Abstract
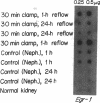
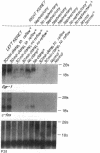
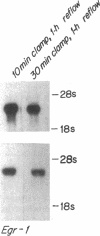
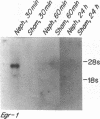
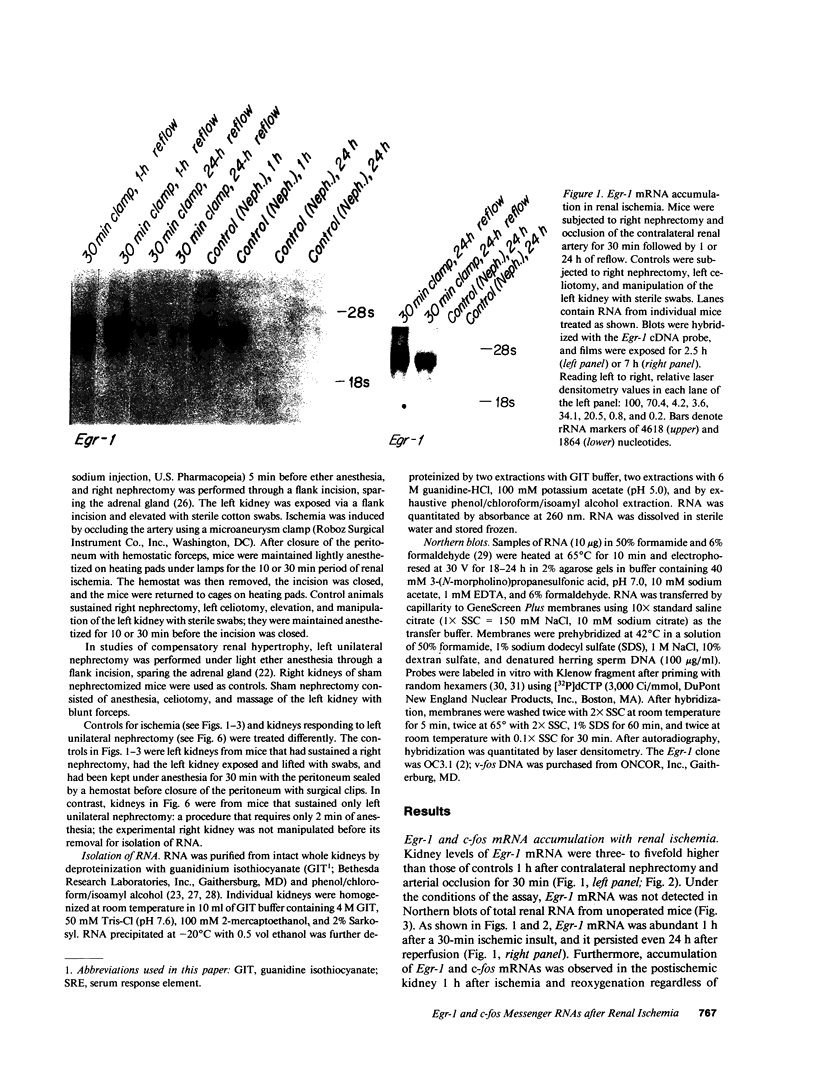
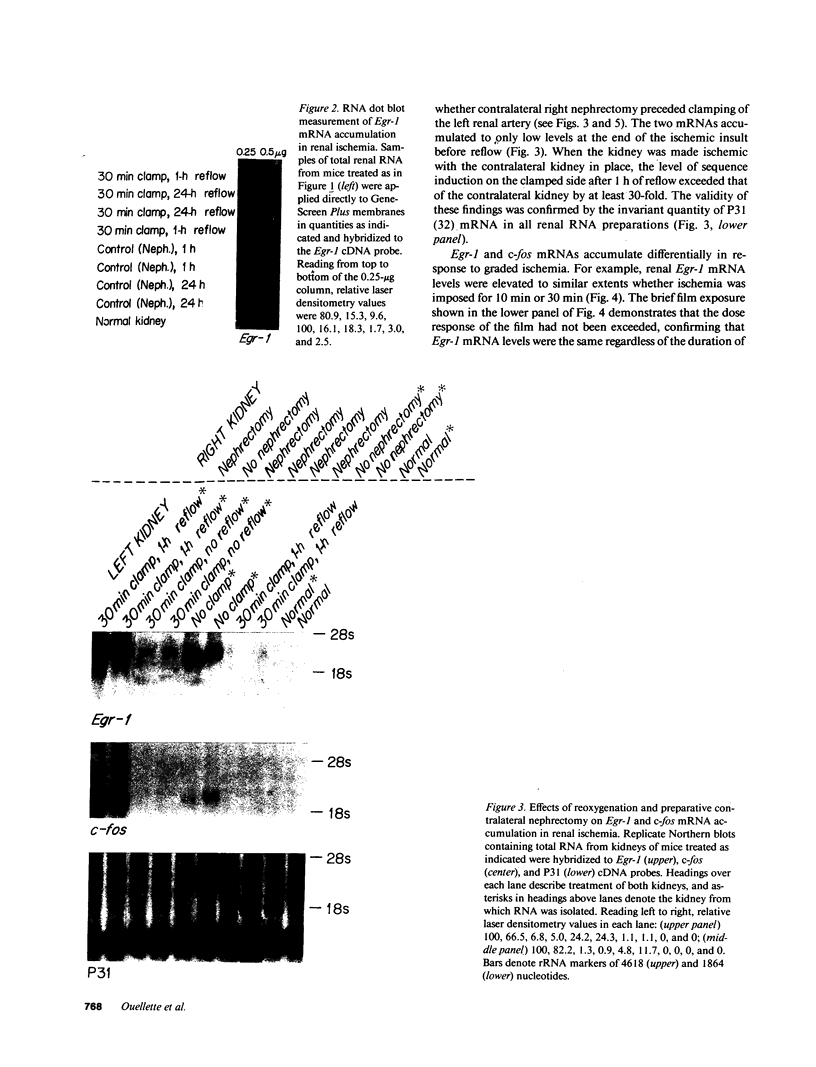
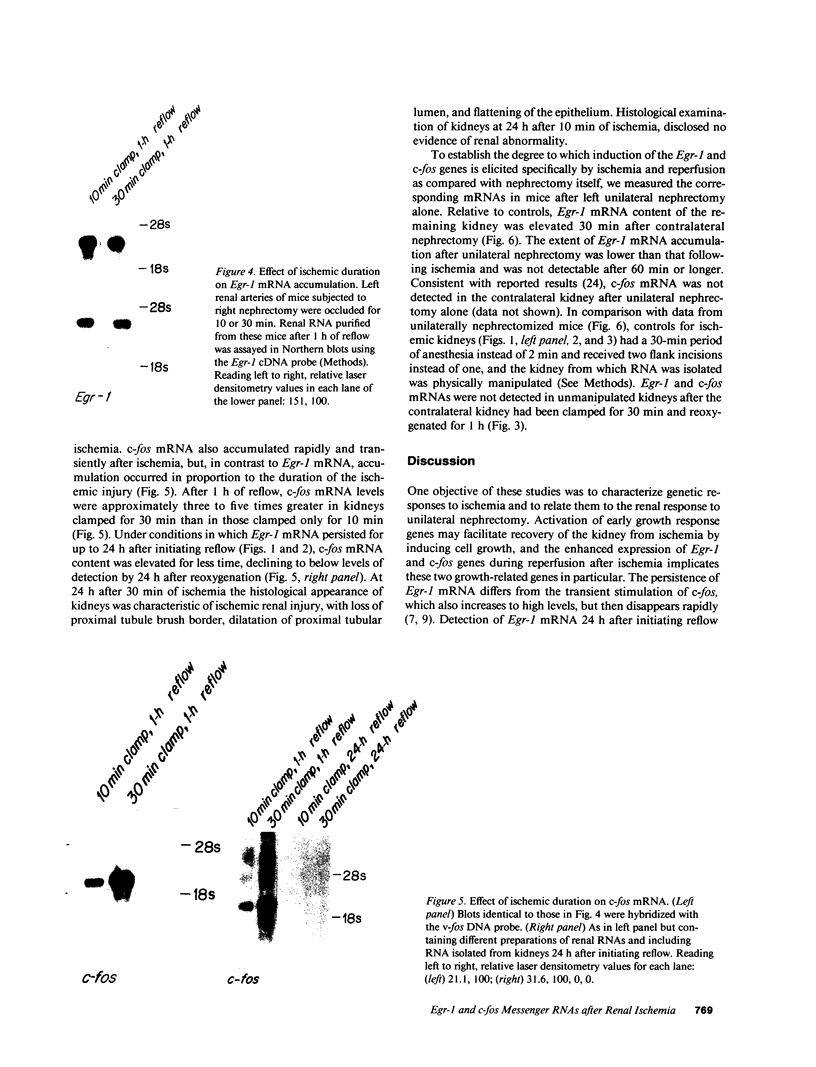
To identify specific genetic regulatory mechanisms associated with renal ischemia, we measured the accumulation of Egr-1 and c-fos mRNAs in the mouse kidney after occlusion of the renal artery and reperfusion. At 1 h after right nephrectomy and arterial occlusion of the contralateral kidney for 10 or 30 min, Egr-1 mRNA levels were three to five times greater in these kidneys as compared with those in control animals that had sustained unilateral nephrectomy alone and were much greater than levels in the normal organ. Whether ischemia was imposed for 10 or for 30 min, renal Egr-1 mRNA contents were equivalent and remained elevated after 24 h of reperfusion subsequent to 30 min of ischemia. Although c-fos mRNA also accumulated in response to ischemia and reperfusion, the pattern differed from that of Egr-1 in that c-fos mRNA content varied with the duration of ischemia and was undetectable 24 h after injury. Contralateral nephrectomy was not necessary to see the marked accumulation of Egr-1 and c-fos mRNAs with unilateral ischemia. Reflow was necessary, however, since only minimal sequence accumulation occurred by the end of the ischemic period. After left uninephrectomy alone, Egr-1 mRNA levels in the remaining kidney were maximal 30 min after surgery, but were not detectable thereafter; c-fos mRNA levels did not change after unilateral nephrectomy. Differential expression of early growth-related genes implicated in transcriptional activation may influence tissue recovery after renal ischemia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arendshorst W. J., Finn W. F., Gottschalk C. W. Pathogenesis of acute renal failure following temporary renal ischemia in the rat. Circ Res. 1975 Nov;37(5):558–568. doi: 10.1161/01.res.37.5.558. [DOI] [PubMed] [Google Scholar]
