Abstract
Introduction:
This study investigated the effect of binge eating on smoking cessation outcomes.
Methods:
Participants (n = 186) reported binge eating status at baseline and at a 6-week postquit evaluation during a larger clinical trial for smoking cessation. Binge eating was defined with a single self-report questionnaire item from the Dieting and Bingeing Severity Scale. Participant groups defined by binge eating status were compared on abstinence rates.
Results:
Among participants, 22% reported binge eating at baseline, 17% denied binge eating at baseline but endorsed binge eating by 6 weeks, and 61% denied binge eating at both timepoints. Participants who reported binge eating prior to or during treatment had lower quit rates at 6-week postquit and at the 24-week follow-up point than those without binge eating; the groups did not differ at the 12-week follow-up point. The group that experienced an emergence of binge eating reported significantly more weight gain than the other groups.
Conclusions:
These results suggest that treatments addressing problematic eating behaviors during smoking cessation are warranted.
Introduction
Smoking cessation is associated with weight gain (Perkins, 1993; Williamson et al., 1991). Research has identified several factors that appear to predict the amount of weight gain following smoking cessation, including gender, initial body weight, length and frequency of smoking behaviors, and diet and physical activity (Klesges, Meyers, Klesges, & La Vasque, 1989). Since weight gain is associated with smoking relapse, it is important to identify factors that put smokers at risk for cessation-related weight gain.
Distinct lines of research suggest that binge eating, or consuming large amounts of food with a subjective sense of loss of control over eating, may be associated with smoking outcomes and with cessation-related weight gain (White, Grilo, O’Malley, & Potenza, 2010). For example, emerging research suggests that excess food intake and smoking may share common mechanisms (e.g., Volkow & Wise, 2005), and research investigating both smoking and binge eating indicates that the presence of both behaviors predicts a worsened clinical profile (White & Grilo, 2007). A large-scale epidemiological study of binge eating in the absence of compensatory behaviors reported increased odds of lifetime smoking among individuals with binge eating compared with controls, which was attenuated in women after adjusting for body mass index (BMI, p = .06; Reichborn-Kjennerud, Bulik, Sullivan, Tambs, & Harris, 2004). One report found marginally increased odds of nicotine dependence among individuals with binge eating disorder compared with obese controls without binge eating (Grucza, Przybeck, & Cloninger, 2007). Our research group found that among obese women with binge eating disorder, a history of cigarette smoking is associated with higher comorbid Axis I psychopathology (White & Grilo, 2006) and a general worsening of eating pathology specific to binge eating (White & Grilo, 2007). Collectively, this research indicates that among overweight individuals with binge eating disorder, cigarette smoking is associated with more severe eating disorder features.
Although the association between smoking cessation and binge eating has been postulated for some time (e.g., Pomerleau et al., 1993), research on the influence of binge eating on smoking outcomes is limited. In a retrospective self-report study, overweight individuals with binge eating reported having gained significantly more weight in the first year following smoking cessation than those who denied binge eating (White, Masheb, & Grilo, 2010). To date, there has been a paucity of prospective research on the effect of binge eating on smoking cessation outcomes. Therefore, the aim of this preliminary study was to investigate the effect of pretreatment binge eating on smoking cessation outcomes. We also investigated the effect of the emergence of binge eating during treatment on smoking outcomes and weight change during treatment.
Method
Participants
This report is a secondary analysis of data from a randomized controlled trial for smoking cessation with sustained-release (SR) bupropion (Toll et al., 2007). Individuals were eligible for participation in the trial if they were at least 18 years of age, smoked 10 cigarettes/day for at least one year, and had a baseline expired carbon monoxide (CO) level of at least 10 parts per million (ppm). The current sample includes all 186 participants who completed the posttreatment (6-week) evaluation. Of these, 63% were overweight (BMI ≥ 25) and 25% were obese (BMI ≥ 30). In relation to the parent trial (Toll et al., 2007), 47.3% of the participants received gain-framed messages and 52.7% received loss-framed messages (described in further detail below). The sample was primarily Caucasian (85%), 54% female, with a mean age of 43.5 years (SD = 11.5). Participants had smoked for an average of 25.8 (SD = 11.1) years. This study was approved by the Institutional Review Board of the Yale University School of Medicine.
