Abstract
Motor learning in the vestibular system can be differentially obtained depending upon the context for which the vestibulo-ocular reflex (VOR) has been exposed. Manipulating head orientation relative to gravity is an example of a contextual cue that can elicit independent VOR gains. We were interested in examining retention of short-term VOR adaptation when the adapting stimulus was paired with a novel contextual cue. Two sets of non-human primate VOR adaptation experiments were designed to assess the influence of head position relative to gravity on retention of the pitch VOR. First, the pitch VOR of three squirrel monkeys was adapted for 3 h using minimizing (×0.45) spectacles and a sum-of-sines stimulus (20°/s at 0.5, 1.1, 2.3, and 3.7 Hz) while the animals were positioned left ear down (LED adaptation). Pitch VOR gains were measured in the adapted position (LED) and two non-adapted positions (upright, UP) or right ear down (RED). In the second set of experiments, the pitch VOR was adapted in an upright head position (same adapting stimulus as used in LED) and tested in UP, LED or RED. No head immobility or darkness restrictions were imposed on the animals after the initial adaptation exposure. The pitch VOR gains were measured during the acceleration (GA) and constant velocity (GV) portions of 1,000°/s2−150°/s step responses and during 0.5, 2.0, and 4.0 Hz sinusoids with velocities varying from 20 to 100°/s. All measures of VOR gain for UP, LED, and RED were done immediately after the adaptation and for three subsequent days and at post-adaptation day 7 (PAD 7). When tested in the adapting position, all experiments showed immediate reduction in GA and GV compared with pre-adaptation levels. For LED adaptation experiments, the pitch GA and GV gains were significantly reduced for as long as 7 days. Some retention of the LED-adapted VOR gain also occurred when testing in the RED position. No retention of pitch VOR GA or GV existed for the UP position after adaptation in LED. After the UP-adapt experiments, no retention of the GA or GV was found when tested in the adapting position. Interestingly, however, some retention of GA and GV did exist when the UP-adapted animals were tested in LED or RED. Data from sinusoidal rotations followed a similar adaptation pattern as the step responses. Our findings show that after only 3 h of adaptation exposure, adaptation of the pitch VOR gain is retained for several days. This long-term retention of VOR adaptation after short-term exposure appears to be the result of inducing adaptation with an atypical combination of movement and position for the monkey (LED-adapt). Our results indicate that head orientation relative to gravity is an effective context for retaining learned VOR gains in addition to restricting mobility or keeping animals in the dark. We also show that the adapting head position determines the magnitude of VOR adaptation.
Keywords: VOR adaptation, Context specificity, VOR plasticity, Pitch VOR
Introduction
Various oculomotor systems can be differentially modified depending upon the context for which they have been exposed. In the vestibular system, vertical eye position (Shelhamer et al. 1992), vergence angle (Lewis et al. 2003) and head orientation relative to gravity (Yakushin et al. 2000;Yakushin et al. 2003a) are examples of contextual cues that can elicit different vestibulo-ocular reflex (VOR) gains (-eye velocity/head velocity). As a result, the brain can generate a unique VOR response at the demand of the contextual cue.
Studies that have examined retention of the adapted VOR have identified immobilization (Miles and Eighmy 1980) or light deprivation (Miles and Lisberger 1981) of the animal as efficient means for prolonging the newly acquired VOR gain. Recently, Kuki et al. (2004) demonstrated that VOR memory after short-term adaptation paradigms (< 3 h) decayed quickly. In contrast, animals that were exposed to retinal slip for weeks retained the VOR gain for a longer time period. Kuki et al. (2004) did not measure VOR gains over consecutive days after a short-term exposure. One study measured the VOR for 4 days after short-term VOR adaptation (Yakushin et al. 2003b). Rhesus monkeys underwent 4 h of ear down (interaural axis) pitch VOR adaptation and were found to have a VOR gain that was dependent on head orientation relative to gravity (Yakushin et al. 2003b). The authors reported two main findings: (1) the amount of VOR adaptation progressively decayed as the head moved away from the adapting position, and (2) the adaptation persisted for two or more days when tested in the adapting head orientation (Yakushin et al. 2003b). Similar patterns of adaptation were also reported when rhesus monkeys were tested in different planes of head rotation (Yakushin et al. 2005). Our study differs from this prior work in that we examined VOR retention following more complex adaptation stimuli that included higher frequency components commonly encountered during natural head movements in squirrel monkeys (Armand and Minor 2001). In addition, we measured VOR adaptation across multiple frequencies following adaptation to this broad frequency stimulus. Another difference in our study is we report VOR gain during the acceleration and constant velocity components of a step rotation. This is based on prior work in our laboratory, which has shown behavioral differentiation within the VOR pathway based on the acceleration (phasic component of the VOR which is accentuated at higher frequencies) and velocity (tonic component) content of the head motion (Minor et al. 1999). We conducted VOR behavioral studies in squirrel monkeys and established that the phasic component of the VOR is larger during acceleration steps (GA) than during constant velocity (GV) rotation (Minor et al. 1999). Clendaniel et al. (2002) showed that the two components of the VOR pathway can be selectively modified by altering the frequency and velocity content of the adapting stimulus. We chose to study both GA and GV because our adapting stimulus included frequency and velocity content that would affect both VOR pathways. Finally, we monitored VOR gain changes over 7 days following adaptation, a longer period of time than previous studies (4 days; Yakushin et al. 2003b).
In the presence of vestibular hypofunction or aging, reduced VOR function can have debilitating consequences on daily human activity. Fortunately, the VOR retains its adaptive capabilities in the presence of unilateral hypofunction as well as aging and can be enhanced such that asymmetry between the two sides is reduced (Szturm et al. 1994;Viirre et al. 1998; Paige 1992). Most studies on motor learning within the VOR have used low frequency stimuli yet little is known concerning the effect of broader frequency spectrum VOR gain adaptation, which represents more natural head motion. If we can establish VOR gain adaptation using stimuli that better represent natural motion, and identify factors that improve retention to this broader spectrum of head motion following VOR adaptation, then we can design stimuli that are likely to improve the rehabilitation effect for people with vestibular hypofunction.
We wanted to determine if short-term, broad frequency stimuli pitch VOR gain adaptation in squirrel monkeys, with head orientation relative to gravity as the contextual cue, would lead to long-term VOR adaptation retention. The purpose of this study was:
To examine retention of the adapted pitch VOR when the adapting stimulus was paired with a novel contextual cue [left ear down (LED), rotated about the inter-aural axis (pitch VOR)].
To examine retention of the adapted pitch VOR when the animals were adapted with a more common contextual cue (upright head position, interaural axis pitch VOR).
To determine if the adapted pitch VOR gain transfers to a different head orientation relative to gravity.
To determine the persistence of the adapted pitch VOR gain in different head orientations.