- Beer D. G., Zweifel K. A., Simpson D. P., Pitot H. C. Specific gene expression during compensatory renal hypertrophy in the rat. J Cell Physiol. 1987 Apr;131(1):29–35. doi: 10.1002/jcp.1041310106. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V. Mediators of ischemic renal injury. Annu Rev Med. 1988;39:531–544. doi: 10.1146/annurev.me.39.020188.002531. [DOI] [PubMed] [Google Scholar]
- Büscher M., Rahmsdorf H. J., Litfin M., Karin M., Herrlich P. Activation of the c-fos gene by UV and phorbol ester: different signal transduction pathways converge to the same enhancer element. Oncogene. 1988 Sep;3(3):301–311. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Christy B. A., Lau L. F., Nathans D. A gene activated in mouse 3T3 cells by serum growth factors encodes a protein with "zinc finger" sequences. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7857–7861. doi: 10.1073/pnas.85.21.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen D. R., Curran T. fra-1: a serum-inducible, cellular immediate-early gene that encodes a fos-related antigen. Mol Cell Biol. 1988 May;8(5):2063–2069. doi: 10.1128/mcb.8.5.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Kramer J., Kosic-Smithers J. SSC1, a member of the 70-kDa heat shock protein multigene family of Saccharomyces cerevisiae, is essential for growth. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4156–4160. doi: 10.1073/pnas.84.12.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Evans R. M., Hollenberg S. M. Zinc fingers: gilt by association. Cell. 1988 Jan 15;52(1):1–3. doi: 10.1016/0092-8674(88)90522-3. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ferris D. K., Harel-Bellan A., Morimoto R. I., Welch W. J., Farrar W. L. Mitogen and lymphokine stimulation of heat shock proteins in T lymphocytes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3850–3854. doi: 10.1073/pnas.85.11.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine L. The biology of renal hypertrophy. Kidney Int. 1986 Mar;29(3):619–634. doi: 10.1038/ki.1986.45. [DOI] [PubMed] [Google Scholar]
