Abstract
Thrombopoiesis, the process by which circulating platelets arise from megakaryocytes, remains incompletely understood. Prior studies suggest that megakaryocytes shed platelets in the pulmonary vasculature. To better understand thrombopoiesis and to develop a potential platelet transfusion strategy that is not dependent upon donors, of which there remains a shortage, we examined whether megakaryocytes infused into mice shed platelets. Infused megakaryocytes led to clinically relevant increases in platelet numbers. The released platelets were normal in size, displayed appropriate surface markers, and had a near-normal circulating half-life. The functionality of the donor-derived platelets was also demonstrated in vivo. The infused megakaryocytes mostly localized to the pulmonary vasculature, where they appeared to shed platelets. These data suggest that it may be unnecessary to generate platelets from ex vivo grown megakaryocytes to achieve clinically relevant increases in platelet numbers.
Introduction
While the number of platelet donors is increasing, there is still a significant donor shortage due to the growing population of patients with serious illnesses associated with thrombocytopenia and hemorrhage (1). The use of donor-derived platelets raises the following concerns: variability of quality and quantity, risk of infectious transmission, short lifespan of stored platelets, bacterial contamination during storage, and development of alloantibodies in multi-transfused patients. These problems highlight a need for new strategies to generate platelets for infusion therapy.
Thrombopoiesis, the process by which circulating platelets arise from megakaryocytes remains incompletely understood. In vitro studies suggest that platelets form nodes at tips of proplatelet strands (2). However, direct visualization of live calvaria marrow using multiphoton intravital microscopy suggests that megakaryocytes release large cytoplasmic fragments into the vasculature (3), which must then undergo reorganization into platelets. Studies based on morphologic analysis and quantification of megakaryocyte-like polyploid nuclei in the pulmonary venous system suggested that megakaryocytes release platelets in the lungs (4). Derivation of platelets from megakaryocytes in culture was first reported in 1995 (5) but has been difficult to quantitatively upscale. To date, the best published result from infused in vitro produced platelets used irradiated mice with low platelet counts (~104/μl) (6). Peak percent donor platelet counts were still only 1%–2%. Given the limited success by which platelets have been generated ex vivo, we examined whether infused megakaryocytes release platelets in vivo. We found that by infusing ex vivo generated murine megakaryocytes into mice, we can achieve an approximately 100-fold increase in recipient platelet count over prior published results, achieving clinically relevant levels of donor platelets. These platelets have a slightly shorter half-life than infused platelets, but are normal in size, expression of surface markers, and functionality. Infused megakaryocytes appear to be trapped in the pulmonary bed, where they shed their cytoplasm.
Results and Discussion
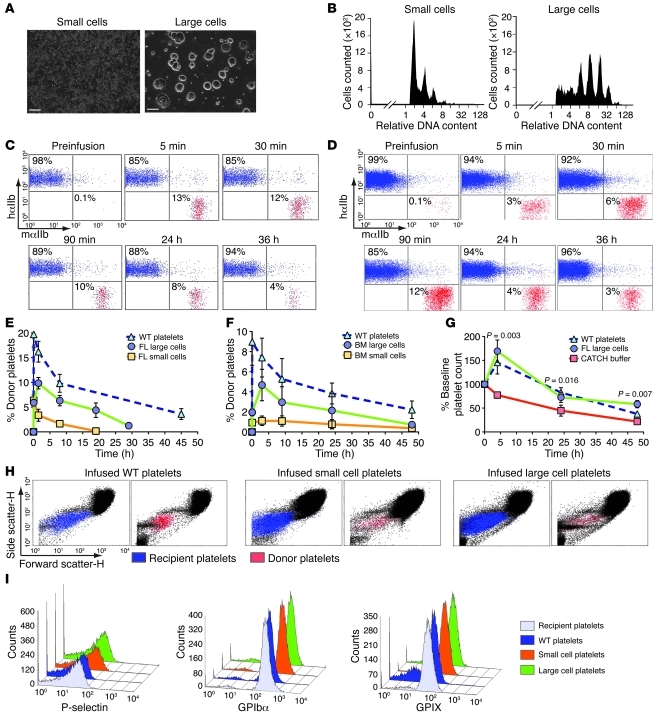
To address whether infused megakaryocytes give rise to circulating platelets, we generated fetal liver– (FL-) and BM-derived magakaryocytes (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI43326DS1) (7). Mature megakaryocytes were separated from other cells and proplatelets using a 2-step density gradient to produce “large cells,” with more than half possessing a diameter greater than 50 μm and with only approximately 2.5:1 proplatelets/cell (Figure 1A). The remaining “small cells” had approximately 10:1 proplatelets/cell (Figure 1A). Ploidy analysis showed FL small cells with low DNA ploidy relative to FL large cells (Figure 1B).
