Abstract
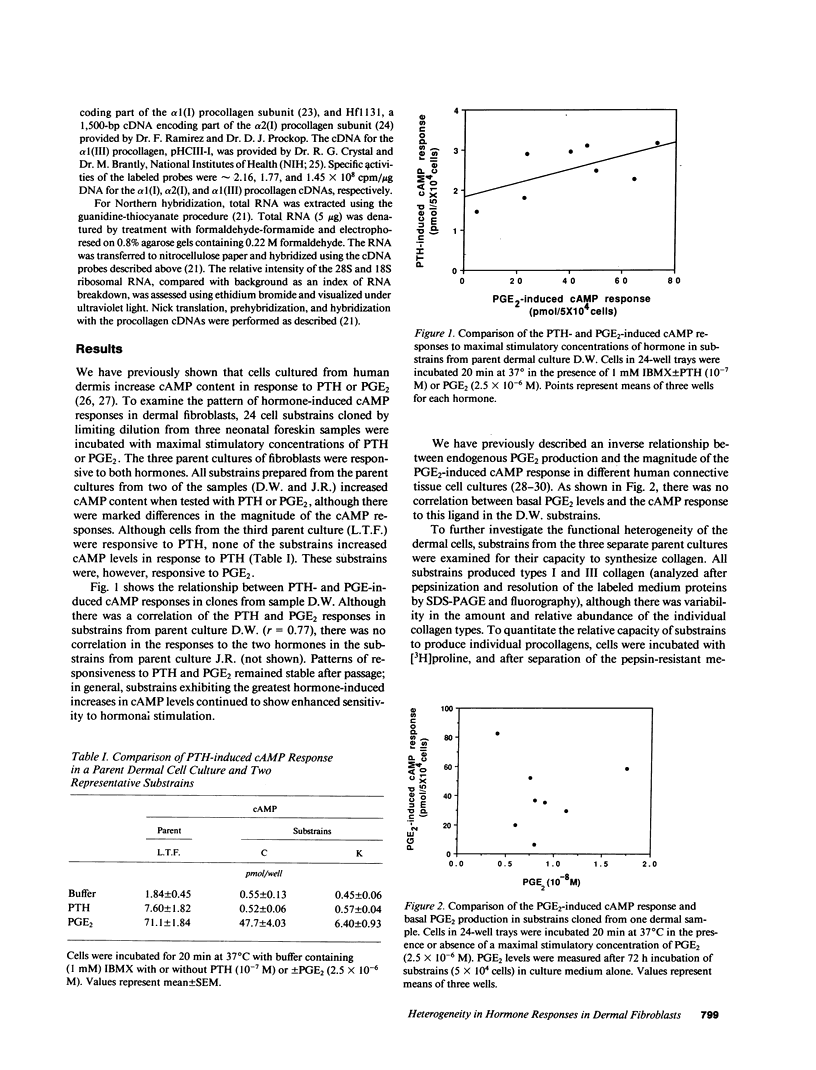
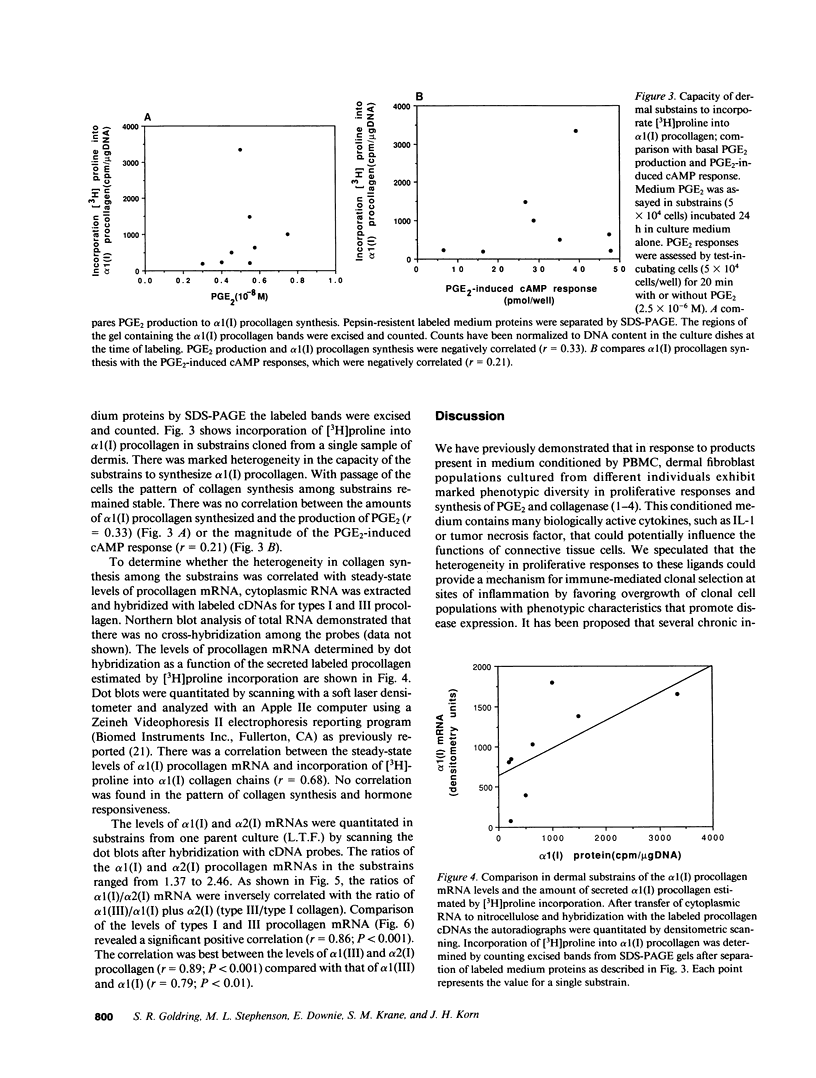
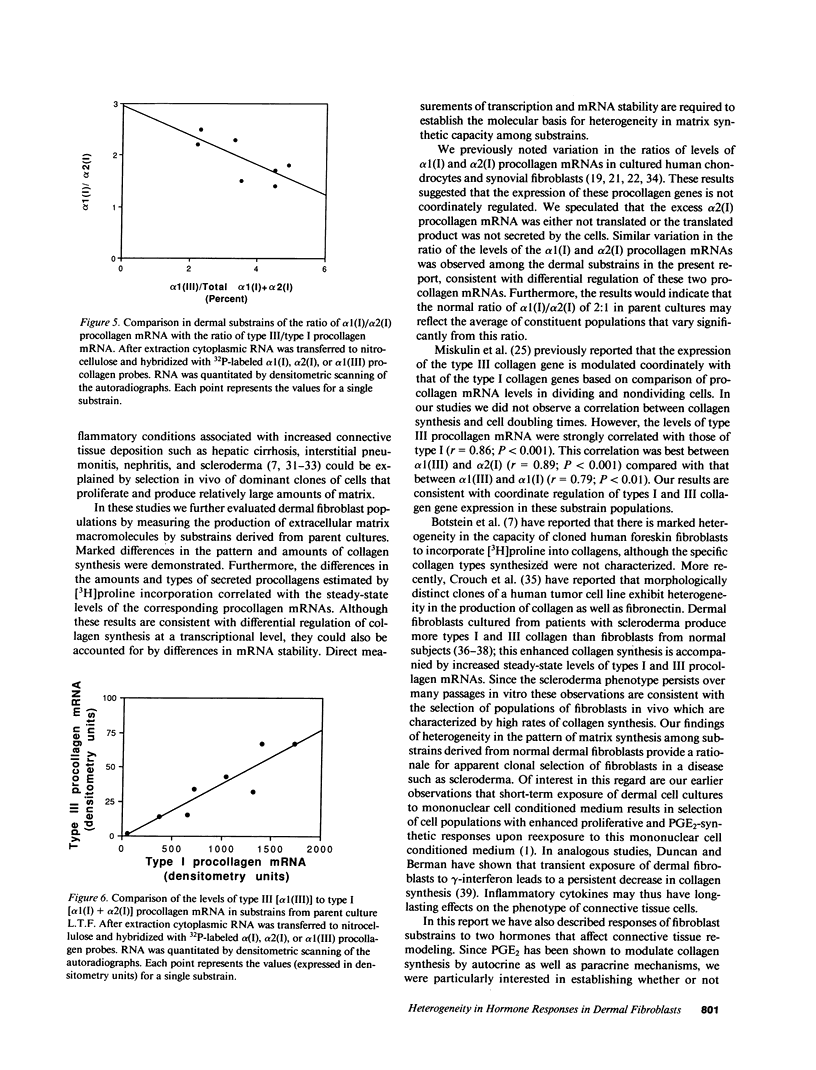
Fibroblasts cultured from normal human dermis are heterogeneous with respect to growth kinetics, synthetic function, and morphologic features. There are many examples of clonal heterogeneity in apparently homogeneous connective tissue cell populations, and it has been suggested that selection of cell populations with particular phenotypic features is the basis for the development of pathologic connective tissue changes in inflammatory disorders. In these studies we report characterization of the pattern of matrix biosynthesis and responses to hormones in cells cloned from normal human dermis. The results indicate that cloned dermal fibroblasts are heterogeneous with respect to synthesis of collagens as well as their responses to prostaglandin E2 and parathyroid hormone. Selective expansion of clonal populations with unique patterns of matrix synthesis and cell surface receptors could provide the basis for abnormal connective tissue remodeling in certain pathologic states.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amento E. P., Bhan A. K., McCullagh K. G., Krane S. M. Influences of gamma interferon on synovial fibroblast-like cells. Ia induction and inhibition of collagen synthesis. J Clin Invest. 1985 Aug;76(2):837–848. doi: 10.1172/JCI112041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benditt E. P., Benditt J. M. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. A., Moss J., Baum B. J., Crystal R. G. Regulation of collagen production by the beta-adrenergic system. J Clin Invest. 1981 May;67(5):1457–1462. doi: 10.1172/JCI110175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard M. P., Myers J. C., Chu M. L., Ramirez F., Eikenberry E. F., Prockop D. J. Structure of a cDNA for the pro alpha 2 chain of human type I procollagen. Comparison with chick cDNA for pro alpha 2(I) identifies structurally conserved features of the protein and the gene. Biochemistry. 1983 Mar 1;22(5):1139–1145. doi: 10.1021/bi00274a023. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Wewers M. D., Rennard S. I., Adelberg S., Crystal R. G. Modulation of alveolar macrophage-driven fibroblast proliferation by alternative macrophage mediators. J Clin Invest. 