Abstract
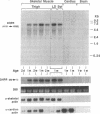
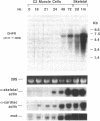
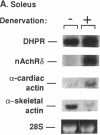
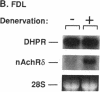
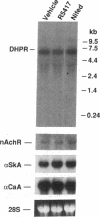
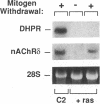
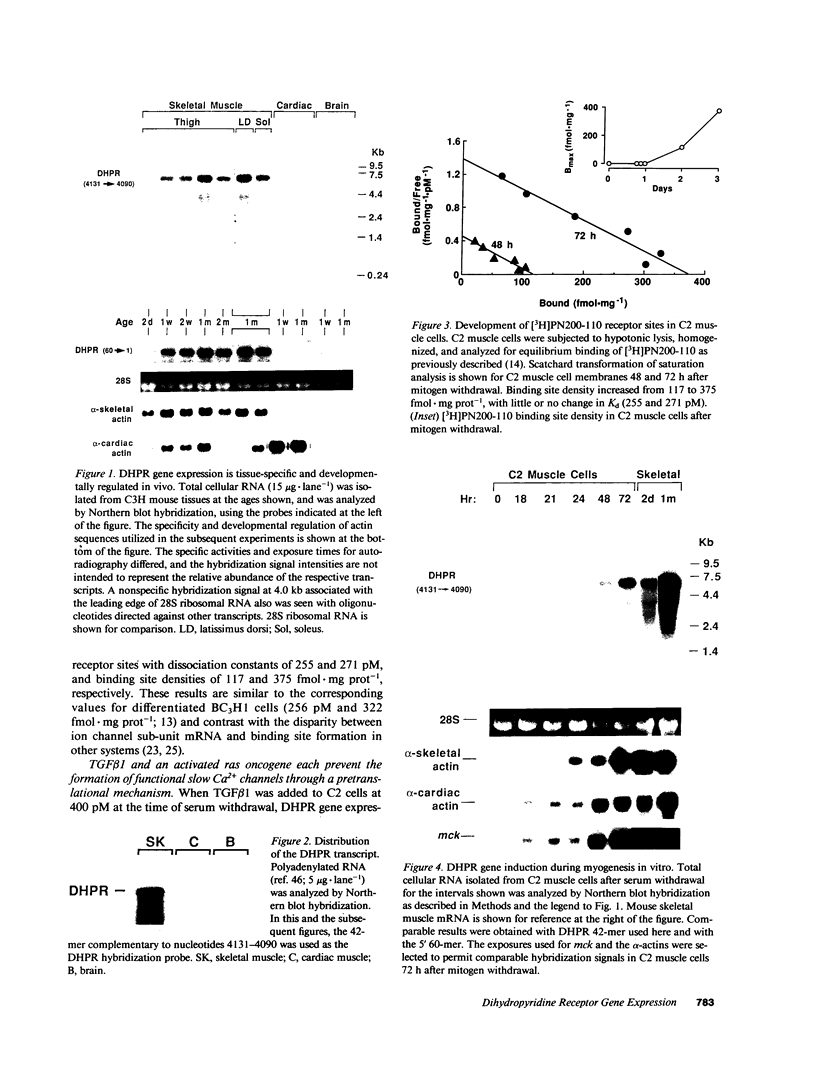
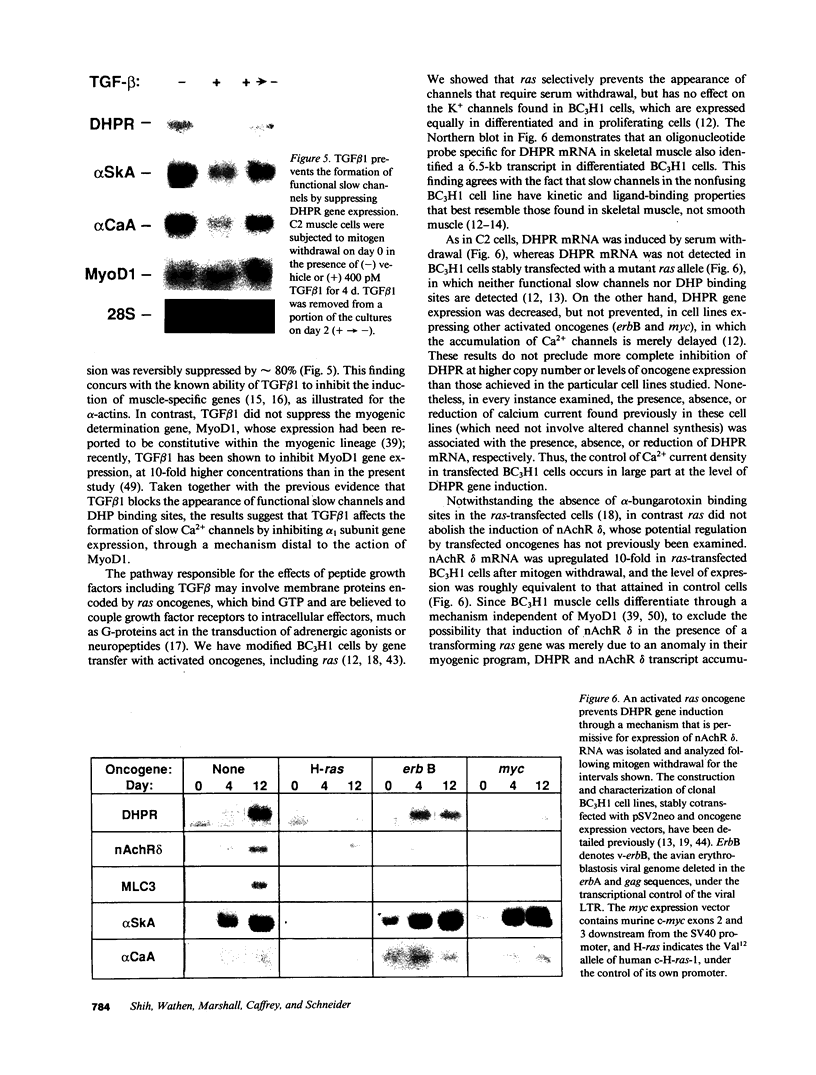
To evaluate developmental and physiological signals that may influence expression of the dihydropyridine-sensitive "slow" Ca2+ channel, we analyzed dihydropyridine receptor (DHPR) mRNA abundance in mouse skeletal muscle. Using synthetic oligonucleotide probes corresponding to the rabbit skeletal muscle DHPR, a 6.5 kb DHPR transcript was identified in postnatal skeletal muscle and differentiated C2 or BC3H1 myocytes, but not cardiac muscle or brain. DHPR gene expression was reversibly suppressed by 0.4 nM transforming growth factor beta-1 or by transfection with a mutant c-H-ras allele, nominal inhibitors of myogenesis that block the appearance of slow channels and DHPR. In contrast, both BC3H1 and C2 myocytes containing the activated ras vector expressed the gene encoding the nicotinic acetylcholine receptor delta subunit, demonstrating that not all muscle-specific genes are extinguished by ras. Denervation stimulated DHPR gene expression less than 0.6-fold, despite 8-fold upregulation of delta-subunit mRNA and reciprocal effects on the skeletal and cardiac alpha-actin genes. Thus, DHPR gene induction is prevented by inhibitors of other muscle-specific genes, whereas, at most, relatively small changes in DHPR mRNA abundance occur during adaptation to denervation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso S., Minty A., Bourlet Y., Buckingham M. Comparison of three actin-coding sequences in the mouse; evolutionary relationships between the actin genes of warm-blooded vertebrates. J Mol Evol. 1986;23(1):11–22. doi: 10.1007/BF02100994. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam K. G., Knudson C. M. Effect of postnatal development on calcium currents and slow charge movement in mammalian skeletal muscle. J Gen Physiol. 1988 Jun;91(6):799–815. doi: 10.1085/jgp.91.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black R., Goldman D., Hochschwender S., Lindstrom J., Hall Z. W. Genetic variants of C2 muscle cells that are defective in synthesis of the alpha-subunit of the acetylcholine receptor. J Cell Biol. 1987 Sep;105(3):1329–1336. doi: 10.1083/jcb.105.3.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey J. M., Brown A. M., Schneider M. D. Ca2+ and Na+ currents in developing skeletal myoblasts are expressed in a sequential program: reversible suppression by transforming growth factor beta-1, an inhibitor of the myogenic pathway. J Neurosci. 1989 Oct;9(10):3443–3453. doi: 10.1523/JNEUROSCI.09-10-03443.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey J. M., Brown A. M., Schneider M. D. Mitogens and oncogenes can block the induction of specific voltage-gated ion channels. Science. 1987 May 1;236(4801):570–573. doi: 10.1126/science.2437651. [DOI] [PubMed] [Google Scholar]