Methods
Surgical procedures and eye movement recording
All surgical and animal care procedures used in this study were done in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and a protocol approved by the Animal Care and Use Committee of the Johns Hopkins University School of Medicine. Surgery was done under sterile conditions in three adult squirrel monkeys anesthetized with inhalation of halothane/nitrous oxide/oxygen. The surgical procedures were similar to those previously described for this laboratory (Minor et al. 1999; Clendaniel et al. 2001). Briefly, a head bolt was cemented to the occiput in order to position the animal 15°pitched nose down relative to the horizontal stereotaxic plane when in the upright head orientation. A frontal scleral search coil (11 mm diameter, three turns) was implanted, followed by a second, smaller search coil (5.6 mm diameter, five turns), implanted ∼90° in relation to the frontal coil (Paige and Tomko 1991). The two coil leads (∼50 cm in length) and connectors were stored in an implanted 2 × 2-cm plastic cylinder with screw lid.
Each animal was seated in a plastic chair with its head restrained by attaching the implanted bolt to a chair-mounted clamp. The chair could be adjusted to align the semicircular canals in the plane of rotation. The chair was connected to a superstructure that was mounted to the top surface of a servo-controlled rotation table capable of generating a peak torque of 125°N-m (Acutronic, Pittsburgh, PA). Nested within the superstructure is a secondary axis and motor (19°N-m) that enabled the animals to be rotated in pitch about an interaural axis (seated in the upright head orientation). The pitch VOR was therefore tested both with the animals positioned ear down (canal only dynamic stimulation) and with the animals positioned in an upright head orientation (canal and otolith dynamic stimulation).
The experimental procedures used for recording eye movements were identical to those previously described for this laboratory (Minor et al. 1999; Migliaccio et al. 2004). Briefly, three pairs of field coils (45 cm side length) were rigidly attached to the superstructure and moved with the animal. The three magnetic fields were orthogonal and aligned with the X (naso-occipital), Y (inter-aural), and Z (cephalo-caudal) coordinate axes. The X, Y, and Z fields oscillated at 100, 50, and 75 kHz respectively. The three frequency signals induced across each coil were demodulated (Remmel 1984) to produce three voltages roughly proportional to the angles between the coil and each magnetic field (Straumann et al. 1995). The peak-to-peak noise at the output of the circuit was equivalent to an eye movement of 0.02°. All signals transducing motion of the head or the eye were passed through eight-pole Butterworth antialiasing filters with a corner frequency of 100 Hz. These signals were digitized at a sampling rate of 1 kHz.
We calibrated the eye-coil system with a method similar to one previously described (Robinson 1963; Migliaccio et al. 2004). To ensure the demodulated voltage signals generated by each implanted coil were due only to the interaction between the coil and the magnetic fields, we first cancelled the offset voltages (Straumann et al. 1995). Next, the squirrel monkey and chair were placed inside a non-ferromagnetic cage (tin box and lid), nullifying the interaction between the implanted coils and the magnetic field. We electronically offset the residual voltage signals to zero. The relative peak magnetic field strengths were determined by placing an identical coil in a Fick gimbal positioned at the center of the three magnetic fields and rotating the coil until it was perpendicular to the X, Y, or Z magnetic field. Maximum voltages were recorded respectively, allowing us to determine a three-dimensional angular eye position. This process was repeated for each channel.
Angular rotations were expressed as rotation vectors with roll, pitch, and yaw coordinates (Haslwanter 1995; Migliaccio and Todd 1999). The angular velocity vectors of eye-in-head (head and superstructure) were calculated from the corresponding rotation vectors (Hepp 1990). The resolution of the coil system was 0.2° (tested over the angular range of ±25° combined yaw, pitch, and roll positions) when measured with a Fick gimbal. The accuracy of the gimbal was 0.1°. The differentiated signal noise was approximately 2.5°/s.
Rotational testing
The VOR was always measured when animals were in complete darkness. Both step responses and single frequency stimuli were used to measure the VOR. Stimuli for the step responses consisted of 1,000°/s2 accelerations to a peak velocity of 150°/s followed by a plateau of head velocity lasting 2.0–2.2 s and then deceleration at 1,000°/s2 to rest. The direction of acceleration was pseudo-randomly applied from one trial to the next, in order to ensure an equal number of trials in each direction. Sinusoid head rotations (interaural pitch axes in upright and ear down head positions) consisted of 0.5, 2.0, and 4.0 Hz. For the 0.5 Hz, velocities measured included 20, 50, and 100°/s. For 2.0°Hz, velocities measured included 20, 47, and 91°/s. At 4°Hz, the velocity limit of our equipment for interaural pitch rotations was 20 and 47°/s. Each stimulus frequency was given for at least 30 s. We chose to examine the VOR across these frequencies, as this encompassed the adaptive stimuli.
Adaptation paradigms
For all adaptation experiments, a solution of d-amphetaminesulfate (0.3 mg/kg) and audio alerting techniques were used throughout the adaptation to ensure alertness. Eye traces were continually monitored in attempt to prevent sleeping. Animals recovered for 3–4 weeks before participation in the complementary adaptation paradigm. Participation in either the experimental or control conditions was randomized across the monkeys.
Experimental condition
The pitch VOR of three squirrel monkeys was adapted with minimizing lenses (×0.45) for 3 h using a sum of sines stimuli (peak velocity 20°/s at 0.5, 1.1, 2.3, and 3.7°Hz) while positioned left ear down (LED). Upon completion of the adaptation, animals were returned to their home cages for normal light–dark cycles and normal freedom of movement. No further adaptation was done beyond the initial 3 h.
Control condition
The pitch VOR gain of the same three squirrel monkeys was adapted while the animals were positioned upright for 3 h with minimizing lenses (×0.45) using the same sum-of-sines stimulus as in the experimental condition. Upon completion of the adaptation, animals were returned to their home cages for normal light–dark cycles and normal freedom of movement. No further adaptation was done beyond the initial 3 h.
Data collection
Each of the animals was tested before and immediately after the adaptation paradigms (pre and post). Additionally, the animals were subsequently tested on post-adaptation days 1 through 3 (PAD 1, PAD 2, PAD 3) and finally on PAD 7. For the experimental conditions, animals were tested in the adapted position (left ear down, LED) and two non-adapted positions: right ear down (RED) and upright (UP). For the control experiments, animals were tested in the adapted position (UP) and in 1 non-adapted position (either RED or LED). The order of head position tested was varied for both experimental and control experiments.
Data analysis
The data were analyzed off-line using software written in the Matlab (The Math Works) and LabVIEW (National Instruments) programming environments. Onset of head rotation was measured by using the 3-SD method, where mean and standard deviation of eye and head velocity signals were measured during the 20-ms interval before the onset of the command signal to the rotation table. The onset of the head rotation was determined to be the point where the velocity signal deviated from the mean value measured before onset of the stimulus for head velocity by >3 SD (Minor et al. 1999).
Measurements of the gain of the VOR for the 1,000°/s2−150°/s steps were made during two components of the stimulus: the acceleration portion of the stimulus and after the plateau of head velocity had been reached (Minor et al. 1999; Migliaccio et al. 2004). The acceleration gain of the VOR (GA) was measured for each trial as the ratio of the slope of a line (best fit) through the eye-velocity points to the slope of a line through the head-velocity points during a 20 ms period starting 20 ms after the onset of the stimulus (head rotation). The velocity gain of the VOR (GV) was measured from the ratio of the mean slow component eye velocity and head velocity evaluated 200–400 ms after the plateau head velocity had been reached for each trial. Responses from 10 to 15 trials in each direction were averaged to determine mean and SD gain values. Determination of VOR gain during the acceleration and velocity components of the step response was done off-line (Minor et al. 1999; Migliaccio et al. 2004).