1986 Mar;77(3):700–708. doi: 10.1172/JCI112364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botstein G. R., Sherer G. K., Leroy E. C. Fibroblast selection in scleroderma. An alternative model of fibrosis. Arthritis Rheum. 1982 Feb;25(2):189–195. doi: 10.1002/art.1780250212. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff C. E., Nagel J. E. Collagenase production by cloned populations of rabbit synovial fibroblasts. Coll Relat Res. 1981 Sep;1(5):433–444. doi: 10.1016/s0174-173x(81)80027-1. [DOI] [PubMed] [Google Scholar]
- Chu M. L., Myers J. C., Bernard M. P., Ding J. F., Ramirez F. Cloning and characterization of five overlapping cDNAs specific for the human pro alpha 1(I) collagen chain. Nucleic Acids Res. 1982 Oct 11;10(19):5925–5934. doi: 10.1093/nar/10.19.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotran R. S. Monocytes, proliferation, and glomerulonephritis. J Lab Clin Med. 1978 Dec;92(6):837–840. [PubMed] [Google Scholar]
- Crouch E. C., Stone K. R., Bloch M., McDivitt R. W. Heterogeneity in the production of collagens and fibronectin by morphologically distinct clones of a human tumor cell line: evidence for intratumoral diversity in matrix protein biosynthesis. Cancer Res. 1987 Nov 15;47(22):6086–6092. [PubMed] [Google Scholar]
- Danks J. A., Ebeling P. R., Hayman J., Chou S. T., Moseley J. M., Dunlop J., Kemp B. E., Martin T. J. Parathyroid hormone-related protein: immunohistochemical localization in cancers and in normal skin. J Bone Miner Res. 1989 Apr;4(2):273–278. doi: 10.1002/jbmr.5650040221. [DOI] [PubMed] [Google Scholar]
- Duncan M. R., Berman B. Persistence of a reduced-collagen-producing phenotype in cultured scleroderma fibroblasts after short-term exposure to interferons. J Clin Invest. 1987 May;79(5):1318–1324. doi: 10.1172/JCI112956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring M. B., Birkhead J., Sandell L. J., Kimura T., Krane S. M. Interleukin 1 suppresses expression of cartilage-specific types II and IX collagens and increases types I and III collagens in human chondrocytes. J Clin Invest. 1988 Dec;82(6):2026–2037. doi: 10.1172/JCI113823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring M. B., Krane S. M. Modulation by recombinant interleukin 1 of synthesis of types I and III collagens and associated procollagen mRNA levels in cultured human cells. J Biol Chem. 1987 Dec 5;262(34):16724–16729. [PubMed] [Google Scholar]