- Chen C. F., Corbley M. J., Roberts T. M., Hess P. Voltage-sensitive calcium channels in normal and transformed 3T3 fibroblasts. Science. 1988 Feb 26;239(4843):1024–1026. doi: 10.1126/science.2449730. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chua M., Dulhunty A. F. Inactivation of excitation-contraction coupling in rat extensor digitorum longus and soleus muscles. J Gen Physiol. 1988 May;91(5):737–757. doi: 10.1085/jgp.91.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooperman S. S., Grubman S. A., Barchi R. L., Goodman R. H., Mandel G. Modulation of sodium-channel mRNA levels in rat skeletal muscle. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8721–8725. doi: 10.1073/pnas.84.23.8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Delorme E. M., McGee R., Jr Regulation of voltage-dependent Ca2+ channels of neuronal cells by chronic changes in membrane potential. Brain Res. 1986 Nov 5;397(1):189–192. doi: 10.1016/0006-8993(86)91385-5. [DOI] [PubMed] [Google Scholar]
- Denis N., Blanc S., Leibovitch M. P., Nicolaiew N., Dautry F., Raymondjean M., Kruh J., Kitzis A. c-myc oncogene expression inhibits the initiation of myogenic differentiation. Exp Cell Res. 1987 Sep;172(1):212–217. doi: 10.1016/0014-4827(87)90107-8. [DOI] [PubMed] [Google Scholar]
- Dillmann W. H., Barrieux A., Neeley W. E., Contreras P. Influence of thyroid hormone on the in vitro translational activity of specific mRNAs in the rat heart. J Biol Chem. 1983 Jun 25;258(12):7738–7745. [PubMed] [Google Scholar]
- Dulhunty A. F., Gage P. W. Asymmetrical charge movement in slow- and fast-twitch mammalian muscle fibres in normal and paraplegic rats. J Physiol. 1983 Aug;341:213–231. doi: 10.1113/jphysiol.1983.sp014802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D. G., Olson E. N. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989 May;3(5):628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- Ellis S. B., Williams M. E., Ways N. R., Brenner R., Sharp A. H., Leung A. T., Campbell K. P., McKenna E., Koch W. J., Hui A. Sequence and expression of mRNAs encoding the alpha 1 and alpha 2 subunits of a DHP-sensitive calcium channel. Science. 1988 Sep 23;241(4873):1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- Endo T., Nadal-Ginard B. Three types of muscle-specific gene expression in fusion-blocked rat skeletal muscle cells: translational control in EGTA-treated cells. Cell. 1987 May 22;49(4):515–526. doi: 10.1016/0092-8674(87)90454-5. [DOI] [PubMed] [Google Scholar]
- Falcone G., Tatò F., Alemà S. Distinctive effects of the viral oncogenes myc, erb, fps, and src on the differentiation program of quail myogenic cells. Proc Natl Acad Sci U S A. 1985 Jan;82(2):426–430. doi: 10.1073/pnas.82.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Goldman D., Brenner H. R., Heinemann S. Acetylcholine receptor alpha-, beta-, gamma-, and delta-subunit mRNA levels are regulated by muscle activity. Neuron. 1988 Jun;1(4):329–333. doi: 10.1016/0896-6273(88)90081-5. [DOI] [PubMed] [Google Scholar]