- Finn W. F., Chevalier R. L. Recovery from postischemic acute renal failure in the rat. Kidney Int. 1979 Aug;16(2):113–123. doi: 10.1038/ki.1979.112. [DOI] [PubMed] [Google Scholar]
- Gilman M. Z. The c-fos serum response element responds to protein kinase C-dependent and -independent signals but not to cyclic AMP. Genes Dev. 1988 Apr;2(4):394–402. doi: 10.1101/gad.2.4.394. [DOI] [PubMed] [Google Scholar]
- Goyette M., Petropoulos C. J., Shank P. R., Fausto N. Regulated transcription of c-Ki-ras and c-myc during compensatory growth of rat liver. Mol Cell Biol. 1984 Aug;4(8):1493–1498. doi: 10.1128/mcb.4.8.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Halliburton I. W., Thomson R. Y. Chemical aspects of compensatory renal hypertrophy. Cancer Res. 1965 Dec;25(11):1882–1887. [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Ziff E. The role of the leucine zipper in the fos-jun interaction. Nature. 1988 Dec 15;336(6200):646–651. doi: 10.1038/336646a0. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire P., Revelant O., Bravo R., Charnay P. Two mouse genes encoding potential transcription factors with identical DNA-binding domains are activated by growth factors in cultured cells. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4691–4695. doi: 10.1073/pnas.85.13.4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinsky N. G. Pathophysiology of acute renal failure. N Engl J Med. 1977 Jun 23;296(25):1453–1458. doi: 10.1056/NEJM197706232962509. [DOI] [PubMed] [Google Scholar]
- Malt R. A., Lemaitre D. A. Accretion and turnover of RNA in the renoprival kidney. Am J Physiol. 1968 May;214(5):1041–1047. doi: 10.1152/ajplegacy.1968.214.5.1041. [DOI] [PubMed] [Google Scholar]
- Malt R. A., Lemaittre D. A. Nucleic acids in fetal kidney after maternal nephrectomy. Proc Soc Exp Biol Med. 1969 Feb;130(2):539–542. doi: 10.3181/00379727-130-35600. [DOI] [PubMed] [Google Scholar]