For the sinusoid rotations, eye-position data was differentiated using a four-point central difference algorithm to obtain eye velocity. Saccades were separated from the slow component eye rotations based on their velocities being greater than the peak stimulus velocity and their direction being anti-compensatory. This was done manually through an interactive program written in Matlab 7.0.1. Gains for eye with respect to head velocity were expressed with the convention that a unity gain implied a perfectly compensatory VOR (Minor et al. 1999).
Statistical analysis
Repeated measures multi-way ANOVA (MANOVA) was used to assess differences across sinusoid and step profile VOR gains with respect to direction (head up, head down), day (pre, post, PAD 1–3, PAD 7), frequency, velocity, adaptive training (LED, UP), and test position (LED, RED, UP). We binned the sine VOR gain values from the 47 and 91°/s head velocities with 50 and 100°/s, respectively. When the overall effects of the model were significant, ANOVA and post hoc t test were used to assess significance between the pre-adaptation and PAD1–PAD3 and PAD7 VOR gain values. All levels of significance were assessed at P < 0.05. VOR gains are presented as means ± 1 SD.
Results
Responses after left ear down adaptation
Acceleration steps in LED
There was no difference in acceleration gain (GA) or velocity gain (GV) for head up or head down rotations in the LED test position across day of test (repeated measures MANOVA P = 0.76). The mean pre-adaptation GA value in the LED position was 0.78 ± 0.15 (1.0, 0.70, and 0.65 for animals M1, M2, and M3, respectively). Immediately post-adaptation, the mean GA was reduced by 49% to 0.40 ± 0.20 (0.66, 0.16, and 0.39, respectively, for monkeys M1, M2, and M3). The mean pre-adaptation GV was 0.69 ± 0.14 (0.88, 0.58, and 0.60 for animals M1, M2, and M3). Immediately post-adaptation, mean GV was reduced by 30% to 0.48 ± 0.10 (0.62, 0.44, and 0.39 for the respective monkeys). The changes in GA and GV immediately post-adaptation were significant for each monkey (paired t tests, P < 0.05), Fig. 1.
Fig. 1.
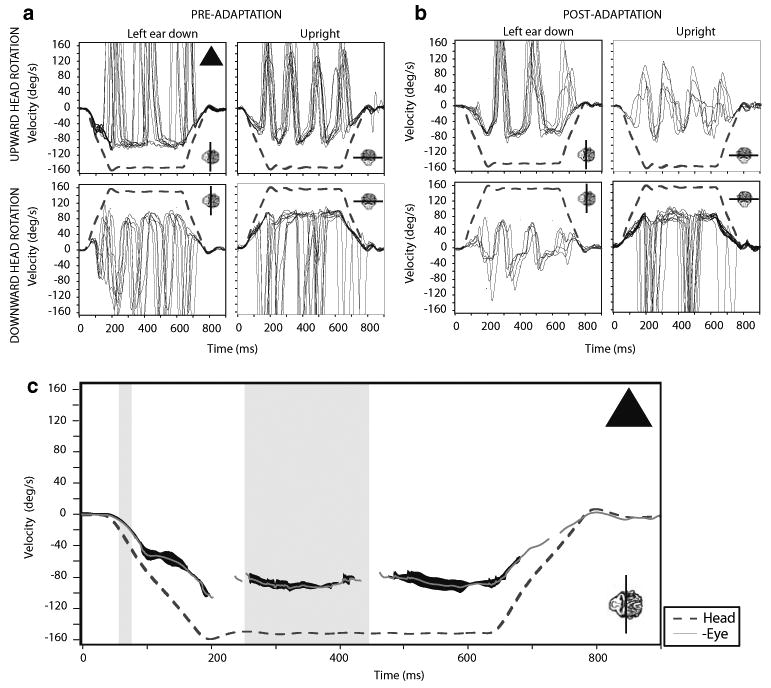
Eye responses to steps of whole body rotation at 1,000°/s2 in left ear down and upright head positions before and after left ear down (LED) adaptation in one monkey. Head (whole body) velocity indicated by the dashed line and eye velocity by the solid lines. a Data collected before the adaptation, while b includes data collected after the adaptation on the same day. Line plotted with monkey head inset indicates the axis of rotation. c displays the averages of the responses from left ear down, pre-adaptation (triangle). Averages of these responses are represented by the gray line with 1-SD indicated by dark shading around this line. The narrow vertical shaded region indicates the 20–40 ms interval from head velocity onset used to calculate the GA. The wider vertical shaded region indicates the 200–400 ms interval from head velocity onset, used to calculate GV
When tested in the adapting head orientation (LED) on subsequent days, there was an effect of test day on GA, indicating retention of the adapted response (repeated measures MANOVA, P < 0.01). Post hoc analyses of GA revealed that there was a reduction (P < 0.05) in gain through PAD7 as compared to pre-adaptation levels. Gain values for both GA and GV were then normalized to a Pre adaptation gain value of 1.0. The normalized means for GA over time are plotted in Fig. 2a. The effect of test day on GV was also significant (P < 0.05), indicating this component of the adapted response was retained beyond the immediate post-adaptation period. Post hoc analyses indicated that there was a reduction of GV through PAD3 (P < 0.05), as compared to the pre-adaptation values. By PAD7, however, there was no difference in GV (pre-adaptation: 0.69, PAD7 0.60, P = 0.12). The normalized means for GV over time are plotted in Fig. 2b.
Fig. 2.
