PKGI affinity for cGMP, the residues/structures that provide for these processes and those that create motifs that selectively target PKGIα and PKGIβ to particular proteins or subcellular compartments differ.
In some instances, the unique chemical signatures on the faces of the respective leucine zippers of PKGI isozymes provide for interaction with cGMP-dependent protein kinase-interacting proteins (GKIPs) that can localize PKG to certain regions of the cell and that may also be PKG substrates (Fig. 4). The GKIP acronym is employed because PKGI-interacting proteins are not always anchors in the context of permanently tethering PKG to a subcellular domain. Characteristics of GKIPs differ significantly from those of the well studied anchoring proteins for PKA (AKAPs) (Gold et al., 2006; Carnegie et al., 2008; Carnegie et al., 2009), and the two groups of proteins do not appear to be entirely parallel in function. Almost all known GKIPs are PKGI substrates, and unlike in AKAPs, common motifs in GKIPs or their PKG partners that provide for interaction have not been convincingly defined. Moreover, in some instances, association of a PKGI with a GKIP requires activation by cGMP and perhaps translocation, features that are also unlike the dynamics involved in many PKA and AKAP interactions. The requirement for cGMP activation of PKGI in certain instances suggests that the GKIP-interactive face of the respective leucine zippers in PKGs is either sequestered in the inactive state or undergoes a conformational change induced by enzyme activation; alternatively, cGMP binding could expose a new interface (other than the leucine zipper) on PKG that then binds to particular GKIPs (Surks et al., 1999; Ammendola et al., 2001; Casteel et al., 2005; Fiedler et al., 2006; Zhang et al., 2007a; Sharma et al., 2008). Reversal of these cGMP-dependent interactions would require a decline in cGMP, cGMP dissociation from the PKG, and reversal of the purported conformational change. The list of GKIPs continues to grow, and PKGI substrate GKIPs are listed in Table 1; they include the myosin-binding subunit of myosin light-chain phosphatase (Surks et al., 1999), TFII-I (Casteel et al., 2005), IRAG (Ammendola et al., 2001; Schlossmann and Desch, 2009), phosphodiesterase-5 (Thomas et al., 1990b), vimentin (Wyatt et al., 1991; Pryzwansky et al., 1995), troponin (Yuasa et al., 1999), the regulator of G-protein signaling-2 (RGS2) (Tang et al., 2003; Osei-Owusu et al., 2007), formin homology domain protein-1 (Wang et al., 2004), and cysteine-rich protein 2 (Huber et al., 2000; Zhang et al., 2007a).
TABLE 1.
Putative physiological substrates phosphorylated by PKGI and their functions
| Substrate | Function of Substrate Phosphorylation | Reference(s) |
|---|---|---|
| ATP-sensitive K+ channel | Increases channel activity | Han et al., 2001 |
| Bad | Protects against neuronal apoptosis | Johlfs and Fiscus, 2010 |
| Battenin (CLN3) | Targets this lysosomal protein to substrates | Michalewski et al., 1998 |
| β3-adrenergic receptor | Negates receptor effects, causing relaxation | Angelone et al., 2008 |
| Ca2+ channel (voltage-dependent; Cav1.2 L-type) α1C subunit | Affects cardiac action potential duration; modulates pacemaker activity; inhibits channel activity | Yang et al., 2007 |
| Ca2+ channel (voltage-dependent; Cav1.2 L-type) β2 subunit | Affects cardiac action potential duration; modulates pacemaker activity; inhibits channel activity | Yang et al., 2007 |
| Ca2+ channel, L-type, α1c subunit | Inhibits Ca2+ current | Jiang et al., 2000 |
| Ca2+-activated K+ channel (α-subunit, cslo-α) | Opens channel, causing muscle relaxation | Fukao et al., 1999 |
| Ca2+-sensitive K+ channel (Hslo) | Activates channel | Alioua et al., 1998 |
| Calponin homology-associated smooth muscle protein (CHASM) | Desensitizes protein to Ca2+ | Borman et al., 2004 |
| cAMP-dependent protein kinase regulatory subunit type I (PKA RI) | Disrupts inhibitory interaction with catalytic subunit; affects cAMP binding | Geahlen et al., 1981 |
| cGMP-dependent protein kinase anchoring protein 42 (GKAP42) | Promotes germ cell developmenta | Yuasa et al., 2000b |
| cGMP-dependent protein kinase type I-α (PKGIα) | Relieves autoinhibition; sensitizes kinase to cAMP and cGMP binding; targets PKGIα for ubiquitination | Hofmann and Flockerzi, 1983; Busch et al., 2002; Dey et al., 2009 |
| cGMP-dependent protein kinase type I-β (PKGIβ) | Increases basal activity; increases sensitivity to cGMP and cAMP; controls kinase localization | Smith et al., 1996; Collins and Uhler, 1999 |
| Cysteine-rich protein 2 (CRP2) [also known as cysteine-rich LIM-only protein 4 (CRP4)]b | Regulates cytoskeletal organizationa and pain perception; increases transcription of smooth-muscle related genes | Huber et al., 2000; Zhang et al., 2007; Schmidtko et al., 2008 |
| Cystic fibrosis transmembrane conductance regulator (CFTR) | Activates Cl− channel | Picciotto et al., 1992 |
| Formin homology-domain containing protein-1 (FHOD-1) | Affects cytoskeletal arrangement | Wang et al., 2004 |
| GABA-A receptor | —a | Leidenheimer et al., 1996; Nugent et al., 2009 |
| G-septin | Facilitates neuronal signal transductiona | Xue et al., 2000 |
| G-substratec | Participates in cerebellar function; activates protein phosphatase inhibitor; promotes long-term depression | Aitken et al., 1981; Endo et al., 1999, 2003 |
| Guanylyl cyclase, soluble | Desensitizes guanylyl cyclase to activators | Zhou et al., 2008 |
| 27-kDa Heat shock protein (HSP27) | Effects platelet function | Butt et al., 2001 |
| 60- and 70-kDa Heat shock proteins (HSP60, HSP70) | Prevents apoptosis; inhibits peroxynitrite production | Li et al., 2005; Chan et al., 2007 |
| Histone | —a | Glass and Krebs, 1979, 1982 |
| Inositol triphosphate receptor (IP3R) | Decreases Ca2+ release from IP3-receptive storehouses | Haug et al., 1999; Murthy and Zhou, 2003 |
| Inositol triphosphate receptor-associated PKG substrate isoform A (IRAGa)c | Decreases Ca2+ release from IP3-receptive storehouses | Schlossman et al., 2000; Ammendola et al., 2001 |
| IRAGb | Decreases Ca2+ release from IP3-receptive storehouses | Casteel et al., 2008 |
| Large conductance calcium-activated potassium channel (BKCa)c | Increases probability of channel opening; hyperpolarizes cells | Sausbier et al., 2000 |
| LIM and SH3 domain protein (LASP-1) | Induces translocation from membrane to cytosol; reduces interaction with actin | Butt et al., 2003; Keicher et al., 2004 |
| MEKK1 | Activates MEKK1 kinase activity | Soh et al., 2001 |
| Myosin phosphatase small regulatory subunit (M20) | Impedes myosin phosphatase interaction with phospholipids | Nakamura et al., 1999 |
| Myosin phosphatase large regulatory subunit (MYPT1)c | Impedes myosin phosphatase interaction with phospholipids; inhibits Rho kinase thereby inhibiting myosin phosphatase; decreases Ca2+ sensitization | Nakamura et al., 1999; Wooldridge et al., 2004 |
| Na+/K+ ATPase α-subunit | Stimulates ATPase activity | Fotis et al., 1999 |
| NC-1.1 receptor | Regulates natural cytotoxicitya | Holmgreen et al., 1997 |
| p21-activated kinase 1 (Pak) | Regulates cell morphology and Pak localization | Fryer et al., 2006 |
| Phosphodiesterase 5c | Increases cGMP hydrolysis; regulates cGMP binding to allosteric and catalytic binding sites | Corbin et al., 2000; Murthy et al., 2001; Rybalkin et al., 2002; Wilson et al., 2008 |
| Phospholambanc | Increase Ca2+ uptake by Serca [sarco(endo)plasmic reticulum Ca2+-ATPase] | Lalli et al., 1999 |
| Phospholipase A2 (PLA2) | Protects against PLA2-induced cell death | Chalimoniuk et al., 2009 |
| Phospholipase C β2 (PLCβ2) | —a | Xia et al., 2001 |
| Phospholipase C β3 (PLCβ3) | Reduces IP3 production | Xia et al., 2001 |
| pH-sensitive K+ channel | Hyperpolarizes basal forebrain cholinergic neurons | Kang et al., 2007 |
| Protein phosphatase inhibitor 1 (PPI-1) | Inhibits phosphatase activity | Tokui et al., 1996 |
| Rap1 GTP-ase activating protein 2-α (Rap1GAP2α)b | No detected effect in GTPase activity | Schultess et al., 2005 |
| Rap1GAP2βb | No detected effect in GTPase activity | Schultess et al., 2005 |
| Regulator of G protein signaling 2 (RGS2)c | Increases GTPase activity; inhibits IP3 production | Tang et al., 2003; Sun et al., 2005 |
| RGS4 | Decreases GTPase activity and phosphoinositol (PI) hydrolysis | Huang et al., 2007 |
| RhoAc | Decreases RhoA activity; regulates RhoA translocation; decreases contraction; controls vesicle trafficking; reduces myosin light chain (MLC) phosphorylation | Sawada et al., 2001; Ellerbroek et al., 2003; Murthy et al., 2003 |
| Ryanodine receptor | Regulates ryanodine binding to receptor | Takasago et al., 1991 |
| Septin-3b | Regulates septin localizationa | Xue et al., 2004 |
| Serotonin transporter (SERT)b | Increases serotonin (5-HT) uptake | Ramamoorthy et al., 2007; Zhang et al., 2007 |
| Smooth muscle light chain phosphatase (SMPP-1M) | Facilitates relaxation of smooth muscle | Lee et al., 1997 |
| Smoothelin-like protein 1 (SMTNL1) | Affects muscle fiber response to exercise | Wooldridge et al., 2008 |
| Splicing factor 1 | Interferes with pre-mRNA intron splicing | Wang et al., 1999 |
| Steroidogenic acute regulatory protein (StAR) | Increases androgen production | Andric et al., 2007 |
| Telokinc | Inhibits myosin light chain kinase (MLCK) activity; destabilizes myosin; mediates cGMP-induced relaxation | Walker et al., 2001 |
| Thromboxane A2 receptor 1α (TPα)b | Desensitizes receptor to thromboxane stimulationa | Yamamoto et al., 2001; Kelley-Hickie et al., 2007 |
| Titin N2BA and N2B | Affects sarcomere rigidity | Krüger et al., 2009 |
| Transient receptor potential Ca2+ channel isoform 3 (TRPC3)b | Decreases Ca2+ influx | Kwan et al., 2004 |
| Transient receptor potential Ca2+ channel isoform 6 (TRPC6) | Decrease Ca2+ influx | Koitabashi et al., 2010 |
| Troponin I | Desensitizes protein to Ca2+ | Layland et al., 2002 |
| Tyrosine hydroxylase | Decreases catecholamine synthesisa | Rodríguez-Pascual et al., 1999 |
| Vasodilator-stimulated phosphoprotein (VASP) | Regulates actin cytoskeleton and vesicle trafficking; controls K+ efflux from cells through ATP-sensitive channel | Butt et al., 1994; Cook and Haynes, 2007 |
| Vimentinc | Controls cytoskeleton intermediate filament dynamics | Wyatt et al., 1991; Pryzwansky et al., 1995 |
Function unknown or not confirmed.
PKGI substrates identified in heterologous systems (Hofmann et al., 2009).
Established PKGI substrates (Hofmann et al., 2009).
3. Autoinhibition and Activation.
In the absence of cGMP, PKG activity is suppressed by autoinhibitory contacts. Monomeric forms of PKGI generated by N-terminal truncation contain their autoinhibitory sequences and, in the absence of cGMP, exhibit low catalytic activity like that in full-length dimeric PKGs (Wolfe et al., 1989b). Catalytic site residues directly contact an autoinhibitory subdomain that is located ∼50 to 75 residues from the N terminus (Francis et al., 1996; Francis et al., 2005). This subdomain includes an amino acid sequence that mimics a PKG phosphorylation site, although it lacks a phosphorylatable residue and is known as a pseudosubstrate sequence; the sequences in PKGIα and PKGIβ are 59TRQAIS63 and 74KRQAIS78, respectively [pseudosubstrate sequences are underlined with the phosphorylation site position (P0 for a typical substrate) in bold]. Studies with synthetic peptides suggest that the “ideal” sequence for a PKGI phosphorylation site is (R/K2–3)(X/K)(S/T)X (Lincoln et al., 1976; Glass and Krebs, 1979; Tegge et al., 1995). Basic residues at second and third positions N-terminal (P−2 and P−3, respectively) to the phosphorylated residue (P0) improve peptide substrate affinity for PKGI (Glass and Krebs, 1982; Tegge et al., 1995; Dostmann et al., 1999). Serine is the preferred phospho-acceptor. The pseudosubstrate site in PKGIα has a basic residue only at P−2 and is not an “ideal” substrate-like sequence. Several residues located within and near to the pseudosubstrate sequences also contribute to autoinhibition, including Ser-64 in PKGIα and Ser-79, Arg-75, and Ile-78 in PKGIβ (Francis et al., 1996; Collins and Uhler, 1999; Yuasa et al., 2000a; Busch et al., 2002). Deletion of the pseudosubstrate sequence does not fully activate PKGIs, indicating that regions C-terminal to the pseudosubstrate site contribute to maintaining the inactive state.
PKGI activation involves cGMP binding to both allosteric sites and is associated with a marked molecular elongation (∼27%) (Zhao et al., 1997; Wall et al., 2003; Alverdi et al., 2008). The affinities of the two allosteric cGMP-binding sites on each subunit of PKGIα differ by ∼10-fold, and cGMP-mediated activation exhibits strong positive cooperativity. In PKGIα, occupation of the N-terminal site produces partial activation (Corbin and Døskeland, 1983; Francis et al., 2005). The effect of the interaction of PKGI with GKIPs on PKGI cGMP-binding affinity and activation has not been explored, but it seems plausible that there could be important influences. Where cGMP activation of PKG is required for association with GKIPs, the principle of reciprocity would predict that the GKIP would in turn enhance cGMP-binding affinity (Weber, 1975). Such an effect could increase cGMP-binding affinity of PKGIβ to within a range of responsiveness more in line with cGMP levels that are thought to exist in most cells. Similar effects could also affect PKGIα sensitivity to cGMP. These possibilities should be studied to develop a more accurate picture of the action of these cGMP targets in their physiological settings.
Several reports indicate that oxidative processes could provide a NO/cGMP-independent mechanism for PKGIα activation. Reactive oxygen species have been implicated in down-regulation of PKGI in vascular smooth muscle (Liu et al., 2007). Landgraf et al. (1991) reported that PKGIα is reversibly activated and rendered largely cGMP-independent by metal ion-mediated oxidation; this treatment catalyzes formation of two intermonomer disulfide cross-links involving 1) Cys-117 and Cys-195 and 2) Cys-312 and Cys-518. The authors conclude that one or both cross-links are involved in decreasing cGMP-dependence of PKGIα, although the maximum catalytic rate of the oxidized enzyme is similar to that of cGMP-activated PKG; the relevance of this effect for PKGI regulation in intact cells is questionable. More recently, Burgoyne et al. (2007) reported that a hydrogen peroxide-induced disulfide cross-link between Cys-42 in PKGIα monomers increases affinity for the peptide substrate (RKRSRAE), does not significantly increase catalytic rate, and promotes PKGIα redistribution to the microsomal fraction. This activation profile is in contrast to the classic cGMP-mediated activation that increases catalytic rate with little effect on affinity for substrates. The authors of this study suggest that endothelially derived oxidants such as hydrogen peroxide or superoxide anions may act physiologically to increase PKG-mediated vasorelaxation that is independent of NO/cGMP. The Cys-42 cross-link between PKGIα monomers was first reported in purified native bovine lung PKGIα 30 years ago (Monken and Gill, 1980). The purified PKGI preparations that these investigators used typically have a strong dependence on cGMP for activation. The physiological and pathophysiological impact of oxidant-mediated regulation of PKG activity compared with that of the NO/cGMP system is not yet clear. Short-term and long-term effects of oxidants applied to whole cells on cGMP/PKG signaling must also consider effects on NO-synthases, NO-GC, cGMP-hydrolyzing PDEs, cGMP-regulated channels, contractile proteins, GKIPs, etc. Mechanisms for reversal of these changes under physiological conditions must also be taken into consideration if this is a readily dynamic process.
4. Autophosphorylation.
PKGIα and PKGIβ undergo autophosphorylation at multiple sites in the divergent N-terminal region (Fig. 4) (Lincoln et al., 1978; Aitken et al., 1984; Hofmann et al., 1985; Smith et al., 1996; Busch et al., 2002). Most of the sites do not closely resemble canonical PKG phosphorylation site sequences. Autophosphorylation of certain sites increases cGMP-binding affinity and basal phosphotransferase activity, but the roles of modifications at other sites are unknown. Autophosphorylation in vitro is typically slow, occurs by an intrasubunit process, and is increased by cGMP or cAMP (Smith et al., 1996; Busch et al., 2002; Francis et al., 2005). PKG autophosphorylation is implicated in both a “feed-forward” effect on cGMP signaling and a negative feedback effect. The autophosphorylation-induced increase in cGMP affinity increases PKG activation by low cGMP acutely and perhaps prolongs signaling. Autophosphorylation of PKGIα Ser-64 partially activates catalysis and enhances affinity for cGMP but also promotes PKGIα degradation by the ubiquitin/26S proteosomal pathway (Dey et al., 2009); the latter effect would act chronically as a negative feedback regulation.
Little is known about the rate and extent of PKGI autophosphorylation in vivo or conditions that favor these modifications. Consequently, prediction of the absolute affinities and functions of PKGI isozymes in intact cells is difficult because they may exist in various states of autophosphorylation, in different compartments where cGMP levels may vary, and in association with other proteins that may alter activation status and affinity for cGMP. The time course required for significant autophosphorylation of PKGI isozymes in cells or whether stoichiometric phosphorylation is achieved at particular sites is not known. PKG autophosphorylation sites are located close to the N-terminal leucine zipper, and it is possible that introduction of phosphates in this region could influence colocalization of PKGI with other proteins, including substrates. PKGIα is efficiently dephosphorylated by phosphoprotein phosphatase-1 in vitro, but the phosphatase(s) responsible for dephosphorylation in intact cells have not been defined (Chu et al., 1997; Francis et al., 2005). Improved quantification of PKGIs in tissues and characterization of PKGI autophosphorylation status, biochemical characteristics under various conditions, and susceptibility to degradation are needed for developing fuller understanding of the physiological functions of cGMP signaling through PKGI and changes that may be evoked under various pharmacological regimens, such as prolonged treatment with nitrovasodilators or PDE inhibitors.
C. Substrates for cGMP-Dependent Protein Kinase I Isozymes
The PKA type I regulatory subunit (Geahlen and Krebs, 1980), PKGIα regulatory domain (Glass and Smith, 1983; Aitken et al., 1984), PDE5 (Thomas et al., 1990b), G-substrate in cerebellum (Aitken et al., 1981), vimentin (Wyatt et al., 1991), and the ryanodine receptor (Takasago et al., 1991) were among the first proteins found to be PKG substrates. Many more protein substrates have been identified recently by in vitro peptide studies, in vitro 32P-labeling, or heterologous expression system analysis (Table 1), but only a fraction of these have been confirmed to be PKG substrates in vivo. NO-mediated elevation in cGMP for activation of PKGI and phosphorylation of many of these is implicated in regulation of functions in neurons or smooth muscle cells, but the spectrum of PKGI actions in diverse tissues continues to expand.
1. cGMP-Dependent Protein Kinase I Phosphorylation Sites in Intact Proteins.
Identification of potential PKGI phosphorylation sites by analyzing the amino acid sequence of a protein is highly problematic. The primary sequence around a PKG phosphorylation site frequently lacks the complete characteristics of a PKGI “consensus” phosphorylation sequence or, when it has a full consensus sequence, it may be described as a PKA phosphorylation site. There are well documented cases in which either kinase can readily phosphorylate a particular site. A majority of known PKG substrates (identified either in vitro or confirmed in vivo) have basic residues at P−3 and P−2 (Table 2). Fifteen of the proteins contain a total of 20 phosphorylation sites that have Arg at both P−3 and P−2, which is the preferred sequence for PKA substrates. However, 22 sites located in 16 of the substrate proteins shown in Table 2 contain an Arg at P−3 and a Lys at P−2, which suggests that either sequence in proteins is favorable for PKG phosphorylation in vivo. Fourteen substrates have phosphorylation sequences with a basic residue at only one of the P−3 or P−2 positions [e.g., the phosphorylation sites on IRAG (isoform A), the myosin-binding subunit, and RGS4)]. Some phosphorylation sites lack a basic residue at both P−3 or P−2; these include the site (Thr-276) in the serotonin transporter (SERT); PKGIα autophosphorylation sites (Ser-1, Ser-50, Ser-64, Ser-72), one PKGIβ autophosphorylation site (Ser-79), and type I regulatory subunit of PKA (Geahlen and Krebs, 1980; Aitken et al., 1984; Smith et al., 1996; Francis et al., 2005; Ramamoorthy et al., 2007; Zhang et al., 2007b). For PKGI autophosphorylation, proximity of the catalytic site may foster autophosphorylation at unusual sites. Likewise, PKGI colocalization with some proteins could foster heterophosphorylation at nonconsensus sequences simply as a result of increased proximity.
TABLE 2.
Phosphorylation sites in PKGI substrates
Phosphorylated residues are underlined.
| Substrate | Phosphorylation Site | Substrate Sequence | GenBank Accession Number | Species | Reference(s) |
|---|---|---|---|---|---|
| Consensus sequence | Ser or Thr | XRRXSXXX | |||
| ATP-sensitive K+ channel | N.D. | N.D. | Han et al., 2001; Chai and Lin, 2008 | ||
| Bad (Bcl-2 associated death promoter) | Ser155 | LRRMSDEF | AAC15100 | Rattus norvegicus | Johlfs and Fiscus, 2010 |
| Battenin (CLN3) | N.D. | N.D. | Michalewski et al., 1998 | ||
| β3-adrenergic receptor | N.D. | N.D. | Angelone et al., 2008 | ||
| Ca2+ channel (voltage-dependent; Cav1.2 L-type) α1C subunit | Ser1928 | GRRASFHL | Yang et al., 2007 | ||
| Ca2+ channel (voltage-dependent; Cav1.2 L-type) β2 subunit | Ser496 | SRGLSRQE | Yang et al., 2007 | ||
| Ca2+ channel, L-type, α1c subunit | Ser533 | KSKFSRYW | Jiang et al., 2000 | ||
| Ser1371 | VKLLSRGE | Jiang et al., 2000 | |||
| Ca2+-activated K+ channel (α-subunit, cslo-α) | Ser1072 | SKKSSSVH | Fukao et al., 1999 | ||
| Ca2+-sensitive K+ channel (Hslo) | N.D. | N.D. | Alioua et al., 1998 | ||
| Calponin homology-associated smooth muscle protein (CHASM) | Ser301 | ERRVSAPS | Borman, et al., 2004 | ||
| cAMP-dependent protein kinase regulatory subunit type I (PKA RI) | Ser100 | RGAISAEV | Geahlen and Krebs, 1980; Hashimoto et al., 1981 | ||
| cGMP-dependent protein kinase anchoring protein 42 (GKAP42) | Ser106 | AQKESREE | Yuasa et al., 2000 | ||
| cGMP-dependent protein kinase type I-α (PKGIα) | Ser1 | SEL | Aitken et al., 1984 | ||
| Ser50 | LPVPSTHI | Aitken et al., 1984 | |||
| Thr58 | GPRTTRAQ | Glass and Smith, 1983; Aitken et al., 1984 | |||
| Ser64 | AQGISAEP | Aitken et al., 1984 | |||
| Ser72 | QTYRSFHD | Aitken et al., 1984 | |||
| Thr84 | FRKFTKSE | Aitken et al., 1984 | |||
| cGMP-dependent protein kinase type I-β (PKGIβ) | Ser63 | AQKQSAST | NP_035290 | Mus musculus | Francis et al., 1996 |
| Ser79 | RQAISAEP | Smith et al., 1996 | |||
| Cysteine-rich protein 2 (CRP2) [also known as cysteine-rich LIM-only protein 4 (CRP4)]a | Ser104 | ERKTSGPP | Huber et al., 2000; Zhang et al., 2007; Schmidtko et al., 2008 | ||
| Cystic fibrosis transmembrane conductance regulator (CFTR) | Ser660 | ERRNSILT | Picciotto et al., 1992 | ||
| Ser700 | KRKNSILN | Picciotto et al., 1992 | |||
| Ser737 | ERRLSLVP | Picciotto et al., 1992 | |||
| Ser768 | RRRQSVL | Picciotto et al., 1992 | |||
| Ser795 | TRKVSLA | Picciotto et al., 1992 | |||
| Ser813 | SRRLSQET | Picciotto et al., 1992 | |||
| Formin homology domain-containing protein (FHOD1) | Ser1131 | ERKRSRGN | NP_037373 | Homo sapiens | Wang et al., 2004 |
| GABA-A receptor | Ser409 | RKPLSSRE | NP_000803 | H. sapiens | Leidenhemier, 1996; McDonald and Moss, 1997 |
| G-septin | Ser91 | SRKASSWN | Xue et al., 2000 | ||
| G-substrateb | Thr68 | RRKDTPAL | Oryctolagus cuniculus | Aitken et al., 1981; Aswad and Greengard, 1981; Endo et al., 1999 | |
| Thr72 | RRKDTPAL | R. norvegicus | Endo et al., 2003 | ||
| Thr119 | RRKDTPAL | O. cuniculus | Aitken et al., 1981; Aswad and Greengard, 1981; Endo et al., 1999 | ||
| Thr123 | RRKDTPAL | R. norvegicus | Endo et al., 2003 | ||
| Guanylyl cyclase, soluble | Ser64 | QRKTSRNR | Murthy, 2001; Zhou et al., 2008 | ||
| 27-kDa Heat shock protein (HSP 27) | Thr143 | TRKYTLPP | AAAA62175 | H. sapiens | Butt et al., 2001 |
| Histone 2B | Ser32 | RKRSRKE | Glass and Krebs, 1979; Glass and Krebs, 1982 | ||
| Ser36 | RKESYSV | Glass and Krebs, 1979 | |||
| Inositol triphosphate receptor (IP3R) | Ser1589 | ARRDSVLA | Komalavilas and Lincoln, 1994; Haug et al., 1999; Murthy and Zhou, 2003; Wagner et al., 2003 | ||
| Ser1755 | GRRESLTS | Komalavilas and Lincoln, 1994; Haug et al., 1999; Murthy and Zhou, 2003; Wagner et al., 2003 | |||
| Inositol triphosphate receptor-associated PKG substrate isoform A (IRAGa)# | Ser683 | ARSMSLSL | Schlossmann et al., 2000; Geiselhöringer et al., 2004 | ||
| Ser696 | RRRVSVAV | Schlossmann et al., 2000; Ammendola et al., 2001 | |||
| IRAGb | Ser644 | RRRVSVAV | AAF61203 | Bos taurus | Casteel et al., 2008 |
| Large conductance calcium activated potassium channel (BKCa)b | N.D. | N.D. | Sausbier et al., 2000 | ||
| LIM and SH3 domain protein (LASP-1) | Ser61 | YPKQSFTM | AAK28338 | R. norvegicus | Keicher et al., 2004 |
| Thr156 | YRRPTEQQ | AAK28338 | R. norvegicus | Keicher et al., 2004 | |
| Ser146 | ERRDSQDG | NP_006139 | H. sapiens | Butt et al., 2003 | |
| MEKK1 | N.D. | N.D. | Soh et al., 2001 | ||
| Myosin phosphatase small regulatory subunit (M20) | N.D. | N.D. | Nakamura et al., 1999 | ||
| Myosin phosphatase large regulatory subunit (MYPT1)b | Ser695 | QSRRSTQG | Nakamura et al., 1999; Wooldridge et al., 2004 | ||
| Na+/K+ ATPase α-subunit | N.D. | N.D. | Fotis et al., 1999 | ||
| NC-1.1 receptor | N.D. | N.D. | Holmgreen et al., 1997 | ||
| p21-activated kinase 1 (Pak) | Ser21 | MRNTSTMI | Fryer et al., 2006 | ||
| Phosphodiesterase 5b | Ser92 | TRKISASE | Thomas et al., 1990 | ||
| Phospholambanb | Ser16 | IRRASTIE | NP_002658 | H. sapiens | Raeymaekers et al., 1988; Cornwell et al., 1991 |
| Phospholipase A2 (PLA2) | N.D. | N.D. | Chalimoniuk et al., 2009 | ||
| Phospholipase C β2 (PLCβ2) | N.D. | N.D. | Xia et al., 2001 | ||
| Phospholipase C β3 (PLCβ3) | Ser26 | LRRGSKEI | Xia et al., 2001 | ||
| Ser1105 | KRHNSISE | Xia et al., 2001 | |||
| pH-sensitive K+ channel | N.D. | N.D. | Kang et al., 2007 | ||
| Protein phosphatase inhibitor 1 (PPI-1) | Thr35 | RRPTATL | Tokui et al., 1996 | ||
| Rap1 GTP-ase activating protein 2-α (Rap1GAP2α)a | Ser7 | GRKRSVSF | CAF31653 | H. sapiens | Schultess et al., 2005 |
| Rap1GAP2βa | Ser7 | GRKRSVSF | CAF31652 | H. sapiens | Schultess et al., 2005 |
| Regulator of G protein signaling 2 (RGS2)b | Ser46 | KTRLSYFL | Tang et al., 2003; Sun et al., 2005 | ||
| Ser64 | GKKSKQQ | Tang et al., 2003; Sun et al., 2005 | |||
| RGS4 | Ser52 | QRVSQEE | BAA20400 | M. musculus | Huang et al., 2007 |
| RhoAb | Ser188 | GKKKSGCL | AAM21117 | H. sapiens | Sawada et al., 2001; Ellerbroek et al., 2003; Murthy and Zhou, 2003 |
| Ryanodine receptor | N.D. | N.D. | Takasago et al., 1991 | ||
| Septin-3a | Ser91 | SRKASSWN | NP_062248 | R. norvegicus | Xue et al., 2004 |
| Serotonin transporter (SERT)a | Thr276 | KGVKTSGK | CAA71909 | R. norvegicus | Ramamoorthy et al., 2007; Zhang et al., 2007 |
| Smooth myosin phosphatase light chain phosphatase (SMPP-1M) | N.D. | N.D. | Wu et al., 1996; Lee et al., 1997 | ||
| Smoothelin-like protein 1 (SMTNL1) | Ser301 | RRVSARS | NP_001099035 | M. musculus | Wooldridge et al., 2008 |
| Splicing factor 1 | Ser20 | RKRSRWNQ | Wang et al., 1999 | ||
| Steroidogenic acute regulatory protein (StAR) | N.D. | N.D. | Andric et al., 2007 | ||
| Telokinb | Ser13 | GRKSSTGS | AAN63946 | M. musculus | Walker et al., 2001 |
| Thromboxane A2 receptor 1α (Tpα)a | Ser331 | PRSLSLQP | Yamamoto et al., 2001; Reid and Kinsella, 2003 | ||
| Titin N2BA and N2B | Ser469 | GAKTSLQE | Kruger et al., 2009 | ||
| Transient receptor potential | Thr11 | LRRMTVMR | NP_003296 | H. sapiens | Kwan et al., 2004 |
| Ca2+ channel isoform 3 (TRPC3)& | Ser263 | YRKLSMQC | NP_003296 | H. sapiens | Kwan et al., 2004 |
| Transient receptor potential | Thr70 | HRRQTVLR | NP_004612 | H. sapiens | Koitabashi et al., 2010 |
| Ca2+ channel isoform 6 (TRPC6) | Ser322 | YKKLSMQC | NP_004612 | H. sapiens | Koitabashi et al., 2010 |
| Troponin I | N.D. | N.D. | Layland et al., 2002 | ||
| Tyrosine hydroxylase | N.D. | N.D. | Rodríguez-Pascual et al., 1999 | ||
| Vasodilator-stimulated phosphoprotein (VASP) | Ser157 | ERRVSNAG | Smolenski et al., 1998 | ||
| Ser239 | LRKVSKQE | Butt et al., 1994 | |||
| Thr278 | RRKATQVG | Cook and Haynes, 2007 | |||
| Vimentinb | N.D. | N.D. | Wyatt et al., 1991; Pryzwansky et al., 1995 |
N.D., not determined or not identified.
PKGI substrates identified in heterologous systems (Hofmann et al., 2009).
Established PKGI substrates (Hofmann et al., 2009).
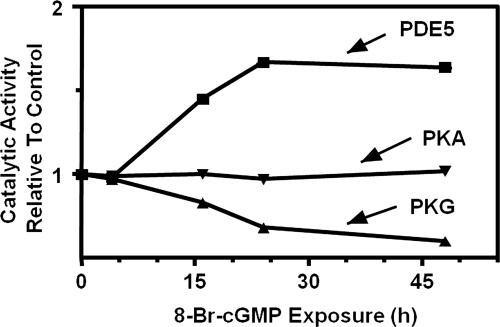
PKGI-mediated phosphorylation at consensus phosphorylation sequences can be enhanced by interactions outside the phosphorylation site. Phosphorylation of bovine PDE5 at Ser-92 in the canonical PKG phosphorylation site (89RKISASE93) occurs with ∼25-fold higher affinity than that for phosphorylation of the synthetic heptapeptide containing that sequence (Liu et al., 2002), thus emphasizing the importance of additional contacts for PKGI/PDE5 interaction. In addition, the apparent requirement for physical colocalization of PKG with some substrates for phosphorylation to occur even at consensus phosphorylation sequences may also support the importance of contacts outside the phosphorylation site (Gudi et al., 1997; Surks et al., 1999; Casteel et al., 2008; Steiner et al., 2009). This is discussed in greater detail in section II.D.