Figure 1. Characterization and infusion of megakaryocytes.
(A) Representative fields of small and large cells. Scale bars: 100 μm. (B) Representative analysis of DNA content of FL small and large cells. (C) Flow cytometry from recipient mouse before and after infusion of 108 WT platelets or (D) 106 FL large cells. (E) Flow cytometric percentage of 106 infused FL large cells and (F) 106 infused adult BM cells. n = 5 for WT platelets, n = 9 for FL cells, n = 5 for BM studies. (G) Percent platelet rise in irradiated thrombocytopenic mice after infusion. n = 5 per arm. Mean ± 1 SD are shown. Initial platelet counts (108/ml) in the 3 groups were: CATCH buffer, 1.8 ± 0.2; platelets, 1.9 ± 0.3; large cells, 1.0 ± 0.2. (H) Size determination of circulating recipient (blue) and infused platelets (red) by forward versus side scatter analysis. (I) Representative flow cytometric analysis of infused and FL-derived platelets comparing P-selectin, GPIbα, and GPIX.
Positive control WT platelets were isolated and infused into hαIIb+ recipient mice, with only human αIIb on their platelet surface (8), to allow flow cytometric detection of infused platelets using species-specific anti-αIIb (CD41) Abs. After infusion, WT platelets were detected in hαIIb+ recipient mice immediately (i.e., 5-minute time point), with an overall half-life of approximately 36 hours (Figure 1, C, E, and F). In contrast, infused FL large cells resulted in delayed platelets, with a peak at approximately 90 minutes (Figure 1, D and E). These platelets had a shorter overall half-life of approximately 20 hours. Based on the number of cells infused, the peak increase in platelet count, and the recipient mouse blood volume of approximately 2 ml, we calculated 100–200 platelets from each large cell, assuming all cells gave rise to platelets. Infused FL small cells enriched with proplatelets gave rise to an immediate peak similar to infused WT platelets; however, these platelets had a truncated half-life of approximately 2 hours (Figure 1E). Infusing adult BM megakaryocytes resulted in a similarly delayed appearance of platelets as with FL-derived megakaryocytes, but with a slightly longer half-life of 24 hours (Figure 1F). To simulate clinical thrombocytopenia, we irradiated mice and infused CATCH buffer (1× PBS, 1.5% BSA, 1 mM adenosine, 2 mM theophylline, and 0.38% sodium citrate), WT platelets, or FL large cells near the induced platelet nadir and observed that both platelets and megakaryocytes significantly increased the platelet count relative to CATCH buffer over a time course of more than 24 hours (Figure 1G).
To understand the shortened half-life of platelets derived from infused large cells, we examined their size distribution and microparticles compared with infused WT platelets and found no differences (Figure 1H). Another indicator of platelet activation is the expression of surface P-selectin (9). Flow cytometric analysis showed that surface P-selectin levels were similar in platelets derived from infused FL cells and infused WT platelets (Figure 1I). ADAM17 is a metalloproteinase found in cultures that shortens platelet half-life (10) and cleaves the glycocalicin extracellular portion of GPIbα, inactivating the GPIb/IX receptor without altering receptor density (11). Platelets derived from infused FL cells and infused WT platelets displayed similar ratios of extracellular GPIb to GPIX (Figure 1I). Thus, the slightly shortened half-life of the platelets derived from infused large cells does not appear to be due to ADAM17 activity.
