Abstract
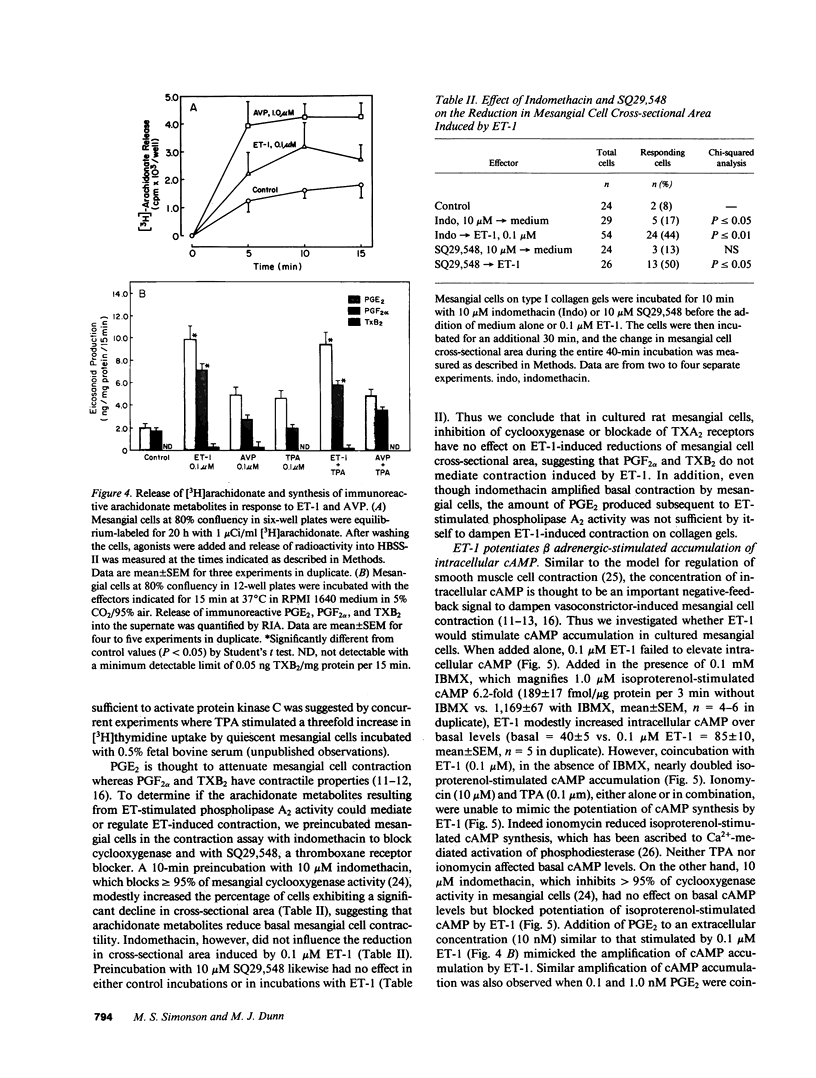
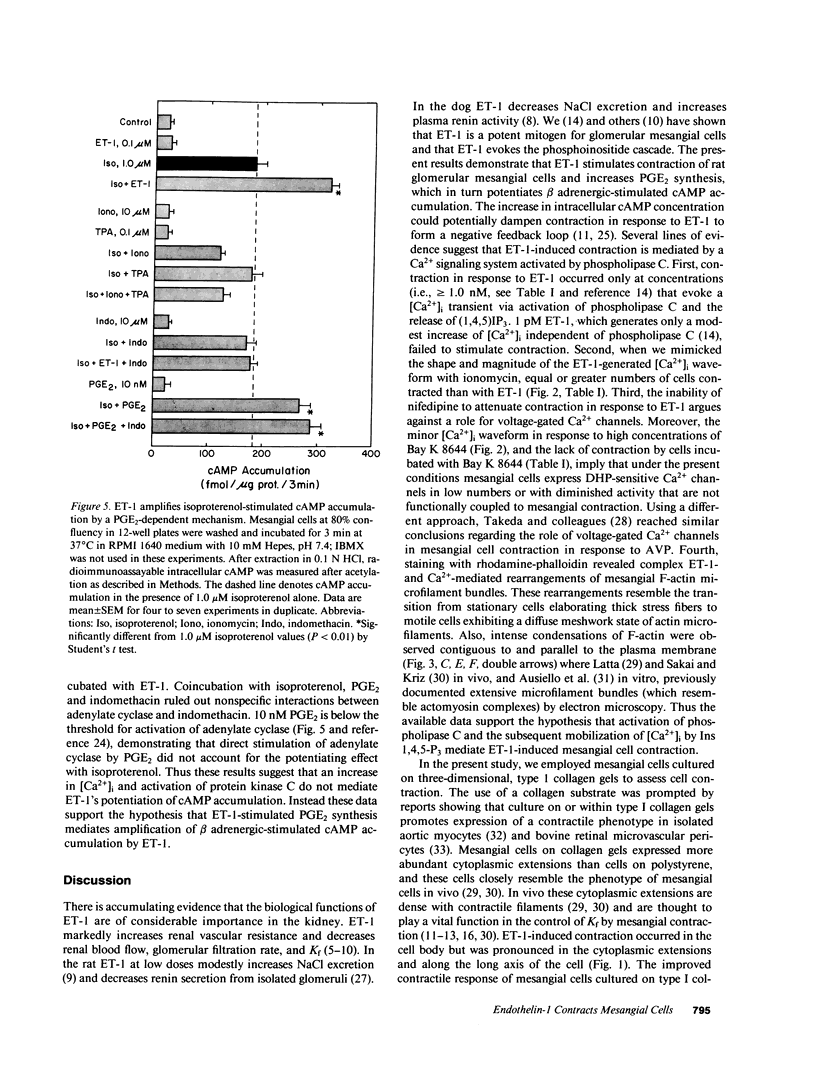
The newly isolated peptide, endothelin-1 (ET-1), is a potent pressor agent that reduces GFR and the glomerular ultrafiltration coefficient. Recent evidence demonstrates that ET-1 mobilizes intracellular Ca2+ [( Ca2+]i) in glomerular mesangial cells by activating the phosphoinositide cascade. The present experiments were designed to examine whether ET-1 stimulates mesangial cell contraction and regulates the synthesis of PGE2 and cAMP, which dampen vasoconstrictor-induced mesangial contraction. ET-1 (greater than or equal to 1 nM) reduced the cross-sectional area of rat mesangial cells cultured on three-dimensional gels of collagen type I. ET-1 also caused complex rearrangements of F-actin microfilaments consistent with a motile response. Contraction in response to ET-1 occurred only at concentrations that activate phospholipase C, and contraction was unaffected by blockade of dihydropyridine-sensitive Ca2+ channels. Elevation of [Ca2+]i with ionomycin, to equivalent concentrations of [Ca2+]i achieved with ET-1, also reduced mesangial cell cross-sectional area. ET-1 (0.1 microM) also evoked [3H]arachidonate release and a fivefold increase in PGE2 synthesis as well as increased synthesis of PGF2 alpha and small changes of TXB2. ET-1 caused a minor increase in intracellular cAMP accumulation only in the presence of 3-isobutyl-1-methylxanthine. ET-1 also amplified cAMP production in response to isoproterenol. TPA and ionomycin, alone and in combination, failed to mimic the potentiating effect of ET-1; however, indomethacin blocked ET-1-induced potentiation of isoproterenol-stimulated cAMP, which was restored by addition of exogenous 10 nM PGE2. Thus the present data demonstrate that ET-1 stimulates mesangial cell contraction via pharmacomechanical coupling and activates phospholipase A2 to produce PGE2, PGF2 alpha, and TXB2. ET-1 also amplified beta adrenergic-stimulated cAMP accumulation by a PGE2-dependent mechanism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ausiello D. A., Kreisberg J. I., Roy C., Karnovsky M. J. Contraction of cultured rat glomerular cells of apparent mesangial origin after stimulation with angiotensin II and arginine vasopressin. J Clin Invest. 1980 Mar;65(3):754–760. doi: 10.1172/JCI109723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badr K. F., Murray J. J., Breyer M. D., Takahashi K., Inagami T., Harris R. C. Mesangial cell, glomerular and renal vascular responses to endothelin in the rat kidney. Elucidation of signal transduction pathways. J Clin Invest. 