2. Potential Problems with Prediction of cGMP-Dependent Protein Kinase I Phosphorylation Sites.
A protein may be phosphorylated by PKGI in vitro, but not in vivo, as seems to occur with the voltage-dependent Cav1.2 L-type Ca2+ channel α1C subunit (Yang et al., 2007) and LIM and SH3 domain protein (LASP-1) (Keicher et al., 2004). This could be due to inaccessibility of a particular site in vivo as a result of different compartmentation of the protein and PKG, association of the protein in question with proteins that block the phosphorylation site, conformational differences between the purified protein substrate and that in the cell, and myriad other processes. Conversely, proteins that seem to be PKG substrates in intact cells may not be phosphorylated by PKG in vitro because PKG may effect the phosphorylation in intact cells by phosphorylating another kinase that actually carries out the phosphorylation reaction (i.e., acting as a “kinase kinase”), or the PKG-catalyzed phosphorylation may require prior phosphorylation at another site by another kinase (i.e., hierarchal phosphorylation). In addition, because PKA and PKG have similar consensus sequences, a protein identified in vitro as a PKGI substrate may primarily be a PKA substrate in vivo and vice versa (Butt et al., 1994a; Rodríguez-Pascual et al., 1999; Schultess et al., 2005; Lincoln et al., 2006). Furthermore, a PKGI phosphorylation site in one species may be absent in another; PKGI phosphorylates Thr-156 in mouse LASP-1 (Keicher et al., 2004), but a Leu occupies this position in human LASP-1.
D. Requirement for Selective Role of cGMP-dependent Protein Kinase I Isozymes and Subcellular Targeting for Phosphorylation of Substrates
The shared specifications for consensus sequence in phosphorylation of synthetic peptides by PKGI isozymes suggests that either could phosphorylate a target protein in intact cells, but this is now a controversial concept (Wolfe et al., 1989a; Casteel et al., 2005; Francis et al., 2005; Christensen and Mendelsohn, 2006; Weber et al., 2007). A number of compelling studies have shown that a specific PKGI or specific localization of PKGIα or PKGIβ is required for phosphorylation of certain substrates and associated physiological effects (Surks et al., 1999; Yuasa et al., 1999; Feil et al., 2002; Schröder et al., 2003; Christensen and Mendelsohn, 2006; Fiedler et al., 2006; Zhang et al., 2007a; Casteel et al., 2008; Sharma et al., 2008; Wilson et al., 2008; Steiner et al., 2009). In contrast to the diverse family of AKAPs that accounts for localization of PKA near many of its substrates, a similarly diverse family of PKGI anchoring proteins has not been identified. Where the requirements for PKGI localization have been studied in detail, the characteristics of the respective N-terminal leucine zippers seem to dictate selective targeting of PKGIα or PKGIβ. For example, selective interaction of PKGIα, but not PKGIβ, with SERT in certain neuronal cells suggests involvement of the PKGIα leucine zipper, but this has not been experimentally documented (Steiner et al., 2009). Other contacts may contribute to selective PKGI targeting, and in some instances either PKGI isozyme can dock with a protein, which implies interaction involving features that are common to both PKGs (Zhang et al., 2007a).
Insight into the selectivity of interactions between PKGs and proteins to which they are targeted provides the potential for development of peptides that could disrupt these interfaces; this could significantly advance studies of PKGI action or lead to development of a new class of drugs. In some instances, specific interactions or PKGI translocation increases when PKGI is activated by cGMP. This suggests that previously unexposed regions of PKG, including changes in the leucine zipper region, account for PKGI localization with target proteins (Chu et al., 1997; Wall et al., 2003; Alverdi et al., 2008). Whether post-transcriptional modifications of the target protein or PKG (e.g., phosphorylation, nitrosylation, or cross-linking of cysteines) alter the contacts that contribute to these unique interactions and whether interaction in all cases is restricted to the PKGI leucine zippers are not known.
1. Colocalization of cGMP-Dependent Protein Kinase Iα with Substrates and Heterophosphorylation of Substrates.
Residues on the leucine zipper face of the PKGIα dimer selectively form multiple contacts with residues on the face of the coiled-coiled and/or leucine zipper region of the dimerized myosin-binding subunit of myosin light chain phosphatase to form a heterotetramer that is tightly associated (Lee et al., 2007; Sharma et al., 2008). This interaction is purportedly required for PKG phosphorylation of the myosin-binding subunit and activation of myosin light chain phosphatase (Surks et al., 1999). The leucine zipper of PKGIα also mediates binding to RGS2, resulting in phosphorylation and activation of this protein (Tang et al., 2003; Sun et al., 2005; Osei-Owusu et al., 2007). Phosphorylation of RGS2 increases its association with the plasma membrane, where it increases the GTPase activity of Gqα, thereby interfering with and decreasing signaling by Gq-coupled receptors. Increased cellular cGMP and PKG activation promotes phosphorylation of SERT, and phosphorylation of Thr-276, in particular, is associated with increased activity of SERT to 5-hydroxytryptamine. It is not known whether phosphorylation of SERT is directly mediated by PKG or involves its action on another kinase, such as the p38-mitogen-activated protein kinase, and whether the effect involves an intermediary protein (Zhu et al., 2004; Ramamoorthy et al., 2007; Steiner et al., 2009).
2. Colocalization of cGMP-Dependent Protein Kinase Iβ with Substrates and Heterophosphorylation of Substrates.
Casteel et al. (2005) have used site-directed mutagenesis combined with secondary structure predictions and direct binding studies to identify a motif that provides for PKGIβ-specific localization with and phosphorylation of two substrates, TFII-I and IRAG. One of the predicted surfaces of the PKGIβ leucine zipper α-helix displays negatively charged amino acids (Asp and Glu) that have been proposed to interact with a cluster of positively charged residues (Lys and Arg) in the regions of TFII-I or IRAG that bind PKGIβ (Ammendola et al., 2001; Casteel et al., 2005). Mutation of several of the negatively charged residues on PKGIβ to positively charged residues found at homologous positions in PKGIα has no effect on PKGIβ dimerization but disrupts interactions with TFII-I and IRAG (Casteel et al., 2005). Likewise, mutations of several positively charged residues in the segments of TFII-I or IRAG that are required for interaction with PKGIβ disrupt interaction with PKGIβ. Despite similarities in the motifs for formation of complexes between PKGIβ and either TFII-I or IRAG, there are clear differences; a single mutation (E27K) eliminates interaction of PKGIβ and TFII-I but has no measurable effect on that with IRAG (Casteel et al., 2005). These differences highlight subtleties in the interaction of PKGs with target proteins and potentially provide a basis for a spectrum of selective physiological effects mediated by PKGI isozymes.
In studies of PKGIβ function and localization in vascular smooth muscle cells that lack IRAG, the intracellular localization of PKGIβ is similar to that found in wild-type controls (Desch et al., 2010). This suggests that interactions with other proteins contribute to its subcellular distribution. However, in support of the importance of PKGIβ localization to its function, in cells that either lack IRAG or express a mutant IRAG that still binds PKGIβ but no longer interacts with the IP3R1, the NO/cGMP-dependent inhibition of calcium transients is impaired (Geiselhöringer et al., 2004; Desch et al., 2010). This is presumably due to displacement of PKGIβ from the macromolecular complex involving IRAG and IP3R1.
The requirement or advantage for colocalization of PKGI isozymes with certain substrates could be due to a number of factors (Koller et al., 2003). Proximity of PKGI to the target residue could increase efficiency of phosphate transfer, as has been suggested for autophosphorylation events described in section II.C.1. Colocalization increases the concentration of the kinase and its substrate in that locale, which would increase the rate of phosphorylation assuming that the substrate concentration in the unbound state is below that required to achieve Vmax. Alternatively, interaction of the target protein with PKGI could elicit a conformational change that uncovers or approximates the phosphorylatable residue. Selective localization of PKGI proteins with substrates in distinct microcompartments of cells and differential control of cGMP levels may allow particular PKGI populations in cells to respond differently to a range of changes in cGMP.
3. Regulation of cGMP-Dependent Protein Kinase I Colocalization with Substrates; Translocation among Cellular Compartments.
Interactions between GKIPs and PKGI isozymes are frequently stable, but some are transient and altered by cGMP activation of PKGI (Yuasa et al., 1999; Wang et al., 2004). In some instances, PKGI isozymes reversibly relocate in response to changes in cGMP and can thus phosphorylate protein substrate(s) at the new location (Wyatt et al., 1991; Pryzwansky et al., 1995; Fiedler et al., 2006; Casteel et al., 2008). Molecular mechanisms that provide for these transient PKG targeting events are not known. A cGMP-induced exposure of a site(s) on PKG could either work independently or in combination with contacts provided by the leucine zipper to foster the localization. A nuclear translocation signal (404KIIRKKHI411) in the ATP-binding subdomain is exposed upon cGMP activation and mediates nuclear translocation of PKGI isozymes, which occurs in some cells (Gudi et al., 1997; Pilz and Broderick, 2005). PKG exit from the nucleus is slow, and it is not known if nuclear GKIPs bind the activated PKG, thereby preserving nuclear localization, or how reversal of this translocation is regulated.
The distribution of PKGI isozymes among microdomains within a cell is undoubtedly influenced by GKIPs that either selectively or nonselectively complex with PKGIα or PKGIβ. Nuclear translocation of PKGIβ is restrained by its high-affinity interaction with IRAG, which is tightly associated with the endoplasmic reticulum (Casteel et al., 2008; Haas et al., 2009). The impact of GKIPs such as IRAG could be influenced by 1) the relative abundance of GKIPs and PKGs, 2) the affinities of the GKIPs for the PKGIs, 3) changes in those affinities as a result of post-translational modifications, or 4) the cellular environment. The effect of NO to modify proteins by nitrosylation or nitration is well established, and the NO/cGMP/PKG pathway is influenced by kinases and phosphatases involved in other pathways (Antl et al., 2007; Costa et al., 2008; Das et al., 2009; Koitabashi et al., 2010). Consequently, it is entirely plausible that a number of events could alter characteristics of proteins (e.g., GKIPs, PDEs, cGMP-gated channels, and substrates) involved in the NO/cGMP/PKG pathway along with changes evoked by PKG activation. These possibilities add substantially to the potential complexity of the cellular response to a NO signal.
4. Caveats to the Role of cGMP-Dependent Protein Kinase I Colocalization with Substrates.
Despite the findings cited above in sections II.D.1 and II.D.2, the functional consequences of selective colocalization of PKGI isozymes with particular substrates in intact tissues are still not well clarified. Weber et al. (2007) reported that reconstitution of either PKGIα or PKGIβ in vascular smooth muscle and gastrointestinal smooth muscle of PKGI knockout mice [PKG(−/−)] rescues basic smooth muscle functions in these animals; the expression level of the PKGI proteins compares well with PKGI levels in wild-type controls, and restored functions include intestinal motility, relaxation of both vascular and gastrointestinal smooth muscle, and cGMP-dependent inhibition of calcium transients. Although systemic blood pressure in PKG(−/−) mice is elevated, that in mice expressing either PKGI is similar to that of wild-type control mice. The discordance between reports such as this one and others that suggest strict specificity between a PKGI isozyme and its substrate is difficult to reconcile. At the outset, it suggests that PKG substrate selectivity in the intact animal may be less rigorously controlled than in isolated cultured cells. However, given the complexity of mechanisms associated with PKGI function and the diverse cell types that use NO/cGMP/PKGI signaling, there are likely to be contributing factors, of which we are currently unaware, that will allow better understanding of these differences. On the surface, the apparent lack of PKGI isozyme specificity seems to seriously compromise potential benefits of medications that might target one or the other PKGI or its anchoring. Nevertheless, in cells in which one isozyme vastly predominates, as occurs in human platelets (PKGIβ ⋙ PKGIα) or the pulmonary vasculature (PKGIα ⋙ PKGIβ) (Lincoln et al., 1977; Eigenthaler et al., 1992; Francis and Corbin, 1994), peptidomimetics that pharmacologically interfere with the association of that PKGI with target proteins could eventually prove to be useful.
III. Major Modes of cGMP-Dependent Protein Kinase I Action in Response to Nitric Oxide-Mediated Increases in cGMP
A number of mechanisms for NO/cGMP/PKGI-signaling action in both smooth muscle and other cells have been determined. However, understanding of the full impact of NO/cGMP/PKGI signaling in most cells is rudimentary. It is clear that functions of this pathway are integrally intertwined with many other signaling pathways, and the influence of these other pathways will almost assuredly differ to some extent among cell types. Full treatment of these topics is beyond the scope of this review. Moreover, NO/cGMP/PKGI effects may differ substantially among cells within an intact tissue or in the same cells prepared as primary cultures or multiply passaged cells in culture (Lincoln et al., 2006; Weinmeister et al., 2008). Described sections III.A–III.G is a modest compilation of mechanisms that have been shown to involve input from the NO/cGMP/PKGI pathway, and the number and complexity of these continue to expand.
Major mechanisms that have been established for NO/cGMP/PKGI action in smooth muscle include decreasing contractile tone by concomitantly lowering intracellular calcium and desensitizing proteins to the effects of calcium; this signaling pathway also powerfully influences smooth muscle cell phenotype by regulation of gene expression. The contractile state of smooth muscle is largely determined by the state of phosphorylation and activity of the myosin light chain; phosphorylation of the myosin light chain at Ser-19 is mediated by the myosin light-chain kinase and leads to increased myosin ATPase activity and muscle contraction (Kamm and Stull, 1985; Somlyo and Somlyo, 2003; Ding et al., 2009). Myosin light-chain phosphatase reverses this by catalyzing the dephosphorylation of the myosin light chain. The balance between the kinase and phosphatase activities is a critical determinant in the contractile status of smooth muscle, and the functions of both are affected by NO/PKG/cGMP signaling (Wu et al., 1996; Lee et al., 1997; Somlyo and Somlyo, 2003; Hofmann, 2005; Berridge, 2008; Mizuno et al., 2008). Upon elevation of intracellular calcium and increased formation of the calcium/calmodulin complex, myosin light-chain kinase binds calcium/calmodulin and is activated. When calcium is lowered as a result of increased intracellular sequestration and/or extrusion, the kinase and the myosin light chain are less active, thereby favoring smooth muscle relaxation. Moreover, in smooth muscle cells containing members of the calcium/calmodulin-activated PDE1 family, which hydrolyzes both cGMP and cAMP, an increase in cellular calcium would promote lowering of cGMP as well as cAMP, both of which promote smooth muscle relaxation.
A. Modulation of Intracellular Calcium
Amplification at every step in the NO/cGMP/PKGI signaling pathway converts the signal initiated by a modest amount of NO into a marked lowering of intracellular free calcium, desensitization of contractile proteins to calcium, and decrease in the contractile state of smooth muscle or in platelet activation. Numerous protein targets for PKGI phosphorylation are implicated in modulating cellular calcium (Fig. 5), but many studies imply that the contribution of each of these targets may vary substantially among cell types.
PKGI phosphorylation of G-protein-activated phospholipase-Cβ3 at Ser-26 and Ser-1105 inhibits the activity of this enzyme, thereby decreasing generation of IP3 and calcium mobilization (Xia et al., 2001).
NO/cGMP/PKGI signaling promotes increased sequestration and decreased release of calcium from intracellular stores as a result of phosphorylation of IRAG, which regulates the InsP3 receptor and therefore InsP3-mediated calcium release from intracellular stores (Schlossmann et al., 2000; Ammendola et al., 2001; Geiselhöringer et al., 2004; Antl et al., 2007; Vanderheyden et al., 2009). PKGIβ is bound tightly to IRAG, which anchors it with the IP3 receptor. In response to a NO-mediated inrease in cGMP, PKGIβ phosphorylates human IRAG at Ser-696, which converts IRAG to an inhibitor of IP3-receptor activity, thereby suppressing release of calcium from intracellular stores.
PKGI phosphorylation/activation of large-conductance calcium-activated (BKCa) potassium channels promotes channel opening, thereby allowing for loss of intracellular potassium, hyperpolarization of the plasma membrane, and decreased calcium influx through L-type calcium channels (White et al., 1993; Zhou et al., 1996; Alioua et al., 1998; Fukao et al., 1999; Hall and Armstrong, 2000). A recent report indicates that L-type channels expressed in human embryonic kidney cells or cardiomyocytes are phosphorylated by PKGI, which is also associated with inhibition of channel function (Yang et al., 2007). Whether this mechanism significantly affects calcium current in smooth muscle cells has not been determined.
Phosphorylation of phospholamban at Ser-16 abrogates its inhibitory effect on the sarcoplasmic reticulum calcium/ATPase pump, thereby increasing calcium sequestration (Raeymaekers et al., 1988; Colyer, 1998; Schlossmann et al., 2000; Koller et al., 2003).
Signaling through certain receptors, the actions of which are mediated through calcium, can also be attenuated by direct PKGI action (Christensen and Mendelsohn, 2006). Thromboxane A2 signaling through the TPα isoform of the thromboxane receptor activates phospholipase-Cβ to increase IP3 production and mobilization of intracellular calcium. The calcium in turn increases eNOS activity and signaling through the NO/cGMP/PKG pathway. The subsequent PKGI phosphorylation of TPα receptor at Ser-331 desensitizes the receptor, thereby decreasing intracellular calcium (Reid and Kinsella, 2003; Kelley-Hickie et al., 2007). Likewise, PKGI phosphorylates the transient receptor potential canonical (TRPC)-6 channel at Thr-70 and Ser-322, which inactivates the associated inward calcium current (Koitabashi et al., 2010).
Signaling through other receptors whose actions are also mediated through calcium can be blunted through the action of the NO/cGMP-activated PKGI to phosphorylate RGS proteins that alter receptor coupling to their G-protein partners (Tang et al., 2003; Osei-Owusu et al., 2007; Schlossmann and Desch, 2009). PKGI phosphorylates and activates RGS proteins, thereby promoting translocation to the plasma membrane where phospho-RGS increases the GTPase activity of G proteins and interferes with their interaction with G-protein coupled receptors (Sun et al., 2005; Huang et al., 2007; Osei-Owusu et al., 2007). TPα signaling through RhoA is also diminished by NO/cGMP activation of PKGI, but whether the mechanism of this effect relates to processes described above or immediately below is not clear (Wikström et al., 2008).
Fig. 5.
Role of PKGI in modulating intracellular calcium level to promote smooth muscle relaxation. cGMP activation of PKGI in response to nitric oxide elicits multiple phosphorylations of cellular proteins that result in lowering of cellular calcium either through decreased mobilization from intracellular stores or decreased entry from the extracellular space. Solid arrows leading from PKG to a protein indicate that the PKGI-mediated phosphorylation of that protein stimulates its biological activity; for example PKG phosphorylation of RGS proteins activates these proteins, leading to their effect to impair coupling between G proteins and G protein-coupled receptors (GPCRs). An inhibitory bar indicates that the PKGI-mediated phosphorylation impairs the biological function of that protein; for example, PKGI phosphorylation of phospholamban decreases its inhibitory effect on the sarcoplasmic reticulum calcium/ATPase, thereby allowing increased calcium sequestration. PLCβ, phospholipase Cβ. [Adapted from Francis SH and Corbin JD (2005) Phosphodiesterase-5 inhibition: the molecular biology of erectile function and dysfunction. Urol Clin North Am 32:419–429. Copyright © 2005 Elsevier Ltd. Used with permission.]
B. Effect on Functions of Ras Homolog Gene Family Member A
Another major mode of action of NO/cGMP/PKGI signaling involves PKGI-mediated inactivation of the Ras homolog gene family member A (RhoA). PKGI phosphorylation of RhoA at Ser-188 blocks the action of this protein in myriad processes; these include signaling through Rho-associated protein kinases to foster increased contraction in vascular smooth muscle, regulation of cellular adhesion, and relief of inhibition of insulin receptor substrate-1 to enhance insulin signaling through activation of the downstream phosphoinositide 3-kinase-Akt cascade (Ellerbroek et al., 2003; Murthy et al., 2003; Dhaliwal et al., 2007; Weinmeister et al., 2008; Haas et al., 2009; Nossaman and Kadowitz, 2009). In vascular smooth muscle in the absence of cGMP signaling, RhoA translocates from the cytosol to the plasma membrane, where it increases the activity of the Rho-associated protein kinase. The activated Rho-kinase then phosphorylates Thr-696 of the myosin-binding subunit of myosin light-chain phosphatase, thereby inhibiting phosphatase activity toward myosin light chain and fostering contraction (Amano et al., 1996; Kimura et al., 1996; Kureishi et al., 1997; Feng et al., 1999; Wooldridge et al., 2004). PKGI phosphorylation of RhoA promotes its dissociation from the plasma membrane and its return to the cytosol. Activated PKG also catalyzes phosphorylation of Ser-695 on the myosin light-chain phosphatase regulatory subunit, which in turn blocks Rho kinase inactivation of the phosphatase (Nakamura et al., 2007).
C. Gene Regulation
Activation of PKGI by NO signaling alters gene expression in a number of tissues (Gudi et al., 1997; Collins and Uhler, 1999; Pilz and Broderick, 2005; Zhao et al., 2005; Zhang et al., 2007a). In smooth muscle cells, increased cGMP and PKGI activity influence expression of smooth muscle-specific contractile proteins, levels of proteins in the NO/cGMP signaling pathway (e.g., NO-GC), down-regulation of the matrix proteins osteopontin and thrombospondin-1 to limit smooth muscle cell migration and phenotype, and differentiation of cells, including the transition of the smooth muscle cell from a contractile phenotype to a synthetic phenotype (Lincoln et al., 2006; Zhang et al., 2007a). PKGI can phosphorylate cAMP response element-binding protein in vitro, but whether this occurs physiologically is unclear (Colbran et al., 1992). NO-mediated increases in cGMP and PKG activation are reported to increase mitochondrial biogenesis and abundance of the uncoupling protein in brown fat (Haas et al., 2009). PKGI-mediated regulation of gene expression and protein levels is implicated in some models of neuronal plasticity and learning, as well as in pathological conditions such as Alzheimer's disease and schizophrenia (Monfort et al., 2002; Monfort and Felipo, 2005; van der Staay et al., 2008; Nugent et al., 2009).
Nuclear factor of activated T cells (NFAT) translocation between the cytosol and nucleus is regulated by phosphorylation/dephosphorylation of serines in its N-terminal segment (Beals et al., 1997a,b; Crabtree and Olson, 2002; Sheridan et al., 2002). Phospho-NFAT is cytosolic; when dephosphorylated, it translocates to the nucleus and promotes gene transcription, which in the case of cardiomyocytes seem to be pro-hypertrophic genes (Hogan et al., 2003; Koitabashi et al., 2010). NFAT is dephosphorylated by calcineurin, a calcium-activated phosphoprotein phosphatase; increased activity of the cGMP/PKGI signaling pathway lowers cellular calcium, thereby inhibiting the pro-hypertrophic effects of NFAT/calciuneurin signaling (Fiedler et al., 2002; Wollert et al., 2002). Activation of TRPC channels either by stretch or a Gq-coupled agonist increases influx of calcium into the cell and concomitantly activates many proteins that can dephosphorylate NFAT (Crabtree and Olson, 2002; Nilius et al., 2005, 2007). In addition, the promoter for TRPC6 contains NFAT-response elements; TRPC6 expression is significantly up-regulated in models of cardiac hypertrophy and is associated with positive regulation of the calcineurin/NFAT signaling pathway (Kuwahara et al., 2006). Increased nuclear NFAT has been proposed to directly promote expression of proteins that are associated with cardiac hypertrophy as well as to activate a feed-forward mechanism for increased expression of TRPC6, which would further increase intracellular calcium and foster persistent nuclear NFAT relocation (Kuwahara et al., 2006). Activation of the NO/cGMP/PKGI pathway counters this effect: first, PKGI has recently been shown to phosphorylate two sites (Ser-322 and Thr-70) on TRPC6 (Takahashi et al., 2008; Koitabashi et al., 2010), thereby decreasing calcium entry into the cell; second, the effect of NO/cGMP/PKGI signaling to lower intracellular calcium decreases calcineurin activity, thereby favoring retention of phospho-NFAT in the cytosol (Kwan et al., 2004; Takahashi et al., 2008; Koitabashi et al., 2010).
D. Regulation of Vasodilator-Stimulated Phosphoprotein Functions
The effect of PKGI to phosphorylate vasodilator-stimulated phosphoprotein (VASP) in platelets and smooth muscle was documented early in the history of cGMP signaling. VASP is phosphorylated by PKG and PKA (Walter and Gambaryan, 2009); PKGI preferentially phosphorylates VASP at Ser-239. Phosphorylation of VASP is correlated with 1) changes in platelet cytoskeletal organization, 2) inhibition of platelet activation (Horstrup et al., 1994; Nolte et al., 1994), 3) decreased association of VASP with F-actin and VASP-driven actin filament formation (Benz et al., 2008; Benz et al., 2009), 4) enhanced angiogenesis (Chen et al., 2004, 2008), and 5) induction of long-term potentiation (Wang et al., 2005). Increased phosphorylation of VASP also causes a change in its intracellular location (Lohmann and Walter, 2005; Walter and Gambaryan, 2009).
E. Regulation of Phosphodiesterase-5
PKGI phosphorylates and activates the cGMP-specific PDE5, the first recognized physiological substrate of known enzymatic function, in platelets, smooth muscle cells, and cerebellar Purkinje cells (Wyatt et al., 1998; Corbin et al., 2000; Rybalkin et al., 2002; Shimizu-Albergine et al., 2003; Koesling et al., 2005; Wilson et al., 2008). Phosphorylation of PDE5 at a single serine (Ser-102 in human PDE5; Ser-92 in bovine PDE5) in the regulatory domain increases the affinity of the enzyme for the substrate (Thomas et al., 1990b; Corbin et al., 2000; Rybalkin et al., 2002), thereby increasing cGMP hydrolysis at subsaturating concentrations. It also increases the Vmax of the enzyme and affinity of the allosteric sites for cGMP (Wyatt et al., 1998; Corbin et al., 2000; Rybalkin et al., 2002). Both cGMP binding to allosteric sites in PDE5 and its phosphorylation by PKGI contribute importantly to a powerful negative feedback on NO/cGMP/PKGI signaling (see section IV.D.4) (Corbin and Francis, 1999; Rybalkin et al., 2002; Mullershausen et al., 2003; Francis et al., 2009).
F. Cytoprotective Processes
Increased NO/cGMP signaling is cytoprotective in many cell types, but whether all of these protections require PKGI action is not known. Cytoprotective effects have been observed in heart cells (Qin et al., 2004; Kukreja et al., 2005; Das et al., 2008), neurons and glia (Barger et al., 1995; Nakamizo et al., 2003; Zhang et al., 2003; Thippeswamy et al., 2005; Korkmaz et al., 2009a,b; Paquet-Durand et al., 2009) and epithelial cells (Chan and Fiscus, 2003). Precise mechanisms for these protective effects are undoubtedly complex. One mechanism in cardiac tissues involves NO/cGMP/PKGI signaling to increase opening of mitochondrial K+/ATP channels and thereby diminish damage incurred in response to ischemia-reperfusion or myocardial infarcts (Ockaili et al., 2002; Das et al., 2004; Qin et al., 2004; Costa et al., 2005; Kukreja et al., 2005; Costa et al., 2008; Salloum et al., 2008, 2009), activate ERK signaling, and inhibit glycogen synthase kinase 3β (Das et al., 2008). Another mechanism that acts through PKGI blocks the proapoptotic effects of p38 mitogen-activated protein kinase (p38 MAPK) (Ge et al., 2002; Tanno et al., 2003), and yet another involves PKGI phosphorylation of Bad, a pro-apoptotic protein (Zha and Reed, 1997; Johlfs and Fiscus, 2010). A number of studies have demonstrated that signaling through PKGI decreases ischemia/reperfusion-induced cardiac damage (Qin et al., 2004; Kukreja et al., 2005; Das et al., 2008; Kasseckert et al., 2009). Evidence suggests that an increase in cGMP and activation of PKGI in cardiomyocytes causes PKG-mediated phosphorylation of an unknown protein (or proteins) on the outer mitochondrial membrane; this event triggers a signal that activates a PKCε that is in complex with and phosphorylates the mitochondrial potassium channel on the inner mitochondrial membrane (Costa et al., 2005, 2008; Jabůrek et al., 2006). PKC-mediated phosphorylation and activation of this channel produces rapid potassium influx into the mitochondria and increased production of reactive oxygen species (e.g., H2O2 and hydroxide radical). The reactive oxygen species purportedly activates another pool of PKCε, which inhibits the mitochondrial permeability transition pore and decreases cell damage. Although the precise mechanism by which cGMP/PKGI signaling brings about this protective effect is not known, many studies using diverse tissues support the critical involvement of PKGI in this process, which has been demonstrated in mitochondria from diverse tissues.
Major tissue damage to intact hearts or cardiomyocytes subjected to ischemia and reperfusion conditions is mediated in part by the proapoptotic pathway involving p38 MAPK (Ma et al., 1999; Yue et al., 2000). Under conditions of ischemia/reperfusion, p38 MAPK associates with the scaffold protein TAB1 (transforming growth factor-β-activated protein kinase 1 binding protein 1), where it undergoes autophosphorylation and activation (Ge et al., 2002; Tanno et al., 2003; Fiedler et al., 2006). As shown by coimmunoprecipitation and mutagenesis experiments, the leucine zipper region of cGMP-activated PKGI forms a complex with p38 MAPK, thereby blocking p38 MAPK interaction with TAB1 and activation of proapoptotic pathways; by this process, activation of PKGI decreases cell damage both in intact heart and isolated cardiomyocytes (Fiedler et al., 2006). No PKGI-mediated phosphorylation is apparently required for this effect, which suggests that PKGI acts by physically displacing another protein. If so, this is a heretofore unreported mechanism for cGMP-dependent PKGI action and may occur in other instances as well.
An antiapoptotic effect of PKGIα activation has been reported in neural cells. A part of this protection may be mediated by PKGI phosphorylation of Bad, an apoptosis-regulating Bcl-2 family member, at Ser-155 (Zha and Reed, 1997; Johlfs and Fiscus, 2010). Phosphorylation at this site by PKA and other kinases has been correlated with decreased apoptosis and increased cell viability. Ser-155 is located in the region that is required for dimerization with and blocking of Bcl-xL action, a cytoprotective protein. Phosphorylation at Ser-155 blocks Bad binding to Bcl-xL, thereby fostering cytoprotection (Datta et al., 2000; Tan et al., 2000; Zhou et al., 2000).
G. Proapoptotic Effects
Elevation of cGMP and activation of PKGI or overexpression of constitutively active PKG in colon cancer cells promotes apoptosis (Thompson et al., 2000; Deguchi et al., 2004). PKGI activation in colon cancer cells results in phosphorylation and activation of the mitogen-activated protein kinase kinase (MEKK1), activation of the stress-activated protein/ERK kinase 1 (SEK1), and activation of the c-Jun NH2-terminal kinase 1 (JNK1) pathway (Soh et al., 2000, 2001). Elevation of cGMP and activation of PKGI also suppresses growth and promotes apoptosis in some endothelial cells and human colon tumor HT29 cells (Zhu et al., 2005, 2009; Zhu and Strada, 2007). These effects have prompted efforts to use inhibitors of cGMP-hydrolyzing PDEs for treatment of certain types of cancers.
IV. Factors That Modulate Nitric Oxide/cGMP/cGMP-Dependent Protein Kinase I Signaling
With such a complex system, there are myriad possibilities for physiological or pathophysiological factors to impact the signaling process; these factors may vary among tissues. Regulation or impairment of NO-signaling through cGMP and PKGI can involve alterations in NO production and bioavailability, level and sensitivity of NO-GC activity, level and function of effector proteins (e.g., PKGs and cGMP-gated channels), localization/activity of cGMP-hydrolyzing PDEs, and phosphoprotein phosphatase activity. In some instances, alteration of function at one point in the pathway may elicit changes at another point, thereby emphasizing the interconnectedness of these components and complicating understanding of mechanisms in play.
A. Relationship between the Time Course of cGMP Elevation and Impact of Levels of cGMP and Intracellular cGMP-Binding Sites on Activation of the cGMP/cGMP-Dependent Protein Kinase Signaling Pathway
1. Time-Course of cGMP Elevation in Response to Nitric Oxide.
Elevation of cGMP and activation of PKG in response to NO donors are typically rapid. However, in some cases, the biological effects are slower than would be predicted if cGMP equilibration throughout the cell (estimated to require ∼0.2 s for a typical cell) were based only on the diffusion rate for cN (200–800 μm2/s) (Chen et al., 1999; Conti and Beavo, 2007). After treatment with NO donors, cGMP has been shown to peak at 5 to 30 s in platelets (Mullershausen et al., 2001, 2003) and at ∼50 s in vascular smooth muscle cells (Wyatt et al., 1998; Cawley et al., 2007). Activation of PKG after elevation of cGMP typically elicits phosphorylation of protein substrates such as PDE5 and VASP within seconds (Eigenthaler et al., 1992; Wyatt et al., 1998; Rybalkin et al., 2002). In cerebellar Purkinje cells, PDE5 phosphorylation, which is considered to be a good indicator for PKG activation, peaks at ∼20 min (Shimizu-Albergine et al., 2003). Microdomains that are either architecturally and/or enzymatically defined are likely to affect the temporal response of cells to the NO signal. Signals that stimulate PKGI-mediated phosphorylations are countered by actions of phosphoprotein phosphatases and PDEs that reverse the phosphorylations or terminate the signals, respectively.
2. Impact of Levels of cGMP and Intracellular cGMP-Binding Sites on Action of the cGMP/cGMP-Dependent Protein Kinase I Signaling Pathway.