Measures
Body Mass Index
BMI is an index of weight-for-height that is commonly used to classify underweight, overweight, and obesity in adults. It is defined as the weight in kilograms divided by height in meters square (kg/m2). Overweight is classified by BMI ≥25 and obesity by BMI ≥30 (World Health Organization, 2000). Height and weight without shoes were measured on a standing mechanical scale. Height was collected at baseline only, and weight was collected at each evaluation.
Dieting and Bingeing Severity Scale
The Dieting and Bingeing Severity Scale (DBSS; Kurth, Krahn, Nairn, & Drewnowski, 1995) is a 25-item survey evaluating the frequency and severity of dieting and binge eating behaviors. The DBSS measures behavioral features of eating disorders, including binge eating. Participants completed the DBSS at baseline, 6-week postquit, and the 12- and 24-week follow-up evaluations.
Procedure
All participants received bupropion SR (300 mg/daily) for a 7-week period (1-week prequit and 6-week postquit) and were randomized to receive gain- or loss-framed messages. For 6 weeks after their quit date, participants attended research appointments on a biweekly basis. Questionnaires were administered, and framed video and print messages encouraging smoking abstinence (e.g., air freshener with printed slogans on them) were provided at these appointments. These messages focused on either the benefits of quitting smoking (gain framed) or the costs of continuing to smoke (loss framed). Participants were recontacted for follow-up assessments at 12- and 24-week postquit.
Smoking was assessed using timeline followback (TLFB) methodology at each biweekly appointment (Sobell & Sobell, 1992). Baseline TLFB data were gathered for the 30 days prior to their first screening session, and at all postquit biweekly appointments, individuals were verbally asked to indicate the number of cigarettes they consumed each day for the preceding weeks. CO levels were obtained to verify smoking abstinence with CO less than or equal to 10 ppm coded as abstinent. Participants who dropped out or missed multiple appointments were considered to be smoking. Participants were allowed to miss one appointment and still be coded as abstinent as long as the appointment before and after the missed appointment had a CO-verified report of abstinence from smoking (Toll et al., 2007).
Statistical Analysis
Patterns of outcomes were collapsed across message framing conditions in order to examine the unique effect of binge eating on abstinence rates. Participants were categorized according to binge eating status at baseline and at the 6-, 12-, and 24-week evaluations. When data for a follow-up point were not available, positive binge classification from the previous evaluation point was carried forward. Therefore, individuals who endorsed binge eating at a previous evaluation point were classified as binge eating in the subsequent point to allow for inclusion of these participants in the analysis. Binge eating status was determined according to categorical response (yes/no) to the DBSS question of eating “a large amount of food in a discrete period of time—an eating binge.” In this manner, we were able to adequately capture individuals with pretreatment binge eating as well as those whose binge eating emerged within 6-week treatment period or within the 24-week follow-up evaluation period. Of note, classification of a binge episode was left up to the interpretation of participants—it was not possible to confirm whether the amount of food consumed is consistent with the diagnostic criterion of an objectively large amount of food.
Smoking abstinence was defined as self-reported abstinence (no smoking and not even a puff), on the TLFB at 6-, 12-, and 24-week postquit, verified by an exhaled CO level ≤10 ppm (SRNT Subcommittee on Biochemical Verification, 2002). Logistic regression compared abstinence rates across binge eating groups. Logistic regression models also tested the effect of binge eating after controlling for treatment condition. Analysis of covariance examined weight change from baseline to the 6-week evaluation, controlling for baseline BMI, across binge eating groups.
Results
Frequency of Binge Eating
Among participants who completed the pretreatment and posttreatment (6 weeks) evaluations, 22% endorsed binge eating at baseline. An additional 17% denied binge eating at baseline but reported binge eating by 6-week postquit, and 61% denied binge eating at both timepoints. The frequency of participants endorsing binge eating increased over the duration of the follow-up period, with 53% reporting binge eating by 12-week postquit and 62% endorsing binge eating by the 24-week postquit follow-up point. Of those who reported binge eating at baseline, 65% reported binge eating episodes at a frequency of at least once per month and 25% reported episodes occurring at least three to four times per month. At 6-week postquit, the proportion increased to 79% reporting at least one to two episodes per month and 32% reporting episodes three to four times (or more) per month. The proportion of individuals reporting binge eating did not differ across study groups in the larger trial (i.e., gain-framed vs. loss-framed messages), χ2 = 0.2, p = .64. The frequency of binge eating did not differ across male (42%) and female (37%) participants, χ2 = 0.5, p = .50.