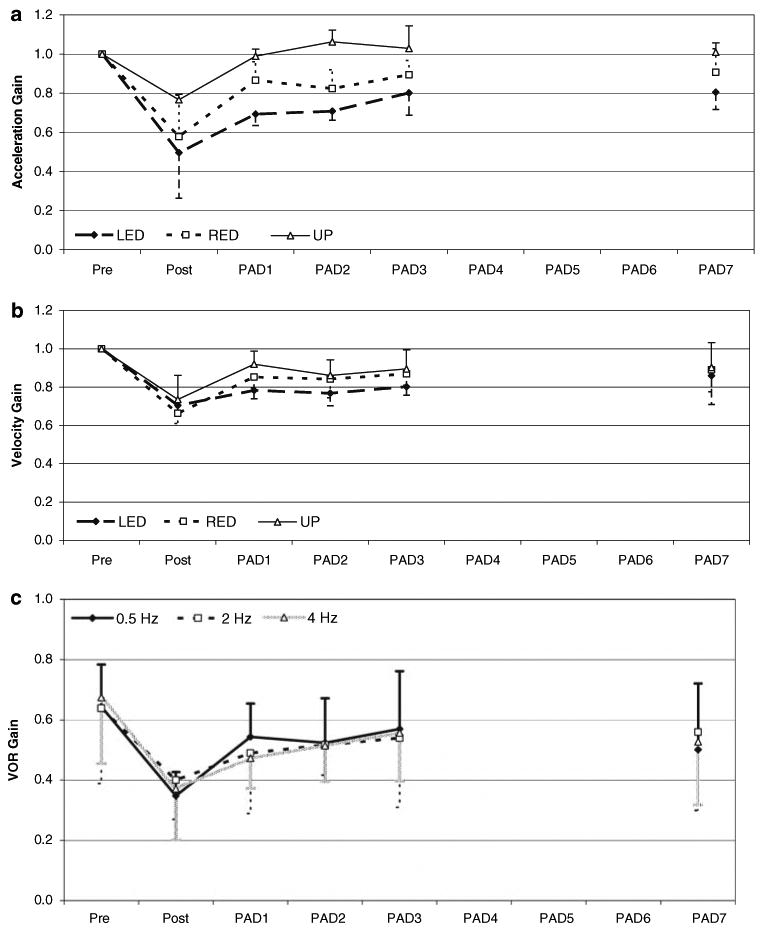
VOR gain during a 1,000°/s2 step response and sinusoidal rotation in LED after LED adaptation. Plots a, b represent normalized mean VOR gains during the acceleration (a) and peak velocity (b) components of a 1,000°/s2 step response over time after LED adaptation. a Acceleration gain component is a 20 ms period determined 20 ms after the onset of the stimulus. b Velocity gain component determined from the ratio of the mean slow component eye velocity to head velocity evaluated 200–400 ms after the plateau head velocity had been reached for each trial. Responses from 10 to 15 trials for each day for each monkey were averaged to determine mean ± 1 SD. c Mean ± 1 SD sinusoid VOR gains over time after LED adaptation at 20°/s for 0.5, 2.0 and 4.0 Hz. There was no difference in VOR gain across the other tested velocities. Reduction in sinusoidal gain persisted through PAD7 (paired t test, P < 0.01). Pre- and post-measures occurred on same day. Post-adaptation days 1–3 and PAD 7 reflect retention of VOR gain
Acceleration steps in RED
Immediately after LED adaptation, mean GA value in the RED head orientation was reduced by 39% from 0.77 ± 0.18 to 0.47 ± 0.23 (M1: 1.03–0.78, M2: 0.61–0.21, and M3: 0.68–0.43). Similarly, the mean GV was reduced by 34% from 0.73 ± 0.13 to 0.48 ± 0.06 (M1: 0.90–0.56, M2: 0.58–0.42, and M3: 0.70–0.45). The changes in GA and GV for RED immediately post-adaptation were significant (paired t tests, P < 0.05).
Similar to the findings with LED, when tested in the RED position on subsequent days, there was retention of the adapted response beyond the immediate post-adaptation period (significant effect of test day on GA, repeated measures MANOVA, P < 0.05). Post hoc analyses of GA revealed that there was a reduction (P < 0.01) in gain through PAD3 as compared to pre-adaptation levels. By PAD7, the GA values had returned to pre-adaptation levels (pre-adaptation: 0.77; PAD7: 0.70, P = 0.13). The effect of test day on GV was also significant (P < 0.05), suggesting that this component of the adapted response was retained beyond the immediate post-adaptation period. Post hoc analyses indicated that there was a reduction of GV through PAD3 (P < 0.05), as compared to the pre-adaptation values. By PAD7, however, there was no difference in GV (pre-adaptation: 0.73; PAD7: 0.64, P = 0.11). Normalized mean GA and GV over time are plotted in Fig. 2a and b.
Acceleration steps in upright
The mean pre-adaptation GA value in the upright (UP) position was 0.65 ± 0.12 (0.81, 0.62, and 0.53, respectively, for monkeys M1, M2, and M3). Immediately post-adaptation, the mean GA was reduced by 23% to 0.50 ± 0.10 (0.64, 0.48, and 0.39 for M1, M2, and M3). Mean GV pre-adaptation was 0.69 ± 0.10 (0.83, 0.59, and 0.66, respectively, for monkeys M1, M2, and M3). Post-adaptation the mean GV was reduced by 28% to 0.50 ± 0.02 (0.49, 0.48, and 0.53 for M1, M2, and M3). The changes in GA and GV immediately post-adaptation were significant (paired t tests, P < 0.05). When tested in UP on subsequent days, there was no effect of time on either GA or GV (repeated measures MANOVA, P > 0.05), indicating there was no retention of the adapted gain in the upright head orientation (Fig. 2a, b).
Sinusoidal rotations in LED
Regardless of sine frequency, direction of rotation (clockwise or counterclockwise), PAD, adapting head orientation relative to gravity (LED, UP) or test condition (LED, RED, UP), there was no difference in the VOR gain values among the different peak velocities (repeated measures MANOVA, P = 0.29). Consequently, for ease of discussion, only the sinusoidal stimuli with peak velocities of 20°/s across the various frequencies are reported.
The results of testing with sinusoidal rotations in the LED position following LED adaptation, revealed responses similar to those seen with the steps of acceleration under the same conditions. The overall VOR gain was reduced by 43%, 0.65 ± 0.18 pre-adaptation to 0.37 ± 0.12 immediately post-adaptation (paired t test, P < 0.01), across the tested frequencies (46%, 37% and 45% reductions for 0.5, 2.0, 4.0 Hz stimuli, respectively). With subsequent testing in the adapting position, there was a persistent reduction in gain (repeated measures ANOVA, main effect of test day P < 0.01), indicating that the adapted gain was retained past the immediate post-adaptation period. Post hoc analyses revealed that the reduction in sinusoidal gain persisted through PAD7, at which point the sinusoidal gain was 0.53 ± 0.21 (paired t test, P < 0.01). The mean data for 0.5, 2 and 4 Hz are plotted in Fig. 2c.
Sinusoidal rotations in RED
When animals adapted in LED were tested in RED immediately following the adaptation paradigm, the overall VOR gain was reduced by 42%, 0.63 ± 0.10 pre-adaptation to 0.37 ± 0.10 post-adaptation across the testing frequencies. When subsequently tested in RED, there was an effect of test day (repeated measures MANOVA, P < 0.05), again, indicating retention of the reduced gain beyond the immediate post-adaptation time frame. Post hoc analyses revealed that reduction in sinusoidal gain was significant (paired t tests, P < 0.01) at PAD1 (gain = 0.57 ± 0.12) and PAD3 (gain = 0.57 ± 0.13). On PAD2 (gain = 0.60 ± 0.12) and PAD7 (gain = 0.62 ± 0.17) there were no significant reductions in sinusoidal gain values. These results indicate that the retention of the reduced gain, while present, was not as robust as in the adapting position.
Sinusoidal rotations in upright
When the animals were tested with their heads in an upright head orientation immediately following the LED adaptation paradigm, the mean VOR gain was reduced across frequencies by 34% (0.53 ± 0.15 to 0.35 ± 0.13) (P < 0.002). There was a 29% reduction at both 0.5 and 2.0 Hz, and a 49% reduction at 4.0 Hz. Repeated measures MANOVA revealed an effect of test frequency (P < 0.01). Post hoc analysis of the test frequency effect revealed that there were reductions in sinusoidal gain with increasing frequency of testing. The gain at 0.5 Hz, across time (pre-adaptation, PAD1, PAD2, PAD3, and PAD7) was on average 0.61 ± 0.14, which was greater than that at 2.0 Hz (0.51 ± 0.12; P < 0.01). The gain at 4.0 Hz was 0.41 ± 0.11, which was lower than the gain at 2.0 Hz (P < 0.001). We did not find an effect of PAD test day or interaction between test frequency and test day (P > 0.746). These results indicate that there was no retention of the adapted gain over time in the upright condition.