Several points must be considered when discussing what is deemed to be a “physiologically relevant” change in cGMP. The affinities and cellular concentration of cGMP-binding sites on cGMP-binding proteins (PKGs and cGMP-binding PDEs) that compete for cGMP are key factors in determining cGMP action, but these points are rarely discussed in the literature. Moreover, both cGMP-binding sites on PKGI monomers contribute to activation of PKG phosphotransferase activity so that significant occupation of the sites is necessary to achieve optimum phosphotransferase activity (Francis and Corbin, 1999). The population of PKGI isozymes in a cell is also an important factor because of the difference in their cGMP affinities and the fact that PKGIα is partially activated when one cGMP is bound per two cGMP-binding sites of each monomer.
Studies have suggested that a ∼3- to 4-fold elevation of basal cGMP in vascular smooth muscle is sufficient to fully activate PKGI (Francis et al., 1988; Jiang et al., 1992). In addition, a 2- to 3-fold elevation of cGMP in murine platelets or aorta causes a substantial increase in phospho-VASP, which is considered a ubiquitous target for activated PKGI (Gambaryan et al., 2008; Walter and Gambaryan, 2009). However, NO donors produce ∼30- and 150-fold increases in cGMP in platelets and rat aortic strips, respectively (Eigenthaler et al., 1992; Mullershausen et al., 2001). In mouse platelets subjected to a maximal NO stimulus, intracellular cGMP (based on whole cell volume) has been estimated to transiently rise to 50 to 100 μM or higher. This concentration would be much higher if cGMP were synthesized/retained in particular microdomains. These levels of cGMP seem to be extraordinarily high compared with the affinities of most cellular cGMP receptors for cGMP. The catalytic sites of cGMP-hydrolyzing sites of some PDEs (PDE1C, PDE3, PDE5, PDE9, and PDE11) and the allosteric cGMP-binding sites on PKGs and certain PDEs have affinities for cGMP in the 0.02 to 3 μM range (Francis et al., 2005; Bender and Beavo, 2006a; Conti and Beavo, 2007).
The concentration of intracellular receptors for cGMP varies widely. In cells with a large amount of PKG and/or other cGMP-binding proteins, a greater excursion in cGMP would be required to activate a significant portion of the PKG; the extent of PKG activation that is required to elicit different biological processes may also vary within the same cell. In porcine vascular smooth muscle, PKGI (dimer) has been determined to be 0.06 to 0.13 μM, which would contain 0.24 to 0.52 μM cGMP-binding sites (i.e., four allosteric cGMP-binding sites per dimer) (Francis et al., 1988, 2005; Gopal et al., 2001). However, PKGI in platelets (∼3.7 μM dimer or 14.8 μM cGMP-binding sites) is much higher (Eigenthaler et al., 1992). Based on these considerations and a basal cGMP concentration in vascular smooth muscle cells of ∼0.1 μM, ∼6-fold elevation in cGMP would maximally activate PKG. However, if the basal level of cGMP in platelets were also ∼0.1 μM, then a 150-fold increase in cGMP would be required to saturate the sites (14.8 μM). This calculation does not consider the quantity of the allosteric cGMP-binding sites of the cGMP-binding PDEs and cGMP-gated channels or the catalytic sites of cGMP-hydrolyzing PDEs in those cells. Signaling through cGMP and PKG plays a pivotal role in both cell types, but the absolute concentrations of these molecules and the magnitude of change in cGMP required to activate cGMP signaling could differ markedly. cGMP-gated cation channels are typically much lower in abundance than PKGs; therefore, the amount of cGMP associated with these channels is predicted to minimally alter free intracellular cGMP. However, that is not the case for cGMP-binding PDEs such as PDE5 (Gopal et al., 2001).
B. Generation of the Nitric Oxide/cGMP Signal
A number of problems can decrease generation of the NO signal. This can result from a deficit of arginine (the substrate for NO synthases), although there is no clear evidence that arginine deficiency contributes significantly to low NO production in patients suffering from maladies associated with dysfunction in the NO/cGMP/PKGI pathway. A decrease in the level/activity of NO synthases or an increase in scavenging of NO can occur in instances in which the health and function of the tissue source of NO is compromised or stressed; these changes have been implicated in endothelial dysfunction or deterioration/disruption of nonadrenergic/noncholinergic nerves as occurs in diabetes, metabolic syndrome, spinal cord damage, and aging (Celermajer et al., 1993; Meurer et al., 2009; Musicki et al., 2009; Napoli and Ignarro, 2009). However, development of drugs (particularly by Bayer Schering Pharma AG) that directly activate NO-GC, thereby circumventing the NO-generation step, shows promise for treatment of patients who are plagued with problems in this portion of the pathway.
Compromised function and alterations in level of the NO-GC have also been implicated in diminished responsiveness to NO (Li et al., 2001; Laber et al., 2002; Witte et al., 2002; Friebe and Koesling, 2003; Münzel et al., 2003; Sayed et al., 2007, 2008). NO-GC is inactivated by NO-mediated nitrosation of its cysteines, and physiologically relevant levels of glutathione protect against this (Sayed et al., 2008; Mayer et al., 2009). Studies suggest that tolerance to nitrovasodilators could be due in part to desensitization of NO-GC, decreased metabolic processing of nitrovasodilators to generate NO, or increased cGMP breakdown. NO-mediated vasodilation that is blunted in a model of chronic hypoxia is associated with decreased NO-GC catalytic activity, although the NO-GC protein level is unchanged (Pearce et al., 2009). In this study, no significant changes were observed in PDE activity or relaxation of the vessels elicited by cGMP-analog activators of PKGI; this suggests that, in this model, impaired NO-GC function accounts for the hypoxia-induced desensitization to NO. In addition, the redox status of the NO-GC heme or loss of that heme can influence NO-GC function, and decreased responsiveness of NO-GC to NO under certain conditions has been reported (Meurer et al., 2009).
C. Temporal Regulation of cGMP Level
The cGMP signal produced in response to NO is typically short-lived as a result of substrate depletion, rapid breakdown/scavenging of NO, and strong negative feedback mechanisms (Corbin and Francis, 1999; Mullershausen et al., 2001). In platelets or smooth muscle exposed to NO, the peak of cGMP elevation is short-lived and declines to near basal level by ∼40 to 60 s (Mullershausen et al., 2001, 2003). Tight regulation of cGMP level, as with any biological signal, is required to maintain the sensitivity of the response to the incoming signal and rapid adjustment to changes in that signal. An increase in cGMP sufficient to saturate its intracellular receptors (i.e., PKG, cGMP-gated cation channels, and PDEs) would quickly overwhelm the potential for meaningful adjustments in the reactions generated by this pathway. Under such conditions, the signaling pathway would be functioning at full throttle and would be temporarily unresponsive to changes in the incoming signal, such as a decline in NO production. It is noteworthy that cGMP concentration in response to NO in platelets and vascular smooth muscle is biphasic and quickly returns to near basal levels primarily by the action of cGMP-hydrolyzing PDEs. The action of PDE5, in particular, plays a prominent role in lowering cGMP levels in these two cell types (Mullershausen et al., 2001, 2006; Farrow et al., 2008). Moreover, cGMP binds to and can remain bound to allosteric sites of certain PDEs (e.g., PDE2 and PDE5), thereby keeping them in the activated state for more than an hour. While bound to the PDEs, cGMP is protected against breakdown (Kotera et al., 2004a).
D. Termination or Blunting of cGMP Signaling by cGMP-Hydrolyzing Phosphodiesterases
Decline in the NO signal along with actions of PDEs are the major players in dynamically controlling cellular cGMP and terminating cGMP signaling. With waning of the signal, the NO dissociates from NO-GC, which then rapidly reverts to the inactive state. The response of cellular PDEs to increasing cGMP level is fast, likely to involve several PDEs, and frequently persists for extended periods of time because of regulatory features of the PDEs.
cGMP interacts with the catalytic site in cGMP-hydrolyzing PDEs, where it is broken down to 5′-GMP, which is inactive in this signaling pathway. cGMP-hydrolyzing PDEs occur in all cell types; the collection and abundance of PDE families in particular cell types vary and frequently differ among species. The 11 mammalian PDE families are derived from 21 genes, and PDE families 1, 2, 3, 5, 6, 9, 10, and 11 hydrolyze cGMP (Table 3) (Bender and Beavo, 2006a); with the exception of PDE6, isozymes of each of these families are widely distributed and contribute importantly to control of cellular cGMP (Fig. 6). Because of the additional presence of splice-variants, a given organism is estimated to contain more than 50 cGMP-hydrolyzing PDEs (Bender and Beavo, 2006a; Conti and Beavo, 2007). PDE catalytic function can be altered by numerous factors, including different physiological and pathophysiological processes that affect PDE protein expression, PDE protein breakdown, localization of PDEs, or the Vmax and/or Km of these enzymes. Therefore, interpretation of results involving PDE action should seriously consider these factors before making extrapolations to prospects for development of potential therapies that would intersect with these pathways. With some exceptions, the absolute concentration of PDEs within a cell is low because the catalytic rate for most PDEs is quite high (kcat values range from 0.5 to 4000 s−1 (Bender and Beavo, 2006a; Omori and Kotera, 2007). Moreover, the Km values for hydrolysis of cGMP by different PDEs cover a broad range of concentrations (0.2–40 μM). The biological advantage of such a diverse group of enzymes with broadly similar function is not clear, but selective compartmentation of some PDEs within cells, the apparent actions of these PDEs on particular pools of cGMP, and the capacity for diverse population of PDEs to deal with a broad range of cellular cGMP concentrations are now recognized (Conti and Beavo, 2007). Unlike other proteins in the NO/cGMP/PKG signaling pathway, there are few knockout models for the PDEs; the PD1B, PDE3, and PDE11 isozymes are the only cGMP-hydrolyzing PDEs that have thus far been studied in this way (Masciarelli et al., 2004; Choi et al., 2006; Siuciak et al., 2007; Sun et al., 2007; Kelly et al., 2010). Overlap in affinity of various PDEs for cGMP and the uncertainties about compartmentation of these enzymes make it difficult to assign exclusive function to one PDE even when relatively selective PDE inhibitors are used. Little is known about the efficiency with which PDE inhibitors access the interior of the cell and whether they are selectively localized in particular regions of those cells (Thompson, 1991; Wunder et al., 2005). This problem continues to confound studies of PDE actions.
TABLE 3.
Substrate specificities and kinetic characteristics of mammalian cGMP-hydrolyzing PDEs
Values compiled from Bender and Beavo (2006a). The range of values is likely to be due to the different assay conditions and preparations of the respective enzymes in various laboratories.
| Isoenzyme | Substrate Preference |
Km |
Vmax |
||
|---|---|---|---|---|---|
| cGMP | cAMP | cGMP | cAMP | ||
| μM | μmol/min/mg | ||||
| PDE1A | cGMP > cAMP | 3–4 | 73–120 | 50–300 | 70–750 |
| PDE1B | cGMP > cAMP | 1–6 | 10–24 | 30 | 10 |
| PDE1C | cGMP = cAMP | 1–2 | 0.3–1 | N.D. | N.D. |
| PDE2A | cGMP = cAMP | 10 | 30 | 123 | 120 |
| PDE3A | cGMP < cAMP | 0.02–0.2 | 0.2 | 0.3 | 3–6 |
| PDE3B | cGMP < cAMP | 0.3 | 0.4 | 2 | 9 |
| PDE5A | cGMP ≫ cAMP | 1–6 | 90 | 1–3 | 1–3 |
| PDE6A/B | cGMP ≫ cAMP | 15 | 700 | 2300 | N.D. |
| PDE6C | cGMP ≫ cAMP | 17 | 610 | 1400 | N.D. |
| PDE9A | cGMP ≫ cAMP | 0.2–0.7 | 230 | N.D. | N.D. |
| PDE10A | cGMP < cAMP | 13 | 0.2–1 | N.D. | N.D. |
| PDE11A | cGMP = cAMP | 0.4–2 | 0.5–3 | N.D. | N.D. |
N.D., no reliable data currently available.
Fig. 6.
Negative feedback mechanisms that blunt or terminate NO and cGMP signaling. cGMP signaling is blunted and/or terminated by multiple points of action that serve to decrease cGMP level by lowering NO-GC activity, activating cGMP-hydrolyzing PDEs, or decreasing levels of the proteins involved in the signaling. PDEs 1, 2, 3, 5, 9, 10, and 11 hydrolyze cGMP and are widespread throughout mammalian tissues. The activities of PDE2 and PDE5 can be further accelerated by allosteric cGMP binding and by phosphorylation for PDE5. [Adapted in part from Francis SH, Corbin JD, and Bischoff E (2009) Cyclic GMP-hydrolyzing phosphodiesterases. Handb Exp Pharmacol 191:367–408. Copyright © 2009 Springer Science+Business Media. Used with permission.]
1. cGMP-Specific Phosphodiesterases.
Three of the 11 mammalian PDE families (PDEs 5, 6, 9) have a ∼100-fold substrate preference for cGMP over cAMP as substrate and are therefore considered to be “cGMP-specific PDEs” (Bender and Beavo, 2006a). As alluded to in section IV.D, PDE6 expression is restricted to photoreceptor and pineal cells. PDE5 and PDE9 are the only “cGMP-specific PDEs” that are expressed in peripheral tissues, and the catalytic sites of both have affinities for cGMP that fall within the physiological range.
A role for PDE5 has now been established in modulating NO/cGMP effects in vascular smooth muscles, heart, platelets, and lower urinary tract organs (Francis et al., 2006, 2009; Kass et al., 2007a; Sandner et al., 2009). Involvement of PDE5 in many other processes continues to be a major point of interest to the scientific and medical communities (Rutten et al., 2005; Burnett et al., 2006; Heymann, 2006; Sandner et al., 2007). The Km of the PDE5 catalytic site for cGMP is 2 to 5 μM, and the kcat is ∼4.3 s−1. Despite low expression of PDE5 protein in many tissues, its role in cGMP signaling can still be quite important.
PDE9 catalytic site has exceptionally high affinity for cGMP (Km<0.2 μM), and its kcat has not been determined. It has been suggested to function as a “housekeeping” PDE to maintain low cellular cGMP (van Staveren et al., 2002). PDE9 expression has been reported in retinal pigment epithelial cells and in certain neurons (Diederen et al., 2007; de Vente et al., 2006; Reyes-Irisarri et al., 2007). In some instances, its expression in neural tissues coincides with that of the NO-GC; consequently, it has been suggested to play a role in NO/cGMP signaling in these cells.
2. Dual-Specificity Phosphodiesterases.
Among dual-specificity PDEs (PDEs 1, 2, 3, 10, and 11), hydrolysis of the respective cN occurs with varying efficacies. The affinity (Km) and selectivity of catalytic sites of dual-specificity PDEs for cGMP and cAMP can vary significantly even within a single PDE family. Among catalytic sites of PDE1 isozymes, affinities for cGMP are 1 μM (PDE1C2), 3 μM (PDE1B1), and 5 μM (PDE1A2), compared with 1, 24, and 113 μM, respectively, for cAMP. Maximum catalytic activities of the PDE1 isozymes for cGMP and cAMP breakdown are similar (Table 3) (Bender and Beavo, 2006a). Affinity of the PDE2 catalytic site for both cNs is low (∼10–30 μM) and above what is considered to be a physiological concentration for either cN; maximum breakdown rates for the cNs are similar (Bender and Beavo, 2006a; Conti and Beavo, 2007). In contrast, the high affinity of PDE3 for cGMP (Km ∼ 0.02–0.3 μM) and cAMP (Km ∼ 0.2–0.4 μM) is within the physiological range for these cNs, but the Vmax for cAMP is 5 to 10 times greater than that for cGMP (Manganiello et al., 1995; Degerman et al., 1997). PDE3 is commonly referred to as the “cGMP-inhibited cAMP-PDE” because as a high-affinity but low-turnover substrate, cGMP effectively competes with cAMP, thereby impeding cAMP hydrolysis. PKA phosphorylation of PDE3 increases its catalytic activity (López-Aparicio et al., 1993; Bender and Beavo, 2006a; Conti and Beavo, 2007), but whether PKGI also phosphorylates and activates PDE3 catalytic activity has not been studied. PDE10 has ∼70-fold higher affinity for cAMP compared with that for cGMP (Fujishige et al., 1999; Loughney et al., 1999; van Staveren et al., 2002). PDE11 hydrolyzes both cNs with similar efficacies and affinities (Km values for cAMP and cGMP are 1 and 1.6 μM, respectively) (Fawcett et al., 2000; Bender and Beavo, 2006a; Weeks et al., 2007).
3. Tissue Distribution and Function of cGMP-Hydrolyzing Phosphodiesterases.
In a given cell type, one commonly finds representatives of three or more cGMP-hydrolyzing PDE families. However, the population of PDEs in any given portion of a cell or even in cells of the same type in the same or different species can vary (Shimizu-Albergine et al., 2003; Vasta et al., 2005; Bender and Beavo, 2006a). In some cells, one PDE isozyme may predominate; examples of this include the zona glomerulosa cells of the adrenal cortex [in which PDE2 accounts for >90% of the PDE activity (MacFarland et al., 1991)], platelets [in which PDE5 activity is the major cGMP-hydrolyzing PDE (Ito et al., 1996)], and photoreceptor cells [in which PDE6 is abundant (Cote, 2006)]. Some PDEs are primarily located in the cytosol (e.g., PDE3A, PDE5, PDE9A5) (Liu and Maurice, 1998; Palmer and Maurice, 2000; Shakur et al., 2001; Wang et al., 2003; Francis et al., 2006); one has been reported to be in the nucleus (PDE9A1); some are primarily associated with particulate components of the cell (e.g., PDE3B, PDE10A2) (Kotera et al., 1999b); and others can be found in both regions (e.g., PDE3A) but with preferential location to one or the other venue (Bender and Beavo, 2006a; Conti and Beavo, 2007; Omori and Kotera, 2007). Evidence suggests that the action of each of these PDEs is important to physiological control of cGMP and perhaps particular cGMP pools (Castro et al., 2006; Takimoto et al., 2007; Wilson et al., 2008).
Development of compounds that selectively block the catalytic function of particular PDEs continues to be a major interest of the pharmaceutical industry, academicians, and clinicians. The potential impact of such compounds has already been demonstrated with the therapeutic and marketing success of three PDE5-selective inhibitors, sildenafil, vardenafil, and tadalafil, which bind to the PDE5 catalytic site, thereby blocking access and hydrolysis of cGMP (Jeremy et al., 1997; Francis and Corbin, 2003, 2005; Carson, 2005; Porst et al., 2006; Francis et al., 2009). More of these PDE5 inhibitors are making their way into the commercial market (Kouvelas et al., 2009). The availability of X-ray crystal structures of the catalytic domains of several cGMP-hydrolyzing PDEs, some in complex with an inhibitor or catalytic product, has provided meaningful direction for synthesis of potent and selective new drugs (Sung et al., 2003; Huai et al., 2004; Card et al., 2005; Wang et al., 2006). However, to date, all potent drugs that inhibit cGMP-hydrolyzing PDEs have been identified using the traditional medicinal chemistry approach of modifying a starting scaffold. The X-ray crystal structure of a near full-length PDE is only available for one isozyme (PDE2) (Pandit et al., 2009), and PDE2 is in the inactive state in this structure. Thus, understanding influences of the regulatory domains on catalytic site functions for most PDEs still relies on biochemical approaches.
A subset of PDEs (PDEs 2, 5, and 6) also has allosteric sites that interact with cGMP to regulate PDE function and sequester cGMP (Corbin and Francis, 1999; Bender and Beavo, 2006a; Cote, 2006; Francis et al., 2006; Conti and Beavo, 2007). These allosteric cGMP-binding sites in PDEs 2 and 5 are also considered to be good drug targets, but progress on this front continues to lag behind that made in identifying ligands for catalytic sites. Several structures of the allosteric cGMP-binding sites have now been reported and provide invaluable information about the topography of these pockets (Martinez et al., 2002, 2008; Heikaus et al., 2008, 2009; Pandit et al., 2009).
In addition to the concept of developing compounds that target only one protein, there is potential for development of compounds that act at several points in the cGMP-signaling pathway (as noted in the discussion of cN analogs in section II.B.1). Such compounds could potentially work at low levels and elicit synergistic effects. Examples of this have already been reported: YC-1 and BAY 41-2272, which were developed as NO-GC activators, are also potent inhibitors of PDE5 and act through both mechanisms to increase cGMP (Friebe et al., 1998; Mullershausen et al., 2004). Certain cGMP analogs are both potent PKGI activators and PDE inhibitors and would be predicted to synergistically increase PKGI-mediated phosphorylations (Sekhar et al., 1996).
4. Regulation of cGMP-Hydrolyzing Phosphodiesterases.
PDEs that hydrolyze cGMP have been shown to be subject to diverse regulatory influences, including expression level, cellular calcium level (PDE1), phosphorylation status (PDE3, PDE5, and PDE10), and expression level (PDE1, PDE3, and PDE5) (Liu and Maurice, 1998; Kotera et al., 1999a; Palmer and Maurice, 2000; Bender et al., 2005; Champion et al., 2005; Bender and Beavo, 2006a; Conti and Beavo, 2007; Omori and Kotera, 2007; Farrow et al., 2008; Francis et al., 2009). It is likely that regulatory features will ultimately be demonstrated for other cGMP-hydrolyzing PDEs. Although some cGMP-hydrolyzing PDEs undergo reversible relocation among cellular compartments (Kotera et al., 2004b), this process does not seem to be as common as occurs for certain cAMP-hydrolyzing PDEs (Conti and Beavo, 2007). The PDE5 catalytic site has moderate affinity for cGMP (Thomas et al., 1990a). Either phosphorylation by PKG or PKA in its regulatory domain or cGMP binding to its allosteric site increases catalytic-site affinity and catalytic rate (Corbin et al., 2000; Mullershausen et al., 2001, 2003; Rybalkin et al., 2002; Francis et al., 2006; Bessay et al., 2008).
Overall, little is known about regulation of the levels of PDE proteins, although changes in both the activities and levels of particular cGMP-hydrolyzing PDEs have been demonstrated in association with cellular proliferation and differentiation, as well as with tissue dysfunction (Maclean et al., 1997; Kotera et al., 1999a; Palmer and Maurice, 2000; Bender et al., 2004, 2005; Champion et al., 2005; Wharton et al., 2005; Bender and Beavo, 2006b; Nagel et al., 2006; Schermuly et al., 2007; Farrow et al., 2008; Miller et al., 2009). Both short-term and long-term regulation of the action of PDEs allows for fine control of the amplitude and duration of cGMP elevation and provides for refined blunting or enhancement of the signal (Gopal et al., 2001; Mullershausen et al., 2001, 2003; Rybalkin et al., 2002, 2003; Bender et al., 2004, 2005; Champion et al., 2005; Takimoto et al., 2005a; Fischmeister et al., 2006; Kass et al., 2007a; Omori and Kotera, 2007; Zhu and Strada, 2007; Muzaffar et al., 2008; Pokreisz et al., 2009; Zhu et al., 2009).
The affinities of the catalytic sites of certain PDE families (PDEs 1, 3, 5, 9, 10, and 11) for cGMP fall within what is accepted to be the range of cellular cGMP concentration (0.01–10 μM) in the basal and/or elevated state. As a result, under many conditions, the hydrolytic rate of PDEs would be less than maximal and increased cellular cGMP would be predicted to increase hydrolytic rate of these PDEs simply as a result of mass action. PDE2 has low affinity for cGMP (Km > 10 μM), which might suggest limited function in the “physiological” range of cGMP levels found in most cells, but its function has been shown to be physiologically relevant in a number of instances (MacFarland et al., 1991; Bender and Beavo, 2006a; Conti and Beavo, 2007; Surapisitchat et al., 2007; Masood et al., 2008, 2009).
PDE1, PDE2, PDE3, and PDE5 have been reported to be present in cardiomyocytes although there are still controversies in this area (see below). Members of the PDE1 and PDE3 families account for the greatest proportion of cGMP-PDE activity in the heart (Wallis et al., 1999; Vandeput et al., 2009). The cGMP-hydrolyzing activity of PDE1 is largely determined by intracellular calcium level and perhaps by specific pools of calcium that enter the cell in response to different stimuli (Miller et al., 2009). Although precise subcellular localization of PDE1 is not known, it apparently targets a pool of cGMP other than that targeted by PDE5 (Miller et al., 2009), and action of cGMP/PKGI to lower intracellular calcium would blunt PDE1 action by negative feedback. At least a portion of the cardiomyocyte PDE2 is presumed to be located near the cell membrane because it largely accounts for breakdown of cGMP pools in this compartment. PDE5, whose presence in cardiomyocytes continues to be a matter of debate, is largely cytosolic and apparently more involved in breakdown of cytosolic cGMP pools generated by NO-GC activation (Palmer and Maurice, 2000; Corbin et al., 2005; Castro et al., 2006; Fischmeister et al., 2006; Lukowski et al., 2010). The catalytic activities of the PDE3 family have long been recognized as having important impact on lipolysis, glycogenolysis, insulin secretion, and cardiac function (Degerman et al., 1997; Thompson et al., 2007). PDE3 was the target of the first pharmacological agent (milrinone) approved for blocking PDE action (Seino et al., 1995). Phosphorylation of PDE3B by either PKA or PKB activates catalysis, although different sites are modified; phosphorylation by PKA fosters association with 14-3-3 protein, and in this complex, phospho-PDE3B is resistant to dephosphorylation by phosphoprotein phosphatases (Palmer et al., 2007). The end result is a powerful negative feedback loop in which increased cAMP directly activates PDE3 through PKA action, and association of phospho-3B with the 14-3-3 protein prolongs the activated state of PDE3B.
Increases in PDE1 and PDE5 activities in cardiomyocytes have been reported to be associated with cardiac hypertrophy, and suppression of these activities produces an antihypertrophic effect (Corbin et al., 2003; Yanaka et al., 2003; Takimoto et al., 2005b; Nagayama et al., 2008; Zhang et al., 2008; Miller et al., 2009; Tsai and Kass, 2009). Most studies suggest that in the normal heart, PDE5 represents <30% of the cGMP-hydrolyzing activity, and many studies in both healthy persons and patients with a variety of cardiac maladies have shown that PDE5-selective inhibitors have little or no effect on cardiac function (Jackson, 2004, 2005; Carson, 2005; Jackson et al., 2006). However, studies probing cardiac functions in rodent models of the stressed heart indicate that imbalances in PDE5 activity and NO/cGMP/PKG signaling are involved in cardiac hypertrophy, work-load induced damage, reperfusion ischemic response, apoptosis, and modulation of acute β-adrenergic stimulation; certain stresses are reported to increase PDE5 level in cardiomyocytes (Kukreja et al., 2005; Takimoto et al., 2005a,b, 2007; Kass et al., 2007a,b; Khairallah et al., 2008; Zhang et al., 2008; Ziolo et al., 2008; Kasseckert et al., 2009; Miller et al., 2009; Nagayama et al., 2009a,b; Wang et al., 2009).
Blocking PDE5 activity in cardiomyocytes by use of sildenafil (or other PDE5 inhibitors) has been suggested to increase cGMP, activate PKG, and prevent and reverse cardiac hypertrophy induced by pressure overload (Takimoto et al., 2005b), but this interpretation is still controversial (Lukowski et al., 2010). The effect of sildenafil is suggested to be mediated at least in part by PKGI-mediated activation of RGS2, which suppresses Gq-coupled signaling through phospholipase Cβ, although other mechanisms may also be involved (Hsu et al., 2009; Takimoto et al., 2009). Using immunohistochemistry, the Kass group (Takimoto et al., 2005a,b; Zhang et al., 2008; Wang et al., 2009) found that PDE5 is primarily localized to the z-bands in cardiomyocytes; this localization is fostered by NO signaling. Persistence of PDE5 localization at the z-band in the absence of NO signaling is insufficient for the antiadrenergic effect of sildenafil. Because the pharmacological effect of sildenafil in this rodent model requires continued NOS activity, this could suggest that the associated increase in cGMP-binding to PDE5 allosteric sites and/or increased phosphorylation of PDE5 by activated PKG mediates the antihypertrophic effect of sildenafil. The antihypertrophic effect of cGMP-hydrolyzing PDE inhibitors is consistent with reports that NO-mediated activation of PKGI or overexpression of PKGI is also antihypertrophic (Calderone et al., 1998; Fiedler et al., 2002; Wollert et al., 2002).
In another model of cardiomyopathy, overexpression of NO-GC or sildenafil inhibition of PDE5 in dystrophin-deficient hearts improves contractile function, protects cardiomyocytes against workload-induced damage of the sarcolemma, improves the profile of mitochondrial metabolism, and mitigates progression of cardiac disease (Khairallah et al., 2008). Evidence is mounting that cGMP activation of PKGI and the resulting reduction in intracellular calcium blunts expression of genes associated with cardiomyocyte hypertrophy, perhaps in part through diminished calcineurin activity (Hogan et al., 2003; Koitabashi et al., 2010); however, other pathways may also participate in these antihypertrophic effects (Hsu et al., 2009).
The role of cGMP/PKG/PDE5 signaling in models of cardiac hypertrophy is highly controversial. Although it is generally agreed that elevation of cGMP in heart tissues is frequently associated with antihypertrophic and antifibrotic effects, the role of PKGI in mediating these effects is still unclear, and the PDE(s) that are responsible for controlling cGMP level is also unclear. Some have suggested that effects of agents (e.g., PDE5 inhibitors, natriuretic factors) that increase cGMP signaling and blunt cardiac hypertrophy may result at least in part from effects on cells other than cardiomyocytes (e.g., myofibroblasts or coronary artery smooth muscle cells) or through inhibition of other cGMP-hydrolyzing PDEs (Conti and Beavo, 2007; Vandeput et al., 2009; Lukowski et al., 2010). Lukowski et al. (2010) have examined the proposed importance of PKGI and PDE5 in cardiac hypertrophy induced by either isoproterenol or transverse aortic constriction using PKGI knockout mice [PKGI(−/−)] that lack PKG globally, mice that express PKGIβ only in smooth muscle (PKGIβ rescue mice), and control mice. This group found that absence of PKGI in cardiomyocytes does not increase the hypertrophy induced by either process and does not increase basal heart size; this suggests that proteins other than PKGI may mediate the antihypertrophic effects of cGMP. Moreover, no changes in cGMP-hydrolyzing PDE activities or protein levels in these heart tissues could be discerned, and no PDE5 activity or protein could be detected in cardiomyocytes from any of these animals. These results raise questions about the mode of action reported for the antihypertrophic effects of PDE5 inhibitors and are difficult to reconcile with the well documented reports of others who have provided compelling evidence for the functional importance of PKGI and PDE5 in cardiomyocytes. However, differences in the models and conditions that have been used to study these processes and the overall complexity of signaling in the heart in response to various agonists and functional challenges may account for some of the conflicting results. It is clear that the role of PKGI and PDE5 in mediating/modulating effects of cGMP and/or PDE inhibitors in the heart remains an open question.
cGMP elevation in platelets is physiologically critical in inhibiting platelet aggregation (Maurice, 2005; Walter and Gambaryan, 2009). Platelets contain cGMP-hydrolyzing PDE2, PDE3, and abundant amounts of PDE5, as well as a high level of PKGIβ. A portion of platelet PDE5 that has low catalytic activity is localized to a complex composed of PKGIβ, IP3R1, and IRAG (Wilson et al., 2008). After elevation of cGMP and activation of PKG, the PDE5 in this complex is phosphorylated and activated, thereby decreasing cGMP and countering changes in intracellular calcium; the PDE5 activity in the cytosol is reportedly unaffected. The authors suggest that each pool of PDE5 is involved in controlling cGMP in select microdomains, and that localized, rather than global, changes in cGMP in response to NO (or PDE5 inhibitors) selectively affect calcium mobilization and its proaggregatory effects.
5. Allosteric cGMP-Binding Sites on Phosphodiesterases.
Allosteric cGMP-binding sites of PDEs 2, 5, and 6 are located in a GAF subdomain in the N-terminal portion of the respective proteins. cGMP is bound at these sites with strong preference over cAMP (Fig. 7, A and B); the cGMP-binding affinities of these sites, which range from 0.02 μM for PDE2 (Wu et al., 2004) and 0.03 to 0.2 μM for PDE5 (Francis et al., 2006), to 0.1 μM for PDE6 (Cote, 2006), are much higher than those of the respective PDE catalytic sites. This suggests that cGMP would occupy the allosteric sites and induce the more active state before significant interaction of cGMP with the respective catalytic sites. PDE2 and PDE5 are expressed in many tissues involved in NO/cGMP/PKG signaling, but PDE6 is found exclusively in photoreceptor cells, the pineal gland, and some types of melanoma cells (Cote, 2006; Bazhin et al., 2010). The allosteric sites of PDE2 and PDE5 would be predicted to continue to bind cGMP well after cGMP has fallen below concentrations that would significantly interact with the respective catalytic sites. However, the PDE allosteric sites would be predicted to interact with cGMP within the range of concentrations that affect PKGI activity. Because allosteric cGMP binding to PDEs activates the catalytic sites, this would sustain an activated state of cGMP breakdown for a significant time, thereby contributing to negative feedback action (Mullershausen et al., 2003; Bender and Beavo, 2006a). Moreover, the cGMP-binding affinities of some of the allosteric sites (e.g., PDE5) are enhanced by ligand occupation of the catalytic site, phosphorylation of the regulatory domain, oxidation/reduction, and other processes, and the impact of these affinity shifts within cells is not yet fully appreciated (Corbin et al., 2000; Mullershausen et al., 2001; Bender and Beavo, 2006a; Cote, 2006; Francis et al., 2006; Conti and Beavo, 2007; Bessay et al., 2008). These allosteric sites on PDEs also have the potential to act to sequester cGMP from the cytosol, and bound cGMP is protected against breakdown (Corbin and Francis, 1999; Gopal et al., 2001; Kotera et al., 2004a; Francis et al., 2005). As the cGMP signal declines, the bound form of cGMP could then be released to foster continued cGMP signaling.