- Goldring M. B., Sandell L. J., Stephenson M. L., Krane S. M. Immune interferon suppresses levels of procollagen mRNA and type II collagen synthesis in cultured human articular and costal chondrocytes. J Biol Chem. 1986 Jul 5;261(19):9049–9055. [PubMed] [Google Scholar]
- Goldring S. R., Dayer J. M., Krane S. M. Rheumatoid synovial cell hormone responses modulated by cell-cell interactions. Inflammation. 1984 Mar;8(1):107–121. doi: 10.1007/BF00918358. [DOI] [PubMed] [Google Scholar]
- Goldring S. R., Dayer J. M., Russell R. G., Mankin H. J., Krane S. M. Response to hormones of cells cultured from human giant cell tumors of bone. J Clin Endocrinol Metab. 1978 Mar;46(3):425–433. doi: 10.1210/jcem-46-3-425. [DOI] [PubMed] [Google Scholar]
- Goldring S. R., Mahaffey J. E., Rosenblatt M., Dayer J. M., Potts J. T., Jr, Krane S. M. Parathyroid hormone inhibitors: comparison of biological activity in bone- and skin-derived tissue. J Clin Endocrinol Metab. 1979 Apr;48(4):655–659. doi: 10.1210/jcem-48-4-655. [DOI] [PubMed] [Google Scholar]
- Goldring S. R., Roelke M. S., Bringhurst F. R., Rosenblatt M. Differential effects of parathyroid hormone responsive cultured human cells on biological activity of parathyroid hormone and parathyroid hormone inhibitory analogues. Biochemistry. 1985 Jan 15;24(2):513–518. doi: 10.1021/bi00323a040. [DOI] [PubMed] [Google Scholar]
- Goldring S. R., Roelke M. S., Petrison K. K., Bhan A. K. Human giant cell tumors of bone identification and characterization of cell types. J Clin Invest. 1987 Feb;79(2):483–491. doi: 10.1172/JCI112838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring S. R., Tyler G. A., Krane S. M., Potts J. T., Jr, Rosenblatt M. Photoaffinity labeling of parathyroid hormone receptors: comparison of receptors across species and target tissues and after desensitization to hormone. Biochemistry. 1984 Jan 31;23(3):498–502. doi: 10.1021/bi00298a015. [DOI] [PubMed] [Google Scholar]
- Graves P. N., Weiss I. K., Perlish J. S., Fleischmajer R. Increased procollagen mRNA levels in scleroderma skin fibroblasts. J Invest Dermatol. 1983 Feb;80(2):130–132. doi: 10.1111/1523-1747.ep12532908. [DOI] [PubMed] [Google Scholar]