Responses after upright adaptation
Acceleration steps in upright
There was no difference in GA between head up and head down rotations for the upright test condition (repeated measures MANOVA P = 0.76). We found a difference in GV between head up and head down rotations (repeated measures MANOVA P = 0.002). Post hoc analysis revealed one animal (M3) had larger GV values for pitch up head rotations across PAD1-3, and PAD7 (ANOVA P = 0.009, paired t tests, P < 0.05). Although significant, the differences between pitch-up and down head rotations were minimal (mean 7%). There was no difference between pitch-up and down head rotations for the PRE-adaptation condition in this one animal.
The mean pre-adaptation GA value in the upright (UP) position was 0.62 ± 0.08 (0.71 0.62, and 0.52 for the animals M1, M2, and M3, respectively). Immediately post-adaptation, the mean GA was reduced by 28.1% to 0.44 ± 0.04 (0.49, 0.44, and 0.40, respectively, for monkeys M1, M2, and M3), Fig. 3a. Mean velocity gain, GV, pre-adaptation was 0.64 ± 0.06 (0.70, 0.56, and 0.66 for animals M1, M2, and M3). Immediately post-adaptation, the mean GV was reduced by 26.6% to 0.47 ± 0.05 (0.54, 0.44, and 0.43 for monkeys M1, M2, and M3). The changes in GA and GV were significant (paired t tests, P < 0.05).
Fig. 3.
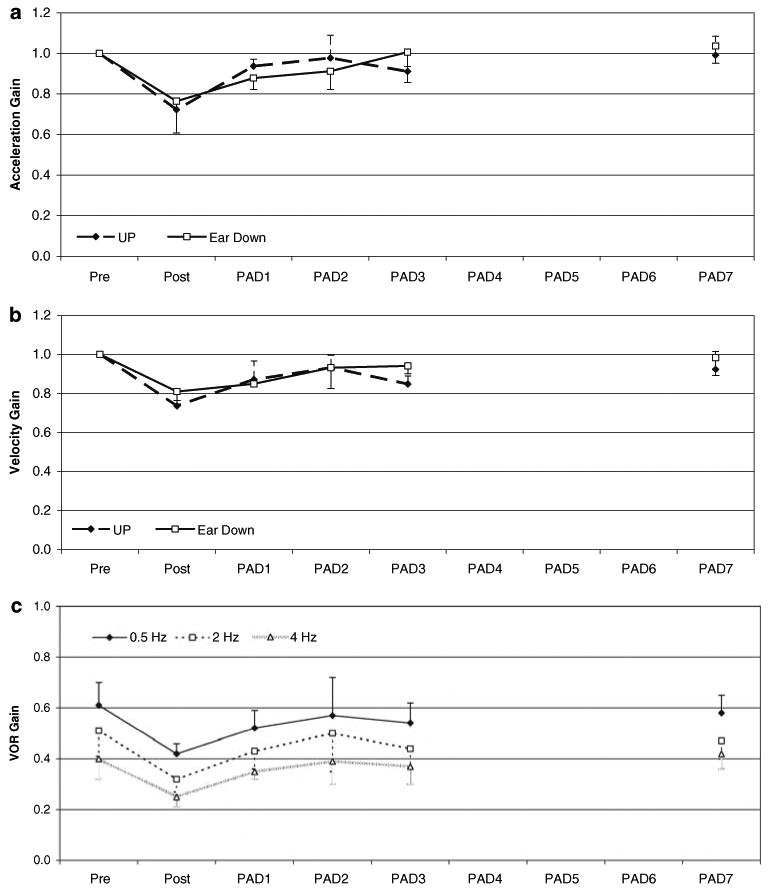
VOR gain during a 1,000°/s2 step response and sinusoidal rotation in UP after upright adaptation. Plots a, b represent normalized mean VOR gains during the acceleration (a) and peak velocity (b) components of a 1,000°/s2 step response over time after UP adaptation. Acceleration and velocity gains determined as described in Fig. 2. c Mean ± 1 SD sinusoid VOR gains over time after UP adaptation at 20°/s for 0.5, 2.0 and 4.0 Hz. We found a progressive reduction in VOR gain as frequency of the head rotation increased (P < 0.001). There was no retention of the adapted sinusoid gain over time in the UP condition (P = 0.746)
When subsequently tested in the adapting position (UP) there was no difference in pitch VOR GA values between pre-adaptation and any of the post-adaptation values (excluding the immediate post-adaptation GA gains), indicating that there was no retention of the adapted gains (repeated measures MANOVA, P > 0.5). Similarly, there was no change in GV from pre-adaptation levels when the monkeys were tested on subsequent days (repeated measures MANOVA, P > 0.1), see Fig. 3b.
Acceleration steps in ear down
There was no difference in GA or GV between clockwise and counterclockwise head rotations for the ear down test head orientation (repeated measures MANOVA, P = 0.76). The mean pre-adaptation GA value in an ear down (ED) orientation was 0.69 ± 0.03 (0.73 0.68, and 0.67 for the animals M1, M2, and M3, respectively). Immediately following upright adaptation, the post-adaptation mean GA was reduced by 23.1% to 0.53 ± 0.11 (0.69, 0.45, and 0.46 in M1, M2, and M3). Pre-adaptation, the mean GV in ED was 0.66 ± 0.06 (0.70, 0.58, and 0.70 for animals M1, M2, and M3). Immediately post-adaptation, GV was reduced by 18.7% to 0.54 ± 0.07 (0.59, 0.44, and 0.58 for the 3 animals). Paired t tests of pre-adaptation and immediate post-adaptation measures of GA showed a reduction in gain that was not significant (P = 0.058). A closer inspection of the data revealed that animals M2 and M3 had reductions in GA of 34 and 31%. Animal M1, however, had only a 5% reduction in acceleration gain. The relatively modest change in GA for animal M1 likely accounts for the lack of statistically significant change immediately post-adaptation. Similar paired t tests for GV demonstrated a decrease in gain immediately post-adaptation (P < 0.01).
When subsequently tested in ED (the non-adapting position) on subsequent days, there was an effect of test day on GV (P < 0.05); GA values however, were not significantly different (P = 0.08). Post hoc analyses of GV demonstrated a significant reduction in gain from pre-adaptation levels to PAD1 (0.66 ± 0.06 to 0.56 ± 0.04: P < 0.01). By PAD2, however, there was no difference in GV (P > 0.1), Fig. 3a and b.