Fig. 7.
Working model of PDE2 and PDE5. A, PDE2 hydrolyzes cGMP and cAMP, and binding of cGMP to GAF-B stimulates cGMP breakdown at the catalytic site, resulting in a negative feedback action of cGMP level. Zn2+ and another divalent metal ion (perhaps Mg2+ or Mn2+) comprise a binuclear metal binding site where a hydroxyl ion from water is polarized for breaking the cyclic phosphate ring. B, PDE5 has overall structure similar to that of PDE2. PKGI phosphorylates Ser-102 located near the amino terminus; both phosphorylation and allosteric cGMP binding, which occurs at GAF-A, increase cGMP breakdown at subsaturating cGMP level. [Adapted from Francis SH and Corbin JD (2005) Phosphodiesterase-5 inhibition: the molecular biology of erectile function and dysfunction. Urol Clin North Am 32:419–429. Copyright © 2005 Elsevier Ltd. Used with permission.]
From these considerations, it is evident that cGMP produced within a given cell or a particular microdomain in that cell is likely to encounter multiple sites that compete for cGMP binding based on affinity and abundance. The total concentration of the respective binding sites will also affect the cGMP distribution, thereby influencing signaling efficacy and persistence of the signal. For instance, in the penile corpus cavernosum, total concentration of allosteric cGMP-binding sites derived from PKGI and PDE5 is >240 nM compared with a low basal level (∼20 nM) of cGMP (Gopal et al., 2001). Because the affinities of these proteins for cGMP are in the same range, cGMP would be predicted to immediately distribute into all of these sites.
E. Therapeutic Use of Inhibitors of cGMP-Hydrolyzing Phosphodiesterases
A number of inhibitors of cGMP-hydrolyzing PDEs are currently in clinical use for treatment of maladies associated with cardiovascular health. Cilostazol (Pletal), a relatively selective inhibitor of PDE3, exhibits vasodilatory and antiaggregatory effects on vascular smooth muscle and platelets, respectively, and is marketed to treat intermittent claudication (Kambayashi et al., 2006); it also blocks adenosine uptake and has weak inhibitory potency for PDE5, which is abundant in its target tissues. Milrinone (Primacor) is also a selective inhibitor of PDE3 and is used in treatment of acute heart failure (Stehlik and Movsesian, 2006). Dipyridamole, a weak inhibitor of three cGMP-hydrolyzing PDEs (PDE5, PDE10, and PDE11), also blocks adenosine uptake and is used for prevention of ischemic events after stroke (Schaper, 2005). The antiaggregatory effect of dipyridamole in platelets involves its effect on adenosine uptake, but at therapeutic levels achieved in plasma, it could also inhibit PDE5 activity (Serebruany et al., 2009). Both effects would promote platelet disaggregation. The PDE5-selective inhibitors (vardenafil, sildenafil, and tadalafil) have gained wide visibility as treatments for erectile dysfunction. Sildenafil (marketed as Revatio) and tadalafil (marketed as Adcirca) are also approved for treatment of pulmonary hypertension (Francis et al., 2009; Falk et al., 2010). PDE5 inhibitors have also been shown to be effective in treatment of Raynaud's disease, although they are currently not approved for this use (Fries et al., 2005; Levien, 2006).
The outstanding safety profiles of the PDE5 inhibitors have encouraged consideration of use of these medications for treatment of other maladies. In fact, a clinical trial entitled “PDE-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure (RELAX)” and sponsored by the National Heart, Lung, and Blood Institute is currently enrolling participants. Given the strong basic science background for effects of these inhibitors in models of ischemic/reperfusion cardiac damage described above in section III.F, it is likely that there will soon be trials examining potential clinical applications for this problem. PDE5 inhibitors may also be useful in treatment of benign prostate syndrome and lower urinary tract symptoms (LUTS), which are common maladies in aging men. PDE5 is expressed in human prostate and bladder, and PDE5 inhibitors elicit relaxation of tone in tissues from both organs. Preliminary trials with PDE5 inhibitors decreased LUTS in some patients (Sandner et al., 2009). Investigators are testing the efficacy of PDE5-selective inhibitors for reducing symptoms associated with these maladies. Symptoms of overactive bladder syndrome and urge urinary incontinence occur in both male and female patients and are related to those that occur in LUTS; inhibitors that block PDE5 and/or PDE1 activity have shown some promise in relieving symptoms and are considered potential therapies for these problems (Gacci et al., 2007). In addition, use of PDE inhibitors is being tested for treatment of premature ejaculation, Peyronie's disease, and ureteral relaxation for eased passage of kidney stones (Gratzke et al., 2007; Sandner et al., 2009). There is some evidence that elevation of cGMP by use of PDE5 inhibitors is atheroprotective in the vasculature (Kemp-Harper and Schmidt, 2009) and may improve endothelial health (Rosano et al., 2005; Aversa et al., 2007). Despite the small effect of the approved PDE5 inhibitors on systemic blood pressure, investigators still consider development of pharmacotherapies that increase cGMP in the vasculature good candidates for treatment of hypertension (Kemp-Harper and Schmidt, 2009), and there continues to be some interest in usefulness of these therapies for treatment of female sexual dysfunction. Finally, reports have suggested that PDE5 inhibitors may speed circadian adaptation to changes in light schedules (as experienced in transcontinental travel) (Agostino et al., 2007), are neuroprotective in stroke models in rodents (Zhang et al., 2002), and have procognitive functions (Prickaerts et al., 2002). An inhibitor for PDE10 that hydrolyzes both cGMP and cAMP is considered a promising target for treatment of schizophrenia (Schmidt et al., 2008). Thus, all proteins involved in the NO/cGMP/PKG/PDE pathway are now recognized as potential drug targets for modulating this signaling.
V. Feedback Mechanisms That Affect cGMP Signaling
A. Negative Feedback
Negative feedback regulation is a common control for accelerating the demise of second messenger signals, including that of cGMP. In addition to the normal mass action effect of cGMP elevation, this process is mediated by several mechanisms in the cGMP-signaling pathway: 1) increased breakdown of cGMP as a result of cGMP activation of catalytic activities by binding to allosteric sites of certain PDEs (PDEs 2 and 5) (Figs. 6 and 7) (Bender and Beavo, 2006a; Conti and Beavo, 2007); 2) increased breakdown of cGMP resulting from phosphorylation of the regulatory domain (PDE3 and PDE5) (Ahmad et al., 2000; Koesling et al., 2005; Francis et al., 2009); 3) increased binding of cGMP to allosteric sites in PDEs resulting in sequestration of cGMP (PDEs 2 and 5) (Gopal et al., 2001; Kotera et al., 2004a); 4) desensitization of NO-GC (Browner et al., 2004; Mayer et al., 2009), 5) down-regulation of PKG protein level (Lincoln et al., 2001; Browner et al., 2004; Dey et al., 2009), and 5) up-regulation of PDEs such as PDE1 and PDE5 (T. Kono, S. Francis, and J. Corbin, unpublished observations; Kim et al., 2001).
PDE1 and PDE5 play major roles in negative feedback regulation of cGMP signaling in vascular and airway smooth muscle (Corbin and Francis, 1999; Kim et al., 2001; Mullershausen et al., 2006; Farrow et al., 2008; Wilson et al., 2008). Under basal conditions, cGMP level is well below the Km of both PDEs, so that as cGMP synthesis increases, cGMP hydrolysis accelerates simply as a result of mass action. In addition, cGMP binds to the allosteric cGMP-binding site provided by GAF-A on PDE5. This binding increases catalytic site affinity for cGMP, thereby increasing the catalytic rate at subsaturating cGMP. It also increases phosphorylation of Ser-102 by PKG, which further increases catalytic site affinity for cGMP and also enhances allosteric site affinity for cGMP; this increases cGMP sequestration away from the free cytosolic pool. In concert, these events increase the PDE5 efficiency to thwart the NO-initiated increase in cellular cGMP level. These events occur within seconds after NO-stimulation and are pivotal in returning cGMP to near-basal levels in the short term. Moreover, these same events increase the affinity of the PDE5 catalytic site for inhibitors, which then facilitates potency and efficacy of the pharmacological action of these compounds (Blount et al., 2004; Bessay et al., 2008).
B. Persistent Effects of Nitric Oxide/cGMP Signaling
In platelets, vascular smooth muscle and airway smooth muscle, the effects of increases in cGMP and activation of the negative feedback pathway through PDE5 activation are, in effect, “remembered” for more than an hour (Fig. 8) (Wyatt et al., 1998; Mullershausen et al., 2001, 2003). In these instances, prior exposure to a stimulus that elevates cGMP causes rapid activation of PDE5 that persists and desensitizes the cell to subsequent challenges by the activator. In both vascular smooth muscle cells and platelets, evidence indicates that cGMP binding to the PDE5 allosteric site is sufficient to activate the enzyme and that this cGMP dissociates slowly so that the activation of catalytic function is sustained. Because the affinity of the allosteric cGMP-binding sites of both PDE5 and PDE2 for cGMP is ∼10- to 50-fold greater than that of the respective catalytic sites, cGMP may remain associated with these allosteric sites well after cGMP has fallen below that required for interaction with the PDE catalytic sites. This cGMP-bound form of PDE5 and PDE2 would preserve the activated state that is primed to counter subsequent increases in cGMP. Responsiveness to a second NO signal is slowly recovered as indicated by the progressive increase in both the magnitude of the increase in cGMP and the time course of its elevation (Fig. 8). However, in patients taking nitroglycerin, NO exposure would be ongoing and could contribute to NO resistance.
Fig. 8.
Desensitization of cGMP accumulation in response to NO. Strips of aorta were preincubated in the absence [Control (No Pretreatment)] or presence of a NO donor for 10 min; tissue was washed to remove the NO donor. After either 30 or 60 min, the tissue was rechallenged with high levels of the NO donor. Tissues were harvested at indicated times and analyzed for cGMP content. [Adapted from Mullershausen F, Lange A, Mergia E, Friebe A, and Koesling D (2006) Desensitization of NO/cGMP signaling in smooth muscle: blood vessels versus airways. Mol Pharmacol 69:1969–1974. Copyright © 2006 American Society for Pharmacology and Experimental Therapeutics.]
Phosphorylation of PDE5 enhances the desensitization but is not necessary for the persistent and diminished changes in cGMP in response to a NO signal (Mullershausen et al., 2001, 2003, 2005). However, phospho-PDE5 persists for at least an hour in some instances, indicating that phosphoprotein phosphatases do not rapidly dephosphorylate the enzyme under these conditions (Wyatt et al., 1998; Corbin and Francis, 1999; Mullershausen et al., 2001, 2003, 2006; Koesling et al., 2005; Francis et al., 2006). Agents that directly activate PKG potently relax the tissues, indicating that the desensitization is not due to changes in PKG function and is consistent with the interpretation that desensitization is due to an effect on PDE5 (Mullershausen et al., 2006).
In some instances, prolonged exposure to NO and increased cGMP lead to more persistent changes at several steps in the NO/cGMP/PKG signaling pathway, including down-regulation of PKG and up-regulation of PDEs. Long-term exposure of vascular tissues to NO elicits down-regulation of PKG activity, PKG protein, and PKG mRNA (Gao et al., 2004). Tolerance to NO derived from nitrovasodilators is associated with up-regulation of PDE1A mRNA, PDE1A protein, and PDE1A activity levels (Kim et al., 2001); PDE5 protein and mRNA levels are unchanged. In results from our lab, PDE5 activity in vascular smooth muscle cells incubated with 8-Br-cGMP increases over time (T. Kono, S. Francis, and J. Corbin, unpublished observations) (Fig. 9). This is accompanied by a decline in PKGI activity. A similar decline in PKGI protein as well as PKGI mRNA has been reported by Browner et al. (2004) in cultured bovine aortic smooth muscle cells exposed to inflammatory cytokines, NO-donors, or cGMP analogs.
Fig. 9.
Long-term regulation of PDE5 and PKGI in response to persistent elevation of cGMP. Cultured rat vascular smooth muscle cells were exposed to 0.1 mM 8-Br-cGMP for various times. PDE5 catalytic activity was measured using 0.3 μM cGMP as substrate ± 0.1 μM sildenafil as described previously (Corbin et al., 2005). PKG and PKA catalytic activities were determined as described previously (Jiang et al., 1992). cAMP-PDE activity [measured at either 0.3 or 30 μM cAMP as described previously (Corbin et al., 2005)] and activity of PDE 1, 3, or 4, determined on the basis of effects of family-selective PDE inhibitors, was unchanged. Prolonged exposure to 0.1 mM 8-Br-cAMP had no effect. Data represent the mean ± S.E. (n = 3–7).
Suppression of PKGI is associated with and dependent upon increased NO-GC activity and high cGMP, which cross-activates PKA to alter PKGI gene expression. Sellak et al. (2002) had earlier demonstrated that NO-induced suppression of PKG expression in vascular smooth muscle cells is mediated by PKA action to interfere with Sp1-dependent gene transcription. Likewise, promoters that drive expression of PDE5A are up-regulated by either cAMP or cGMP, which would be consistent with effects of prolonged exposure of cells to either cN analogs or NO (Kotera et al., 1999a; Lin et al., 2001a,b). The molecular basis for these changes in PKGI and PDE5 are still poorly understood and are likely to be mediated by a number of processes, including changes in gene transcription, mRNA stabilization, protein synthesis, protein degradation, or state of activation of the respective proteins. For PKG, prolonged activation fosters autophosphorylation, which has been shown to promote ubiquitination and protein breakdown (Dey et al., 2009). PKG expression is also down-regulated by RhoA and up-regulated by Rac1, but whether these processes are in play with prolonged elevation of cGMP is not known (Zeng et al., 2006). These changes in PDEs and PKG in response to sustained increases in the NO/cGMP/PKG signaling pathway suggest a classic feedback response to blunt or terminate a signal. Nevertheless, unlike the tolerance associated with NO therapies, no tachyphylaxis after use of PDE5 inhibitors for treatment of erectile dysfunction has been reported despite prolonged and regular use in many instances. So far, however, most of these inhibitors have been taken sporadically (once or twice a week). A daily regimen has been approved for tadalafil so that drug exposure will be more sustained; long-term follow-up on these patients with particular attention to an increase in tachyphylaxis would be prudent.
An unexpected persistence of the pharmacological effects of PDE5 inhibitors has been reported in men being treated for erectile dysfunction and in experimental protocols that model cardiac damage. In many men treated with PDE5 inhibitors (sildenafil or tadalafil) for relief of erectile dysfunction, improvement of the erectile response persists beyond the time required for plasma clearance of the drugs (t1/2 values of 4 and 18 h, respectively) to subtherapeutic levels (Porst et al., 2003; Moncada et al., 2004; Young et al., 2005; Shabsigh et al., 2006). This suggests that sustained high level of the inhibitors in plasma is not required for continued therapeutic efficacy. However, the molecular mechanism underlying this phenomenon is not understood (Francis et al., 2008). One possibility is that the biochemical processes and changes elicited by elevation of cGMP in penile vascular smooth muscle are retained for many hours after PDE5 inhibitors are cleared. This scenario is supported by results reported by Kukreja and colleagues (Salloum et al., 2003; Kukreja et al., 2005) in their studies of the cardioprotective effects of sildenafil in hearts exposed to ischemia-reperfusion conditions or induction of myocardial infarction. A single dose of sildenafil in these mice has cardioprotective effects even at 24 h. Hearts from sildenafil-treated animals have significantly elevated levels of eNOS and inducible NOS proteins, the activities of which are implicated in the protective effects (Salloum et al., 2003; Kukreja et al., 2005); cGMP level in hearts of sildenafil-treated animals is elevated, which would foster increased PKGI-mediated processes (Das et al., 2002).
Alternatively, tissue sensitivity to a subsequent cGMP signal may be enhanced by a feed-forward process. These inhibitors have very high affinity for PDE5 (0.1–4 nM), and because of this tight binding to the enzyme, they may be retained in smooth muscle beyond the plasma clearance time (Francis et al., 2008). Exposure of purified PDE5 to sildenafil, vardenafil, or tadalafil for 5 to 12 h causes a time-dependent conversion of PDE5 to a form that has higher affinity for these compounds (Blount et al., 2007). Sildenafil, vardenafil, and tadalafil reach peak plasma concentration (and presumably peak cellular concentration) at ∼1 to 2 h after dosing, and the t1/2 for plasma clearance of these drugs is ∼4, ∼4, and ∼18 h, respectively. Thus, the time of PDE5 exposure to these medications is within the range of the time required for PDE5 conversion to a higher-affinity conformation. Moreover, the smooth muscle in the penile vasculature of a man who has been sexually aroused after taking one of these medications will experience an increase in cGMP synthesis as a result of NO released from penile nerves and endothelial cells and a decrease in cGMP breakdown as a result of these medications to block PDE5 activity. PDE5 conversion to a higher-affinity form would be enhanced not only by the time of exposure to the inhibitors before and after sexual arousal but also by the NO-induced rise in cGMP in response to sexual arousal. This would foster increased cGMP binding to PDE5 allosteric sites and phosphorylation at Ser-102 (human) in its regulatory site, both of which increase the affinity of PDE5 interaction with the respective inhibitors (Corbin et al., 2000; Blount et al., 2004, 2007; Bessay et al., 2008). All components would favor a conformation of PDE5 that has significantly higher affinity for the inhibitors. As these medications are cleared from the plasma, the amount that is free in the cytosol will decline in parallel. However, the component that is bound to PDE5 (both higher and lower affinity states) will dissociate slowly; once dissociated, it is likely to be bound again either by the same PDE5 molecule or by another PDE5 that has a free catalytic site, thereby retarding clearance of the drug from the cell and providing for persistence of its inhibition of PDE5 beyond the predicted therapeutic time frame based on plasma clearance time (Francis et al., 2008). Thus, in contrast to the NO-desensitization associated with prior NO exposure that has been described in section IV.B for platelets and aortic vascular smooth muscle, in this setting, the penile vascular smooth muscle seems to have enhanced responsiveness to a subsequent NO signal from penile nerves and endothelium. This apparently disparate response of different smooth muscles to NO-induced increased cGMP may indicate that there are distinct mechanisms at play even when tissues are closely related. The fact that vascular smooth muscle cells in the penile corpus cavernosum are continually exposed to a low level of endothelially derived NO, as are other vascular smooth muscle cells and platelets, but experience a brief surge of neuronally derived NO during sexual arousal may be a partial explanation for this different response.
VI. Conclusions
Many of the mechanisms involved in NO signaling through cGMP and PKG are now well established, and roles of this pathway in physiological and pathological processes continue to be discovered. The impact of the action of cGMP-hydrolyzing PDEs on this signaling pathway is also well established, and the potential for therapeutic intervention has been demonstrated by the success of PDE5 inhibitors sildenafil, tadalafil, and vardenafil for treatment of erectile dysfunction and pulmonary hypertension. There are many opportunities and challenges to fully understand this complex NO/cGMP-signaling pathway. A full grasp of its mechanisms will undoubtedly open new avenues for treating medical consequences of its dysfunction.
Acknowledgments.
This work was supported by the National Institutes of Health National Institutes of Diabetes and Digestive and Kidney Diseases [Grant DK40029].
This article is available online at http://pharmrev.aspetjournals.org.
doi:10.1124/pr.110.002907.
- AKAP
- anchoring protein for PKA
- BAY 41-2272
- 5-cyclopropyl-2-[1-(2-fluoro-benzyl)-1H-pyrazolo[3,4-b]pyridine-3-yl] pyrimidin-4-ylamine
- BAY 58-2667
- cinaciguat
- CAP
- catabolite gene-activator protein
- cN
- cyclic nucleotide
- DT-2
- membrane permeable peptide inhibitor for PKGIα
- eNOS
- endothelial nitric-oxide synthase
- EPAC
- exchange proteins activated by cAMP
- GAF
- protein domain conserved in cGMP-binding PDEs/Anabaena adenylyl cyclases/Escherichia coli FhlA
- GKIP
- cGMP-dependent protein kinase-interacting protein
- IP3
- inositol 1,4,5-trisphosphate
- IRAG
- IP3 receptor-associated PKG substrate
- LASP
- LIM and SH3 domain protein
- LUTS
- lower urinary tract symptoms
- MAPK
- mitogen-activated protein kinase
- NFAT
- nuclear factor of activated T cells
- nNOS
- neuronal nitric-oxide synthase
- NO
- nitric oxide
- NO-GC
- NO-activated or soluble guanylyl cyclase
- NOS
- nitric-oxide synthase
- PDE
- phosphodiesterase
- PKA
- cAMP-dependent protein kinase
- PKG
- cGMP-dependent protein kinase
- RGS
- regulator of G-protein signaling
- SERT
- serotonin transporter
- TFII-I
- transcription factor II-I
- TPα
- α isoform of the thromboxane A2 receptor
- TRPC
- transient receptor potential canonical
- VASP
- vasodilator-stimulated phosphoprotein.
References
- Agostino et al., 2007.Agostino PV, Plano SA, Golombek DA. (2007) Sildenafil accelerates reentrainment of circadian rhythms after advancing light schedules. Proc Natl Acad Sci USA 104:9834–9839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad et al., 2000.Ahmad F, Cong LN, Stenson Holst L, Wang LM, Rahn Landstrom T, Pierce JH, Quon MJ, Degerman E, Manganiello VC. (2000) Cyclic nucleotide phosphodiesterase 3B is a downstream target of protein kinase B and may be involved in regulation of effects of protein kinase B on thymidine incorporation in FDCP2 cells. J Immunol 164:4678–4688 [DOI] [PubMed] [Google Scholar]
- Aitken et al., 1981.Aitken A, Bilham T, Cohen P, Aswad D, Greengard P. (1981) A specific substrate from rabbit cerebellum for guanosine-3′:5′- monophosphate-dependent protein kinase. III. Amino acid sequences at the two phosphorylation sites. J Biol Chem 256:3501–3506 [PubMed] [Google Scholar]
- Aitken et al., 1984.Aitken A, Hemmings BA, Hofmann F. (1984) Identification of the residues on cyclic GMP-dependent protein kinase that are autophosphorylated in the presence of cyclic AMP and cyclic GMP. Biochim Biophys Acta 790:219–225 [DOI] [PubMed] [Google Scholar]
- Aizawa et al., 2003.Aizawa T, Wei H, Miano JM, Abe J, Berk BC, Yan C. (2003) Role of phosphodiesterase 3 in NO/cGMP-mediated antiinflammatory effects in vascular smooth muscle cells. Circ Res 93:406–413 [DOI] [PubMed] [Google Scholar]
- Alderton et al., 2001.Alderton WK, Cooper CE, Knowles RG. (2001) Nitric oxide synthases: structure, function and inhibition. Biochem J 357:593–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alioua et al., 1998.Alioua A, Tanaka Y, Wallner M, Hofmann F, Ruth P, Meera P, Toro L. (1998) The large conductance, voltage-dependent, and calcium-sensitive K+ channel, Hslo, is a target of cGMP-dependent protein kinase phosphorylation in vivo. J Biol Chem 273:32950–32956 [DOI] [PubMed] [Google Scholar]
- Alverdi et al., 2008.Alverdi V, Mazon H, Versluis C, Hemrika W, Esposito G, van den Heuvel R, Scholten A, Heck AJ. (2008) cGMP-binding prepares PKG for substrate binding by disclosing the C-terminal domain. J Mol Biol 375:1380–1393 [DOI] [PubMed] [Google Scholar]
- Amano et al., 1996.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. (1996) Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 271:20246–20249 [DOI] [PubMed] [Google Scholar]
- Ammendola et al., 2001.Ammendola A, Geiselhöringer A, Hofmann F, Schlossmann J. (2001) Molecular determinants of the interaction between the inositol 1,4,5-trisphosphate receptor-associated cGMP kinase substrate (IRAG) and cGMP kinase Ibeta. J Biol Chem 276:24153–24159 [DOI] [PubMed] [Google Scholar]
- Andric et al., 2007.Andric SA, Janjic MM, Stojkov NJ, Kostic TS. (2007) Protein kinase G-mediated stimulation of basal Leydig cell steroidogenesis. Am J Physiol Endocrinol Metab 293:E1399–E1408 [DOI] [PubMed] [Google Scholar]
- Angelone et al., 2008.Angelone T, Filice E, Quintieri AM, Imbrogno S, Recchia A, Pulera E, Mannarino C, Pellegrino D, Cerra MC. (2008) Beta3-adrenoceptors modulate left ventricular relaxation in the rat heart via the NO-cGMP-PKG pathway. Acta Physiol (Oxf) 193:229–239 [DOI] [PubMed] [Google Scholar]
- Antl et al., 2007.Antl M, von Brühl ML, Eiglsperger C, Werner M, Konrad I, Kocher T, Wilm M, Hofmann F, Massberg S, Schlossmann J. (2007) IRAG mediates NO/cGMP-dependent inhibition of platelet aggregation and thrombus formation. Blood 109:552–559 [DOI] [PubMed] [Google Scholar]
- Aswad and Greengard, 1981.Aswad DW, Greengard P. (1981) A specific substrate from rabbit cerebellum for guanosine 3′:5′-monophosphate-dependent protein kinase. I. Purification and characterization. J Biol Chem 256:3487–3493 [PubMed] [Google Scholar]
- Aversa et al., 2007.Aversa A, Bruzziches R, Vitale C, Marazzi G, Francomano D, Barbaro G, Spera G, Rosano GM. (2007) Chronic sildenafil in men with diabetes and erectile dysfunction. Expert Opin Drug Metab Toxicol 3:451–464 [DOI] [PubMed] [Google Scholar]
- Barber and Butcher, 1981.Barber R, Butcher RW. (1981) The quantitative relationship between intracellular concentration and egress of cyclic AMP from cultured cells. Mol Pharmacol 19:38–43 [PubMed] [Google Scholar]
- Barger et al., 1995.Barger SW, Fiscus RR, Ruth P, Hofmann F, Mattson MP. (1995) Role of cyclic GMP in the regulation of neuronal calcium and survival by secreted forms of beta-amyloid precursor. J Neurochem 64:2087–2096 [DOI] [PubMed] [Google Scholar]
- Barman et al., 2003.Barman SA, Zhu S, Han G, White RE. (2003) cAMP activates BKCa channels in pulmonary arterial smooth muscle via cGMP-dependent protein kinase. Am J Physiol Lung Cell Mol Physiol 284:L1004–L1111 [DOI] [PubMed] [Google Scholar]
- Bazhin et al., 2010.Bazhin AV, Tambor V, Dikov B, Philippov PP, Schadendorf D, Eichmüller SB. (2010) cGMP-phosphodiesterase 6, transducin and Wnt5a/Frizzled-2-signaling control cGMP and Ca2+ homeostasis in melanoma cells. Cell Mol Life Sci 67:817–828 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Beals et al., 1997a.Beals CR, Clipstone NA, Ho SN, Crabtree GR. (1997a) Nuclear localization of NF-ATc by a calcineurin-dependent, cyclosporin-sensitive intramolecular interaction. Genes Dev 11:824–834 [DOI] [PubMed] [Google Scholar]
- Beals et al., 1997b.Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR. (1997b) Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275:1930–1934 [DOI] [PubMed] [Google Scholar]
- Beavo et al., 2006.Beavo J, Houslay MD, Francis SH. (2006) Cyclic nucleotide phosphodiesterase superfamily, in Cyclic Nucleotide Phosphodiesterases in Health and Disease (Beavo J, Francis SH, Houslay MD. eds) pp 3–17, CRC Press, Boca Raton [Google Scholar]
- Beebe et al., 1988a.Beebe SJ, Beasley-Leach A, Corbin JD. (1988a) cAMP analogs used to study low-Km, hormone-sensitive phosphodiesterase. Methods Enzymol 159:531–540 [DOI] [PubMed] [Google Scholar]
- Beebe et al., 1988b.Beebe SJ, Blackmore PF, Chrisman TD, Corbin JD. (1988b) Use of synergistic pairs of site-selective cAMP analogs in intact cells. Methods Enzymol 159:118–139 [DOI] [PubMed] [Google Scholar]
- Beltman et al., 1995.Beltman J, Becker DE, Butt E, Jensen GS, Rybalkin SD, Jastorff B, Beavo JA. (1995) Characterization of cyclic nucleotide phosphodiesterases with cyclic GMP analogs: topology of the catalytic domains. Mol Pharmacol 47:330–339 [PubMed] [Google Scholar]
- Bender and Beavo, 2006a.Bender AT, Beavo JA. (2006a) Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58:488–520 [DOI] [PubMed] [Google Scholar]
- Bender and Beavo, 2006b.Bender AT, Beavo JA. (2006b) PDE1B2 regulates cGMP and a subset of the phenotypic characteristics acquired upon macrophage differentiation from a monocyte. Proc Natl Acad Sci USA 103:460–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender et al., 2004.Bender AT, Ostenson CL, Giordano D, Beavo JA. (2004) Differentiation of human monocytes in vitro with granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor produces distinct changes in cGMP phosphodiesterase expression. Cell Signal 16:365–374 [DOI] [PubMed] [Google Scholar]
- Bender et al., 2005.Bender AT, Ostenson CL, Wang EH, Beavo JA. (2005) Selective up-regulation of PDE1B2 upon monocyte-to-macrophage differentiation. Proc Natl Acad Sci USA 102:497–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz et al., 2008.Benz PM, Blume C, Moebius J, Oschatz C, Schuh K, Sickmann A, Walter U, Feller SM, Renné T. (2008) Cytoskeleton assembly at endothelial cell-cell contacts is regulated by alphaII-spectrin-VASP complexes. J Cell Biol 180:205–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz et al., 2009.Benz PM, Blume C, Seifert S, Wilhelm S, Waschke J, Schuh K, Gertler F, Münzel T, Renné T. (2009) Differential VASP phosphorylation controls remodeling of the actin cytoskeleton. J Cell Sci 122:3954–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge, 2008.Berridge MJ. (2008) Smooth muscle cell calcium activation mechanisms. J Physiol 586:5047–5061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessay et al., 2008.Bessay EP, Blount MA, Zoraghi R, Beasley A, Grimes KA, Francis SH, Corbin JD. (2008) Phosphorylation increases affinity of the phosphodiesterase-5 catalytic site for tadalafil. J Pharmacol Exp Ther 325:62–68 [DOI] [PubMed] [Google Scholar]