1989 Jan;83(1):336–342. doi: 10.1172/JCI113880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benovic J. L., Bouvier M., Caron M. G., Lefkowitz R. J. Regulation of adenylyl cyclase-coupled beta-adrenergic receptors. Annu Rev Cell Biol. 1988;4:405–428. doi: 10.1146/annurev.cb.04.110188.002201. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V., Swidler M. Calcium dependency of prostaglandin E2 production in rat glomerular mesangial cells. Evidence that protein kinase C modulates the Ca2+-dependent activation of phospholipase A2. J Clin Invest. 1988 Jul;82(1):168–176. doi: 10.1172/JCI113566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brooker G., Harper J. F., Terasaki W. L., Moylan R. D. Radioimmunoassay of cyclic AMP and cyclic GMP. Adv Cyclic Nucleotide Res. 1979;10:1–33. [PubMed] [Google Scholar]
- Brunton L. L., Mayer S. E. Extrusion of cyclic AMP from pigeon erythrocytes. J Biol Chem. 1979 Oct 10;254(19):9714–9720. [PubMed] [Google Scholar]
- Daly J. W., Padgett W., Seamon K. B. Activation of cyclic AMP-generating systems in brain membranes and slices by the diterpene forskolin: augmentation of receptor-mediated responses. J Neurochem. 1982 Feb;38(2):532–544. doi: 10.1111/j.1471-4159.1982.tb08660.x. [DOI] [PubMed] [Google Scholar]
- Danoff S. K., Young J. M. Is histamine potentiation of adenosine-stimulated cyclic AMP accumulation in guinea-pig cerebral cortical slices mediated by products of inositol phospholipid breakdown? Biochem Pharmacol. 1987 Apr 1;36(7):1177–1179. doi: 10.1016/0006-2952(87)90432-1. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Heasley L. E., Brunton L. L. Prostaglandin A1 metabolism and inhibition of cyclic AMP extrusion by avian erythrocytes. J Biol Chem. 1985 Sep 25;260(21):11514–11519. [PubMed] [Google Scholar]
- Heasley L. E., Watson M. J., Brunton L. L. Putative inhibitor of cyclic AMP efflux: chromatography, amino acid composition, and identification as a prostaglandin A1-glutathione adduct. J Biol Chem. 1985 Sep 25;260(21):11520–11523. [PubMed] [Google Scholar]
- Itoh Y., Yanagisawa M., Ohkubo S., Kimura C., Kosaka T., Inoue A., Ishida N., Mitsui Y., Onda H., Fujino M. Cloning and sequence analysis of cDNA encoding the precursor of a human endothelium-derived vasoconstrictor peptide, endothelin: identity of human and porcine endothelin. FEBS Lett. 1988 Apr 25;231(2):440–444. doi: 10.1016/0014-5793(88)80867-6. [DOI] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu Rev Pharmacol Toxicol. 1985;25:593–620. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- Kelley C., D'Amore P., Hechtman H. B., Shepro D. Microvascular pericyte contractility in vitro: comparison with other cells of the vascular wall. J Cell Biol. 1987 Mar;104(3):483–490. doi: 10.1083/jcb.104.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A. J., Brenner B. M., Anderson S. Endothelin: a potent renal and systemic vasoconstrictor peptide. Am J Physiol. 1989 Jun;256(6 Pt 2):F1051–F1058. doi: 10.1152/ajprenal.1989.256.6.F1051. [DOI] [PubMed] [Google Scholar]