- Milbrandt J. A nerve growth factor-induced gene encodes a possible transcriptional regulatory factor. Science. 1987 Nov 6;238(4828):797–799. doi: 10.1126/science.3672127. [DOI] [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Tremblay J. M., Cline M. J., Verma I. M. Differential expression of cellular oncogenes during pre- and postnatal development of the mouse. Nature. 1982 Oct 14;299(5884):640–644. doi: 10.1038/299640a0. [DOI] [PubMed] [Google Scholar]
- Norman J. T., Bohman R. E., Fischmann G., Bowen J. W., McDonough A., Slamon D., Fine L. G. Patterns of mRNA expression during early cell growth differ in kidney epithelial cells destined to undergo compensatory hypertrophy versus regenerative hyperplasia. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6768–6772. doi: 10.1073/pnas.85.18.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellette A. J., Moonka R., Zelenetz A. D., Malt R. A. Regulation of ribosome synthesis during compensatory renal hypertrophy in mice. Am J Physiol. 1987 Oct;253(4 Pt 1):C506–C513. doi: 10.1152/ajpcell.1987.253.4.C506. [DOI] [PubMed] [Google Scholar]
- Ouellette A. J., Watson R. K., Billmire K., Dygert M. K., Ingwall J. S. Protein synthesis in the cultured fetal mouse heart: effects of deprivation of oxygen and oxidizable substrate. Biochemistry. 1983 Mar 1;22(5):1201–1207. doi: 10.1021/bi00274a033. [DOI] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Cohen D. R., Curran T., Bos T. J., Vogt P. K., Bohmann D., Tjian R., Franza B. R., Jr Fos-associated protein p39 is the product of the jun proto-oncogene. Science. 1988 May 20;240(4855):1010–1016. doi: 10.1126/science.3130660. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer K. A., Ganote C. E., Jennings R. B. Alterations in renal cortex following ischemic injury. 3. Ultrastructure of proximal tubules after ischemia or autolysis. Lab Invest. 1972 Apr;26(4):347–363. [PubMed] [Google Scholar]