- Blount et al., 2004.Blount MA, Beasley A, Zoraghi R, Sekhar KR, Bessay EP, Francis SH, Corbin JD. (2004) Binding of tritiated sildenafil, tadalafil, or vardenafil to the phosphodiesterase-5 catalytic site displays potency, specificity, heterogeneity, and cGMP stimulation. Mol Pharmacol 66:144–152 [DOI] [PubMed] [Google Scholar]
- Blount et al., 2007.Blount MA, Zoraghi R, Bessay EP, Beasley A, Francis SH, Corbin JD. (2007) Conversion of phosphodiesterase-5 (PDE5) catalytic site to higher affinity by PDE5 inhibitors. J Pharmacol Exp Ther 323:730–737 [DOI] [PubMed] [Google Scholar]
- Borman et al., 2004.Borman MA, MacDonald JA, Haystead TA. (2004) Modulation of smooth muscle contractility by CHASM, a novel member of the smoothelin family of proteins. FEBS Lett 573:207–213 [DOI] [PubMed] [Google Scholar]
- Bouallegue et al., 2007.Bouallegue A, Daou GB, Srivastava AK. (2007) Nitric oxide attenuates endothelin-1-induced activation of ERK1/2, PKB, and Pyk2 in vascular smooth muscle cells by a cGMP-dependent pathway. Am J Physiol Heart Circ Physiol 293:H2072–H2079 [DOI] [PubMed] [Google Scholar]
- Browner et al., 2004.Browner NC, Dey NB, Bloch KD, Lincoln TM. (2004) Regulation of cGMP-dependent protein kinase expression by soluble guanylyl cyclase in vascular smooth muscle cells. J Biol Chem 279:46631–46636 [DOI] [PubMed] [Google Scholar]
- Bryan et al., 2009.Bryan NS, Bian K, Murad F. (2009) Discovery of the nitric oxide signaling pathway and targets for drug development. Front Biosci 14:1–18 [DOI] [PubMed] [Google Scholar]
- Buga et al., 1989.Buga GM, Gold ME, Wood KS, Chaudhuri G, Ignarro LJ. (1989) Endothelium-derived nitric oxide relaxes nonvascular smooth muscle. Eur J Pharmacol 161:61–72 [DOI] [PubMed] [Google Scholar]
- Burgoyne et al., 2007.Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schröder E, Browning DD, Eaton P. (2007) Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science 317:1393–1397 [DOI] [PubMed] [Google Scholar]
- Burkhardt et al., 2000.Burkhardt M, Glazova M, Gambaryan S, Vollkommer T, Butt E, Bader B, Heermeier K, Lincoln TM, Walter U, Palmetshofer A. (2000) KT5823 inhibits cGMP-dependent protein kinase activity in vitro but not in intact human platelets and rat mesangial cells. J Biol Chem 275:33536–33541 [DOI] [PubMed] [Google Scholar]
- Burnett, 2006.Burnett AL. (2006) The role of nitric oxide in erectile dysfunction: implications for medical therapy. J Clin Hypertens (Greenwich) 8 (12 Suppl 4):53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnett et al., 2006.Burnett AL, Bivalacqua TJ, Champion HC, Musicki B. (2006) Feasibility of the use of phosphodiesterase type 5 inhibitors in a pharmacologic prevention program for recurrent priapism. J Sex Med 3:1077–1084 [DOI] [PubMed] [Google Scholar]
- Burnette and White, 2006.Burnette JO, White RE. (2006) PGI2 opens potassium channels in retinal pericytes by cyclic AMP-stimulated, cross-activation of PKG. Exp Eye Res 83:1359–1365 [DOI] [PubMed] [Google Scholar]
- Busch et al., 2002.Busch JL, Bessay EP, Francis SH, Corbin JD. (2002) A conserved serine juxtaposed to the pseudosubstrate site of type I cGMP-dependent protein kinase contributes strongly to autoinhibition and lower cGMP affinity. J Biol Chem 277:34048–34054 [DOI] [PubMed] [Google Scholar]
- Butt et al., 1994a.Butt E, Abel K, Krieger M, Palm D, Hoppe V, Hoppe J, Walter U. (1994a) cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J Biol Chem 269:14509–14517 [PubMed] [Google Scholar]
- Butt et al., 2000.Butt E, Bernhardt M, Smolenski A, Kotsonis P, Fröhlich LG, Sickmann A, Meyer HE, Lohmann SM, Schmidt HH. (2000) Endothelial nitric-oxide synthase (type III) is activated and becomes calcium independent upon phosphorylation by cyclic nucleotide-dependent protein kinases. J Biol Chem 275:5179–5187 [DOI] [PubMed] [Google Scholar]
- Butt et al., 1994b.Butt E, Eigenthaler M, Genieser HG. (1994b) (Rp)-8-pCPT-cGMPS, a novel cGMP-dependent protein kinase inhibitor. Eur J Pharmacol 269:265–268 [DOI] [PubMed] [Google Scholar]
- Butt et al., 2003.Butt E, Gambaryan S, Göttfert N, Galler A, Marcus K, Meyer HE. (2003) Actin binding of human LIM and SH3 protein is regulated by cGMP- and cAMP-dependent protein kinase phosphorylation on serine 146. J Biol Chem 278:15601–15607 [DOI] [PubMed] [Google Scholar]
- Butt et al., 2001.Butt E, Immler D, Meyer HE, Kotlyarov A, Laass K, Gaestel M. (2001) Heat shock protein 27 is a substrate of cGMP-dependent protein kinase in intact human platelets: phosphorylation-induced actin polymerization caused by HSP27 mutants. J Biol Chem 276:7108–7113 [DOI] [PubMed] [Google Scholar]
- Butt et al., 1992.Butt E, Nolte C, Schulz S, Beltman J, Beavo JA, Jastorff B, Walter U. (1992) Analysis of the functional role of cGMP-dependent protein kinase in intact human platelets using a specific activator 8-para-chlorophenylthio-cGMP. Biochem Pharmacol 43:2591–2600 [DOI] [PubMed] [Google Scholar]
- Calderone et al., 1998.Calderone A, Thaik CM, Takahashi N, Chang DL, Colucci WS. (1998) Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J Clin Invest 101:812–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card et al., 2005.Card GL, Blasdel L, England BP, Zhang C, Suzuki Y, Gillette S, Fong D, Ibrahim PN, Artis DR, Bollag G, et al. (2005) A family of phosphodiesterase inhibitors discovered by cocrystallography and scaffold-based drug design. Nat Biotechnol 23:201–207 [DOI] [PubMed] [Google Scholar]
- Carnegie et al., 2009.Carnegie GK, Means CK, Scott JD. (2009) A-kinase anchoring proteins: from protein complexes to physiology and disease. IUBMB Life 61:394–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnegie et al., 2008.Carnegie GK, Soughayer J, Smith FD, Pedroja BS, Zhang F, Diviani D, Bristow MR, Kunkel MT, Newton AC, Langeberg LK, et al. (2008) AKAP-Lbc mobilizes a cardiac hypertrophy signaling pathway. Mol Cell 32:169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson, 2005.Carson CC., 3rd (2005) Cardiac safety in clinical trials of phosphodiesterase 5 inhibitors. Am J Cardiol 96:37M–41M [DOI] [PubMed] [Google Scholar]
- Cary et al., 2006.Cary SP, Winger JA, Derbyshire ER, Marletta MA. (2006) Nitric oxide signaling: no longer simply on or off. Trends Biochem Sci 31:231–239 [DOI] [PubMed] [Google Scholar]
- Casteel et al., 2005.Casteel DE, Boss GR, Pilz RB. (2005) Identification of the interface between cGMP-dependent protein kinase Ibeta and its interaction partners TFII-I and IRAG reveals a common interaction motif. J Biol Chem 280:38211–38218 [DOI] [PubMed] [Google Scholar]
- Casteel et al., 2008.Casteel DE, Zhang T, Zhuang S, Pilz RB. (2008) cGMP-dependent protein kinase anchoring by IRAG regulates its nuclear translocation and transcriptional activity. Cell Signal 20:1392–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro et al., 2006.Castro LR, Verde I, Cooper DM, Fischmeister R. (2006) Cyclic guanosine monophosphate compartmentation in rat cardiac myocytes. Circulation 113:2221–2228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley et al., 2007.Cawley SM, Sawyer CL, Brunelle KF, van der Vliet A, Dostmann WR. (2007) Nitric oxide-evoked transient kinetics of cyclic GMP in vascular smooth muscle cells. Cell Signal 19:1023–1033 [DOI] [PubMed] [Google Scholar]
- Celermajer et al., 1993.Celermajer DS, Cullen S, Deanfield JE. (1993) Impairment of endothelium-dependent pulmonary artery relaxation in children with congenital heart disease and abnormal pulmonary hemodynamics. Circulation 87:440–446 [DOI] [PubMed] [Google Scholar]
- Chai and Lin, 2008.Chai Y, Lin YF. (2008) Dual regulation of the ATP-sensitive potassium channel by activation of cGMP-dependent protein kinase. Pflugers Arch 456:897–915 [DOI] [PubMed] [Google Scholar]
- Chalimoniuk et al., 2009.Chalimoniuk M, Stolecka A, Ziemińska E, Stepień A, Langfort J, Strosznajder JB. (2009) Involvement of multiple protein kinases in cPLA2 phosphorylation, arachidonic acid release, and cell death in in vivo and in vitro models of 1-methyl-4-phenylpyridinium-induced parkinsonism—the possible key role of PKG. J Neurochem 110:307–317 [DOI] [PubMed] [Google Scholar]
- Champion et al., 2005.Champion HC, Bivalacqua TJ, Takimoto E, Kass DA, Burnett AL. (2005) Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci USA 102:1661–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan et al., 2007.Chan JY, Cheng HL, Chou JL, Li FC, Dai KY, Chan SH, Chang AY. (2007) Heat shock protein 60 or 70 activates nitric-oxide synthase (NOS) I- and inhibits NOS II-associated signaling and depresses the mitochondrial apoptotic cascade during brain stem death. J Biol Chem 282:4585–4600 [DOI] [PubMed] [Google Scholar]
- Chan and Fiscus, 2003.Chan SL, Fiscus RR. (2003) Guanylyl cyclase inhibitors NS2028 and ODQ and protein kinase G (PKG) inhibitor KT5823 trigger apoptotic DNA fragmentation in immortalized uterine epithelial cells: anti-apoptotic effects of basal cGMP/PKG. Mol Hum Reprod 9:775–783 [DOI] [PubMed] [Google Scholar]
- Chen et al., 1999.Chen C, Nakamura T, Koutalos Y. (1999) Cyclic AMP diffusion coefficient in frog olfactory cilia. Biophys J 76:2861–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al., 2008.Chen H, Levine YC, Golan DE, Michel T, Lin AJ. (2008) Atrial natriuretic peptide-initiated cGMP pathways regulate vasodilator-stimulated phosphoprotein phosphorylation and angiogenesis in vascular endothelium. J Biol Chem 283:4439–4447 [DOI] [PubMed] [Google Scholar]
- Chen et al., 2004.Chen L, Daum G, Chitaley K, Coats SA, Bowen-Pope DF, Eigenthaler M, Thumati NR, Walter U, Clowes AW. (2004) Vasodilator-stimulated phosphoprotein regulates proliferation and growth inhibition by nitric oxide in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 24:1403–1408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi et al., 2006.Choi YH, Park S, Hockman S, Zmuda-Trzebiatowska E, Svennelid F, Haluzik M, Gavrilova O, Ahmad F, Pepin L, Napolitano M, et al. (2006) Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B-null mice. J Clin Invest 116:3240–3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen et al., 2003.Christensen AE, Selheim F, de Rooij J, Dremier S, Schwede F, Dao KK, Martinez A, Maenhaut C, Bos JL, Genieser HG, et al. (2003) cAMP analog mapping of Epac1 and cAMP kinase. Discriminating analogs demonstrate that Epac and cAMP kinase act synergistically to promote PC-12 cell neurite extension. J Biol Chem 278:35394–35402 [DOI] [PubMed] [Google Scholar]
- Christensen and Mendelsohn, 2006.Christensen EN, Mendelsohn ME. (2006) Cyclic GMP-dependent protein kinase Ialpha inhibits thrombin receptor-mediated calcium mobilization in vascular smooth muscle cells. J Biol Chem 281:8409–8416 [DOI] [PubMed] [Google Scholar]
- Chu et al., 1997.Chu DM, Corbin JD, Grimes KA, Francis SH. (1997) Activation by cyclic GMP binding causes an apparent conformational change in cGMP-dependent protein kinase. J Biol Chem 272:31922–31928 [DOI] [PubMed] [Google Scholar]
- Colbran et al., 1992.Colbran JL, Roach PJ, Fiol CJ, Dixon JE, Andrisani OM, Corbin JD. (1992) cAMP-dependent protein kinase, but not the cGMP-dependent enzyme, rapidly phosphorylates delta-CREB, and a synthetic delta-CREB peptide. Biochem Cell Biol 70:1277–1282 [DOI] [PubMed] [Google Scholar]
- Collins and Uhler, 1999.Collins SP, Uhler MD. (1999) Cyclic AMP- and cyclic GMP-dependent protein kinases differ in their regulation of cyclic AMP response element-dependent gene transcription. J Biol Chem 274:8391–8404 [DOI] [PubMed] [Google Scholar]
- Colyer, 1998.Colyer J. (1998) Phosphorylation states of phospholamban. Ann NY Acad Sci 853:79–91 [DOI] [PubMed] [Google Scholar]
- Conti and Beavo, 2007.Conti M, Beavo J. (2007) Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem 76:481–511 [DOI] [PubMed] [Google Scholar]
- Cook and Haynes, 2007.Cook AL, Haynes JM. (2007) Phosphorylation of the PKG substrate, vasodilator-stimulated phosphoprotein (VASP), in human cultured prostatic stromal cells. Nitric Oxide 16:10–17 [DOI] [PubMed] [Google Scholar]
- Corbin et al., 2003.Corbin J, Rannels S, Neal D, Chang P, Grimes K, Beasley A, Francis S. (2003) Sildenafil citrate does not affect cardiac contractility in human or dog heart. Curr Med Res Opin 19:747–752 [DOI] [PubMed] [Google Scholar]
- Corbin et al., 2005.Corbin JD, Beasley A, Blount MA, Francis SH. (2005) High lung PDE5: a strong basis for treating pulmonary hypertension with PDE5 inhibitors. Biochem Biophys Res Commun 334:930–938 [DOI] [PubMed] [Google Scholar]
- Corbin and Døskeland, 1983.Corbin JD, Døskeland SO. (1983) Studies of two different intrachain cGMP-binding sites of cGMP-dependent protein kinase. J Biol Chem 258:11391–11397 [PubMed] [Google Scholar]
- Corbin and Francis, 1999.Corbin JD, Francis SH. (1999) Cyclic GMP phosphodiesterase-5: target of sildenafil. J Biol Chem 274:13729–13732 [DOI] [PubMed] [Google Scholar]
- Corbin et al., 2000.Corbin JD, Turko IV, Beasley A, Francis SH. (2000) Phosphorylation of phosphodiesterase-5 by cyclic nucleotide-dependent protein kinase alters its catalytic and allosteric cGMP-binding activities. Eur J Biochem 267:2760–2767 [DOI] [PubMed] [Google Scholar]
- Cornwell et al., 1991.Cornwell TL, Pryzwansky KB, Wyatt TA, Lincoln TM. (1991) Regulation of sarcoplasmic reticulum protein phosphorylation by localized cyclic GMP-dependent protein kinase in vascular smooth muscle cells. Mol Pharmacol 40:923–931 [PubMed] [Google Scholar]
- Costa et al., 2005.Costa AD, Garlid KD, West IC, Lincoln TM, Downey JM, Cohen MV, Critz SD. (2005) Protein kinase G transmits the cardioprotective signal from cytosol to mitochondria. Circ Res 97:329–336 [DOI] [PubMed] [Google Scholar]
- Costa et al., 2008.Costa AD, Pierre SV, Cohen MV, Downey JM, Garlid KD. (2008) cGMP signalling in pre- and post-conditioning: the role of mitochondria. Cardiovasc Res 77:344–352 [DOI] [PubMed] [Google Scholar]
- Cote, 2006.Cote RH. (2006) Photoreceptor phosphodiesterase (PDE6): a G-protein-activated PDE regulating visual excitation in rod and cone photoreceptor cells, in Cyclic Nucleotide Phosphodiesterases in Health and Disease (Beavo J, Francis SH, Houslay MD. eds) pp 105–193, CRC Press, Boca Raton [Google Scholar]
- Crabtree and Olson, 2002.Crabtree GR, Olson EN. (2002) NFAT signaling: choreographing the social lives of cells. Cell 109 (Suppl):S67–S79 [DOI] [PubMed] [Google Scholar]
- Dao et al., 2006.Dao KK, Teigen K, Kopperud R, Hodneland E, Schwede F, Christensen AE, Martinez A, Døskeland SO. (2006) Epac1 and cAMP-dependent protein kinase holoenzyme have similar cAMP affinity, but their cAMP domains have distinct structural features and cyclic nucleotide recognition. J Biol Chem 281:21500–21511 [DOI] [PubMed] [Google Scholar]
- Das et al., 2004.Das A, Ockaili R, Salloum F, Kukreja RC. (2004) Protein kinase C plays an essential role in sildenafil-induced cardioprotection in rabbits. Am J Physiol Heart Circ Physiol 286:H1455–H1460 [DOI] [PubMed] [Google Scholar]
- Das et al., 2009.Das A, Salloum FN, Xi L, Rao YJ, Kukreja RC. (2009) ERK phosphorylation mediates sildenafil-induced myocardial protection against ischemia-reperfusion injury in mice. Am J Physiol Heart Circ Physiol 296:H1236–H1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das et al., 2008.Das A, Xi L, Kukreja RC. (2008) Protein kinase G-dependent cardioprotective mechanism of phosphodiesterase-5 inhibition involves phosphorylation of ERK and GSK3beta. J Biol Chem 283:29572–29585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das et al., 2002.Das S, Maulik N, Das DK, Kadowitz PJ, Bivalacqua TJ. (2002) Cardioprotection with sildenafil, a selective inhibitor of cyclic 3′,5′-monophosphate-specific phosphodiesterase 5. Drugs Exp Clin Res 28:213–219 [PubMed] [Google Scholar]
- Datta et al., 2000.Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, Greenberg ME. (2000) 14–3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell 6:41–51 [PubMed] [Google Scholar]
- de Vente et al., 2006.de Vente J, Markerink-van Ittersum M, Vles JS. (2006) The role of phosphodiesterase isoforms 2, 5, and 9 in the regulation of NO-dependent and NO-independent cGMP production in the rat cervical spinal cord. J Chem Neuroanat 31:275–303 [DOI] [PubMed] [Google Scholar]
- Degerman et al., 1997.Degerman E, Belfrage P, Manganiello VC. (1997) Structure, localization, and regulation of cGMP-inhibited phosphodiesterase (PDE3). J Biol Chem 272:6823–6826 [DOI] [PubMed] [Google Scholar]
- Deguchi et al., 2004.Deguchi A, Thompson WJ, Weinstein IB. (2004) Activation of protein kinase G is sufficient to induce apoptosis and inhibit cell migration in colon cancer cells. Cancer Res 64:3966–3973 [DOI] [PubMed] [Google Scholar]
- Derbyshire and Marletta, 2009.Derbyshire ER, Marletta MA. (2009) Biochemistry of soluble guanylate cyclase. Handb Exp Pharmacol 191:17–31 [DOI] [PubMed] [Google Scholar]
- Desch et al., 2010.Desch M, Sigl K, Hieke B, Salb K, Kees F, Bernhard D, Jochim A, Spiessberger B, Höcherl K, Feil R, et al. (2010) IRAG determines nitric oxide- and atrial natriuretic peptide-mediated smooth muscle relaxation. Cardiovasc Res 86:496–505 [DOI] [PubMed] [Google Scholar]
- Dey et al., 2009.Dey NB, Busch JL, Francis SH, Corbin JD, Lincoln TM. (2009) Cyclic GMP specifically suppresses type-Ialpha cGMP-dependent protein kinase expression by ubiquitination. Cell Signal 21:859–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaliwal et al., 2007.Dhaliwal JS, Casey DB, Greco AJ, Badejo AM, Jr., Gallen TB, Murthy SN, Nossaman BD, Hyman AL, Kadowitz PJ. (2007) Rho kinase and Ca2+ entry mediate increased pulmonary and systemic vascular resistance in L-NAME-treated rats. Am J Physiol Lung Cell Mol Physiol 293:L1306–L1313 [DOI] [PubMed] [Google Scholar]
- Diederen et al., 2007.Diederen RM, La Heij EC, Markerink-van Ittersum M, Kijlstra A, Hendrikse F, de Vente J. (2007) Selective blockade of phosphodiesterase types 2, 5 and 9 results in cyclic 3′5′ guanosine monophosphate accumulation in retinal pigment epithelium cells. Br J Ophthalmol 91:379–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding et al., 2009.Ding HL, Ryder JW, Stull JT, Kamm KE. (2009) Signaling processes for initiating smooth muscle contraction upon neural stimulation. J Biol Chem 284:15541–15548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostmann et al., 1999.Dostmann WR, Nickl C, Thiel S, Tsigelny I, Frank R, Tegge WJ. (1999) Delineation of selective cyclic GMP-dependent protein kinase Ialpha substrate and inhibitor peptides based on combinatorial peptide libraries on paper. Pharmacol Ther 82:373–387 [DOI] [PubMed] [Google Scholar]
- Dostmann et al., 2000.Dostmann WR, Taylor MS, Nickl CK, Brayden JE, Frank R, Tegge WJ. (2000) Highly specific, membrane-permeant peptide blockers of cGMP-dependent protein kinase Ialpha inhibit NO-induced cerebral dilation. Proc Natl Acad Sci USA 97:14772–14777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrascu et al., 2006.Dumitrascu R, Weissmann N, Ghofrani HA, Dony E, Beuerlein K, Schmidt H, Stasch JP, Gnoth MJ, Seeger W, Grimminger F, et al. (2006) Activation of soluble guanylate cyclase reverses experimental pulmonary hypertension and vascular remodeling. Circulation 113:286–295 [DOI] [PubMed] [Google Scholar]
- Egemnazarov et al., 2009.Egemnazarov B, Sydykov A, Schermuly RT, Weissmann N, Stasch JP, Sarybaev AS, Seeger W, Grimminger F, Ghofrani HA. (2009) Novel soluble guanylyl cyclase stimulator BAY 41–2272 attenuates ischemia-reperfusion-induced lung injury. Am J Physiol Lung Cell Mol Physiol 296:L462–L469 [DOI] [PubMed] [Google Scholar]
- Eigenthaler et al., 1992.Eigenthaler M, Nolte C, Halbrügge M, Walter U. (1992) Concentration and regulation of cyclic nucleotides, cyclic-nucleotide-dependent protein kinases and one of their major substrates in human platelets. Estimating the rate of cAMP- regulated and cGMP-regulated protein phosphorylation in intact cells. Eur J Biochem 205:471–481 [DOI] [PubMed] [Google Scholar]
- Ellerbroek et al., 2003.Ellerbroek SM, Wennerberg K, Burridge K. (2003) Serine phosphorylation negatively regulates RhoA in vivo. J Biol Chem 278:19023–19031 [DOI] [PubMed] [Google Scholar]
- Endo et al., 2003.Endo S, Nairn AC, Greengard P, Ito M. (2003) Thr123 of rat G-substrate contributes to its action as a protein phosphatase inhibitor. Neurosci Res 45:79–89 [DOI] [PubMed] [Google Scholar]
- Endo et al., 1999.Endo S, Suzuki M, Sumi M, Nairn AC, Morita R, Yamakawa K, Greengard P, Ito M. (1999) Molecular identification of human G-substrate, a possible downstream component of the cGMP-dependent protein kinase cascade in cerebellar Purkinje cells. Proc Natl Acad Sci USA 96:2467–2472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin et al., 2006.Erwin PA, Mitchell DA, Sartoretto J, Marletta MA, Michel T. (2006) Subcellular targeting and differential S-nitrosylation of endothelial nitric-oxide synthase. J Biol Chem 281:151–157 [DOI] [PubMed] [Google Scholar]
- Falk et al., 2010.Falk JA, Philip KJ, Schwarz ER. (2010) The emergence of oral tadalafil as a once-daily treatment for pulmonary arterial hypertension. Vasc Health Risk Manag 6:273–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow et al., 2008.Farrow KN, Groh BS, Schumacker PT, Lakshminrusimha S, Czech L, Gugino SF, Russell JA, Steinhorn RH. (2008) Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ Res 102:226–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett et al., 2000.Fawcett L, Baxendale R, Stacey P, McGrouther C, Harrow I, Soderling S, Hetman J, Beavo JA, Phillips SC. (2000) Molecular cloning and characterization of a distinct human phosphodiesterase gene family: PDE11A. Proc Natl Acad Sci USA 97:3702–3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feil et al., 2002.Feil R, Gappa N, Rutz M, Schlossmann J, Rose CR, Konnerth A, Brummer S, Kühbandner S, Hofmann F. (2002) Functional reconstitution of vascular smooth muscle cells with cGMP-dependent protein kinase I isoforms. Circ Res 90:1080–1086 [DOI] [PubMed] [Google Scholar]
- Feletou et al., 2008.Feletou M, Tang EH, Vanhoutte PM. (2008) Nitric oxide the gatekeeper of endothelial vasomotor control. Front Biosci 13:4198–4217 [DOI] [PubMed] [Google Scholar]
- Feng et al., 1999.Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T. (1999) Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem 274:37385–37390 [DOI] [PubMed] [Google Scholar]
- Fiedler et al., 2006.Fiedler B, Feil R, Hofmann F, Willenbockel C, Drexler H, Smolenski A, Lohmann SM, Wollert KC. (2006) cGMP-dependent protein kinase type I inhibits TAB1–p38 mitogen-activated protein kinase apoptosis signaling in cardiac myocytes. J Biol Chem 281:32831–32840 [DOI] [PubMed] [Google Scholar]
- Fiedler et al., 2002.Fiedler B, Lohmann SM, Smolenski A, Linnemuller S, Pieske B, Schroder F, Molkentin JD, Drexler H, Wollert KC. (2002) Inhibition of calcineurin-NFAT hypertrophy signaling by cGMP-dependent protein kinase type I in cardiac myocytes. Proc Natl Acad Sci USA 99:11363–11368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmeister et al., 2006.Fischmeister R, Castro LR, Abi-Gerges A, Rochais F, Jurevicius J, Leroy J, Vandecasteele G. (2006) Compartmentation of cyclic nucleotide signaling in the heart: the role of cyclic nucleotide phosphodiesterases. Circ Res 99:816–828 [DOI] [PubMed] [Google Scholar]
- Fisslthaler and Fleming, 2009.Fisslthaler B, Fleming I. (2009) Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res 105:114–127 [DOI] [PubMed] [Google Scholar]
- Fleming and Busse, 2003.Fleming I, Busse R. (2003) Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 284:R1–R12 [DOI] [PubMed] [Google Scholar]
- Foster et al., 2009.Foster MW, Hess DT, Stamler JS. (2009) Protein S-nitrosylation in health and disease: a current perspective. Trends Mol Med 15:391–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotis et al., 1999.Fotis H, Tatjanenko LV, Vasilets LA. (1999) Phosphorylation of the alpha-subunits of the Na+/K+-ATPase from mammalian kidneys and Xenopus oocytes by cGMP-dependent protein kinase results in stimulation of ATPase activity. Eur J Biochem 260:904–910 [DOI] [PubMed] [Google Scholar]
- Francis et al., 2005.Francis SH, Blount MA, Zoraghi R, Corbin JD. (2005) Molecular properties of mammalian proteins that interact with cGMP: protein kinases, cation channels, phosphodiesterases, and multi-drug anion transporters. Front Biosci 10:2097–2117 [DOI] [PubMed] [Google Scholar]
- Francis and Corbin, 1994.Francis SH, Corbin JD. (1994) Structure and function of cyclic nucleotide-dependent protein kinases. Annu Rev Physiol 56:237–272 [DOI] [PubMed] [Google Scholar]
- Francis and Corbin, 1999.Francis SH, Corbin JD. (1999) Cyclic nucleotide-dependent protein kinases: intracellular receptors for cAMP and cGMP action. Crit Rev Clin Lab Sci 36:275–328 [DOI] [PubMed] [Google Scholar]
- Francis and Corbin, 2003.Francis SH, Corbin JD. (2003) Molecular mechanisms and pharmacokinetics of phosphodiesterase-5 antagonists. Curr Urol Rep 4:457–465 [DOI] [PubMed] [Google Scholar]
- Francis and Corbin, 2005.Francis SH, Corbin JD. (2005) Phosphodiesterase-5 inhibition: the molecular biology of erectile function and dysfunction. Urol Clin North Am 32:419–429 [DOI] [PubMed] [Google Scholar]
- Francis et al., 2009.Francis SH, Corbin JD, Bischoff E. (2009) Cyclic GMP-hydrolyzing phosphodiesterases. Handb Exp Pharmacol 191:367–408 [DOI] [PubMed] [Google Scholar]
- Francis et al., 2008.Francis SH, Morris GZ, Corbin JD. (2008) Molecular mechanisms that could contribute to prolonged effectiveness of PDE5 inhibitors to improve erectile function. Int J Impot Res 20:333–342 [DOI] [PubMed] [Google Scholar]
- Francis et al., 1988.Francis SH, Noblett BD, Todd BW, Wells JN, Corbin JD. (1988) Relaxation of vascular and tracheal smooth muscle by cyclic nucleotide analogs that preferentially activate purified cGMP-dependent protein kinase. Mol Pharmacol 34:506–517 [PubMed] [Google Scholar]
- Francis et al., 1996.Francis SH, Smith JA, Colbran JL, Grimes K, Walsh KA, Kumar S, Corbin JD. (1996) Arginine 75 in the pseudosubstrate sequence of type Ibeta cGMP-dependent protein kinase is critical for autoinhibition, although autophosphorylated serine 63 is outside this sequence. J Biol Chem 271:20748–20755 [PubMed] [Google Scholar]
- Francis et al., 2006.Francis SH, Zoraghi R, Kotera J, Ke H, Bessay EP, Blount MA, Corbin JD. (2006) Phosphodiesterase-5: molecular characteristics relating to structure, function, and regulation, in Cyclic Nucleotide Phosphodiesterases in Health and Disease (Beavo JA, Francis SH, Houslay MD. eds), pp 131–164, CRC Press, Boca Raton [Google Scholar]
- Friebe and Koesling, 2003.Friebe A, Koesling D. (2003) Regulation of nitric oxide-sensitive guanylyl cyclase. Circ Res 93:96–105 [DOI] [PubMed] [Google Scholar]
- Friebe et al., 2007.Friebe A, Mergia E, Dangel O, Lange A, Koesling D. (2007) Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase. Proc Natl Acad Sci USA 104:7699–7704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe et al., 1998.Friebe A, Müllershausen F, Smolenski A, Walter U, Schultz G, Koesling D. (1998) YC-1 potentiates nitric oxide- and carbon monoxide-induced cyclic GMP effects in human platelets. Mol Pharmacol 54:962–967 [DOI] [PubMed] [Google Scholar]
- Fries et al., 2005.Fries R, Shariat K, von Wilmowsky H, Böhm M. (2005) Sildenafil in the treatment of Raynaud's phenomenon resistant to vasodilatory therapy. Circulation 112:2980–2985 [DOI] [PubMed] [Google Scholar]
- Fryer et al., 2006.Fryer BH, Wang C, Vedantam S, Zhou GL, Jin S, Fletcher L, Simon MC, Field J. (2006) cGMP-dependent protein kinase phosphorylates p21-activated kinase (Pak) 1, inhibiting Pak/Nck binding and stimulating Pak/vasodilator-stimulated phosphoprotein association. J Biol Chem 281:11487–11495 [DOI] [PubMed] [Google Scholar]
- Fujishige et al., 1999.Fujishige K, Kotera J, Michibata H, Yuasa K, Takebayashi S, Okumura K, Omori K. (1999) Cloning and characterization of a novel human phosphodiesterase that hydrolyzes both cAMP and cGMP (PDE10A). J Biol Chem 274:18438–18445 [DOI] [PubMed] [Google Scholar]
- Fukao et al., 1999.Fukao M, Mason HS, Britton FC, Kenyon JL, Horowitz B, Keef KD. (1999) Cyclic GMP-dependent protein kinase activates cloned BKCa channels expressed in mammalian cells by direct phosphorylation at serine 1072. J Biol Chem 274:10927–10935 [DOI] [PubMed] [Google Scholar]
- Furchgott, 1995.Furchgott RF. (1995) A research trail over half a century. Annu Rev Pharmacol Toxicol 35:1–27 [DOI] [PubMed] [Google Scholar]
- Furchgott, 1998.Furchgott RF. (1998) Nitric oxide: from basic research on isolated blood vessels to clinical relevance in diabetes. An R Acad Nac Med (Madr) 115:317–331 [PubMed] [Google Scholar]
- Furchgott, 1999.Furchgott RF. (1999) Endothelium-derived relaxing factor: discovery, early studies, and identification as nitric oxide. Biosci Rep 19:235–251 [DOI] [PubMed] [Google Scholar]
- Furchgott et al., 1987.Furchgott RF, Carvalho MH, Khan MT, Matsunaga K. (1987) Evidence for endothelium-dependent vasodilation of resistance vessels by acetylcholine. Blood Vessels 24:145–149 [DOI] [PubMed] [Google Scholar]
- Furchgott and Jothianandan, 1991.Furchgott RF, Jothianandan D. (1991) Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels 28:52–61 [DOI] [PubMed] [Google Scholar]
- Gacci et al., 2007.Gacci M, Del Popolo G, Macchiarella A, Celso M, Vittori G, Lapini A, Serni S, Sandner P, Maggi M, Carini M. (2007) Vardenafil improves urodynamic parameters in men with spinal cord injury: results from a single dose, pilot study. J Urol 178:2040–2043 [DOI] [PubMed] [Google Scholar]