- Gonoi T., Hasegawa S. Post-natal disappearance of transient calcium channels in mouse skeletal muscle: effects of denervation and culture. J Physiol. 1988 Jul;401:617–637. doi: 10.1113/jphysiol.1988.sp017183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett L. A., Zhang W., Olson E. N. Dexamethasone-dependent inhibition of differentiation of C2 myoblasts bearing steroid-inducible N-ras oncogenes. J Cell Biol. 1988 Jun;106(6):2127–2137. doi: 10.1083/jcb.106.6.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Hall Z. W. Immunological evidence for a change in subunits of the acetylcholine receptor in developing and denervated rat muscle. Neuron. 1988 Apr;1(2):117–125. doi: 10.1016/0896-6273(88)90195-x. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988 Jan;85(2):339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazoglou T., Schmid A., Renaud J. F., Lazdunski M. Ontogenic appearance of Ca2+ channels characterized as binding sites for nitrendipine during development of nervous, skeletal and cardiac muscle systems in the rat. FEBS Lett. 1983 Nov 28;164(1):75–79. doi: 10.1016/0014-5793(83)80022-2. [DOI] [PubMed] [Google Scholar]
- Konieczny S. F., Drobes B. L., Menke S. L., Taparowsky E. J. Inhibition of myogenic differentiation by the H-ras oncogene is associated with the down regulation of the MyoD1 gene. Oncogene. 1989 Apr;4(4):473–481. [PubMed] [Google Scholar]
- Kraus W. E., Bernard T. S., Williams R. S. Interactions between sustained contractile activity and beta-adrenergic receptors in regulation of gene expression in skeletal muscles. Am J Physiol. 1989 Mar;256(3 Pt 1):C506–C514. doi: 10.1152/ajpcell.1989.256.3.C506. [DOI] [PubMed] [Google Scholar]
- La Rocca S. A., Grossi M., Falcone G., Alemà S., Tatò F. Interaction with normal cells suppresses the transformed phenotype of v-myc-transformed quail muscle cells. Cell. 1989 Jul 14;58(1):123–131. doi: 10.1016/0092-8674(89)90409-1. [DOI] [PubMed] [Google Scholar]
- LaPolla R. J., Mayne K. M., Davidson N. Isolation and characterization of a cDNA clone for the complete protein coding region of the delta subunit of the mouse acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7970–7974. doi: 10.1073/pnas.81.24.7970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb G. D., Walsh T. Calcium currents, charge movement and dihydropyridine binding in fast- and slow-twitch muscles of rat and rabbit. J Physiol. 1987 Dec;393:595–617. doi: 10.1113/jphysiol.1987.sp016843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassar A. B., Thayer M. J., Overell R. W., Weintraub H. Transformation by activated ras or fos prevents myogenesis by inhibiting expression of MyoD1. Cell. 1989 Aug 25;58(4):659–667. doi: 10.1016/0092-8674(89)90101-3. [DOI] [PubMed] [Google Scholar]
- Leader D. P., Gall I., Campbell P. C. The structure of a cDNA clone corresponding to mouse cardiac muscle actin mRNA. Biosci Rep. 1986 Aug;6(8):741–747. doi: 10.1007/BF01116542. [DOI] [PubMed] [Google Scholar]