- Lippton H., Goff J., Hyman A. Effects of endothelin in the systemic and renal vascular beds in vivo. Eur J Pharmacol. 1988 Oct 11;155(1-2):197–199. doi: 10.1016/0014-2999(88)90424-4. [DOI] [PubMed] [Google Scholar]
- Mené P., Dunn M. J. Eicosanoids and control of mesangial cell contraction. Circ Res. 1988 May;62(5):916–925. doi: 10.1161/01.res.62.5.916. [DOI] [PubMed] [Google Scholar]
- Miller W. L., Redfield M. M., Burnett J. C., Jr Integrated cardiac, renal, and endocrine actions of endothelin. J Clin Invest. 1989 Jan;83(1):317–320. doi: 10.1172/JCI113876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabika T., Nara Y., Yamori Y., Lovenberg W., Endo J. Angiotensin II and phorbol ester enhance isoproterenol- and vasoactive intestinal peptide (VIP)-induced cyclic AMP accumulation in vascular smooth muscle cells. Biochem Biophys Res Commun. 1985 Aug 30;131(1):30–36. doi: 10.1016/0006-291x(85)91765-6. [DOI] [PubMed] [Google Scholar]
- Rakugi H., Nakamaru M., Saito H., Higaki J., Ogihara T. Endothelin inhibits renin release from isolated rat glomeruli. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1244–1247. doi: 10.1016/s0006-291x(88)81273-7. [DOI] [PubMed] [Google Scholar]
- Rindler M. J., Bashor M. M., Spitzer N., Saier M. H., Jr Regulation of adenosine 3':5'-monophosphate efflux from animal cells. J Biol Chem. 1978 Aug 10;253(15):5431–5436. [PubMed] [Google Scholar]
- Rozengurt E., Murray M., Zachary I., Collins M. Protein kinase C activation enhances cAMP accumulation in Swiss 3T3 cells: inhibition by pertussis toxin. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2282–2286. doi: 10.1073/pnas.84.8.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Kriz W. The structural relationship between mesangial cells and basement membrane of the renal glomerulus. Anat Embryol (Berl) 1987;176(3):373–386. doi: 10.1007/BF00310191. [DOI] [PubMed] [Google Scholar]
- Schlondorff D. The glomerular mesangial cell: an expanding role for a specialized pericyte. FASEB J. 1987 Oct;1(4):272–281. doi: 10.1096/fasebj.1.4.3308611. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley D. R., Jeffs R. A., Daniel K., Nambi P., Lefkowitz R. J. Phorbol diester treatment promotes enhanced adenylate cyclase activity in frog erythrocytes. Arch Biochem Biophys. 1986 Jan;244(1):373–381. doi: 10.1016/0003-9861(86)90126-8. [DOI] [PubMed] [Google Scholar]
- Siegl A. M., Daly J. W., Smith J. B. Inhibition of aggregation and stimulation of cyclic AMP generation in intact human platelets by the diterpene forskolin. Mol Pharmacol. 1982 May;21(3):680–687. [PubMed] [Google Scholar]