- Gambaryan et al., 2008.Gambaryan S, Kobsar A, Hartmann S, Birschmann I, Kuhlencordt PJ, Müller-Esterl W, Lohmann SM, Walter U. (2008) NO-synthase-/NO-independent regulation of human and murine platelet soluble guanylyl cyclase activity. J Thromb Haemost 6:1376–1384 [DOI] [PubMed] [Google Scholar]
- Gao et al., 2004.Gao Y, Dhanakoti S, Trevino EM, Wang X, Sander FC, Portugal AD, Raj JU. (2004) Role of cGMP-dependent protein kinase in development of tolerance to nitric oxide in pulmonary veins of newborn lambs. Am J Physiol Lung Cell Mol Physiol 286:L786–L792 [DOI] [PubMed] [Google Scholar]
- Ge et al., 2002.Ge B, Gram H, Di Padova F, Huang B, New L, Ulevitch RJ, Luo Y, Han J. (2002) MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science 295:1291–1294 [DOI] [PubMed] [Google Scholar]
- Geahlen et al., 1981.Geahlen RL, Allen SM, Krebs EG. (1981) Effect of phosphorylation on the regulatory subunit of the type I cAMP-dependent protein kinase. J Biol Chem 256:4536–4540 [PubMed] [Google Scholar]
- Geahlen and Krebs, 1980.Geahlen RL, Krebs EG. (1980) Regulatory subunit of the type I cAMP-dependent protein kinase as an inhibitor and substrate of the cGMP-dependent protein kinase. J Biol Chem 255:1164–1169 [PubMed] [Google Scholar]
- Geiselhöringer et al., 2004.Geiselhöringer A, Werner M, Sigl K, Smital P, Wörner R, Acheo L, Stieber J, Weinmeister P, Feil R, Feil S, et al. (2004) IRAG is essential for relaxation of receptor-triggered smooth muscle contraction by cGMP kinase. EMBO J 23:4222–4231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass and Krebs, 1979.Glass DB, Krebs EG. (1979) Comparison of the substrate specificity of adenosine 3′:5′-monophosphate- and guanosine 3′:5′-monophosphate-dependent protein kinases. Kinetic studies using synthetic peptides corresponding to phosphorylation sites in histone H2B. J Biol Chem 254:9728–9738 [PubMed] [Google Scholar]
- Glass and Krebs, 1982.Glass DB, Krebs EG. (1982) Phosphorylation by guanosine 3′:5′-monophosphate-dependent protein kinase of synthetic peptide analogs of a site phosphorylated in histone H2B. J Biol Chem 257:1196–1200 [PubMed] [Google Scholar]
- Glass and Smith, 1983.Glass DB, Smith SB. (1983) Phosphorylation by cyclic GMP-dependent protein kinase of a synthetic peptide corresponding to the autophosphorylation site in the enzyme. J Biol Chem 258:14797–14803 [PubMed] [Google Scholar]
- Gold et al., 2006.Gold MG, Lygren B, Dokurno P, Hoshi N, McConnachie G, Taskén K, Carlson CR, Scott JD, Barford D. (2006) Molecular basis of AKAP specificity for PKA regulatory subunits. Mol Cell 24:383–395 [DOI] [PubMed] [Google Scholar]
- Gopal et al., 2001.Gopal VK, Francis SH, Corbin JD. (2001) Allosteric sites of phosphodiesterase-5 (PDE5). A potential role in negative feedback regulation of cGMP signaling in corpus cavernosum. Eur J Biochem 268:3304–3312 [DOI] [PubMed] [Google Scholar]
- Gratzke et al., 2010.Gratzke C, Angulo J, Chitaley K, Dai YT, Kim NN, Paick JS, Simonsen U, Uckert S, Wespes E, Andersson KE, et al. (2010) Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med 7:445–475 [DOI] [PubMed] [Google Scholar]
- Gratzke et al., 2007.Gratzke C, Uckert S, Kedia G, Reich O, Schlenker B, Seitz M, Becker AJ, Stief CG. (2007) In vitro effects of PDE5 inhibitors sildenafil, vardenafil and tadalafil on isolated human ureteral smoth muscle: a basic research approach. Urol Res 35:49–54 [DOI] [PubMed] [Google Scholar]
- Groneberg et al., 2010.Groneberg D, König P, Wirth A, Offermanns S, Koesling D, Friebe A. (2010) Smooth muscle-specific deletion of nitric oxide-sensitive guanylyl cyclase is sufficient to induce hypertension in mice. Circulation 121:401–409 [DOI] [PubMed] [Google Scholar]
- Gudi et al., 1997.Gudi T, Lohmann SM, Pilz RB. (1997) Regulation of gene expression by cyclic GMP-dependent protein kinase requires nuclear translocation of the kinase: identification of a nuclear localization signal. Mol Cell Biol 17:5244–5254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas et al., 2009.Haas B, Mayer P, Jennissen K, Scholz D, Diaz MB, Bloch W, Herzig S, Fässler R, Pfeifer A.(2009) Protein kinase G controls brown fat cell differentiation and mitochondrial biogenesis. Sci Signal 2:ra78. [DOI] [PubMed] [Google Scholar]
- Hall and Armstrong, 2000.Hall SK, Armstrong DL. (2000) Conditional and unconditional inhibition of calcium-activated potassium channels by reversible protein phosphorylation. J Biol Chem 275:3749–3754 [DOI] [PubMed] [Google Scholar]
- Han et al., 2001.Han J, Kim N, Kim E, Ho WK, Earm YE. (2001) Modulation of ATP-sensitive potassium channels by cGMP-dependent protein kinase in rabbit ventricular myocytes. J Biol Chem 276:22140–22147 [DOI] [PubMed] [Google Scholar]
- Han et al., 1999.Han P, Werber J, Surana M, Fleischer N, Michaeli T. (1999) The calcium/calmodulin-dependent phosphodiesterase PDE1C down-regulates glucose-induced insulin secretion. J Biol Chem 274:22337–22344 [DOI] [PubMed] [Google Scholar]
- Hashimoto et al., 1981.Hashimoto E, Takio K, Krebs EG. (1981) Studies on the site in the regulatory subunit of type I cAMP-dependent protein kinase phosphorylated by cGMP-dependent protein kinase. J Biol Chem 256:5604–5607 [PubMed] [Google Scholar]
- Haug et al., 1999.Haug LS, Jensen V, Hvalby O, Walaas SI, Ostvold AC. (1999) Phosphorylation of the inositol 1,4,5-trisphosphate receptor by cyclic nucleotide-dependent kinases in vitro and in rat cerebellar slices in situ. J Biol Chem 274:7467–7473 [DOI] [PubMed] [Google Scholar]
- Heikaus et al., 2009.Heikaus CC, Pandit J, Klevit RE. (2009) Cyclic nucleotide binding GAF domains from phosphodiesterases: structural and mechanistic insights. Structure 17:1551–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikaus et al., 2008.Heikaus CC, Stout JR, Sekharan MR, Eakin CM, Rajagopal P, Brzovic PS, Beavo JA, Klevit RE. (2008) Solution structure of the cGMP binding GAF domain from phosphodiesterase 5: insights into nucleotide specificity, dimerization, and cGMP-dependent conformational change. J Biol Chem 283:22749–22759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymann, 2006.Heymann WR. (2006) Sildenafil for the treatment of Raynaud's phenomenon. J Am Acad Dermatol 55:501–502 [DOI] [PubMed] [Google Scholar]
- Hofmann, 2005.Hofmann F. (2005) The biology of cyclic GMP-dependent protein kinases. J Biol Chem 280:1–4 [DOI] [PubMed] [Google Scholar]
- Hofmann et al., 2009.Hofmann F, Bernhard D, Lukowski R, Weinmeister P. (2009) cGMP regulated protein kinases (cGK). Handb Exp Pharmacol 191:137–162 [DOI] [PubMed] [Google Scholar]
- Hofmann and Flockerzi, 1983.Hofmann F, Flockerzi V. (1983) Characterization of phosphorylated and native cGMP-dependent protein kinase. Eur J Biochem 130:599–603 [DOI] [PubMed] [Google Scholar]
- Hofmann et al., 1985.Hofmann F, Gensheimer HP, Göbel C. (1985) cGMP-dependent protein kinase. Autophosphorylation changes the characteristics of binding site 1. Eur J Biochem 147:361–365 [DOI] [PubMed] [Google Scholar]
- Hogan et al., 2003.Hogan PG, Chen L, Nardone J, Rao A. (2003) Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev 17:2205–2232 [DOI] [PubMed] [Google Scholar]
- Holmgreen et al., 1997.Holmgreen SP, Wang X, Clarke GR, Noltorp RS, Roberts TK, Burton RC, Robinson PJ, Smart YC. (1997) Phosphorylation of the NC-1.1 receptor and regulation of natural cytotoxicity by protein kinase C and cyclic GMP-dependent protein kinase. J Immunol 158:2035–2041 [PubMed] [Google Scholar]
- Horstrup et al., 1994.Horstrup K, Jablonka B, Hönig-Liedl P, Just M, Kochsiek K, Walter U. (1994) Phosphorylation of focal adhesion vasodilator-stimulated phosphoprotein at Ser157 in intact human platelets correlates with fibrinogen receptor inhibition. Eur J Biochem 225:21–27 [DOI] [PubMed] [Google Scholar]
- Hsu et al., 2009.Hsu S, Nagayama T, Koitabashi N, Zhang M, Zhou L, Bedja D, Gabrielson KL, Molkentin JD, Kass DA, Takimoto E. (2009) Phosphodiesterase 5 inhibition blocks pressure overload-induced cardiac hypertrophy independent of the calcineurin pathway. Cardiovasc Res 81:301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huai et al., 2004.Huai Q, Liu Y, Francis SH, Corbin JD, Ke H. (2004) Crystal structures of phosphodiesterases 4 and 5 in complex with inhibitor 3-isobutyl-1-methylxanthine suggest a conformation determinant of inhibitor selectivity. J Biol Chem 279:13095–13101 [DOI] [PubMed] [Google Scholar]
- Huang et al., 2007.Huang J, Zhou H, Mahavadi S, Sriwai W, Murthy KS. (2007) Inhibition of Galphaq-dependent PLC-beta1 activity by PKG and PKA is mediated by phosphorylation of RGS4 and GRK2. Am J Physiol Cell Physiol 292:C200–C208 [DOI] [PubMed] [Google Scholar]
- Huang et al., 1993.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. (1993) Targeted disruption of the neuronal nitric oxide synthase gene. Cell 75:1273–1286 [DOI] [PubMed] [Google Scholar]
- Huber et al., 2000.Huber A, Neuhuber WL, Klugbauer N, Ruth P, Allescher HD. (2000) Cysteine-rich protein 2, a novel substrate for cGMP kinase I in enteric neurons and intestinal smooth muscle. J Biol Chem 275:5504–5511 [DOI] [PubMed] [Google Scholar]
- Ignarro, 1999.Ignarro LJ. (1999) Nitric oxide: a unique endogenous signaling molecule in vascular biology. Biosci Rep 19:51–71 [DOI] [PubMed] [Google Scholar]
- Ignarro, 2005.Ignarro LJ. (2005) Nitric oxide. Curr Top Med Chem 5:595. [DOI] [PubMed] [Google Scholar]
- Ignarro et al., 1988.Ignarro LJ, Buga GM, Byrns RE, Wood KS, Chaudhuri G. (1988) Endothelium-derived relaxing factor and nitric oxide possess identical pharmacologic properties as relaxants of bovine arterial and venous smooth muscle. J Pharmacol Exp Ther 246:218–226 [PubMed] [Google Scholar]
- Ignarro et al., 1987.Ignarro LJ, Byrns RE, Buga GM, Wood KS. (1987) Endothelium-derived relaxing factor from pulmonary artery and vein possesses pharmacologic and chemical properties identical to those of nitric oxide radical. Circ Res 61:866–879 [DOI] [PubMed] [Google Scholar]
- Ignarro et al., 1999.Ignarro LJ, Cirino G, Casini A, Napoli C. (1999) Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol 34:879–886 [DOI] [PubMed] [Google Scholar]
- Ignarro et al., 2002.Ignarro LJ, Napoli C, Loscalzo J. (2002) Nitric oxide donors and cardiovascular agents modulating the bioactivity of nitric oxide: an overview. Circ Res 90:21–28 [DOI] [PubMed] [Google Scholar]
- Ignarro et al., 1982.Ignarro LJ, Wood KS, Wolin MS. (1982) Activation of purified soluble guanylate cyclase by protoporphyrin IX. Proc Natl Acad Sci USA 79:2870–2873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito et al., 1996.Ito M, Nishikawa M, Fujioka M, Miyahara M, Isaka N, Shiku H, Nakano T. (1996) Characterization of the isoenzymes of cyclic nucleotide phosphodiesterase in human platelets and the effects of E4021. Cell Signal 8:575–581 [DOI] [PubMed] [Google Scholar]
- Jabůrek et al., 2006.Jabůrek M, Costa AD, Burton JR, Costa CL, Garlid KD. (2006) Mitochondrial PKC epsilon and mitochondrial ATP-sensitive K+ channel copurify and coreconstitute to form a functioning signaling module in proteoliposomes. Circ Res 99:878–883 [DOI] [PubMed] [Google Scholar]
- Jackson, 2004.Jackson G. (2004) Treatment of erectile dysfunction in patients with cardiovascular disease : guide to drug selection. Drugs 64:1533–1545 [DOI] [PubMed] [Google Scholar]
- Jackson, 2005.Jackson G. (2005) Hemodynamic and exercise effects of phosphodiesterase 5 inhibitors. Am J Cardiol 96:32M–36M [DOI] [PubMed] [Google Scholar]
- Jackson et al., 2006.Jackson G, Montorsi P, Cheitlin MD. (2006) Cardiovascular safety of sildenafil citrate (Viagra): an updated perspective. Urology 68:47–60 [DOI] [PubMed] [Google Scholar]
- Jang et al., 1993.Jang EK, Davidson MM, Crankshaw D, Haslam RJ. (1993) Synergistic inhibitory effects of atriopeptin II and isoproterenol on contraction of rat aortic smooth muscle: roles of cGMP and cAMP. Eur J Pharmacol 250:477–481 [DOI] [PubMed] [Google Scholar]
- Jedlitschky et al., 2000.Jedlitschky G, Burchell B, Keppler D. (2000) The multidrug resistance protein 5 functions as an ATP-dependent export pump for cyclic nucleotides. J Biol Chem 275:30069–30074 [DOI] [PubMed] [Google Scholar]
- Jedlitschky et al., 2004.Jedlitschky G, Tirschmann K, Lubenow LE, Nieuwenhuis HK, Akkerman JW, Greinacher A, Kroemer HK. (2004) The nucleotide transporter MRP4 (ABCC4) is highly expressed in human platelets and present in dense granules, indicating a role in mediator storage. Blood 104:3603–3610 [DOI] [PubMed] [Google Scholar]
- Jeremy et al., 1997.Jeremy JY, Ballard SA, Naylor AM, Miller MA, Angelini GD. (1997) Effects of sildenafil, a type-5 cGMP phosphodiesterase inhibitor, and papaverine on cyclic GMP and cyclic AMP levels in the rabbit corpus cavernosum in vitro. Br J Urol 79:958–963 [DOI] [PubMed] [Google Scholar]
- Jiang et al., 1992.Jiang H, Colbran JL, Francis SH, Corbin JD. (1992) Direct evidence for cross-activation of cGMP-dependent protein kinase by cAMP in pig coronary arteries. J Biol Chem 267:1015–1019 [PubMed] [Google Scholar]
- Jiang et al., 2000.Jiang LH, Gawler DJ, Hodson N, Milligan CJ, Pearson HA, Porter V, Wray D. (2000) Regulation of cloned cardiac L-type calcium channels by cGMP-dependent protein kinase. J Biol Chem 275:6135–6143 [DOI] [PubMed] [Google Scholar]
- Johlfs and Fiscus, 2010.Johlfs MG, Fiscus RR. (2010) Protein kinase G type-Ialpha phosphorylates the apoptosis-regulating protein Bad at serine 155 and protects against apoptosis in N1E-115 cells. Neurochem Int 56:546–553 [DOI] [PubMed] [Google Scholar]
- Kambayashi et al., 2006.Kambayashi J, Shakur Y, Liu Y. (2006) Bench to bedside: multiple actions of the PDE3 inhibitor cilostazol, in Cyclic Nucleotide Phosphodiesterases in Health and Disease (Beavo J, Francis SH, Houslay MD. eds) pp 627–648,7 CRC Press, Boca Raton [Google Scholar]
- Kamm and Stull, 1985.Kamm KE, Stull JT. (1985) The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol 25:593–620 [DOI] [PubMed] [Google Scholar]
- Kang et al., 2007.Kang Y, Dempo Y, Ohashi A, Saito M, Toyoda H, Sato H, Koshino H, Maeda Y, Hirai T. (2007) Nitric oxide activates leak K+ currents in the presumed cholinergic neuron of basal forebrain. J Neurophysiol 98:3397–3410 [DOI] [PubMed] [Google Scholar]
- Kass et al., 2007a.Kass DA, Champion HC, Beavo JA. (2007a) Phosphodiesterase type 5: expanding roles in cardiovascular regulation. Circ Res 101:1084–1095 [DOI] [PubMed] [Google Scholar]
- Kass et al., 2007b.Kass DA, Takimoto E, Nagayama T, Champion HC. (2007b) Phosphodiesterase regulation of nitric oxide signaling. Cardiovasc Res 75:303–314 [DOI] [PubMed] [Google Scholar]
- Kasseckert et al., 2009.Kasseckert SA, Schäfer C, Kluger A, Gligorievski D, Tillmann J, Schlüter KD, Noll T, Sauer H, Piper HM, Abdallah Y. (2009) Stimulation of cGMP signalling protects coronary endothelium against reperfusion-induced intercellular gap formation. Cardiovasc Res 83:381–387 [DOI] [PubMed] [Google Scholar]
- Ke and Wang, 2007.Ke H, Wang H. (2007) Crystal structures of phosphodiesterases and implications on substrate specificity and inhibitor selectivity. Curr Top Med Chem 7:391–403 [DOI] [PubMed] [Google Scholar]
- Keicher et al., 2004.Keicher C, Gambaryan S, Schulze E, Marcus K, Meyer HE, Butt E. (2004) Phosphorylation of mouse LASP-1 on threonine 156 by cAMP- and cGMP-dependent protein kinase. Biochem Biophys Res Commun 324:308–316 [DOI] [PubMed] [Google Scholar]
- Kelley-Hickie et al., 2007.Kelley-Hickie LP, O'Keeffe MB, Reid HM, Kinsella BT. (2007) Homologous desensitization of signalling by the α (alpha) isoform of the human thromboxane A2 receptor: a specific role for nitric oxide signalling. Biochim Biophys Acta 1773:970–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly et al., 2010.Kelly MP, Logue SF, Brennan J, Day JP, Lakkaraju S, Jiang L, Zhong X, Tam M, Sukoff Rizzo SJ, Platt BJ, et al. (2010) Phosphodiesterase 11A in brain is enriched in ventral hippocampus and deletion causes psychiatric disease-related phenotypes. Proc Natl Acad Sci USA 107:8457–8462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp-Harper and Schmidt, 2009.Kemp-Harper B, Schmidt HH. (2009) cGMP in the vasculature. Handb Exp Pharmacol 191:447–467 [DOI] [PubMed] [Google Scholar]
- Khairallah et al., 2008.Khairallah M, Khairallah RJ, Young ME, Allen BG, Gillis MA, Danialou G, Deschepper CF, Petrof BJ, Des Rosiers C. (2008) Sildenafil and cardiomyocyte-specific cGMP signaling prevent cardiomyopathic changes associated with dystrophin deficiency. Proc Natl Acad Sci USA 105:7028–7033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al., 2001.Kim D, Rybalkin SD, Pi X, Wang Y, Zhang C, Munzel T, Beavo JA, Berk BC, Yan C. (2001) Upregulation of phosphodiesterase 1A1 expression is associated with the development of nitrate tolerance. Circulation 104:2338–2343 [DOI] [PubMed] [Google Scholar]
- Kimura et al., 1996.Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, et al. (1996) Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 273:245–248 [DOI] [PubMed] [Google Scholar]
- Kleppisch and Feil, 2009.Kleppisch T, Feil R. (2009) cGMP signalling in the mammalian brain: role in synaptic plasticity and behaviour. Handb Exp Pharmacol 191:549–579 [DOI] [PubMed] [Google Scholar]
- Koesling et al., 2005.Koesling D, Mullershausen F, Lange A, Friebe A, Mergia E, Wagner C, Russwurm M. (2005) Negative feedback in NO/cGMP signalling. Biochem Soc Trans 33:1119–1122 [DOI] [PubMed] [Google Scholar]
- Koitabashi et al., 2010.Koitabashi N, Aiba T, Hesketh GG, Rowell J, Zhang M, Takimoto E, Tomaselli GF, Kass DA. (2010) Cyclic GMP/PKG-dependent inhibition of TRPC6 channel activity and expression negatively regulates cardiomyocyte NFAT activation Novel mechanism of cardiac stress modulation by PDE5 inhibition. J Mol Cell Cardiol 48:713–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller et al., 2003.Koller A, Schlossmann J, Ashman K, Uttenweiler-Joseph S, Ruth P, Hofmann F. (2003) Association of phospholamban with a cGMP kinase signaling complex. Biochem Biophys Res Commun 300:155–160 [DOI] [PubMed] [Google Scholar]
- Komalavilas and Lincoln, 1994.Komalavilas P, Lincoln TM. (1994) Phosphorylation of the inositol 1,4,5-trisphosphate receptor by cyclic GMP-dependent protein kinase. J Biol Chem 269:8701–8707 [PubMed] [Google Scholar]
- Korkmaz et al., 2009a.Korkmaz S, Radovits T, Barnucz E, Hirschberg K, Neugebauer P, Loganathan S, Veres G, Páli S, Seidel B, Zöllner S, et al. (2009a) Pharmacological activation of soluble guanylate cyclase protects the heart against ischemic injury. Circulation 120:677–686 [DOI] [PubMed] [Google Scholar]
- Korkmaz et al., 2009b.Korkmaz S, Radovits T, Barnucz E, Neugebauer P, Arif R, Hirschberg K, Loganathan S, Seidel B, Karck M, Szabó G. (2009b) Dose-dependent effects of a selective phosphodiesterase-5-inhibitor on endothelial dysfunction induced by peroxynitrite in rat aorta. Eur J Pharmacol 615:155–162 [DOI] [PubMed] [Google Scholar]
- Kotera et al., 2004a.Kotera J, Francis SH, Grimes KA, Rouse A, Blount MA, Corbin JD. (2004a) Allosteric sites of phosphodiesterase-5 sequester cyclic GMP. Front Biosci 9:378–386 [DOI] [PubMed] [Google Scholar]
- Kotera et al., 1999a.Kotera J, Fujishige K, Imai Y, Kawai E, Michibata H, Akatsuka H, Yanaka N, Omori K. (1999a) Genomic origin and transcriptional regulation of two variants of cGMP-binding cGMP-specific phosphodiesterases. Eur J Biochem 262:866–873 [DOI] [PubMed] [Google Scholar]
- Kotera et al., 1999b.Kotera J, Fujishige K, Yuasa K, Omori K. (1999b) Characterization and phosphorylation of PDE10A2, a novel alternative splice variant of human phosphodiesterase that hydrolyzes cAMP and cGMP. Biochem Biophys Res Commun 261:551–557 [DOI] [PubMed] [Google Scholar]
- Kotera et al., 2004b.Kotera J, Sasaki T, Kobayashi T, Fujishige K, Yamashita Y, Omori K. (2004b) Subcellular localization of cyclic nucleotide phosphodiesterase type 10A variants, and alteration of the localization by cAMP-dependent protein kinase-dependent phosphorylation. J Biol Chem 279:4366–4375 [DOI] [PubMed] [Google Scholar]
- Kouvelas et al., 2009.Kouvelas D, Goulas A, Papazisis G, Sardeli C, Pourzitaki C. (2009) PDE5 inhibitors: in vitro and in vivo pharmacological profile. Curr Pharm Des 15:3464–3475 [DOI] [PubMed] [Google Scholar]
- Krieg et al., 2009.Krieg T, Liu Y, Rütz T, Methner C, Yang XM, Dost T, Felix SB, Stasch JP, Cohen MV, Downey JM. (2009) BAY 58–2667, a nitric oxide-independent guanylyl cyclase activator, pharmacologically post-conditions rabbit and rat hearts. Eur Heart J 30:1607–1613 [DOI] [PubMed] [Google Scholar]
- Krüger et al., 2009.Krüger M, Kötter S, Grützner A, Lang P, Andresen C, Redfield MM, Butt E, dos Remedios CG, Linke WA. (2009) Protein kinase G modulates human myocardial passive stiffness by phosphorylation of the titin springs. Circ Res 104:87–94 [DOI] [PubMed] [Google Scholar]
- Kugiyama et al., 1996.Kugiyama K, Yasue H, Okumura K, Ogawa H, Fujimoto K, Nakao K, Yoshimura M, Motoyama T, Inobe Y, Kawano H. (1996) Nitric oxide activity is deficient in spasm arteries of patients with coronary spastic angina. Circulation 94:266–271 [DOI] [PubMed] [Google Scholar]
- Kukreja et al., 2005.Kukreja RC, Salloum F, Das A, Ockaili R, Yin C, Bremer YA, Fisher PW, Wittkamp M, Hawkins J, Chou E, et al. (2005) Pharmacological preconditioning with sildenafil: Basic mechanisms and clinical implications. Vascul Pharmacol 42:219–232 [DOI] [PubMed] [Google Scholar]
- Kureishi et al., 1997.Kureishi Y, Kobayashi S, Amano M, Kimura K, Kanaide H, Nakano T, Kaibuchi K, Ito M. (1997) Rho-associated kinase directly induces smooth muscle contraction through myosin light chain phosphorylation. J Biol Chem 272:12257–12260 [DOI] [PubMed] [Google Scholar]
- Kurjak et al., 1999.Kurjak M, Fritsch R, Saur D, Schusdziarra V, Allescher HD. (1999) NO releases bombesin-like immunoreactivity from enteric synaptosomes by cross-activation of protein kinase A. Am J Physiol 276:G1521–G1530 [DOI] [PubMed] [Google Scholar]
- Kuwahara et al., 2006.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN. (2006) TRPC6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest 116:3114–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan et al., 2004.Kwan HY, Huang Y, Yao X. (2004) Regulation of canonical transient receptor potential isoform 3 (TRPC3) channel by protein kinase G. Proc Natl Acad Sci USA 101:2625–2630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laber et al., 2002.Laber U, Kober T, Schmitz V, Schrammel A, Meyer W, Mayer B, Weber M, Kojda G. (2002) Effect of hypercholesterolemia on expression and function of vascular soluble guanylyl cyclase. Circulation 105:855–860 [DOI] [PubMed] [Google Scholar]
- Lalli et al., 1999.Lalli MJ, Shimizu S, Sutliff RL, Kranias EG, Paul RJ. (1999) [Ca2+]i homeostasis and cyclic nucleotide relaxation in aorta of phospholamban-deficient mice. Am J Physiol 277:H963–H970 [DOI] [PubMed] [Google Scholar]
- Landgraf et al., 1991.Landgraf W, Regulla S, Meyer HE, Hofmann F. (1991) Oxidation of cysteines activates cGMP-dependent protein kinase. J Biol Chem 266:16305–16311 [PubMed] [Google Scholar]
- Lapp et al., 2009.Lapp H, Mitrovic V, Franz N, Heuer H, Buerke M, Wolfertz J, Mueck W, Unger S, Wensing G, Frey R. (2009) Cinaciguat (BAY 58–2667) improves cardiopulmonary hemodynamics in patients with acute decompensated heart failure. Circulation 119:2781–2788 [DOI] [PubMed] [Google Scholar]
- Layland et al., 2002.Layland J, Li JM, Shah AM. (2002) Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J Physiol 540:457–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al., 2007.Lee E, Hayes DB, Langsetmo K, Sundberg EJ, Tao TC. (2007) Interactions between the leucine-zipper motif of cGMP-dependent protein kinase and the C-terminal region of the targeting subunit of myosin light chain phosphatase. J Mol Biol 373:1198–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee et al., 1997.Lee MR, Li L, Kitazawa T. (1997) Cyclic GMP causes Ca2+ desensitization in vascular smooth muscle by activating the myosin light chain phosphatase. J Biol Chem 272:5063–5068 [DOI] [PubMed] [Google Scholar]
- Leidenheimer, 1996.Leidenheimer NJ. (1996) Effect of PKG activation on recombinant GABAA receptors. Brain Res Mol Brain Res 42:131–134 [DOI] [PubMed] [Google Scholar]
- Levien, 2006.Levien TL. (2006) Phosphodiesterase inhibitors in Raynaud's phenomenon. Ann Pharmacother 40:1388–1393 [DOI] [PubMed] [Google Scholar]
- Li et al., 2001.Li D, Laubach VE, Johns RA. (2001) Upregulation of lung soluble guanylate cyclase during chronic hypoxia is prevented by deletion of eNOS. Am J Physiol Lung Cell Mol Physiol 281:L369–L376 [DOI] [PubMed] [Google Scholar]
- Li et al., 2005.Li FC, Chan JY, Chan SH, Chang AY. (2005) In the rostral ventrolateral medulla, the 70-kDa heat shock protein (HSP70), but not HSP90, confers neuroprotection against fatal endotoxemia via augmentation of nitric-oxide synthase I (NOS I)/protein kinase G signaling pathway and inhibition of NOS II/peroxynitrite cascade. Mol Pharmacol 68:179–192 [DOI] [PubMed] [Google Scholar]
- Lin et al., 2001a.Lin CS, Chow S, Lau A, Tu R, Lue TF. (2001a) Identification and regulation of human PDE5A gene promoter. Biochem Biophys Res Commun 280:684–692 [DOI] [PubMed] [Google Scholar]
- Lin et al., 2001b.Lin CS, Chow S, Lau A, Tu R, Lue TF. (2001b) Regulation of human PDE5A2 intronic promoter by cAMP and cGMP: identification of a critical Sp1-binding site. Biochem Biophys Res Commun 280:693–699 [DOI] [PubMed] [Google Scholar]
- Lincoln et al., 2001.Lincoln TM, Dey N, Sellak H. (2001) Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: from the regulation of tone to gene expression. J Appl Physiol 91:1421–1430 [DOI] [PubMed] [Google Scholar]
- Lincoln et al., 1977.Lincoln TM, Dills WL, Jr., Corbin JD. (1977) Purification and subunit composition of guanosine 3′:5′-monophosphate-dependent protein kinase from bovine lung. J Biol Chem 252:4269–4275 [PubMed] [Google Scholar]
- Lincoln et al., 1978.Lincoln TM, Flockhart DA, Corbin JD. (1978) Studies on the structure and mechanism of activation of the guanosine 3′:5′-monophosphate-dependent protein kinase. J Biol Chem 253:6002–6009 [PubMed] [Google Scholar]
- Lincoln et al., 1976.Lincoln TM, Hall CL, Park CR, Corbin JD. (1976) Guanosine 3′:5′-cyclic monophosphate binding proteins in rat tissues. Proc Natl Acad Sci USA 73:2559–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln et al., 1995.Lincoln TM, Komalavilas P, Boerth NJ, MacMillan-Crow LA, Cornwell TL. (1995) cGMP signaling through cAMP- and cGMP-dependent protein kinases. Adv Pharmacol 34:305–322 [DOI] [PubMed] [Google Scholar]
- Lincoln et al., 2006.Lincoln TM, Wu X, Sellak H, Dey N, Choi CS. (2006) Regulation of vascular smooth muscle cell phenotype by cyclic GMP and cyclic GMP-dependent protein kinase. Front Biosci 11:356–367 [DOI] [PubMed] [Google Scholar]
- Liu and Maurice, 1998.Liu H, Maurice DH. (1998) Expression of cyclic GMP-inhibited phosphodiesterases 3A and 3B (PDE3A and PDE3B) in rat tissues: differential subcellular localization and regulated expression by cyclic AMP. Br J Pharmacol 125:1501–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al., 2002.Liu L, Underwood T, Li H, Pamukcu R, Thompson WJ. (2002) Specific cGMP binding by the cGMP binding domains of cGMP-binding cGMP specific phosphodiesterase. Cell Signal 14:45–51 [DOI] [PubMed] [Google Scholar]
- Liu et al., 2007.Liu S, Ma X, Gong M, Shi L, Lincoln T, Wang S. (2007) Glucose down-regulation of cGMP-dependent protein kinase I expression in vascular smooth muscle cells involves NAD(P)H oxidase-derived reactive oxygen species. Free Radic Biol Med 42:852–863 [DOI] [PubMed] [Google Scholar]
- Lohmann and Walter, 2005.Lohmann SM, Walter U. (2005) Tracking functions of cGMP-dependent protein kinases (cGK). Front Biosci 10:1313–1328 [DOI] [PubMed] [Google Scholar]
- López-Aparicio et al., 1993.López-Aparicio P, Belfrage P, Manganiello VC, Kono T, Degerman E. (1993) Stimulation by insulin of a serine kinase in human platelets that phosphorylates and activates the cGMP-inhibited cAMP phosphodiesterase. Biochem Biophys Res Commun 193:1137–1144 [DOI] [PubMed] [Google Scholar]
- Loughney et al., 1999.Loughney K, Snyder PB, Uher L, Rosman GJ, Ferguson K, Florio VA. (1999) Isolation and characterization of PDE10A, a novel human 3′, 5′-cyclic nucleotide phosphodiesterase. Gene 234:109–117 [DOI] [PubMed] [Google Scholar]
- Lukowski et al., 2010.Lukowski R, Rybalkin SD, Loga F, Leiss V, Beavo JA, Hofmann F. (2010) Cardiac hypertrophy is not amplified by deletion of cGMP-dependent protein kinase I in cardiomyocytes. Proc Natl Acad Sci USA 107:5646–5651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowski et al., 2008.Lukowski R, Weinmeister P, Bernhard D, Feil S, Gotthardt M, Herz J, Massberg S, Zernecke A, Weber C, Hofmann F, et al. (2008) Role of smooth muscle cGMP/cGKI signaling in murine vascular restenosis. Arterioscler Thromb Vasc Biol 28:1244–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma et al., 1999.Ma XL, Kumar S, Gao F, Louden CS, Lopez BL, Christopher TA, Wang C, Lee JC, Feuerstein GZ, Yue TL. (1999) Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation 99:1685–1691 [DOI] [PubMed] [Google Scholar]
- MacFarland et al., 1991.MacFarland RT, Zelus BD, Beavo JA. (1991) High concentrations of a cGMP-stimulated phosphodiesterase mediate ANP-induced decreases in cAMP and steroidogenesis in adrenal glomerulosa cells. J Biol Chem 266:136–142 [PubMed] [Google Scholar]
- Maclean et al., 1997.Maclean MR, Johnston ED, Mcculloch KM, Pooley L, Houslay MD, Sweeney G. (1997) Phosphodiesterase isoforms in the pulmonary arterial circulation of the rat: changes in pulmonary hypertension. J Pharmacol Exp Ther 283:619–624 [PubMed] [Google Scholar]
- Madhusoodanan and Murad, 2007.Madhusoodanan KS, Murad F. (2007) NO-cGMP signaling and regenerative medicine involving stem cells. Neurochem Res 32:681–694 [DOI] [PubMed] [Google Scholar]