Sinusoidal rotations in upright
As noted previously, because there was no difference in the pattern of response across the tested frequencies at the different peak velocities of stimulation, only the sinusoidal stimuli with 20°/s peak velocities are reported here. The results of testing with sinusoid rotations in the UP head orientation following UP adaptation, revealed responses similar to those seen with the steps of acceleration under the same conditions. The overall VOR gain (across frequencies) was reduced by 35%, 0.51 ± 0.12 pre-adaptation to 0.33 ± 0.08 immediately post-adaptation (paired t test, P < 0.01). The gain was reduced by 31, 37, and 37% for the 0.5, 2.0, and 4.0 Hz stimuli, respectively. When subsequently tested in the adapting head orientation, there were significant (P < 0.01) main effects of test day and frequency (repeated measures MANOVA). There was no interaction between test day and test frequency. Post hoc analyses revealed that the reduction in sinusoidal gain was only present through PAD1, at which point the gain was 0.44 ± 0.11 (paired t test, P < 0.01). By PAD2, the gain had increased to 0.49 ± 0.14 (P > 0.1).
Interestingly, there was a consistent reduction in UP VOR gain with increasing frequency regardless of testing day (0.57, 0.47, and 0.38 at 0.5, 2.0, and 4.0 Hz, respectively). The differences between the gains at adjacent testing frequencies were significant (P < 0.01), Fig. 3c. The individual and mean monkey data across frequencies at 20°/s are listed in Table 1.
Table 1.
Individual and mean VOR gains in UP after UP adaptation across days and frequency at 20°/s
| Gain | Hz | Pre | Post | PAD1 | PAD2 | PAD3 | PAD7 |
|---|---|---|---|---|---|---|---|
| M1 | 0.5 | 0.55 ± 0.03 | 0.41 ± 0.03 | 0.57 ± 0.05 | 0.44 ± 0.01 | 0.53 ± 0.04 | 0.59 ± 0.02 |
| M2 | 0.5 | 0.56 ± 0.07 | 0.39 ± 0.09 | 0.47 ± 0.12 | 0.53 ± 0.05 | 0.45 ± 0.05 | 0.50 ± 0.03 |
| M3 | 0.5 | 0.71 ± 0.04 | 0.46 ± 0.06 | –a | 0.74 ± 0.07 | 0.62 ± 0.01 | 0.65 ± 0.01 |
| Mean | 0.61 ± 0.09 | 0.42 ± 0.04 | 0.52 ± 0.07 | 0.57 ± 0.15 | 0.54 ± 0.08 | 0.58 ± 0.07 | |
| M1 | 2.0 | 0.45 ± 0.001 | 0.32 ± 0.001 | 0.47 ± 0.05 | 0.39 ± 0.01 | 0.43 ± 0.1 | 0.45 ± 0.001 |
| M2 | 2.0 | 0.45 ± 0.001 | 0.27 ± 0.03 | 0.31 ± 0.04 | 0.48 ± 0.03 | 0.36 ± 0.002 | 0.40 ± 0.07 |
| M3 | 2.0 | 0.63 ± 0.03 | 0.37 ± 0.03 | 0.52 ± 0.01 | 0.64 ± 0.003 | 0.54 ± 0.01 | 0.57 ± 0.01 |
| Mean | 0.51 ± 0.10 | 0.32 ± 0.05 | 0.43 ± 0.07 | 0.50 ± 0.15 | 0.44 ± 0.08 | 0.47 ± 0.07 | |
| M1 | 4.0 | 0.43 ± 0.02 | 0.28 ± 0.07 | 0.36 ± 0.03 | 0.34 ± 0.02 | 0.38 ± 0.03 | 0.37 ± 0.01 |
| M2 | 4.0 | 0.31 ± 0.01 | 0.20 ± 0.01 | 0.32 ± 0.02 | 0.33 ± 0.04 | 0.29 ± 0.01 | –a |
| M3 | 4.0 | 0.46 ± 0.03 | 0.28 ± 0.03 | 0.37 ± 0.02 | 0.49 ± 0.02 | 0.43 ± 0.06 | 0.46 ± 0.02 |
| Mean | 0.40 ± 0.08 | 0.25 ± 0.04 | 0.35 ± 0.03 | 0.39 ± 0.09 | 0.37 ± 0.07 | 0.42 ± 0.06 |
Missing data
Sinusoidal rotations in ED
When the animals were tested with their heads ED immediately following the UP adaptation paradigm, the mean VOR gain was significantly reduced across frequencies by 35% (0.66 ± 0.07 to 0.43 ± 0.06). There was a 34% reduction in gain at both 0.5 and 4.0 Hz, and a 37% reduction at 2.0 Hz. Repeated measures MANOVA revealed a significant effect of test day and test frequency (P < 0.01) but no interaction between test day and test frequency (P = 0.99). These results indicate that there was some retention of the adapted gain over time that differed across test frequency. Post hoc analyses revealed that there were significant reductions in gain at PAD1 (gain = 0.52 ± 0.05, P < 0.01), PAD2 (0.57 ± 0.11, P < 0.05), and PAD3 (0.59 ± 0.10, P < 0.05). There was no difference between the pre-adaptation and PAD7 sinusoidal ED VOR gains.
Discussion
Effect of head orientation relative to gravity on VOR adaptation and retention
We have shown that a broad spectrum adapting stimulus presented within a unique context (head position) can lead to VOR adaptation that is retained for a time much longer than the adaptation exposure. When the animals were adapted in a more common head orientation (UP), retention in that adapting position did not occur. Interestingly however, adaptation in UP did show some retention during ear down. The retention of the ear down adaptation (after LED adaptation and UP adaptation) is likely due to the behavioral limits of the monkey. Since the monkey does not frequently perform pitch head movements while on its side, there would be little stimulus for de-adaptation. As a result the attenuated VOR gain persisted in unique head positions.
One prior study showed similar retention in macaques adapted in an ear down position (Yakushin et al. 2003b). In humans too, pitch VOR adaptation while lying on one side was retained for 2 days when tested in the adapting position, even though subjects were only exposed to 1 h of adaptation stimuli (Yakushin et al. 2003c). Kuki et al. (2004) reported a longer decay to normal when pitch VOR gain was reduced compared with decay to normal when pitch VOR gain was enhanced. In that study, post-adaptive VOR was only measured for 180 min. Our study agrees with these previous data and extends this knowledge to suggest that VOR gain adaptation can persist for much longer than previously reported, when pairing broad frequency spectrum stimuli with a novel head orientation.
In addition, our data support the notion that any previously adaptive response is negated once subjects return to activity that includes head rotation in the same plane of adaptation (Istl-Lenz et al. 1985; Miles and Eighmy 1980; Miles and Lisberger 1981). These findings may have important implications for motor learning in the vestibular system, particularly in cases of pathology. If we can identify the parameters that contribute to newly acquired VOR memory (retention), then we may be able to develop new strategies for improving hypofunction.