- Hassell T. M., Page R. C., Narayanan A. S., Cooper C. G. Diphenylhydantoin (dilantin) gingival hyperplasia: drug-induced abnormality of connective tissue. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2909–2912. doi: 10.1073/pnas.73.8.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko S. D., Page R. C., Narayanan A. S. Fibroblast heterogeneity and prostaglandin regulation of subpopulations. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3429–3432. doi: 10.1073/pnas.74.8.3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn J. H., Brinckerhoff C. E., Edwards R. L. Synthesis of PGE2, collagenase and tissue factor by fibroblast substrains: substrains are differentially activated for different metabolic products. Coll Relat Res. 1985 Nov;5(5):437–447. doi: 10.1016/s0174-173x(85)80031-5. [DOI] [PubMed] [Google Scholar]
- Korn J. H. Fibroblast prostaglandin E2 synthesis. Persistence of an abnormal phenotype after short-term exposure to mononuclear cell products. J Clin Invest. 1983 May;71(5):1240–1246. doi: 10.1172/JCI110873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn J. H. Substrain heterogeneity in prostaglandin E2 synthesis of human dermal fibroblasts. Differences in prostaglandin E2 synthetic capacity of substrains are not stimulus-restricted. Arthritis Rheum. 1985 Mar;28(3):315–322. doi: 10.1002/art.1780280312. [DOI] [PubMed] [Google Scholar]
- Korn J. H., Torres D., Downie E. Clonal heterogeneity in the fibroblast response to mononuclear cell derived mediators. Arthritis Rheum. 1984 Feb;27(2):174–179. doi: 10.1002/art.1780270208. [DOI] [PubMed] [Google Scholar]
- Krane S. M., Dayer J. M., Simon L. S., Byrne M. S. Mononuclear cell-conditioned medium containing mononuclear cell factor (MCF), homologous with interleukin 1, stimulates collagen and fibronectin synthesis by adherent rheumatoid synovial cells: effects of prostaglandin E2 and indomethacin. Coll Relat Res. 1985 Mar;5(2):99–117. doi: 10.1016/s0174-173x(85)80033-9. [DOI] [PubMed] [Google Scholar]
- Kream B. E., Rowe D. W., Gworek S. C., Raisz L. G. Parathyroid hormone alters collagen synthesis and procollagen mRNA levels in fetal rat calvaria. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5654–5658. doi: 10.1073/pnas.77.10.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kähäri V. M., Vuorio T., Näntö-Salonen K., Vuorio E. Increased type I collagen mRNA levels in cultured scleroderma fibroblasts. Biochim Biophys Acta. 1984 Feb 24;781(1-2):183–186. doi: 10.1016/0167-4781(84)90136-2. [DOI] [PubMed] [Google Scholar]