- Lotan I., Goelet P., Gigi A., Dascal N. Specific block of calcium channel expression by a fragment of dihydropyridine receptor cDNA. Science. 1989 Feb 3;243(4891):666–669. doi: 10.1126/science.2464853. [DOI] [PubMed] [Google Scholar]
- Luff A. R., Atwood H. L. Changes in the sarcoplasmic reticulum and transverse tubular system of fast and slow skeletal muscles of the mouse during postnatal development. J Cell Biol. 1971 Nov;51(21):369–383. doi: 10.1083/jcb.51.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Endo T., Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayoux E., Callens F., Swynghedauw B., Charlemagne D. Adaptational process of the cardiac Ca2+ channels to pressure overload: biochemical and physiological properties of the dihydropyridine receptors in normal and hypertrophied rat hearts. J Cardiovasc Pharmacol. 1988 Oct;12(4):390–396. doi: 10.1097/00005344-198810000-00003. [DOI] [PubMed] [Google Scholar]
- Mikami A., Imoto K., Tanabe T., Niidome T., Mori Y., Takeshima H., Narumiya S., Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989 Jul 20;340(6230):230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Role of ion flux in the control of c-fos expression. Nature. 1986 Aug 7;322(6079):552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- Navarro J. Dihydropyridine [methyl-3H]PN 200-110 binding and myogenesis in intact muscle cells in vitro. J Neurochem. 1986 Apr;46(4):1166–1169. doi: 10.1111/j.1471-4159.1986.tb00632.x. [DOI] [PubMed] [Google Scholar]
- Noda M., Ko M., Ogura A., Liu D. G., Amano T., Takano T., Ikawa Y. Sarcoma viruses carrying ras oncogenes induce differentiation-associated properties in a neuronal cell line. Nature. 1985 Nov 7;318(6041):73–75. doi: 10.1038/318073a0. [DOI] [PubMed] [Google Scholar]
- Offord J., Catterall W. A. Electrical activity, cAMP, and cytosolic calcium regulate mRNA encoding sodium channel alpha subunits in rat muscle cells. Neuron. 1989 May;2(5):1447–1452. doi: 10.1016/0896-6273(89)90190-6. [DOI] [PubMed] [Google Scholar]
- Olson E. N., Spizz G., Tainsky M. A. The oncogenic forms of N-ras or H-ras prevent skeletal myoblast differentiation. Mol Cell Biol. 1987 Jun;7(6):2104–2111. doi: 10.1128/mcb.7.6.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Sternberg E., Hu J. S., Spizz G., Wilcox C. Regulation of myogenic differentiation by type beta transforming growth factor. J Cell Biol. 1986 Nov;103(5):1799–1805. doi: 10.1083/jcb.103.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim R. W., Houenou L., Pincon-Raymond M., Powell J. A., Rieger F., Standish L. J. The development of motoneurons in the embryonic spinal cord of the mouse mutant, muscular dysgenesis (mdg/mdg): survival, morphology, and biochemical differentiation. Dev Biol. 1986 Apr;114(2):426–436. doi: 10.1016/0012-1606(86)90207-1. [DOI] [PubMed] [Google Scholar]