Smoking Abstinence
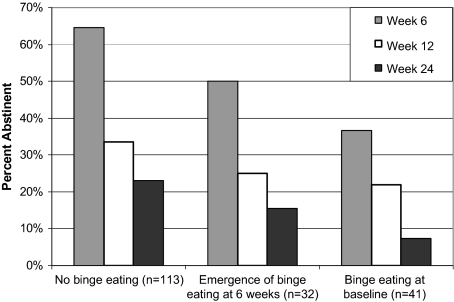
Smoking cessation outcomes for all participants have been presented elsewhere (Toll et al., 2007). Smoking abstinence at 6-week postquit significantly differed across groups depending on the presence or absence of binge eating (odds ratio [OR] = 2.47, p = .003, 95% CI: 1.35–4.52), with individuals with binge eating being significantly less likely to achieve abstinence. At 12- and 24-week postquit, the pattern of relationship was maintained, with the 6-week binge status prospectively predicting outcomes at the follow-up points (12-week OR = 1.67, p = .13, 95% CI: 0.86–3.26 and 24-week OR = 2.43, p = .04, 95% CI: 1.03–5.71). The effect of binge eating on smoking outcomes remained after statistically controlling for study group (gain-framed vs. loss-framed): 6-week OR = 2.46, p = .004, 95% CI: 1.34–4.50; 12-week OR = 1.65, p = .14, 95% CI: 0.84–3.23; and 24-week OR = 2.40, p = .04, 95% CI: 1.01–5.68). The effect of binge eating on smoking outcomes also remained significant after controlling for gender: 6-week OR = 2.49, p = .003, 95% CI: 1.36–4.56; 12-week OR = 1.77, p = .10, 95% CI: 0.90–3.51; and 24-week OR = 2.59, p = .03, 95% CI: 1.09–6.16). Figure 1 shows differences in abstinence rates up to 24 weeks as a function of binge eating at baseline, binge eating that emerged during treatment (i.e., by the 6-week assessment point), or no binge eating at both timepoints. To test the effect of binge status at the follow-up points, we also examined the effect of 12-week eating behavior on the 12-week (concurrent) outcomes and the 24-week (prospective) smoking cessation outcomes. The effect of binge eating at the 12-week follow-up evaluation on smoking outcomes persisted and was highly significant: 12-week OR = 3.32, p = .001, 95% CI: 1.64–6.71 and 24-week OR = 3.55, p = .003, 95% CI: 1.54–8.14. Finally, binge eating status at the 24-week follow-up point predicted concurrent smoking: OR = 3.99, p = .001, 95% CI: 1.76–9.02), indicating that participants who reported binge eating were nearly four times more likely to be smoking at 24 weeks.
Figure 1.
Percent abstinent at 6- and 24-week postquit as a function of binge eating.
Weight Change
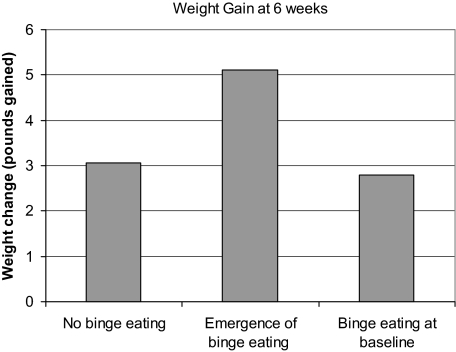
Weight change from baseline to 6-week postquit significantly differed across groups depending on binge eating status (see Figure 2), F(2, 166) = 3.4, p = .04, and remained significant after controlling for baseline BMI [F(2,165) = 3.4, p = .03]. Post-hoc Tukey tests revealed that the group that began binge eating during treatment gained more weight (M = 5.1 pounds and SD = 5.1) than the group that denied binge eating. The group that reported binge eating at baseline (M = 2.8 pounds, SD = 4.6) did not significantly differ from the group that denied binge eating throughout treatment (M = 3.1 pounds, SD = 3.7). Binge eating did not predict weight gain at Week 12 or at Week 24, but this may have been due to a power issue due to attrition at the follow-up points. It is worth noting that the group reporting binge eating at baseline was more likely to dropout of treatment compared with the group that denied binge eating (54% vs. 39% dropout at 12 weeks and 76% vs. 51% dropout at 24 weeks).