- Manganiello et al., 1995.Manganiello VC, Taira M, Degerman E, Belfrage P. (1995) Type III cGMP-inhibited cyclic nucleotide phosphodiesterases (PDE3 gene family). Cell Signal 7:445–455 [DOI] [PubMed] [Google Scholar]
- Marsh and Marsh, 2000.Marsh N, Marsh A. (2000) A short history of nitroglycerine and nitric oxide in pharmacology and physiology. Clin Exp Pharmacol Physiol 27:313–319 [DOI] [PubMed] [Google Scholar]
- Martinez et al., 2002.Martinez SE, Wu AY, Glavas NA, Tang XB, Turley S, Hol WG, Beavo JA. (2002) The two GAF domains in phosphodiesterase 2A have distinct roles in dimerization and in cGMP binding. Proc Natl Acad Sci USA 99:13260–13265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez et al., 2008.Martinez SE, Heikaus CC, Klevit RE, Beavo JA. (2008) The structure of the GAF A domain from phosphodiesterase 6C reveals determinants of cGMP binding, a conserved binding surface, and a large cGMP-dependent conformational change. J Biol Chem 283:25913–25919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masciarelli et al., 2004.Masciarelli S, Horner K, Liu C, Park SH, Hinckley M, Hockman S, Nedachi T, Jin C, Conti M, Manganiello V. (2004) Cyclic nucleotide phosphodiesterase 3A-deficient mice as a model of female infertility. J Clin Invest 114:196–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood et al., 2009.Masood A, Huang Y, Hajjhussein H, Xiao L, Li H, Wang W, Hamza A, Zhan CG, O'Donnell JM. (2009) Anxiolytic effects of phosphodiesterase-2 inhibitors associated with increased cGMP signaling. J Pharmacol Exp Ther 331:690–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masood et al., 2008.Masood A, Nadeem A, Mustafa SJ, O'Donnell JM. (2008) Reversal of oxidative stress-induced anxiety by inhibition of phosphodiesterase-2 in mice. J Pharmacol Exp Ther 326:369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massberg et al., 1999.Massberg S, Sausbier M, Klatt P, Bauer M, Pfeifer A, Siess W, Fässler R, Ruth P, Krombach F, Hofmann F. (1999) Increased adhesion and aggregation of platelets lacking cyclic guanosine 3′,5′-monophosphate kinase I. J Exp Med 189:1255–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice, 2005.Maurice DH. (2005) Cyclic nucleotide phosphodiesterase-mediated integration of cGMP and cAMP signaling in cells of the cardiovascular system. Front Biosci 10:1221–1228 [DOI] [PubMed] [Google Scholar]
- Maurice and Haslam, 1990.Maurice DH, Haslam RJ. (1990) Molecular basis of the synergistic inhibition of platelet function by nitrovasodilators and activators of adenylate cyclase: inhibition of cyclic AMP breakdown by cyclic GMP. Mol Pharmacol 37:671–681 [PubMed] [Google Scholar]
- Mayer et al., 2009.Mayer B, Kleschyov AL, Stessel H, Russwurm M, Münzel T, Koesling D, Schmidt K. (2009) Inactivation of soluble guanylate cyclase by stoichiometric S-nitrosation. Mol Pharmacol 75:886–891 [DOI] [PubMed] [Google Scholar]
- McDonald and Moss, 1997.McDonald BJ, Moss SJ. (1997) Conserved phosphorylation of the intracellular domains of GABA(A) receptor beta2 and beta3 subunits by cAMP-dependent protein kinase, cGMP-dependent protein kinase protein kinase C and Ca2+/calmodulin type II-dependent protein kinase. Neuropharmacology 36:1377–1385 [DOI] [PubMed] [Google Scholar]
- Meurer et al., 2009.Meurer S, Pioch S, Pabst T, Opitz N, Schmidt PM, Beckhaus T, Wagner K, Matt S, Gegenbauer K, Geschka S, et al. (2009) Nitric oxide-independent vasodilator rescues heme-oxidized soluble guanylate cyclase from proteasomal degradation. Circ Res 105:33–41 [DOI] [PubMed] [Google Scholar]
- Michalewski et al., 1998.Michalewski MP, Kaczmarski W, Golabek AA, Kida E, Kaczmarski A, Wisniewski KE. (1998) Evidence for phosphorylation of CLN3 protein associated with Batten disease. Biochem Biophys Res Commun 253:458–462 [DOI] [PubMed] [Google Scholar]
- Miller et al., 2009.Miller CL, Oikawa M, Cai Y, Wojtovich AP, Nagel DJ, Xu X, Xu H, Florio V, Rybalkin SD, Beavo JA, et al. (2009) Role of Ca2+/calmodulin-stimulated cyclic nucleotide phosphodiesterase 1 in mediating cardiomyocyte hypertrophy. Circ Res 105:956–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell et al., 2005.Mitchell DA, Erwin PA, Michel T, Marletta MA. (2005) S-Nitrosation and regulation of inducible nitric oxide synthase. Biochemistry 44:4636–4647 [DOI] [PubMed] [Google Scholar]
- Mizuno et al., 2008.Mizuno Y, Isotani E, Huang J, Ding H, Stull JT, Kamm KE. (2008) Myosin light chain kinase activation and calcium sensitization in smooth muscle in vivo. Am J Physiol Cell Physiol 295:C358–C364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada et al., 2004.Moncada I, Jara J, Subirá D, Castaño I, Hernández C. (2004) Efficacy of sildenafil citrate at 12 hours after dosing: re-exploring the therapeutic window. Eur Urol 46:357–360 [DOI] [PubMed] [Google Scholar]
- Monfort and Felipo, 2005.Monfort P, Felipo V. (2005) Long-term potentiation in hippocampus involves sequential activation of soluble guanylate cyclase, cGMP-dependent protein kinase and cGMP-degrading phosphodiesterase, alterations in hyperammonemia. BMC Pharmacol 5 (Suppl 1):P66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfort et al., 2002.Monfort P, Muñoz MD, Kosenko E, Felipo V. (2002) Long-term potentiation in hippocampus involves sequential activation of soluble guanylate cyclase, cGMP-dependent protein kinase, and cGMP-degrading phosphodiesterase. J Neurosci 22:10116–10122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monken and Gill, 1980.Monken CE, Gill GN. (1980) Structural analysis of cGMP-dependent protein kinase using limited proteolysis. J Biol Chem 255:7067–7070 [PubMed] [Google Scholar]
- Mullershausen et al., 2003.Mullershausen F, Friebe A, Feil R, Thompson WJ, Hofmann F, Koesling D. (2003) Direct activation of PDE5 by cGMP: long-term effects within NO/cGMP signaling. J Cell Biol 160:719–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullershausen et al., 2005.Mullershausen F, Koesling D, Friebe A. (2005) NO-sensitive guanylyl cyclase and NO-induced feedback inhibition in cGMP signaling. Front Biosci 10:1269–1278 [DOI] [PubMed] [Google Scholar]
- Mullershausen et al., 2006.Mullershausen F, Lange A, Mergia E, Friebe A, Koesling D. (2006) Desensitization of NO/cGMP signaling in smooth muscle: blood vessels versus airways. Mol Pharmacol 69:1969–1974 [DOI] [PubMed] [Google Scholar]
- Mullershausen et al., 2004.Mullershausen F, Russwurm M, Friebe A, Koesling D. (2004) Inhibition of phosphodiesterase type 5 by the activator of nitric oxide-sensitive guanylyl cyclase BAY 41–2272. Circulation 109:1711–1713 [DOI] [PubMed] [Google Scholar]
- Mullershausen et al., 2001.Mullershausen F, Russwurm M, Thompson WJ, Liu L, Koesling D, Friebe A. (2001) Rapid nitric oxide-induced desensitization of the cGMP response is caused by increased activity of phosphodiesterase type 5 paralleled by phosphorylation of the enzyme. J Cell Biol 155:271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzel et al., 2003.Münzel T, Feil R, Mülsch A, Lohmann SM, Hofmann F, Walter U. (2003) Physiology and pathophysiology of vascular signaling controlled by guanosine 3′,5′-cyclic monophosphate-dependent protein kinase. Circulation 108:2172–2183 [DOI] [PubMed] [Google Scholar]
- Murad, 1996.Murad F. (1996) The 1996 Albert Lasker Medical Research Awards. Signal transduction using nitric oxide and cyclic guanosine monophosphate. JAMA 276:1189–1192 [PubMed] [Google Scholar]
- Murad et al., 1992.Murad F, Forstermann U, Nakane M, Schmidt H, Pollock J, Sheng H, Matsumoto T, Warner T, Mitchell J, Tracey R. (1992) The nitric oxide-cyclic GMP signal transduction pathway in vascular smooth muscle preparations and other tissues. Jpn J Pharmacol 58 (Suppl 2):150P–157P [PubMed] [Google Scholar]
- Murthy, 2001.Murthy KS. (2001) Activation of phosphodiesterase 5 and inhibition of guanylate cyclase by cGMP-dependent protein kinase in smooth muscle. Biochem J 360:199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy and Zhou, 2003.Murthy KS, Zhou H. (2003) Selective phosphorylation of the IP3R-I in vivo by cGMP-dependent protein kinase in smooth muscle. Am J Physiol Gastrointest Liver Physiol 284:G221–G230 [DOI] [PubMed] [Google Scholar]
- Murthy et al., 2003.Murthy KS, Zhou H, Grider JR, Makhlouf GM. (2003) Inhibition of sustained smooth muscle contraction by PKA and PKG preferentially mediated by phosphorylation of RhoA. Am J Physiol Gastrointest Liver Physiol 284:G1006–G1016 [DOI] [PubMed] [Google Scholar]
- Musicki and Burnett, 2007.Musicki B, Burnett AL. (2007) Endothelial dysfunction in diabetic erectile dysfunction. Int J Impot Res 19:129–138 [DOI] [PubMed] [Google Scholar]
- Musicki et al., 2009.Musicki B, Ross AE, Champion HC, Burnett AL, Bivalacqua TJ. (2009) Posttranslational modification of constitutive nitric oxide synthase in the penis. J Androl 30:352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzaffar et al., 2008.Muzaffar S, Shukla N, Bond M, Sala-Newby GB, Newby AC, Angelini GD, Jeremy JY. (2008) Superoxide from NADPH oxidase upregulates type 5 phosphodiesterase in human vascular smooth muscle cells: inhibition with iloprost and NONOate. Br J Pharmacol 155:847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama et al., 2009a.Nagayama T, Hsu S, Zhang M, Koitabashi N, Bedja D, Gabrielson KL, Takimoto E, Kass DA. (2009a) Pressure-overload magnitude-dependence of the anti-hypertrophic efficacy of PDE5A inhibition. J Mol Cell Cardiol 46:560–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama et al., 2009b.Nagayama T, Hsu S, Zhang M, Koitabashi N, Bedja D, Gabrielson KL, Takimoto E, Kass DA. (2009b) Sildenafil stops progressive chamber, cellular, and molecular remodeling and improves calcium handling and function in hearts with pre-existing advanced hypertrophy caused by pressure overload. J Am Coll Cardiol 53:207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama et al., 2008.Nagayama T, Zhang M, Hsu S, Takimoto E, Kass DA. (2008) Sustained soluble guanylate cyclase stimulation offsets nitric-oxide synthase inhibition to restore acute cardiac modulation by sildenafil. J Pharmacol Exp Ther 326:380–387 [DOI] [PubMed] [Google Scholar]
- Nagel et al., 2006.Nagel DJ, Aizawa T, Jeon KI, Liu W, Mohan A, Wei H, Miano JM, Florio VA, Gao P, Korshunov VA, et al. (2006) Role of nuclear Ca2+/calmodulin-stimulated phosphodiesterase 1A in vascular smooth muscle cell growth and survival. Circ Res 98:777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamizo et al., 2003.Nakamizo T, Kawamata J, Yoshida K, Kawai Y, Kanki R, Sawada H, Kihara T, Yamashita H, Shibasaki H, Akaike A, et al. (2003) Phosphodiesterase inhibitors are neuroprotective to cultured spinal motor neurons. J Neurosci Res 71:485–495 [DOI] [PubMed] [Google Scholar]
- Nakamura et al., 2007.Nakamura K, Koga Y, Sakai H, Homma K, Ikebe M. (2007) cGMP-dependent relaxation of smooth muscle is coupled with the change in the phosphorylation of myosin phosphatase. Circ Res 101:712–722 [DOI] [PubMed] [Google Scholar]
- Nakamura et al., 1999.Nakamura M, Ichikawa K, Ito M, Yamamori B, Okinaka T, Isaka N, Yoshida Y, Fujita S, Nakano T. (1999) Effects of the phosphorylation of myosin phosphatase by cyclic GMP-dependent protein kinase. Cell Signal 11:671–676 [DOI] [PubMed] [Google Scholar]
- Napoli and Ignarro, 2009.Napoli C, Ignarro LJ. (2009) Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Arch Pharmacol Res 32:1103–1108 [DOI] [PubMed] [Google Scholar]
- Nilius et al., 2007.Nilius B, Owsianik G, Voets T, Peters JA. (2007) Transient receptor potential cation channels in disease. Physiol Rev 87:165–217 [DOI] [PubMed] [Google Scholar]
- Nilius et al., 2005.Nilius B, Voets T, Peters J. (2005) TRP channels in disease. Sci STKE 2005:re8. [DOI] [PubMed] [Google Scholar]
- Nolte et al., 1994.Nolte C, Eigenthaler M, Horstrup K, Hönig-Liedl P, Walter U. (1994) Synergistic phosphorylation of the focal adhesion-associated vasodilator-stimulated phosphoprotein in intact human platelets in response to cGMP- and cAMP-elevating platelet inhibitors. Biochem Pharmacol 48:1569–1575 [DOI] [PubMed] [Google Scholar]
- Nossaman and Kadowitz, 2009.Nossaman BD, Kadowitz PJ. (2009) The role of the RhoA/rho-kinase pathway in pulmonary hypertension. Curr Drug Discov Technol 6:59–71 [DOI] [PubMed] [Google Scholar]
- Nugent et al., 2009.Nugent FS, Niehaus JL, Kauer JA. (2009) PKG and PKA signaling in LTP at GABAergic synapses. Neuropsychopharmacology 34:1829–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockaili et al., 2002.Ockaili R, Salloum F, Hawkins J, Kukreja RC. (2002) Sildenafil (Viagra) induces powerful cardioprotective effect via opening of mitochondrial K(ATP) channels in rabbits. Am J Physiol Heart Circ Physiol 283:H1263–H269 [DOI] [PubMed] [Google Scholar]
- Omori and Kotera, 2007.Omori K, Kotera J. (2007) Overview of PDEs and their regulation. Circ Res 100:309–327 [DOI] [PubMed] [Google Scholar]
- Osei-Owusu et al., 2007.Osei-Owusu P, Sun X, Drenan RM, Steinberg TH, Blumer KJ. (2007) Regulation of RGS2 and second messenger signaling in vascular smooth muscle cells by cGMP-dependent protein kinase. J Biol Chem 282:31656–31665 [DOI] [PubMed] [Google Scholar]
- Palmer et al., 2007.Palmer D, Jimmo SL, Raymond DR, Wilson LS, Carter RL, Maurice DH. (2007) Protein kinase A phosphorylation of human phosphodiesterase 3B promotes 14-3-3 protein binding and inhibits phosphatase-catalyzed inactivation. J Biol Chem 282:9411–9419 [DOI] [PubMed] [Google Scholar]
- Palmer and Maurice, 2000.Palmer D, Maurice DH. (2000) Dual expression and differential regulation of phosphodiesterase 3A and phosphodiesterase 3B in human vascular smooth muscle: implications for phosphodiesterase 3 inhibition in human cardiovascular tissues. Mol Pharmacol 58:247–252 [DOI] [PubMed] [Google Scholar]
- Palmer et al., 1987.Palmer RM, Ferrige AG, Moncada S. (1987) Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327:524–526 [DOI] [PubMed] [Google Scholar]
- Pandit et al., 2009.Pandit J, Forman MD, Fennell KF, Dillman KS, Menniti FS. (2009) Mechanism for the allosteric regulation of phosphodiesterase 2A deduced from the X-ray structure of a near full-length construct. Proc Natl Acad Sci USA 106:18225–19230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet-Durand et al., 2009.Paquet-Durand F, Hauck SM, van Veen T, Ueffing M, Ekström P. (2009) PKG activity causes photoreceptor cell death in two retinitis pigmentosa models. J Neurochem 108:796–810 [DOI] [PubMed] [Google Scholar]
- Pearce et al., 2009.Pearce WJ, Williams JM, White CR, Lincoln TM. (2009) Effects of chronic hypoxia on soluble guanylate cyclase activity in fetal and adult ovine cerebral arteries. J Appl Physiol 107:192–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer et al., 1998.Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang GX, Korth M, Aszódi A, Andersson KE, et al. (1998) Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J 17:3045–3051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto et al., 1992.Picciotto MR, Cohn JA, Bertuzzi G, Greengard P, Nairn AC. (1992) Phosphorylation of the cystic fibrosis transmembrane conductance regulator. J Biol Chem 267:12742–12752 [PubMed] [Google Scholar]
- Piggott et al., 2006.Piggott LA, Hassell KA, Berkova Z, Morris AP, Silberbach M, Rich TC. (2006) Natriuretic peptides and nitric oxide stimulate cGMP synthesis in different cellular compartments. J Gen Physiol 128:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilz and Broderick, 2005.Pilz RB, Broderick KE. (2005) Role of cyclic GMP in gene regulation. Front Biosci 10:1239–1268 [DOI] [PubMed] [Google Scholar]
- Pokreisz et al., 2009.Pokreisz P, Vandenwijngaert S, Bito V, Van den Bergh A, Lenaerts I, Busch C, Marsboom G, Gheysens O, Vermeersch P, Biesmans L, et al. (2009) Ventricular phosphodiesterase-5 expression is increased in patients with advanced heart failure and contributes to adverse ventricular remodeling after myocardial infarction in mice. Circulation 119:408–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe et al., 2008.Poppe H, Rybalkin SD, Rehmann H, Hinds TR, Tang XB, Christensen AE, Schwede F, Genieser HG, Bos JL, Doskeland SO, et al. (2008) Cyclic nucleotide analogs as probes of signaling pathways. Nat Methods 5:277–278 [DOI] [PubMed] [Google Scholar]
- Porst et al., 2006.Porst H, Giuliano F, Glina S, Ralph D, Casabé AR, Elion-Mboussa A, Shen W, Whitaker JS. (2006) Evaluation of the efficacy and safety of once-a-day dosing of tadalafil 5mg and 10mg in the treatment of erectile dysfunction: results of a multicenter, randomized, double-blind, placebo-controlled trial. Eur Urol 50:351–359 [DOI] [PubMed] [Google Scholar]
- Porst et al., 2003.Porst H, Padma-Nathan H, Giuliano F, Anglin G, Varanese L, Rosen R. (2003) Efficacy of tadalafil for the treatment of erectile dysfunction at 24 and 36 hours after dosing: a randomized controlled trial. Urology 62:121–125 [DOI] [PubMed] [Google Scholar]
- Potter et al., 2009.Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. (2009) Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol 191:341–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prickaerts et al., 2002.Prickaerts J, van Staveren WC, Sik A, Markerink-van Ittersum M, Niewöhner U, van der Staay FJ, Blokland A, de Vente J. (2002) Effects of two selective phosphodiesterase type 5 inhibitors, sildenafil and vardenafil, on object recognition memory and hippocampal cyclic GMP levels in the rat. Neuroscience 113:351–361 [DOI] [PubMed] [Google Scholar]
- Pryzwansky et al., 1995.Pryzwansky KB, Wyatt TA, Lincoln TM. (1995) Cyclic guanosine monophosphate-dependent protein kinase is targeted to intermediate filaments and phosphorylates vimentin in A23187-stimulated human neutrophils. Blood 85:222–230 [PubMed] [Google Scholar]
- Pryzwansky et al., 1990.Pryzwansky KB, Wyatt TA, Nichols H, Lincoln TM. (1990) Compartmentalization of cyclic GMP-dependent protein kinase in formyl-peptide stimulated neutrophils. Blood 76:612–618 [PubMed] [Google Scholar]
- Qin et al., 2004.Qin Q, Yang XM, Cui L, Critz SD, Cohen MV, Browner NC, Lincoln TM, Downey JM. (2004) Exogenous NO triggers preconditioning via a cGMP- and mitoKATP-dependent mechanism. Am J Physiol Heart Circ Physiol 287:H712–H718 [DOI] [PubMed] [Google Scholar]
- Raeymaekers et al., 1988.Raeymaekers L, Hofmann F, Casteels R. (1988) Cyclic GMP-dependent protein kinase phosphorylates phospholamban in isolated sarcoplasmic reticulum from cardiac and smooth muscle. Biochem J 252:269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy et al., 2007.Ramamoorthy S, Samuvel DJ, Buck ER, Rudnick G, Jayanthi LD. (2007) Phosphorylation of threonine residue 276 is required for acute regulation of serotonin transporter by cyclic GMP. J Biol Chem 282:11639–11647 [DOI] [PubMed] [Google Scholar]
- Reaume and Sokolowski, 2009.Reaume CJ, Sokolowski MB. (2009) cGMP-dependent protein kinase as a modifier of behaviour. Handb Exp Pharmacol 191:423–443 [DOI] [PubMed] [Google Scholar]
- Reed et al., 1996.Reed RB, Sandberg M, Jahnsen T, Lohmann SM, Francis SH, Corbin JD. (1996) Fast and slow cyclic nucleotide-dissociation sites in cAMP-dependent protein kinase are transposed in type Ibeta cGMP-dependent protein kinase. J Biol Chem 271:17570–17575 [DOI] [PubMed] [Google Scholar]
- Reed et al., 1997.Reed RB, Sandberg M, Jahnsen T, Lohmann SM, Francis SH, Corbin JD. (1997) Structural order of the slow and fast intrasubunit cGMP-binding sites of type I alpha cGMP-dependent protein kinase. Adv Second Messenger Phosphoprotein Res 31:205–217 [DOI] [PubMed] [Google Scholar]
- Reid and Kinsella, 2003.Reid HM, Kinsella BT. (2003) The alpha, but not the beta, isoform of the human thromboxane A2 receptor is a target for nitric oxide-mediated desensitization. Independent modulation of Tp alpha signaling by nitric oxide and prostacyclin. J Biol Chem 278:51190–51202 [DOI] [PubMed] [Google Scholar]
- Reyes-Irisarri et al., 2007.Reyes-Irisarri E, Markerink-Van Ittersum M, Mengod G, de Vente J. (2007) Expression of the cGMP-specific phosphodiesterases 2 and 9 in normal and Alzheimer's disease human brains. Eur J Neurosci 25:3332–3338 [DOI] [PubMed] [Google Scholar]
- Richie-Jannetta et al., 2003.Richie-Jannetta R, Francis SH, Corbin JD. (2003) Dimerization of cGMP-dependent protein kinase Ibeta is mediated by an extensive amino-terminal leucine zipper motif, and dimerization modulates enzyme function. J Biol Chem 278:50070–50079 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Pascual et al., 1999.Rodríguez-Pascual F, Ferrero R, Miras-Portugal MT, Torres M. (1999) Phosphorylation of tyrosine hydroxylase by cGMP-dependent protein kinase in intact bovine chromaffin cells. Arch Biochem Biophys 366:207–214 [DOI] [PubMed] [Google Scholar]
- Rosano et al., 2005.Rosano GM, Aversa A, Vitale C, Fabbri A, Fini M, Spera G. (2005) Chronic treatment with tadalafil improves endothelial function in men with increased cardiovascular risk. Eur Urol 47:214–220 [DOI] [PubMed] [Google Scholar]
- Russwurm and Koesling, 2004.Russwurm M, Koesling D. (2004) NO activation of guanylyl cyclase. EMBO J 23:4443–4450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russwurm et al., 2001.Russwurm M, Wittau N, Koesling D. (2001) Guanylyl cyclase/PSD-95 interaction: targeting of the nitric oxide-sensitive alpha2beta1 guanylyl cyclase to synaptic membranes. J Biol Chem 276:44647–44652 [DOI] [PubMed] [Google Scholar]
- Ruth et al., 1991.Ruth P, Landgraf W, Keilbach A, May B, Egleme C, Hofmann F. (1991) The activation of expressed cGMP-dependent protein kinase isozymes I alpha and I beta is determined by the different amino-termini. Eur J Biochem 202:1339–1344 [DOI] [PubMed] [Google Scholar]
- Ruth et al., 1997.Ruth P, Pfeifer A, Kamm S, Klatt P, Dostmann WR, Hofmann F. (1997) Identification of the amino acid sequences responsible for high affinity activation of cGMP kinase Ialpha. J Biol Chem 272:10522–10528 [DOI] [PubMed] [Google Scholar]
- Rutten et al., 2005.Rutten K, Vente JD, Sik A, Ittersum MM, Prickaerts J, Blokland A. (2005) The selective PDE5 inhibitor, sildenafil, improves object memory in Swiss mice and increases cGMP levels in hippocampal slices. Behav Brain Res 164:11–16 [DOI] [PubMed] [Google Scholar]
- Rybalkin et al., 2002.Rybalkin SD, Rybalkina IG, Feil R, Hofmann F, Beavo JA. (2002) Regulation of cGMP-specific phosphodiesterase (PDE5) phosphorylation in smooth muscle cells. J Biol Chem 277:3310–3317 [DOI] [PubMed] [Google Scholar]
- Rybalkin et al., 2003.Rybalkin SD, Rybalkina IG, Shimizu-Albergine M, Tang XB, Beavo JA. (2003) PDE5 is converted to an activated state upon cGMP binding to the GAF A domain. EMBO J 22:469–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salloum et al., 2003.Salloum F, Yin C, Xi L, Kukreja RC. (2003) Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ Res 92:595–597 [DOI] [PubMed] [Google Scholar]
- Salloum et al., 2008.Salloum FN, Abbate A, Das A, Houser JE, Mudrick CA, Qureshi IZ, Hoke NN, Roy SK, Brown WR, Prabhakar S, et al. (2008) Sildenafil (Viagra) attenuates ischemic cardiomyopathy and improves left ventricular function in mice. Am J Physiol Heart Circ Physiol 294:H1398–H406 [DOI] [PubMed] [Google Scholar]
- Salloum et al., 2009.Salloum FN, Chau VQ, Hoke NN, Abbate A, Varma A, Ockaili RA, Toldo S, Kukreja RC. (2009) Phosphodiesterase-5 inhibitor, tadalafil, protects against myocardial ischemia/reperfusion through protein-kinase g-dependent generation of hydrogen sulfide. Circulation 120:S31–S36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandner et al., 2007.Sandner P, Hütter J, Tinel H, Ziegelbauer K, Bischoff E. (2007) PDE5 inhibitors beyond erectile dysfunction. Int J Impot Res 19:533–543 [DOI] [PubMed] [Google Scholar]
- Sandner et al., 2009.Sandner P, Neuser D, Bischoff E. (2009) Erectile dysfunction and lower urinary tract. Handb Exp Pharmacol 191:507–531 [DOI] [PubMed] [Google Scholar]
- Sausbier et al., 2000.Sausbier M, Schubert R, Voigt V, Hirneiss C, Pfeifer A, Korth M, Kleppisch T, Ruth P, Hofmann F. (2000) Mechanisms of NO/cGMP-dependent vasorelaxation. Circ Res 87:825–830 [DOI] [PubMed] [Google Scholar]
- Sawada et al., 2001.Sawada N, Itoh H, Yamashita J, Doi K, Inoue M, Masatsugu K, Fukunaga Y, Sakaguchi S, Sone M, Yamahara K, et al. (2001) cGMP-dependent protein kinase phosphorylates and inactivates RhoA. Biochem Biophys Res Commun 280:798–805 [DOI] [PubMed] [Google Scholar]
- Sayed et al., 2007.Sayed N, Baskaran P, Ma X, van den Akker F, Beuve A. (2007) Desensitization of soluble guanylyl cyclase, the NO receptor, by S-nitrosylation. Proc Natl Acad Sci USA 104:12312–12317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed et al., 2008.Sayed N, Kim DD, Fioramonti X, Iwahashi T, Durán WN, Beuve A. (2008) Nitroglycerin-induced S-nitrosylation and desensitization of soluble guanylyl cyclase contribute to nitrate tolerance. Circ Res 103:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaper, 2005.Schaper W. (2005) Dipyridamole, an underestimated vascular protective drug. Cardiovasc Drugs Ther 19:357–363 [DOI] [PubMed] [Google Scholar]
- Schermuly et al., 2007.Schermuly RT, Pullamsetti SS, Kwapiszewska G, Dumitrascu R, Tian X, Weissmann N, Ghofrani HA, Kaulen C, Dunkern T, Schudt C, et al. (2007) Phosphodiesterase 1 upregulation in pulmonary arterial hypertension: target for reverse-remodeling therapy. Circulation 115:2331–2339 [DOI] [PubMed] [Google Scholar]
- Schlossmann et al., 2000.Schlossmann J, Ammendola A, Ashman K, Zong X, Huber A, Neubauer G, Wang GX, Allescher HD, Korth M, Wilm M, et al. (2000) Regulation of intracellular calcium by a signalling complex of IRAG, IP3 receptor and cGMP kinase Ibeta. Nature 404:197–201 [DOI] [PubMed] [Google Scholar]
- Schlossmann and Desch, 2009.Schlossmann J, Desch M. (2009) cGK substrates. Handb Exp Pharmacol 191:163–193 [DOI] [PubMed] [Google Scholar]
- Schmidt et al., 2008.Schmidt CJ, Chapin DS, Cianfrogna J, Corman ML, Hajos M, Harms JF, Hoffman WE, Lebel LA, McCarthy SA, Nelson FR, et al. (2008) Preclinical characterization of selective phosphodiesterase 10A inhibitors: a new therapeutic approach to the treatment of schizophrenia. J Pharmacol Exp Ther 325:681–690 [DOI] [PubMed] [Google Scholar]
- Schmidt et al., 2003.Schmidt P, Schramm M, Schröder H, Stasch JP. (2003) Mechanisms of nitric oxide independent activation of soluble guanylyl cyclase. Eur J Pharmacol 468:167–174 [DOI] [PubMed] [Google Scholar]
- Schmidtko et al., 2008.Schmidtko A, Gao W, Sausbier M, Rauhmeier I, Sausbier U, Niederberger E, Scholich K, Huber A, Neuhuber W, Allescher HD, et al. (2008) Cysteine-rich protein 2, a novel downstream effector of cGMP/cGMP-dependent protein kinase I-mediated persistent inflammatory pain. J Neurosci 28:1320–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder et al., 2003.Schröder F, Klein G, Fiedler B, Bastein M, Schnasse N, Hillmer A, Ames S, Gambaryan S, Drexler H, Walter U, et al. (2003) Single L-type Ca(2+) channel regulation by cGMP-dependent protein kinase type I in adult cardiomyocytes from PKG I transgenic mice. Cardiovasc Res 60:268–277 [DOI] [PubMed] [Google Scholar]
- Schultess et al., 2005.Schultess J, Danielewski O, Smolenski AP. (2005) Rap1GAP2 is a new GTPase-activating protein of Rap1 expressed in human platelets. Blood 105:3185–3192 [DOI] [PubMed] [Google Scholar]
- Seino et al., 1995.Seino Y, Takano T, Hayakawa H, Kanmatsuse K, Saitoh S, Saitoh T, Kamishima G, Watanabe K, Motomiya T, Murata M, et al. (1995) Hemodynamic effects and pharmacokinetics of oral milrinone for short-term support in acute heart failure. Cardiology 86:34–40 [DOI] [PubMed] [Google Scholar]
- Sekhar et al., 1996.Sekhar KR, Grondin P, Francis SH, Corbin JD. (1996) Design and synthesis of xanthines and cyclic GMP analogues as potent inhibitors of PDE5, in Phosphodiesterase Inhibitors (Schudt C, Dent G, Rabe KF. eds) pp 135–146, Academic Press, New York [Google Scholar]
- Sekhar et al., 1992.Sekhar KR, Hatchett RJ, Shabb JB, Wolfe L, Francis SH, Wells JN, Jastorff B, Butt E, Chakinala MM, Corbin JD. (1992) Relaxation of pig coronary arteries by new and potent cGMP analogs that selectively activate type I alpha, compared with type I beta, cGMP-dependent protein kinase. Mol Pharmacol 42:103–108 [PubMed] [Google Scholar]
- Sellak et al., 2002.Sellak H, Yang X, Cao X, Cornwell T, Soff GA, Lincoln T. (2002) Sp1 transcription factor as a molecular target for nitric oxide- and cyclic nucleotide-mediated suppression of cGMP-dependent protein kinase-Ialpha expression in vascular smooth muscle cells. Circ Res 90:405–412 [DOI] [PubMed] [Google Scholar]
- Serebruany et al., 2009.Serebruany V, Sabaeva E, Booze C, Atar OD, Eisert C, Hanley DAggrenox Compliance Task Force (2009) Distribution of dipyridamole in blood components among post-stroke patients treated with extended release formulation. Thromb Haemost 102:538–543 [DOI] [PubMed] [Google Scholar]
- Shabsigh et al., 2006.Shabsigh R, Seftel AD, Rosen RC, Porst H, Ahuja S, Deeley MC, Garcia CS, Giuliano F. (2006) Review of time of onset and duration of clinical efficacy of phosphodiesterase type 5 inhibitors in treatment of erectile dysfunction. Urology 68:689–696 [DOI] [PubMed] [Google Scholar]
- Shakur et al., 2001.Shakur Y, Holst LS, Landstrom TR, Movsesian M, Degerman E, Manganiello V. (2001) Regulation and function of the cyclic nucleotide phosphodiesterase (PDE3) gene family. Prog Nucleic Acid Res Mol Biol 66:241–277 [DOI] [PubMed] [Google Scholar]
- Sharma et al., 2008.Sharma AK, Zhou GP, Kupferman J, Surks HK, Christensen EN, Chou JJ, Mendelsohn ME, Rigby AC. (2008) Probing the interaction between the coiled coil leucine zipper of cGMP-dependent protein kinase Ialpha and the C terminus of the myosin binding subunit of the myosin light chain phosphatase. J Biol Chem 283:32860–32869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan et al., 2002.Sheridan CM, Heist EK, Beals CR, Crabtree GR, Gardner P. (2002) Protein kinase A negatively modulates the nuclear accumulation of NF-ATc1 by priming for subsequent phosphorylation by glycogen synthase kinase-3. J Biol Chem 277:48664–48676 [DOI] [PubMed] [Google Scholar]