We found that the magnitude of the VOR adaptation generally was greatest when tested in the adapting head orientation (LED or UP). However, for the LED adaptation paradigm we also found that the magnitude of the adaptation was similar when tested in the 180° opposite, non-adapted head orientation (RED). The magnitude change in VOR gain that we report is different than previous VOR adaptation studies in macaques that found the magnitude of adaptation progressively declined as the animals moved away from the adapting position (Yakushin et al. 2000, 2003a, 2005). One explanation for the difference in magnitudes is the difference in the adapting stimuli. We adapted squirrel monkeys with a broad frequency spectrum (0.5–3.7 Hz sum of sines), instead of step responses (60°/s) (Yakushin et al. 2000, 2003a) or single frequency stimuli (0.25 or 0.5 Hz, Yakushin et al. 2003b; 0.2 Hz, Yakushin et al. 2005). The broad frequency spectrum we used in our study may induce a more global adaptation, such that the magnitude persisted irrespective of the ear down head orientation. A second explanation may be related to species differences between macaque and squirrel monkeys. For example, VOR adaptation studies in squirrel monkeys conducted in our laboratory established a non-linearity during the acceleration component of a step response and for rotations greater than 2 Hz. It was found that the gain of the VOR in squirrel monkeys is dependent on frequency and velocity of head rotation at these higher frequencies (Minor et al. 1999). This frequency and velocity dependent nonlinearity in VOR gain has not been found in macaque (Huterer and Cullen 2002). Similar to findings in our study, Yakushin et al. (2003a, 2005) did find some carryover of the adaptation to different head positions, irrespective of the adapting head orientation. The gain in these non-adapted positions followed a cosine function.
Studies that have recorded from central vestibular pathway neurons, using both short and long term VOR adaptation paradigms—implicate brainstem and cerebellar processes as the sites for motor learning (Blasquez et al. 2003; Hirata et al. 2001; Lisberger et al. 1994a, b; Watanabe 1985). Given our stimulus parameters and duration of adaptation, it seems likely that similar processes (sites) are involved for retention of the VOR that we report.
Canal versus otolith afferent input
We found a disparity in the magnitude of adaptation between canal only (ear down 50%) compared with canal + otolith (upright 30%). Two previous studies in monkeys that compared pitch VOR gain adaptation between canal only and canal + otolith stimulation reported similar findings (macaque, ear down vs. upright: Yakushin et al. 2003b; macaque, ear down vs. prone: Yakushin et al. 2005). It has been shown that adaptation of the translational VOR (tVOR) in humans is small and that its response is intrinsically under-compensatory. Rather than gain adaptation, the tVOR relies on other oculomotor mechanisms such as smooth pursuit and saccades (Shelhamer et al. 2000). Therefore we hypothesize that the otolith contribution to the VOR is less modifiable than the canal contribution. If so, then during the pitch VOR, while upright, canal + otolith generated eye movements would be in the same direction, however, after adaptation only the canal component would be reduced. Whereas during the pitch VOR while ED, the eye movement response would be generated mostly by the canals and this component would be reduced after adaptation.
In addition, we report the adapted VOR gain (adaptation and test in UP, same velocity) progressively decreased as the frequency of testing increased, suggesting reduction of the otolith contribution on adaptation as frequency increased. One explanation may be related to differences in otolith responses between lower and higher frequency head rotations. During low frequency and high amplitude pitch head rotations the otoliths interpret tilt, which leads to generation of a vertical eye rotation that is appropriately compensatory for a pitch tilt (Paige and Tomko 1991; Telford et al. 1997; Hess and Angelaki 1999). This otolith mediated response would combine with the canal mediated angular eye rotation to create a larger VOR gain for the lower frequency/higher amplitude pitch rotations (Tomko et al. 1988). During high frequency and low amplitude pitch head rotations, however, the otoliths interpret fore-aft translation and would not generate a vertical eye rotation (Paige and Tomko 1991; Telford et al. 1997).
Conclusions
We have shown that when the context of the adaptation involves a unique head orientation relative to gravity, the brain has the capacity to store adapted VOR gains for a time much longer than the duration of adaptation exposure. This is in addition to previously established immobilization or light deprivation of the animal as effcient means for prolonging the newly acquired VOR gain. Additionally, our data support the notion that the brain can have unique VOR gains coexisting for the same axis of head rotation. In addition, we show that the adapting head position determines the magnitude of VOR adaptation.
Acknowledgments
We thank Mr. David Lasker, MS and Mr. Patpong Jiradejvong, MS for technical expertise. MCS was supported by T32 DC00023 from the National Institute on Deafness and Other Communication Disorders (NIDCD), LBM was supported by NIDCD R01 DC 02390 and RAC was supported by NIDCD R03 DC006363.
Contributor Information
Michael C. Schubert, Email: mschube1@jhmi.edu, Department of Otolaryngology Head and Neck Surgery, Johns Hopkins School of Medicine, 601 N. Caroline St, JHOC Room 6245, Baltimore, MD 21287-0910, USA.
Americo A. Migliaccio, Laboratory of Vestibular Neurophysiology, Department of Otolaryngology Head and Neck Surgery, Johns Hopkins University School of Medicine, Ross Building, Room 710, 720 Rutland Avenue, Baltimore, MD 21205, USA
Lloyd B. Minor, Laboratory of Vestibular Neurophysiology, Department of Otolaryngology Head and Neck Surgery, Johns Hopkins University School of Medicine, Ross Building, Room 710, 720 Rutland Avenue, Baltimore, MD 21205, USA
Richard A. Clendaniel, Department of Community and Family, Medicine, Duke University School of Medicine, Durham, NC, USA
References
- Armand M, Minor LB. Relationship between time- and frequency-domain analyses of angular head movements in the squirrel monkey. J Comput Neurosci. 2001;11(3):217–239. doi: 10.1023/a:1013771014232. [DOI] [PubMed] [Google Scholar]
- Blazquez PM, Hirata Y, Heiney SA, Green AM, Highstein SM. Cerebellar signatures of vestibulo-ocular reflex motor learning. J Neurosci. 2003;23(30):9742–9751. doi: 10.1523/JNEUROSCI.23-30-09742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clendaniel RA, Lasker DM, Minor LB. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. IV. Responses after spectacle-induced adaptation. J Neurophysiol. 2001;86(4):1594–1611. doi: 10.1152/jn.2001.86.4.1594. [DOI] [PubMed] [Google Scholar]