Discussion
Compared with individuals who do not binge eat, individuals who report binge eating are less successful in smoking cessation. In the current trial, individuals without binge eating were three times more likely to achieve abstinence at 6 weeks than those with binge eating problems. In addition, in the current trial, a substantial percentage of smokers began binge eating during smoking cessation. Finally, smokers who experienced an onset of binge eating during their quit attempt were more likely to experience weight gain during the first 6 weeks of treatment. Our results highlight the relevance of binge eating in smoking outcomes. Moreover, these results suggest that some individuals may be at risk for developing eating problems during or shortly after smoking cessation, perhaps due to the behavioral and functional similarity of the two behaviors. For example, both smoking (Nil, 1991) and binge eating (Heatherton & Baumeister, 1991) modulate negative affect, so it is possible that in the absence of smoking, some individuals begin binge eating in an attempt to regulate negative emotions. Our findings are also consistent with the hypothesis that some individuals may effectively use cigarette smoking as a means to suppress urges to binge eat (Pomerleau et al., 1993).
We note certain limitations that should be considered when interpreting these results. First, the measure used to define binge eating relied upon self-report. The definition of “binge eating” was left up to the interpretation of the participant; it is not possible to determine whether binge eating referred to diagnostic definitions of (a) consuming an unusually large amount of food or (b) whether a subjective sense of loss of control accompanied the eating episodes. Future studies should use validated clinical interviews to determine binge eating. In addition, since this was a secondary analysis of individuals participating in a larger clinical trial, our sample size was limited, particularly with regard to weight outcomes at follow-up points. The differential dropout within the binge eating group introduces the possibility of bias, and limited cell sizes precluded analysis of weight outcomes at the 12- and 24-week follow-up points. However, the results pertaining to the smoking abstinence outcomes at the follow-up points were robust and suggest that despite the differential dropout, binge eating was highly predictive of worse abstinence rates. Finally, all study participants were taking bupropion, a drug with appetite-reducing and antidepressant effects, which suggests that the rate of binge eating and weight gain observed in this sample may have been influenced somewhat by the medication. It is possible that the rate of binge eating and weight gain may have been even greater had participants not been taking bupropion.
Despite these limitations, our results indicate significant differences in smoking cessation outcomes as a function of binge eating. Future smoking cessation studies should evaluate and track eating problems throughout the treatment. Attention to eating patterns is important for individuals at all weight ranges because individuals of normal weight are also at risk of eventual weight gain following the emergence or worsening of binge eating. In addition, the differential dropout observed within the binge eating group is suggestive of worse outcomes and the need for additional or different interventions for this group. Since binge eating is likely to result in weight gain and weight gain may predict relapse for smoking, this suggests that additional strategies to prevent the development of binge eating are warranted in smoking cessation treatment.
Funding
This research was supported by National Institutes of Health grants (P50-DA13334, P50-AA15632, R25-DA020515, K12-DA000167, and K23-DK071646) from the National Institute on Alcohol Abuse and Alcoholism, National Institute on Drug Abuse, National Institute on Diabetes and Digestive and Kidney Diseases and by the State of Connecticut, Department of Mental Health and Addictions Services.
Declaration of Interests
None declared.
Figure 2.
Weight gain at 6-week postquit as a function of binge eating.