- Ryder K., Lau L. F., Nathans D. A gene activated by growth factors is related to the oncogene v-jun. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1487–1491. doi: 10.1073/pnas.85.5.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., Dougan S. T., McFadden G., Greenberg M. E. Calcium and growth factor pathways of c-fos transcriptional activation require distinct upstream regulatory sequences. Mol Cell Biol. 1988 Jul;8(7):2787–2796. doi: 10.1128/mcb.8.7.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhatme V. P., Cao X. M., Chang L. C., Tsai-Morris C. H., Stamenkovich D., Ferreira P. C., Cohen D. R., Edwards S. A., Shows T. B., Curran T. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988 Apr 8;53(1):37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Sukhatme V. P., Kartha S., Toback F. G., Taub R., Hoover R. G., Tsai-Morris C. H. A novel early growth response gene rapidly induced by fibroblast, epithelial cell and lymphocyte mitogens. Oncogene Res. 1987 Sep-Oct;1(4):343–355. [PubMed] [Google Scholar]
- Theodor L., Peleg D., Meyuhas O. P31, a mammalian housekeeping protein encoded by a multigene family containing a high proportion of pseudogenes. Biochim Biophys Acta. 1985 Nov 13;826(2-3):137–146. doi: 10.1016/0167-4781(85)90119-8. [DOI] [PubMed] [Google Scholar]
- Toback F. G. Phosphatidylcholine metabolism during renal growth and regeneration. Am J Physiol. 1984 Mar;246(3 Pt 2):F249–F259. doi: 10.1152/ajprenal.1984.246.3.F249. [DOI] [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Tsai-Morris C. H., Cao X. M., Sukhatme V. P. 5' flanking sequence and genomic structure of Egr-1, a murine mitogen inducible zinc finger encoding gene. Nucleic Acids Res. 1988 Sep 26;16(18):8835–8846. doi: 10.1093/nar/16.18.8835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam M. A., Bernard D. B., Donohoe J. F., Levinsky N. G. Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2, and S3 segments. Kidney Int. 1978 Jul;14(1):31–49. doi: 10.1038/ki.1978.87. [DOI] [PubMed] [Google Scholar]
- Wu B. J., Morimoto R. I. Transcription of the human hsp70 gene is induced by serum stimulation. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6070–6074. doi: 10.1073/pnas.82.18.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]