- Siuciak et al., 2007.Siuciak JA, McCarthy SA, Chapin DS, Reed TM, Vorhees CV, Repaske DR. (2007) Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-1B (PDE1B) enzyme. Neuropharmacology 53:113–124 [DOI] [PubMed] [Google Scholar]
- Shimizu-Albergine et al., 2003.Shimizu-Albergine M, Rybalkin SD, Rybalkina IG, Feil R, Wolfsgruber W, Hofmann F, Beavo JA. (2003) Individual cerebellar Purkinje cells express different cGMP phosphodiesterases (PDEs): in vivo phosphorylation of cGMP-specific PDE (PDE5) as an indicator of cGMP-dependent protein kinase (PKG) activation. J Neurosci 23:6452–6459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith et al., 1996.Smith JA, Francis SH, Walsh KA, Kumar S, Corbin JD. (1996) Autophosphorylation of type Ibeta cGMP-dependent protein kinase increases basal catalytic activity and enhances allosteric activation by cGMP or cAMP. J Biol Chem 271:20756–20762 [DOI] [PubMed] [Google Scholar]
- Smolenski et al., 1998.Smolenski A, Bachmann C, Reinhard K, Hönig-Liedl P, Jarchau T, Hoschuetzky H, Walter U. (1998) Analysis and regulation of vasodilator-stimulated phosphoprotein serine 239 phosphorylation in vitro and in intact cells using a phosphospecific monoclonal antibody. J Biol Chem 273:20029–20035 [DOI] [PubMed] [Google Scholar]
- Soh et al., 2000.Soh JW, Mao Y, Kim MG, Pamukcu R, Li H, Piazza GA, Thompson WJ, Weinstein IB. (2000) Cyclic GMP mediates apoptosis induced by sulindac derivatives via activation of c-Jun NH2-terminal kinase 1. Clin Cancer Res 6:4136–4141 [PubMed] [Google Scholar]
- Soh et al., 2001.Soh JW, Mao Y, Liu L, Thompson WJ, Pamukcu R, Weinstein IB. (2001) Protein kinase G activates the JNK1 pathway via phosphorylation of MEKK1. J Biol Chem 276:16406–16410 [DOI] [PubMed] [Google Scholar]
- Somlyo and Somlyo, 2003.Somlyo AP, Somlyo AV. (2003) Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83:1325–1358 [DOI] [PubMed] [Google Scholar]
- Stasch and Hobbs, 2009.Stasch JP, Hobbs AJ. (2009) NO-independent, haem-dependent soluble guanylate cyclase stimulators. Handb Exp Pharmacol 191:277–308 [DOI] [PubMed] [Google Scholar]
- Stasch et al., 2002.Stasch JP, Schmidt P, Alonso-Alija C, Apeler H, Dembowsky K, Haerter M, Heil M, Minuth T, Perzborn E, Pleiss U, et al. (2002) NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle. Br J Pharmacol 136:773–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehlik and Movsesian, 2006.Stehlik J, Movsesian MA. (2006) Inhibitors of cyclic nucleotide phosphodiesterase 3 and 5 as therapeutic agents in heart failure. Expert Opin Investig Drugs 15:733–742 [DOI] [PubMed] [Google Scholar]
- Steiner et al., 2009.Steiner JA, Carneiro AM, Wright J, Matthies HJ, Prasad HC, Nicki CK, Dostmann WR, Buchanan CC, Corbin JD, Francis SH, et al. (2009) cGMP-dependent protein kinase Ialpha associates with the antidepressant-sensitive serotonin transporter and dictates rapid modulation of serotonin uptake. Mol Brain 2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone and Marletta, 1996.Stone JR, Marletta MA. (1996) Spectral and kinetic studies on the activation of soluble guanylate cyclase by nitric oxide. Biochemistry 35:1093–1099 [DOI] [PubMed] [Google Scholar]
- Stout et al., 2007.Stout SL, Wyatt TA, Adams JJ, Sisson JH. (2007) Nitric oxide-dependent cilia regulatory enzyme localization in bovine bronchial epithelial cells. J Histochem Cytochem 55:433–442 [DOI] [PubMed] [Google Scholar]
- Straub et al., 2001.Straub A, Stasch JP, Alonso-Alija C, Benet-Buchholz J, Ducke B, Feurer A, Fürstner C. (2001) NO-independent stimulators of soluble guanylate cyclase. Bioorg Med Chem Lett 11:781–784 [DOI] [PubMed] [Google Scholar]
- Sun et al., 2007.Sun B, Li H, Shakur Y, Hensley J, Hockman S, Kambayashi J, Manganiello VC, Liu Y. (2007) Role of phosphodiesterase type 3A and 3B in regulating platelet and cardiac function using subtype-selective knockout mice. Cell Signal 19:1765–1771 [DOI] [PubMed] [Google Scholar]
- Sun et al., 2005.Sun X, Kaltenbronn KM, Steinberg TH, Blumer KJ. (2005) RGS2 is a mediator of nitric oxide action on blood pressure and vasoconstrictor signaling. Mol Pharmacol 67:631–639 [DOI] [PubMed] [Google Scholar]
- Sung et al., 2003.Sung BJ, Hwang KY, Jeon YH, Lee JI, Heo YS, Kim JH, Moon J, Yoon JM, Hyun YL, Kim E, et al. (2003) Structure of the catalytic domain of human phosphodiesterase 5 with bound drug molecules. Nature 425:98–102 [DOI] [PubMed] [Google Scholar]
- Surapisitchat et al., 2007.Surapisitchat J, Jeon KI, Yan C, Beavo JA. (2007) Differential regulation of endothelial cell permeability by cGMP via phosphodiesterases 2 and 3. Circ Res 101:811–818 [DOI] [PubMed] [Google Scholar]
- Surks et al., 1999.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. (1999) Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase Ialpha. Science 286:1583–1587 [DOI] [PubMed] [Google Scholar]
- Takahashi et al., 2008.Takahashi S, Lin H, Geshi N, Mori Y, Kawarabayashi Y, Takami N, Mori MX, Honda A, Inoue R. (2008) Nitric oxide-cGMP-protein kinase G pathway negatively regulates vascular transient receptor potential channel TRPC6. J Physiol 586:4209–4223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasago et al., 1991.Takasago T, Imagawa T, Furukawa K, Ogurusu T, Shigekawa M. (1991) Regulation of the cardiac ryanodine receptor by protein kinase-dependent phosphorylation. J Biochem 109:163–170 [DOI] [PubMed] [Google Scholar]
- Takimoto et al., 2007.Takimoto E, Belardi D, Tocchetti CG, Vahebi S, Cormaci G, Ketner EA, Moens AL, Champion HC, Kass DA. (2007) Compartmentalization of cardiac beta-adrenergic inotropy modulation by phosphodiesterase type 5. Circulation 115:2159–2167 [DOI] [PubMed] [Google Scholar]
- Takimoto et al., 2005a.Takimoto E, Champion HC, Belardi D, Moslehi J, Mongillo M, Mergia E, Montrose DC, Isoda T, Aufiero K, Zaccolo M, et al. (2005a) cGMP catabolism by phosphodiesterase 5A regulates cardiac adrenergic stimulation by NOS3-dependent mechanism. Circ Res 96:100–109 [DOI] [PubMed] [Google Scholar]
- Takimoto et al., 2005b.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. (2005b) Chronic inhibition of cyclic GMP phosphodiesterase 5A prevents and reverses cardiac hypertrophy. Nat Med 11:214–222 [DOI] [PubMed] [Google Scholar]
- Takimoto et al., 2009.Takimoto E, Koitabashi N, Hsu S, Ketner EA, Zhang M, Nagayama T, Bedja D, Gabrielson KL, Blanton R, Siderovski DP, et al. (2009) Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. J Clin Invest 119:408–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takio et al., 1984.Takio K, Wade RD, Smith SB, Krebs EG, Walsh KA, Titani K. (1984) Guanosine cyclic 3′,5′-phosphate dependent protein kinase, a chimeric protein homologous with two separate protein families. Biochemistry 23:4207–4218 [DOI] [PubMed] [Google Scholar]
- Tan et al., 2000.Tan Y, Demeter MR, Ruan H, Comb MJ. (2000) BAD Ser-155 phosphorylation regulates BAD/Bcl-XL interaction and cell survival. J Biol Chem 275:25865–25869 [DOI] [PubMed] [Google Scholar]
- Tang et al., 2003.Tang KM, Wang GR, Lu P, Karas RH, Aronovitz M, Heximer SP, Kaltenbronn KM, Blumer KJ, Siderovski DP, Zhu Y, et al. (2003) Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nat Med 9:1506–1512 [DOI] [PubMed] [Google Scholar]
- Taniguchi et al., 2006.Taniguchi Y, Tonai-Kachi H, Shinjo K. (2006) Zaprinast, a well-known cyclic guanosine monophosphate-specific phosphodiesterase inhibitor, is an agonist for GPR35. FEBS Lett 580:5003–5008 [DOI] [PubMed] [Google Scholar]
- Tanno et al., 2003.Tanno M, Bassi R, Gorog DA, Saurin AT, Jiang J, Heads RJ, Martin JL, Davis RJ, Flavell RA, Marber MS. (2003) Diverse mechanisms of myocardial p38 mitogen-activated protein kinase activation: evidence for MKK-independent activation by a TAB1-associated mechanism contributing to injury during myocardial ischemia. Circ Res 93:254–261 [DOI] [PubMed] [Google Scholar]
- Taylor et al., 2004.Taylor MS, Okwuchukwuasanya C, Nickl CK, Tegge W, Brayden JE, Dostmann WR. (2004) Inhibition of cGMP-dependent protein kinase by the cell-permeable peptide DT-2 reveals a novel mechanism of vasoregulation. Mol Pharmacol 65:1111–1119 [DOI] [PubMed] [Google Scholar]
- Tegeder et al., 2004.Tegeder I, Del Turco D, Schmidtko A, Sausbier M, Feil R, Hofmann F, Deller T, Ruth P, Geisslinger G. (2004) Reduced inflammatory hyperalgesia with preservation of acute thermal nociception in mice lacking cGMP-dependent protein kinase I. Proc Natl Acad Sci USA 101:3253–3257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegge et al., 1995.Tegge W, Frank R, Hofmann F, Dostmann WR. (1995) Determination of cyclic nucleotide-dependent protein kinase substrate specificity by the use of peptide libraries on cellulose paper. Biochemistry 34:10569–10577 [DOI] [PubMed] [Google Scholar]
- Thippeswamy et al., 2005.Thippeswamy T, McKay JS, Morris R, Quinn J, Wong LF, Murphy D. (2005) Glial-mediated neuroprotection: evidence for the protective role of the NO-cGMP pathway via neuron-glial communication in the peripheral nervous system. Glia 49:197–210 [DOI] [PubMed] [Google Scholar]
- Thomas et al., 1992.Thomas MK, Francis SH, Beebe SJ, Gettys TW, Corbin JD. (1992) Partial mapping of cyclic nucleotide sites and studies of regulatory mechanisms of phosphodiesterases using cyclic nucleotide analogues. Adv Second Messenger Phosphoprotein Res 25:45–53 [PubMed] [Google Scholar]
- Thomas et al., 1990a.Thomas MK, Francis SH, Corbin JD. (1990a) Characterization of a purified bovine lung cGMP-binding cGMP phosphodiesterase. J Biol Chem 265:14964–14970 [PubMed] [Google Scholar]
- Thomas et al., 1990b.Thomas MK, Francis SH, Corbin JD. (1990b) Substrate- and kinase-directed regulation of phosphorylation of a cGMP-binding phosphodiesterase by cGMP. J Biol Chem 265:14971–14978 [PubMed] [Google Scholar]
- Thompson et al., 2007.Thompson PE, Manganiello V, Degerman E. (2007) Re-discovering PDE3 inhibitors—new opportunities for a long neglected target. Curr Top Med Chem 7:421–436 [DOI] [PubMed] [Google Scholar]
- Thompson, 1991.Thompson WJ. (1991) Cyclic nucleotide phosphodiesterases: pharmacology, biochemistry and function. Pharmacol Ther 51:13–33 [DOI] [PubMed] [Google Scholar]
- Thompson et al., 2000.Thompson WJ, Piazza GA, Li H, Liu L, Fetter J, Zhu B, Sperl G, Ahnen D, Pamukcu R. (2000) Exisulind induction of apoptosis involves guanosine 3′,5′-cyclic monophosphate phosphodiesterase inhibition, protein kinase G activation, and attenuated beta-catenin. Cancer Res 60:3338–3342 [PubMed] [Google Scholar]
- Tokui et al., 1996.Tokui T, Brozovich F, Ando S, Ikebe M. (1996) Enhancement of smooth muscle contraction with protein phosphatase inhibitor 1: activation of inhibitor 1 by cGMP-dependent protein kinase. Biochem Biophys Res Commun 220:777–783 [DOI] [PubMed] [Google Scholar]
- Tsai and Kass, 2009.Tsai EJ, Kass DA. (2009) Cyclic GMP signaling in cardiovascular pathophysiology and therapeutics. Pharmacol Ther 122:216–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtcheva et al., 2009.Valtcheva N, Nestorov P, Beck A, Russwurm M, Hillenbrand M, Weinmeister P, Feil R. (2009) The commonly used cGMP-dependent protein kinase type I (cGKI) inhibitor Rp-8-Br-PET-cGMPS can activate cGKI in vitro and in intact cells. J Biol Chem 284:556–562 [DOI] [PubMed] [Google Scholar]
- van der Staay et al., 2008.van der Staay FJ, Rutten K, Bärfacker L, Devry J, Erb C, Heckroth H, Karthaus D, Tersteegen A, van Kampen M, Blokland A, et al. (2008) The novel selective PDE9 inhibitor BAY 73–6691 improves learning and memory in rodents. Neuropharmacology 55:908–918 [DOI] [PubMed] [Google Scholar]
- van Staveren et al., 2002.van Staveren WC, Glick J, Markerink-van Ittersum M, Shimizu M, Beavo JA, Steinbusch HW, de Vente J. (2002) Cloning and localization of the cGMP-specific phosphodiesterase type 9 in the rat brain. J Neurocytol 31:729–741 [DOI] [PubMed] [Google Scholar]
- Vandeput et al., 2009.Vandeput F, Krall J, Ockaili R, Salloum FN, Florio V, Corbin JD, Francis SH, Kukreja RC, Movsesian MA. (2009) cGMP-hydrolytic activity and its inhibition by sildenafil in normal and failing human and mouse myocardium. J Pharmacol Exp Ther 330:884–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderheyden et al., 2009.Vanderheyden V, Devogelaere B, Missiaen L, De Smedt H, Bultynck G, Parys JB. (2009) Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim Biophys Acta 1793:959–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasta et al., 2005.Vasta V, Sonnenburg WK, Yan C, Soderling SH, Shimizu-Albergine M, Beavo JA. (2005) Identification of a new variant of PDE1A calmodulin-stimulated cyclic nucleotide phosphodiesterase expressed in mouse sperm. Biol Reprod 73:598–609 [DOI] [PubMed] [Google Scholar]
- Wagner et al., 2003.Wagner LE, 2nd, Li WH, Yule DI. (2003) Phosphorylation of type-1 inositol 1,4,5-trisphosphate receptors by cyclic nucleotide-dependent protein kinases: a mutational analysis of the functionally important sites in the S2+ and S2- splice variants. J Biol Chem 278:45811–45817 [DOI] [PubMed] [Google Scholar]
- Waldman and Murad, 1988.Waldman SA, Murad F. (1988) Biochemical mechanisms underlying vascular smooth muscle relaxation: the guanylate cyclase-cyclic GMP system. J Cardiovasc Pharmacol 12 (Suppl 5):S115–S118 [PubMed] [Google Scholar]
- Walker et al., 2001.Walker LA, MacDonald JA, Liu X, Nakamoto RK, Haystead TA, Somlyo AV, Somlyo AP. (2001) Site-specific phosphorylation and point mutations of telokin modulate its Ca2+-desensitizing effect in smooth muscle. J Biol Chem 276:24519–24524 [DOI] [PubMed] [Google Scholar]
- Wall et al., 2003.Wall ME, Francis SH, Corbin JD, Grimes K, Richie-Jannetta R, Kotera J, Macdonald BA, Gibson RR, Trewhella J. (2003) Mechanisms associated with cGMP binding and activation of cGMP-dependent protein kinase. Proc Natl Acad Sci USA 100:2380–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis et al., 1999.Wallis RM, Corbin JD, Francis SH, Ellis P. (1999) Tissue distribution of phosphodiesterase families and the effects of sildenafil on tissue cyclic nucleotides, platelet function, and the contractile responses of trabeculae carneae and aortic rings in vitro. Am J Cardiol 83:3C–12C [DOI] [PubMed] [Google Scholar]
- Walter, 1989.Walter U. (1989) Physiological role of cGMP and cGMP-dependent protein kinase in the cardiovascular system. Rev Physiol Biochem Pharmacol 113:41–88 [DOI] [PubMed] [Google Scholar]
- Walter and Gambaryan, 2009.Walter U, Gambaryan S. (2009) cGMP and cGMP-dependent protein kinase in platelets and blood cells. Handb Exp Pharmacol 191:533–548 [DOI] [PubMed] [Google Scholar]
- Wang et al., 2009.Wang H, Kohr MJ, Traynham CJ, Ziolo MT. (2009) Phosphodiesterase 5 restricts NOS3/Soluble guanylate cyclase signaling to L-type Ca2+ current in cardiac myocytes. J Mol Cell Cardiol 47:304–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2006.Wang H, Liu Y, Huai Q, Cai J, Zoraghi R, Francis SH, Corbin JD, Robinson H, Xin Z, Lin G, et al. (2006) Multiple conformations of phosphodiesterase-5: implications for enzyme function and drug development. J Biol Chem 281:21469–21479 [DOI] [PubMed] [Google Scholar]
- Wang et al., 2005.Wang HG, Lu FM, Jin I, Udo H, Kandel ER, de Vente J, Walter U, Lohmann SM, Hawkins RD, Antonova I. (2005) Presynaptic and postsynaptic roles of NO, cGK, and RhoA in long-lasting potentiation and aggregation of synaptic proteins. Neuron 45:389–403 [DOI] [PubMed] [Google Scholar]
- Wang et al., 2003.Wang P, Wu P, Egan RW, Billah MM. (2003) Identification and characterization of a new human type 9 cGMP-specific phosphodiesterase splice variant (PDE9A5). Differential tissue distribution and subcellular localization of PDE9A variants. Gene 314:15–27 [DOI] [PubMed] [Google Scholar]
- Wang et al., 1999.Wang X, Bruderer S, Rafi Z, Xue J, Milburn PJ, Krämer A, Robinson PJ. (1999) Phosphorylation of splicing factor SF1 on Ser20 by cGMP-dependent protein kinase regulates spliceosome assembly. EMBO J 18:4549–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al., 2004.Wang Y, El-Zaru MR, Surks HK, Mendelsohn ME. (2004) Formin homology domain protein (FHOD1) is a cyclic GMP-dependent protein kinase I-binding protein and substrate in vascular smooth muscle cells. J Biol Chem 279:24420–24426 [DOI] [PubMed] [Google Scholar]
- Warner et al., 1994.Warner TD, Mitchell JA, Sheng H, Murad F. (1994) Effects of cyclic GMP on smooth muscle relaxation. Adv Pharmacol 26:171–194 [DOI] [PubMed] [Google Scholar]
- Weber, 1975.Weber G. (1975) Energetics of ligand binding to protein. Adv Protein Chem 29:1–83 [DOI] [PubMed] [Google Scholar]
- Weber et al., 2007.Weber S, Bernhard D, Lukowski R, Weinmeister P, Wörner R, Wegener JW, Valtcheva N, Feil S, Schlossmann J, Hofmann F, et al. (2007) Rescue of cGMP kinase I knockout mice by smooth muscle specific expression of either isozyme. Circ Res 101:1096–1103 [DOI] [PubMed] [Google Scholar]
- Wedel and Garbers, 1998.Wedel BJ, Garbers DL. (1998) Guanylyl cyclases: approaching year thirty. Trends Endocrinol Metab 9:213–219 [DOI] [PubMed] [Google Scholar]
- Weeks et al., 2007.Weeks JL, 2nd, Zoraghi R, Francis SH, Corbin JD. (2007) N-Terminal domain of phosphodiesterase-11A4 (PDE11A4) decreases affinity of the catalytic site for substrates and tadalafil, and is involved in oligomerization. Biochemistry 46:10353–10364 [DOI] [PubMed] [Google Scholar]
- Weinmeister et al., 2008.Weinmeister P, Lukowski R, Linder S, Traidl-Hoffmann C, Hengst L, Hofmann F, Feil R. (2008) Cyclic guanosine monophosphate-dependent protein kinase I promotes adhesion of primary vascular smooth muscle cells. Mol Biol Cell 19:4434–4441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernet et al., 1989.Wernet W, Flockerzi V, Hofmann F. (1989) The cDNA of the two isoforms of bovine cGMP-dependent protein kinase. FEBS Lett 251:191–196 [DOI] [PubMed] [Google Scholar]
- Wharton et al., 2005.Wharton J, Strange JW, Møller GM, Growcott EJ, Ren X, Franklyn AP, Phillips SC, Wilkins MR. (2005) Antiproliferative effects of phosphodiesterase type 5 inhibition in human pulmonary artery cells. Am J Respir Crit Care Med 172:105–113 [DOI] [PubMed] [Google Scholar]
- White et al., 2000.White RE, Kryman JP, El-Mowafy AM, Han G, Carrier GO. (2000) cAMP-dependent vasodilators cross-activate the cGMP-dependent protein kinase to stimulate BK(Ca) channel activity in coronary artery smooth muscle cells. Circ Res 86:897–905 [DOI] [PubMed] [Google Scholar]
- White et al., 1993.White RE, Lee AB, Shcherbatko AD, Lincoln TM, Schonbrunn A, Armstrong DL. (1993) Potassium channel stimulation by natriuretic peptides through cGMP-dependent dephosphorylation. Nature 361:263–266 [DOI] [PubMed] [Google Scholar]
- Wikström et al., 2008.Wikström K, Kavanagh DJ, Reid HM, Kinsella BT. (2008) Differential regulation of RhoA-mediated signaling by the TPalpha and TPbeta isoforms of the human thromboxane A2 receptor: independent modulation of TPalpha signaling by prostacyclin and nitric oxide. Cell Signal 20:1497–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson et al., 2008.Wilson LS, Elbatarny HS, Crawley SW, Bennett BM, Maurice DH. (2008) Compartmentation and compartment-specific regulation of PDE5 by protein kinase G allows selective cGMP-mediated regulation of platelet functions. Proc Natl Acad Sci USA 105:13650–13655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte et al., 2002.Witte K, Jacke K, Stahrenberg R, Arlt G, Reitenbach I, Schilling L, Lemmer B. (2002) Dysfunction of soluble guanylyl cyclase in aorta and kidney of Goto-Kakizaki rats: influence of age and diabetic state. Nitric Oxide 6:85–95 [DOI] [PubMed] [Google Scholar]
- Wolfe et al., 1989a.Wolfe L, Corbin JD, Francis SH. (1989a) Characterization of a novel isozyme of cGMP-dependent protein kinase from bovine aorta. J Biol Chem 264:7734–7741 [PubMed] [Google Scholar]
- Wolfe et al., 1989b.Wolfe L, Francis SH, Corbin JD. (1989b) Properties of a cGMP-dependent monomeric protein kinase from bovine aorta. J Biol Chem 264:4157–4162 [PubMed] [Google Scholar]
- Wollert et al., 2002.Wollert KC, Fiedler B, Gambaryan S, Smolenski A, Heineke J, Butt E, Trautwein C, Lohmann SM, Drexler H. (2002) Gene transfer of cGMP-dependent protein kinase I enhances the antihypertrophic effects of nitric oxide in cardiomyocytes. Hypertension 39:87–92 [DOI] [PubMed] [Google Scholar]
- Wooldridge et al., 2008.Wooldridge AA, Fortner CN, Lontay B, Akimoto T, Neppl RL, Facemire C, Datto MB, Kwon A, McCook E, Li P, et al. (2008) Deletion of the protein kinase A/protein kinase G target SMTNL1 promotes an exercise-adapted phenotype in vascular smooth muscle. J Biol Chem 283:11850–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooldridge et al., 2004.Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA. (2004) Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of serine 695 in response to cyclic nucleotides. J Biol Chem 279:34496–34504 [DOI] [PubMed] [Google Scholar]
- Wooldridge et al., 2004.Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA. (2004) Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J Biol Chem 279:34496–34504 [DOI] [PubMed] [Google Scholar]
- Wörner et al., 2007.Wörner R, Lukowski R, Hofmann F, Wegener JW. (2007) cGMP signals mainly through cAMP kinase in permeabilized murine aorta. Am J Physiol Heart Circ Physiol 292:H237–H244 [DOI] [PubMed] [Google Scholar]
- Wu et al., 2004.Wu AY, Tang XB, Martinez SE, Ikeda K, Beavo JA. (2004) Molecular determinants for cyclic nucleotide binding to the regulatory domains of phosphodiesterase 2A. J Biol Chem 279:37928–37938 [DOI] [PubMed] [Google Scholar]
- Wu et al., 1996.Wu X, Somlyo AV, Somlyo AP. (1996) Cyclic GMP-dependent stimulation reverses G-protein-coupled inhibition of smooth muscle myosin light chain phosphate. Biochem Biophys Res Commun 220:658–663 [DOI] [PubMed] [Google Scholar]
- Wunder et al., 2005.Wunder F, Tersteegen A, Rebmann A, Erb C, Fahrig T, Hendrix M. (2005) Characterization of the first potent and selective PDE9 inhibitor using a cGMP reporter cell line. Mol Pharmacol 68:1775–1781 [DOI] [PubMed] [Google Scholar]
- Wyatt et al., 1991.Wyatt TA, Lincoln TM, Pryzwansky KB. (1991) Vimentin is transiently co-localized with and phosphorylated by cyclic GMP-dependent protein kinase in formyl-peptide-stimulated neutrophils. J Biol Chem 266:21274–21280 [PubMed] [Google Scholar]
- Wyatt et al., 1998.Wyatt TA, Naftilan AJ, Francis SH, Corbin JD. (1998) ANF elicits phosphorylation of the cGMP phosphodiesterase in vascular smooth muscle cells. Am J Physiol 274:H448–H455 [DOI] [PubMed] [Google Scholar]
- Xia et al., 2001.Xia C, Bao Z, Yue C, Sanborn BM, Liu M. (2001) Phosphorylation and regulation of G-protein-activated phospholipase C-beta 3 by cGMP-dependent protein kinases. J Biol Chem 276:19770–19777 [DOI] [PubMed] [Google Scholar]
- Xue et al., 2004.Xue J, Milburn PJ, Hanna BT, Graham ME, Rostas JA, Robinson PJ. (2004) Phosphorylation of septin 3 on Ser-91 by cGMP-dependent protein kinase-I in nerve terminals. Biochem J 381:753–760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue et al., 2000.Xue J, Wang X, Malladi CS, Kinoshita M, Milburn PJ, Lengyel I, Rostas JA, Robinson PJ. (2000) Phosphorylation of a new brain-specific septin, G-septin, by cGMP-dependent protein kinase. J Biol Chem 275:10047–10056 [DOI] [PubMed] [Google Scholar]
- Yamamoto et al., 2001.Yamamoto S, Yan F, Zhou H, Tai HH. (2001) Serine 331 is the major site of receptor phosphorylation induced by agents that activate protein kinase G in HEK 293 cells overexpressing thromboxane receptor alpha. Arch Biochem Biophys 393:97–105 [DOI] [PubMed] [Google Scholar]
- Yanaka et al., 2003.Yanaka N, Kurosawa Y, Minami K, Kawai E, Omori K. (2003) cGMP-phosphodiesterase activity is up-regulated in response to pressure overload of rat ventricles. Biosci Biotechnol Biochem 67:973–979 [DOI] [PubMed] [Google Scholar]
- Yang et al., 2007.Yang L, Liu G, Zakharov SI, Bellinger AM, Mongillo M, Marx SO. (2007) Protein kinase G phosphorylates Cav1.2 alpha1c and beta2 subunits. Circ Res 101:465–474 [DOI] [PubMed] [Google Scholar]
- Young et al., 2005.Young JM, Feldman RA, Auerbach SM, Kaufman JM, Garcia CS, Shen W, Murphy AM, Beasley CM, Jr., Hague JA, Ahuja S. (2005) Tadalafil improved erectile function at twenty-four and thirty-six hours after dosing in men with erectile dysfunction: US trial. J Androl 26:310–318 [DOI] [PubMed] [Google Scholar]
- Yuasa et al., 1999.Yuasa K, Michibata H, Omori K, Yanaka N. (1999) A novel interaction of cGMP-dependent protein kinase I with troponin T. J Biol Chem 274:37429–37434 [DOI] [PubMed] [Google Scholar]
- Yuasa et al., 2000a.Yuasa K, Michibata H, Omori K, Yanaka N. (2000a) Identification of a conserved residue responsible for the autoinhibitiion of cGMP-dependent protein kinase I alpha and beta. FEBS Lett 466:175–178 [DOI] [PubMed] [Google Scholar]
- Yuasa et al., 2000b.Yuasa K, Omori K, Yanaka N. (2000b) Binding and phosphorylation of a novel male germ cell-specific cGMP-dependent protein kinase-anchoring protein by cGMP-dependent protein kinase Ialpha. J Biol Chem 275:4897–4905 [DOI] [PubMed] [Google Scholar]
- Yue et al., 2000.Yue TL, Wang C, Gu JL, Ma XL, Kumar S, Lee JC, Feuerstein GZ, Thomas H, Maleeff B, Ohlstein EH. (2000) Inhibition of extracellular signal-regulated kinase enhances Ischemia/Reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res 86:692–699 [DOI] [PubMed] [Google Scholar]
- Zeng et al., 2006.Zeng Y, Zhuang S, Gloddek J, Tseng CC, Boss GR, Pilz RB. (2006) Regulation of cGMP-dependent protein kinase expression by Rho and Kruppel-like transcription factor-4. J Biol Chem 281:16951–16961 [DOI] [PubMed] [Google Scholar]
- Zha and Reed, 1997.Zha H, Reed JC. (1997) Heterodimerization-independent functions of cell death regulatory proteins Bax and Bcl-2 in yeast and mammalian cells. J Biol Chem 272:31482–31488 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2008.Zhang M, Koitabashi N, Nagayama T, Rambaran R, Feng N, Takimoto E, Koenke T, O'Rourke B, Champion HC, Crow MT, et al. (2008) Expression, activity, and pro-hypertrophic effects of PDE5A in cardiac myocytes. Cell Signal 20:2231–2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 2003.Zhang R, Wang L, Zhang L, Chen J, Zhu Z, Zhang Z, Chopp M. (2003) Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res 92:308–313 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2007a.Zhang T, Zhuang S, Casteel DE, Looney DJ, Boss GR, Pilz RB. (2007a) A cysteine-rich LIM-only protein mediates regulation of smooth muscle-specific gene expression by cGMP-dependent protein kinase. J Biol Chem 282:33367–33380 [DOI] [PubMed] [Google Scholar]
- Zhang et al., 2007b.Zhang YW, Gesmonde J, Ramamoorthy S, Rudnick G. (2007b) Serotonin transporter phosphorylation by cGMP-dependent protein kinase is altered by a mutation associated with obsessive compulsive disorder. J Neurosci 27:10878–10886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al., 2002.Zhang R, Wang Y, Zhang L, Zhang Z, Tsang W, Lu M, Zhang L, Chopp M. (2002) Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke 33:2675–2680 [DOI] [PubMed] [Google Scholar]
- Zhao et al., 1997.Zhao J, Trewhella J, Corbin J, Francis S, Mitchell R, Brushia R, Walsh D. (1997) Progressive cyclic nucleotide-induced conformational changes in the cGMP-dependent protein kinase studied by small angle X-ray scattering in solution. J Biol Chem 272:31929–31936 [DOI] [PubMed] [Google Scholar]
- Zhao et al., 2005.Zhao X, Zhuang S, Chen Y, Boss GR, Pilz RB. (2005) Cyclic GMP-dependent protein kinase regulates CCAAT enhancer-binding protein beta functions through inhibition of glycogen synthase kinase-3. J Biol Chem 280:32683–32692 [DOI] [PubMed] [Google Scholar]
- Zhou et al., 1996.Zhou XB, Ruth P, Schlossmann J, Hofmann F, Korth M. (1996) Protein phosphatase 2A is essential for the activation of Ca2+-activated K+ currents by cGMP-dependent protein kinase in tracheal smooth muscle and Chinese hamster ovary cells. J Biol Chem 271:19760–19767 [DOI] [PubMed] [Google Scholar]
- Zhou et al., 2000.Zhou XM, Liu Y, Payne G, Lutz RJ, Chittenden T. (2000) Growth factors inactivate the cell death promoter BAD by phosphorylation of its BH3 domain on Ser155. J Biol Chem 275:25046–25051 [DOI] [PubMed] [Google Scholar]
- Zhou et al., 2008.Zhou Z, Sayed N, Pyriochou A, Roussos C, Fulton D, Beuve A, Papapetropoulos A. (2008) Protein kinase G phosphorylates soluble guanylyl cyclase on serine 64 and inhibits its activity. Arterioscler Thromb Vasc Biol 28:1803–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al., 2005.Zhu B, Strada S, Stevens T. (2005) Cyclic GMP-specific phosphodiesterase 5 regulates growth and apoptosis in pulmonary endothelial cells. Am J Physiol Lung Cell Mol Physiol 289:L196–L206 [DOI] [PubMed] [Google Scholar]
- Zhu and Strada, 2007.Zhu B, Strada SJ. (2007) The novel functions of cGMP-specific phosphodiesterase 5 and its inhibitors in carcinoma cells and pulmonary/cardiovascular vessels. Curr Top Med Chem 7:437–454 [DOI] [PubMed] [Google Scholar]
- Zhu et al., 2009.Zhu B, Zhang L, Alexeyev M, Alvarez DF, Strada SJ, Stevens T. (2009) Type 5 phosphodiesterase expression is a critical determinant of the endothelial cell angiogenic phenotype. Am J Physiol Lung Cell Mol Physiol 296:L220–L228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al., 2004.Zhu CB, Hewlett WA, Feoktistov I, Biaggioni I, Blakely RD. (2004) Adenosine receptor, protein kinase G, and p38 mitogen-activated protein kinase-dependent up-regulation of serotonin transporters involves both transporter trafficking and activation. Mol Pharmacol 65:1462–1474 [DOI] [PubMed] [Google Scholar]
- Ziolo et al., 2008.Ziolo MT, Kohr MJ, Wang H. (2008) Nitric oxide signaling and the regulation of myocardial function. J Mol Cell Cardiol 45:625–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoraghi et al., 2007.Zoraghi R, Francis SH, Corbin JD. (2007) Critical amino acids in phosphodiesterase-5 catalytic site that provide for high-affinity interaction with cyclic guanosine monophosphate and inhibitors. Biochemistry 46:13554–13563 [DOI] [PubMed] [Google Scholar]