- Simonson M. S., Dunn M. J. Leukotriene C4 and D4 contract rat glomerular mesangial cells. Kidney Int. 1986 Oct;30(4):524–531. doi: 10.1038/ki.1986.217. [DOI] [PubMed] [Google Scholar]
- Simonson M. S., Mené P., Dubyak G. R., Dunn M. J. Identification and transmembrane signaling of leukotriene D4 receptors in human mesangial cells. Am J Physiol. 1988 Dec;255(6 Pt 1):C771–C780. doi: 10.1152/ajpcell.1988.255.6.C771. [DOI] [PubMed] [Google Scholar]
- Simonson M. S., Wann S., Mené P., Dubyak G. R., Kester M., Nakazato Y., Sedor J. R., Dunn M. J. Endothelin stimulates phospholipase C, Na+/H+ exchange, c-fos expression, and mitogenesis in rat mesangial cells. J Clin Invest. 1989 Feb;83(2):708–712. doi: 10.1172/JCI113935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Meyer-Lehnert H., Kim J. K., Schrier R. W. Effect of angiotensin II on Ca2+ kinetics and contraction in cultured rat glomerular mesangial cells. Am J Physiol. 1988 Feb;254(2 Pt 2):F254–F266. doi: 10.1152/ajprenal.1988.254.2.F254. [DOI] [PubMed] [Google Scholar]
- Tomobe Y., Miyauchi T., Saito A., Yanagisawa M., Kimura S., Goto K., Masaki T. Effects of endothelin on the renal artery from spontaneously hypertensive and Wistar Kyoto rats. Eur J Pharmacol. 1988 Aug 2;152(3):373–374. doi: 10.1016/0014-2999(88)90736-4. [DOI] [PubMed] [Google Scholar]
- Travo P., Bodin P., Burnstock G., Stoclet J. C. Quantitative method for the study of the morphological changes induced by vasoactive agents in single aortic myocytes grown in primary cultures. Lab Invest. 1987 Mar;56(3):335–343. [PubMed] [Google Scholar]
- Wright C. E., Fozard J. R. Regional vasodilation is a prominent feature of the haemodynamic response to endothelin in anaesthetized, spontaneously hypertensive rats. Eur J Pharmacol. 1988 Oct 11;155(1-2):201–203. doi: 10.1016/0014-2999(88)90425-6. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M., Inoue A., Ishikawa T., Kasuya Y., Kimura S., Kumagaye S., Nakajima K., Watanabe T. X., Sakakibara S., Goto K. Primary structure, synthesis, and biological activity of rat endothelin, an endothelium-derived vasoconstrictor peptide. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6964–6967. doi: 10.1073/pnas.85.18.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988 Mar 31;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Yoshimasa T., Sibley D. R., Bouvier M., Lefkowitz R. J., Caron M. G. Cross-talk between cellular signalling pathways suggested by phorbol-ester-induced adenylate cyclase phosphorylation. Nature. 1987 May 7;327(6117):67–70. doi: 10.1038/327067a0. [DOI] [PubMed] [Google Scholar]
- de Nucci G., Thomas R., D'Orleans-Juste P., Antunes E., Walder C., Warner T. D., Vane J. R. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]