- Liotta L. A., Rao C. N., Barsky S. H. Tumor invasion and the extracellular matrix. Lab Invest. 1983 Dec;49(6):636–649. [PubMed] [Google Scholar]
- Liotta L. A. Tumor invasion and metastases--role of the extracellular matrix: Rhoads Memorial Award lecture. Cancer Res. 1986 Jan;46(1):1–7. [PubMed] [Google Scholar]
- Martin G. M., Sprague C. A., Norwood T. H., Pendergrass W. R. Clonal selection, attenuation and differentiation in an in vitro model of hyperplasia. Am J Pathol. 1974 Jan;74(1):137–154. [PMC free article] [PubMed] [Google Scholar]
- Miskulin M., Dalgleish R., Kluve-Beckerman B., Rennard S. I., Tolstoshev P., Brantly M., Crystal R. G. Human type III collagen gene expression is coordinately modulated with the type I collagen genes during fibroblast growth. Biochemistry. 1986 Mar 25;25(6):1408–1413. doi: 10.1021/bi00354a033. [DOI] [PubMed] [Google Scholar]
- Nissenson R. A., Diep D., Strewler G. J. Synthetic peptides comprising the amino-terminal sequence of a parathyroid hormone-like protein from human malignancies. Binding to parathyroid hormone receptors and activation of adenylate cyclase in bone cells and kidney. J Biol Chem. 1988 Sep 15;263(26):12866–12871. [PubMed] [Google Scholar]
- Pearson T. A., Dillman J. M., Solex K., Heptinstall R. H. Clonal markers in the study of the origin and growth of human atherosclerotic lesions. Circ Res. 1978 Jul;43(1):10–18. doi: 10.1161/01.res.43.1.10. [DOI] [PubMed] [Google Scholar]
- Pun K. K., Arnaud C. D., Nissenson R. A. Parathyroid hormone receptors in human dermal fibroblasts: structural and functional characterization. J Bone Miner Res. 1988 Aug;3(4):453–460. doi: 10.1002/jbmr.5650030413. [DOI] [PubMed] [Google Scholar]
- Silve C., Santora A., Breslau N., Moses A., Spiegel A. Selective resistance to parathyroid hormone in cultured skin fibroblasts from patients with pseudohypoparathyroidism type Ib. J Clin Endocrinol Metab. 1986 Apr;62(4):640–644. doi: 10.1210/jcem-62-4-640. [DOI] [PubMed] [Google Scholar]
- Silve C., Santora A., Spiegel A. A factor produced by cultured rat Leydig tumor (Rice 500) cells associated with humoral hypercalcemia stimulates adenosine 3',5'-monophosphate production via the parathyroid hormone receptor in human skin fibroblasts. J Clin Endocrinol Metab. 1985 Jun;60(6):1144–1147. doi: 10.1210/jcem-60-6-1144. [DOI] [PubMed] [Google Scholar]