- Clendaniel RA, Lasker DM, Minor LB. Differential adaptation of the linear and nonlinear components of the horizontal vestibuloocular reflex in squirrel monkeys. J Neurophysiol. 2002;88(6):3534–3540. doi: 10.1152/jn.00404.2002. [DOI] [PubMed] [Google Scholar]
- Haslwanter T. Mathematics of three-dimensional eye rotations. Vision Res. 1995;35:1727–1739. doi: 10.1016/0042-6989(94)00257-m. [DOI] [PubMed] [Google Scholar]
- Hepp K. On Listing's law. Commun Math Phys. 1990;132:285–295. [Google Scholar]
- Hess BJ, Angelaki DE. Oculomotor control of primary eye position discriminates between translation and tilt. J Neurophysiol. 1999;81(1):394–398. doi: 10.1152/jn.1999.81.1.394. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Highstein SM. Acute adaptation of the vestibuloocular reflex: signal processing by floccular and ventral parafloccular Purkinje cells. J Neurophysiol. 2001;85(5):2267–2288. doi: 10.1152/jn.2001.85.5.2267. [DOI] [PubMed] [Google Scholar]
- Huterer M, Cullen KE. Vestibuloocular reflex dynamics during high-frequency and high-acceleration rotations of the head on body in rhesus monkey. J Neurophysiol. 2002;88:13–28. doi: 10.1152/jn.2002.88.1.13. [DOI] [PubMed] [Google Scholar]
- Istl-Lenz Y, Hyden D, Schwarz DWF. Response of the human vestibulo-ocular reflex following long-term 2× magnified visual input. Exp Brain Res. 1985;57:448–455. doi: 10.1007/BF00237831. [DOI] [PubMed] [Google Scholar]
- Kuki Y, Hirata Y, Blazquez PM, Heiney SA, Highstein SM. Memory retention of vestibuloocular reflex motor learning in squirrel monkeys. NeuroReport. 2004;15:1007–1011. doi: 10.1097/00001756-200404290-00015. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA, Broussard DM. Neural basis for motor learning in the vestibuloocular reflex of primates. I. Changes in the responses of brain stem neurons. J Neurophysiol. 1994a;72(2):928–953. doi: 10.1152/jn.1994.72.2.928. [DOI] [PubMed] [Google Scholar]
- Lisberger SG, Pavelko TA, Bronte-Stewart HM, Stone LS. Neural basis for motor learning in the vestibuloocular reflex of primates. II. Changes in the responses of horizontal gaze velocity Purkinje cells in the cerebellar flocculus and ventral paraflocculus. J Neurophysiol. 1994b;72(2):954–973. doi: 10.1152/jn.1994.72.2.954. [DOI] [PubMed] [Google Scholar]
- Lewis RF, Clendaniel RA, Zee DS. Vergence-dependent adaptation of the vestibulo-ocular reflex. Exp Brain Res. 2003;152:335–340. doi: 10.1007/s00221-003-1563-9. [DOI] [PubMed] [Google Scholar]
- Migliaccio AA, Todd MJ. Real-time rotation vectors. Australas Phys Eng Sci Med. 1999;22:73–80. [PubMed] [Google Scholar]
- Migliaccio AA, Schubert MC, Jiradejvong P, Lasker DM, Clendaniel RA, Minor LB. The three-dimensional vestibulo-ocular reflex evoked by high-acceleration rotations in the squirrel monkey. Exp Brain Res. 2004;159(4):433–46. doi: 10.1007/s00221-004-1974-2. Epub 2004 September 3. [DOI] [PubMed] [Google Scholar]
- Miles FA, Eighmy BB. Long-term adaptive change in primate vestibuloocular reflex I. Behavioral observations. J Neurophysiol. 1980;43:1406–1425. doi: 10.1152/jn.1980.43.5.1406. [DOI] [PubMed] [Google Scholar]
- Miles FA, Lisberger SG. Plasticity in the vestibulo-ocular reflex: a new hypothesis. Annu Rev Neurosci. 1981;4:273–299. doi: 10.1146/annurev.ne.04.030181.001421. [DOI] [PubMed] [Google Scholar]
- Minor LB, Lasker DM, Backous DD, Hullar TE. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. I. Normal responses. J Neurophysiol. 1999;82:1254–1270. doi: 10.1152/jn.1999.82.3.1254. [DOI] [PubMed] [Google Scholar]
- Paige GD. Senescence of human visual-vestibular interactions 1.Vestibulo-ocular reflex and adaptive plasticity with aging. J Vestib Res. 1992;2:133–151. [PubMed] [Google Scholar]
- Paige GD, Tomko DL. Eye movement responses to linear head motion in the squirrel monkey. I. Basic characteristics. J Neurophysiol. 1991;65:1170–1182. doi: 10.1152/jn.1991.65.5.1170. [DOI] [PubMed] [Google Scholar]
- Remmel RS. An inexpensive eye movement monitor using the scleral search coil technique. IEEE Trans Biomed Eng. 1984;31:388–390. doi: 10.1109/TBME.1984.325352. [DOI] [PubMed] [Google Scholar]
- Robinson DA. A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng. 1963;10:137–145. doi: 10.1109/tbmel.1963.4322822. [DOI] [PubMed] [Google Scholar]
- Shelhamer M, Robinson DA, Tan HS. Context-specific adaptation of the gain of the vestibulo-ocular reflex in humans. J Vestib Res. 1992;2:89–96. [PubMed] [Google Scholar]
- Shelhamer M, Roberts DC, Zee DS. Dynamics of the human linear vestibulo-ocular reflex at medium frequency and modiflcation by short-term training. J Vestib Res. 2000;10(6):271–282. [PubMed] [Google Scholar]
- Straumann D, Zee DS, Solomon D, Lasker AG, Roberts DC. Transient torsion during and after saccades. Vision Res. 1995;35:3321–3334. doi: 10.1016/0042-6989(95)00091-r. [DOI] [PubMed] [Google Scholar]
- Szturm T, Ireland DJ, Lessing-Turner M. Comparison of different exercise programs in the rehabilitation of patients with chronic peripheral vestibular dysfunction. J Vestib Res. 1994;4:461–479. [PubMed] [Google Scholar]
- Telford L, Seidman SH, Paige GD. Dynamics of squirrel monkey linear vestibuloocular reflex and interactions with fixation distance. J Neurophysiol. 1997;78:1775–1790. doi: 10.1152/jn.1997.78.4.1775. [DOI] [PubMed] [Google Scholar]
- Tomko DL, Wall C, III, Robinson FR, Staab JP. Influence of gravity on cat vertical vestibulo-ocular reflex. Exp Brain Res. 1988;69:307–314. doi: 10.1007/BF00247576. [DOI] [PubMed] [Google Scholar]
- Viirre E, Draper M, Gailey C, Miller D, Furness T. Adaptation of the VOR in patients with low VOR gains. J Vestib Res. 1998;8:331–334. [PubMed] [Google Scholar]
- Yakushin SB, Raphan T, Cohen B. Context-specific adaptation of the vertical vestibuloocular reflex with regard to gravity. J Neurophysiol. 2000;84:3067–3071. doi: 10.1152/jn.2000.84.6.3067. [DOI] [PubMed] [Google Scholar]
- Yakushin SB, Raphan T, Cohen B. Gravity-specific adaptation of the angular vestibuloocular reflex: dependence on head orientation with regard to gravity. J Neurophysiol. 2003a;89:571–586. doi: 10.1152/jn.00287.2002. [DOI] [PubMed] [Google Scholar]
- Yakushin SB, Bukharina SE, Raphan T, Büttner-Ennever J, Cohen B. Adaptive Changes in the angular VOR: duration of gain changes and lack of effect of nodulo-uvulectomy. Ann N Y Acad Sci. 2003b;1004:78–93. [PubMed] [Google Scholar]
- Yakushin SB, Palla A, Haslwanter T, Bockisch CJ, Straumann D. Dependence of adaptation of the human vertical angular vestibulo-ocular reflex on gravity. Exp Brain Res. 2003c;152:137–142. doi: 10.1007/s00221-003-1543-0. [DOI] [PubMed] [Google Scholar]
- Yakushin SB, Xiang Y, Raphan T, Cohen B. The role of gravity in adaptation of the vertical angular vestibulo-ocular reflex. Ann N Y Acad Sci. 2005;1039:97–110. doi: 10.1196/annals.1325.010. [DOI] [PubMed] [Google Scholar]
- Watanabe E. Role of the primate flocculus in adaptation of the vestibulo-ocular reflex. Neurosci Res. 1985;3(1):20–38. doi: 10.1016/0168-0102(85)90036-7. [DOI] [PubMed] [Google Scholar]