References
- Grucza RA, Przybeck TR, Cloninger CR. Prevalence and correlates of binge eating disorder in a community sample. Comprehensive Psychiatry. 2007;48:124–131. doi: 10.1016/j.comppsych.2006.08.002. doi:10.1016/j.comppsych.2006.08.002doi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Baumeister RF. Binge eating as escape from self-awareness. Psychological Bulletin. 1991;110:86–108. doi: 10.1037/0033-2909.110.1.86. doi:10.1037/0033-2909.110.1.86. [DOI] [PubMed] [Google Scholar]
- Klesges RC, Meyers AW, Klesges LM, La Vasque ME. Smoking, body weight, and their effects on smoking behavior: A comprehensive review of the literature. Psychological Bulletin. 1989;106:204–230. doi: 10.1037/0033-2909.106.2.204. [DOI] [PubMed] [Google Scholar]
- Kurth C, Krahn D, Nairn K, Drewnowski A. The severity of dieting and bingeing behaviors in college women: Interview validation of survey data. Journal of Psychiatric Research. 1995;29:211–225. doi: 10.1016/0022-3956(95)00002-m. doi:10.1016/0022-3956(95)00002-M. [DOI] [PubMed] [Google Scholar]
- Nil R. A psychopharmacological and psychophysiological evaluation of smoking motives. Reviews on Environmental Health. 1991;9:85–115. doi: 10.1515/reveh.1991.9.2.85. [DOI] [PubMed] [Google Scholar]
- Perkins KA. Weight gain following smoking cessation. Journal of Consulting and Clinical Psychology. 1993;61:768–777. doi: 10.1037//0022-006x.61.5.768. [DOI] [PubMed] [Google Scholar]
- Pomerleau CS, Ehrlich E, Tate JC, Marks JL, Flessland KA, Pomerleau OF. The female weight-control smoker: A profile. Journal of Substance Abuse. 1993;5:391–400. doi: 10.1016/0899-3289(93)90007-x. doi:10.1016/0899-3289(93)90007-X. [DOI] [PubMed] [Google Scholar]
- Reichborn-Kjennerud T, Bulik CM, Sullivan PF, Tambs K, Harris JR. Psychiatric and medical symptoms in binge eating in the absence of compensatory behaviors. Obesity Research. 2004;12:1445–1454. doi: 10.1038/oby.2004.181. doi:10.1038/oby.2004.181. [DOI] [PubMed] [Google Scholar]
- Sobell L, Sobell M. Timeline followback: A technique for assessing self-reported alcohol consumption. In: Litten R, Allen J, editors. Measuring alcohol consumption. Rockville, MD: Humana Press; 1992. pp. 207–224. [Google Scholar]
- SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. doi:10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Toll B, O’Malley S, Katulak N, Wu R, Dubin J, Latimer A, et al. Comparing gain- and loss-framed messages for smoking cessation with bupropion: A randomized controlled trial. Psychology of Addictive Behaviors. 2007;21:534–544. doi: 10.1037/0893-164X.21.4.534. doi: 10.1037/0893-164X.21.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nature Neuroscience. 2005;8:555–560. doi: 10.1038/nn1452. doi:10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- White MA, Grilo CM. Psychiatric comorbidity in binge-eating disorder as a function of smoking history. Journal of Clinical Psychiatry. 2006;67:594–599. doi: 10.4088/jcp.v67n0410. [DOI] [PubMed] [Google Scholar]
- White MA, Grilo CM. Symptom severity in obese women with binge eating disorder as a function of smoking history. International Journal of Eating Disorders. 2007;40:77–81. doi: 10.1002/eat.20334. doi: 10.1002/eat.20334. [DOI] [PubMed] [Google Scholar]
- White MA, Grilo CM, O’Malley SS, Potenza MN. Clinical case discussion: Binge eating disorder, obesity and tobacco smoking. Journal of Addiction Medicine. 2010;4:11–19. doi: 10.1097/ADM.0b013e3181ce38c8. doi:10.1097/ADM.0b013e3181ce38c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MA, Masheb RM, Grilo CM. Self-reported weight gain following smoking cessation: A function of binge eating behavior. International Journal of Eating Disorders. 2010;43:572–575. doi: 10.1002/eat.20729. doi:10.1002/eat.20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. New England Journal of Medicine. 1991;324:739–745. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Obesity: Preventing and managing the global epidemic. Report of a WHO Consultation on Obesity. 2000. World Health Organization: Geneva, Switzerland: 2000. [PubMed] [Google Scholar]