- Payne P. A., Olson E. N., Hsiau P., Roberts R., Perryman M. B., Schneider M. D. An activated c-Ha-ras allele blocks the induction of muscle-specific genes whose expression is contingent on mitogen withdrawal. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8956–8960. doi: 10.1073/pnas.84.24.8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E., Kim H. S., Lacerda A. E., Horne W., Wei X. Y., Rampe D., Campbell K. P., Brown A. M., Birnbaumer L. Induction of calcium currents by the expression of the alpha 1-subunit of the dihydropyridine receptor from skeletal muscle. Nature. 1989 Jul 20;340(6230):233–236. doi: 10.1038/340233a0. [DOI] [PubMed] [Google Scholar]
- Perryman M. B., Kerner S. A., Bohlmeyer T. J., Roberts R. Isolation and sequence analysis of a full-length cDNA for human M creatine kinase. Biochem Biophys Res Commun. 1986 Nov 14;140(3):981–989. doi: 10.1016/0006-291x(86)90732-1. [DOI] [PubMed] [Google Scholar]
- Rampe D., Caffrey J. M., Schneider M. D., Brown A. M. Control of expression of the 1,4-dihydropyridine receptor in BC3H1 cells. Biochem Biophys Res Commun. 1988 Apr 29;152(2):769–775. doi: 10.1016/s0006-291x(88)80104-9. [DOI] [PubMed] [Google Scholar]
- Rieger F., Pinçon-Raymond M., Tassin A. M., Garcia L., Romey G., Fosset M., Lazdunski M. Excitation-contraction uncoupling in the developing skeletal muscle of the muscular dysgenesis mouse embryo. Biochimie. 1987 Apr;69(4):411–417. doi: 10.1016/0300-9084(87)90033-2. [DOI] [PubMed] [Google Scholar]
- Schmid A., Renaud J. F., Fosset M., Meaux J. P., Lazdunski M. The nitrendipine-sensitive Ca2+ channel in chick muscle cells and its appearance during myogenesis in vitro and in vivo. J Biol Chem. 1984 Sep 25;259(18):11366–11372. [PubMed] [Google Scholar]
- Schmidt J. W., Catterall W. A. Biosynthesis and processing of the alpha subunit of the voltage-sensitive sodium channel in rat brain neurons. Cell. 1986 Aug 1;46(3):437–444. doi: 10.1016/0092-8674(86)90664-1. [DOI] [PubMed] [Google Scholar]
- Schneider M. D., Olson E. N. Control of myogenic differentiation by cellular oncogenes. Mol Neurobiol. 1988 Spring;2(1):1–39. doi: 10.1007/BF02935631. [DOI] [PubMed] [Google Scholar]
- Schneider M. D., Perryman M. B., Payne P. A., Spizz G., Roberts R., Olson E. N. Autonomous expression of c-myc in BC3H1 cells partially inhibits but does not prevent myogenic differentiation. Mol Cell Biol. 1987 May;7(5):1973–1977. doi: 10.1128/mcb.7.5.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert D., Harris A. J., Devine C. E., Heinemann S. Characterization of a unique muscle cell line. J Cell Biol. 1974 May;61(2):398–413. doi: 10.1083/jcb.61.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz T. L., Tempel B. L., Papazian D. M., Jan Y. N., Jan L. Y. Multiple potassium-channel components are produced by alternative splicing at the Shaker locus in Drosophila. Nature. 1988 Jan 14;331(6152):137–142. doi: 10.1038/331137a0. [DOI] [PubMed] [Google Scholar]
- Shimizu N., Kamel-Reid S., Zak R. Expression of actin mRNAs in denervated chicken skeletal muscle. Dev Biol. 1988 Aug;128(2):435–440. doi: 10.1016/0012-1606(88)90305-3. [DOI] [PubMed] [Google Scholar]