- Smith J. R., Hayflick L. Variation in the life-span of clones derived from human diploid cell strains. J Cell Biol. 1974 Jul;62(1):48–53. doi: 10.1083/jcb.62.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M. L., Krane S. M., Amento E. P., McCroskery P. A., Byrne M. Immune interferon inhibits collagen synthesis by rheumatoid synovial cells associated with decreased levels of the procollagen mRNAs. FEBS Lett. 1985 Jan 21;180(1):43–50. doi: 10.1016/0014-5793(85)80227-1. [DOI] [PubMed] [Google Scholar]
- Stewart A. F., Wu T., Goumas D., Burtis W. J., Broadus A. E. N-terminal amino acid sequence of two novel tumor-derived adenylate cyclase-stimulating proteins: identification of parathyroid hormone-like and parathyroid hormone-unlike domains. Biochem Biophys Res Commun. 1987 Jul 31;146(2):672–678. doi: 10.1016/0006-291x(87)90581-x. [DOI] [PubMed] [Google Scholar]
- Strewler G. J., Stern P. H., Jacobs J. W., Eveloff J., Klein R. F., Leung S. C., Rosenblatt M., Nissenson R. A. Parathyroid hormonelike protein from human renal carcinoma cells. Structural and functional homology with parathyroid hormone. J Clin Invest. 1987 Dec;80(6):1803–1807. doi: 10.1172/JCI113275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suva L. J., Winslow G. A., Wettenhall R. E., Hammonds R. G., Moseley J. M., Diefenbach-Jagger H., Rodda C. P., Kemp B. E., Rodriguez H., Chen E. Y. A parathyroid hormone-related protein implicated in malignant hypercalcemia: cloning and expression. Science. 1987 Aug 21;237(4817):893–896. doi: 10.1126/science.3616618. [DOI] [PubMed] [Google Scholar]
- Uitto J., Bauer E. A., Eisen A. Z. Scleroderma: increased biosynthesis of triple-helical type I and type III procollagens associated with unaltered expression of collagenase by skin fibroblasts in culture. J Clin Invest. 1979 Oct;64(4):921–930. doi: 10.1172/JCI109558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Whiteside T. L., Ferrarini M., Hebda P., Buckingham R. B. Heterogeneous synthetic phenotype of cloned scleroderma fibroblasts may be due to aberrant regulation in the synthesis of connective tissues. Arthritis Rheum. 1988 Oct;31(10):1221–1229. doi: 10.1002/art.1780311002. [DOI] [PubMed] [Google Scholar]
- Wyler D. J., Prakash S., Libby P. Mesenchymal target cell specificity of egg granuloma-derived fibroblast growth factor in schistosomiasis. J Infect Dis. 1987 Apr;155(4):728–736. doi: 10.1093/infdis/155.4.728. [DOI] [PubMed] [Google Scholar]