- Simoncini L., Block M. L., Moody W. J. Lineage-specific development of calcium currents during embryogenesis. Science. 1988 Dec 16;242(4885):1572–1575. doi: 10.1126/science.2849207. [DOI] [PubMed] [Google Scholar]
- Sternberg E. A., Spizz G., Perry M. E., Olson E. N. A ras-dependent pathway abolishes activity of a muscle-specific enhancer upstream from the muscle creatine kinase gene. Mol Cell Biol. 1989 Feb;9(2):594–601. doi: 10.1128/mcb.9.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Powell J. A., Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988 Nov 10;336(6195):134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- Tapscott S. J., Davis R. L., Thayer M. J., Cheng P. F., Weintraub H., Lassar A. B. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988 Oct 21;242(4877):405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- Taubman M. B., Smith C. W., Izumo S., Grant J. W., Endo T., Andreadis A., Nadal-Ginard B. The expression of sarcomeric muscle-specific contractile protein genes in BC3H1 cells: BC3H1 cells resemble skeletal myoblasts that are defective for commitment to terminal differentiation. J Cell Biol. 1989 May;108(5):1799–1806. doi: 10.1083/jcb.108.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaghy P. L., Striessnig J., Miwa K., Knaus H. G., Itagaki K., McKenna E., Glossmann H., Schwartz A. Identification of a novel 1,4-dihydropyridine- and phenylalkylamine-binding polypeptide in calcium channel preparations. J Biol Chem. 1987 Oct 15;262(29):14337–14342. [PubMed] [Google Scholar]
- Vaidya T. B., Rhodes S. J., Taparowsky E. J., Konieczny S. F. Fibroblast growth factor and transforming growth factor beta repress transcription of the myogenic regulatory gene MyoD1. Mol Cell Biol. 1989 Aug;9(8):3576–3579. doi: 10.1128/mcb.9.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J. A., Reynolds I. J., Weisman H. F., Dudeck P., Weisfeldt M. L., Snyder S. H. Calcium antagonist receptors in cardiomyopathic hamster: selective increases in heart, muscle, brain. Science. 1986 Apr 25;232(4749):515–518. doi: 10.1126/science.3008330. [DOI] [PubMed] [Google Scholar]
- Wagner J. A., Sax F. L., Weisman H. F., Porterfield J., McIntosh C., Weisfeldt M. L., Snyder S. H., Epstein S. E. Calcium-antagonist receptors in the atrial tissue of patients with hypertrophic cardiomyopathy. N Engl J Med. 1989 Mar 23;320(12):755–761. doi: 10.1056/NEJM198903233201202. [DOI] [PubMed] [Google Scholar]
- Wollner D. A., Scheinman R., Catterall W. A. Sodium channel expression and assembly during development of retinal ganglion cells. Neuron. 1988 Oct;1(8):727–737. doi: 10.1016/0896-6273(88)90171-7. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Imoto Y., Reeves J. P., Birnbaumer L., Brown A. M. A G protein directly regulates mammalian cardiac calcium channels. Science. 1987 Nov 27;238(4831):1288–1292. doi: 10.1126/science.2446390. [DOI] [PubMed] [Google Scholar]