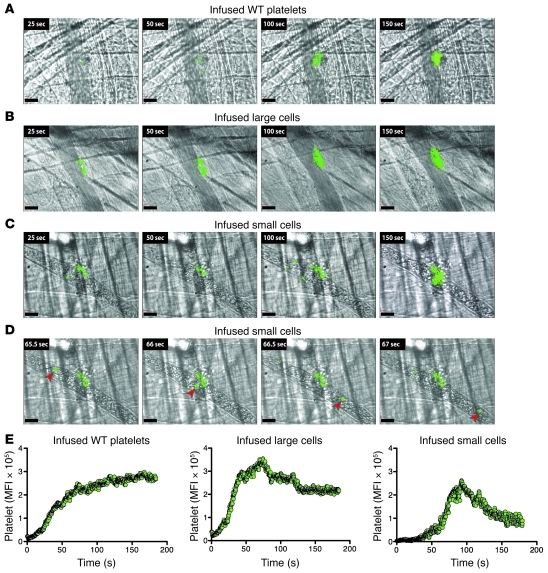
Clinically, platelets are transfused not to increase platelet number, but to reverse or prevent a bleeding diathesis due to thrombocytopenia. In the laser-induced cremaster arteriole injury model, we looked at incorporation of infused WT platelets and platelets derived from infused FL cells into growing clots. In all settings, the platelets readily become incorporated into the developing thrombi. WT platelets and platelets derived from FL large cells were similarly incorporated (Figure 2, A and B). Platelets derived from FL small cells incorporated to a lesser extent, with a distinct population of CD41+ cells that recirculated and rarely incorporated into the clot (Figure 2, C and D, and Supplemental Video 1). This phenomenon was rarely seen after infusion of FL large cells (Figure 2B and Supplemental Video 2), and never with infused WT platelets (Figure 2A and Supplemental Video 3).
Figure 2. Platelet incorporation into arterial clots after laser injury.
(A–C) Representative images of platelets incorporating into clots after infusion of platelets or indicated cells. (A) Donor WT platelets detected using a labeled anti–mouse αIIb Ab. (B and C) Same as in A, but after infusion of FL cells. (D) Sequential stills from left to right noting a recirculating mouse αIIb+ cell (arrowheads) after small cell infusion. Scale bars: 30 μm. (E) Summation of donor platelets incorporated into growing thrombi after infusion of either WT platelets or FL cells. Twenty movies were evaluated per graph.
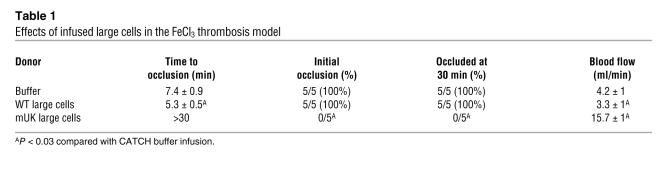
We also examined platelet function using an FeCl3 carotid artery injury model after infusing either CATCH buffer or FL large cells into the hαIIb+ recipient mice, which have a mild bleeding diathesis likely because of low CD41 surface density (12). Infusion of FL large cells significantly shortened time to development of stable occlusion (Table 1). We also infused FL large cells from mUK mice, which ectopically express and store urokinase in their α-granules (13) and are resistant to thrombosis in the FeCl3 carotid artery injury model. Transfusion of mUK platelets into WT mice blocked clot development (14). We reasoned that if infused mUK platelets from FL large cells are functional, they would interfere with clotting in the hαIIb+ recipient mice. Indeed, infused mUK large cells failed to form stable occlusions (Table 1).
Table 1 .
Effects of infused large cells in the FeCl3 thrombosis model
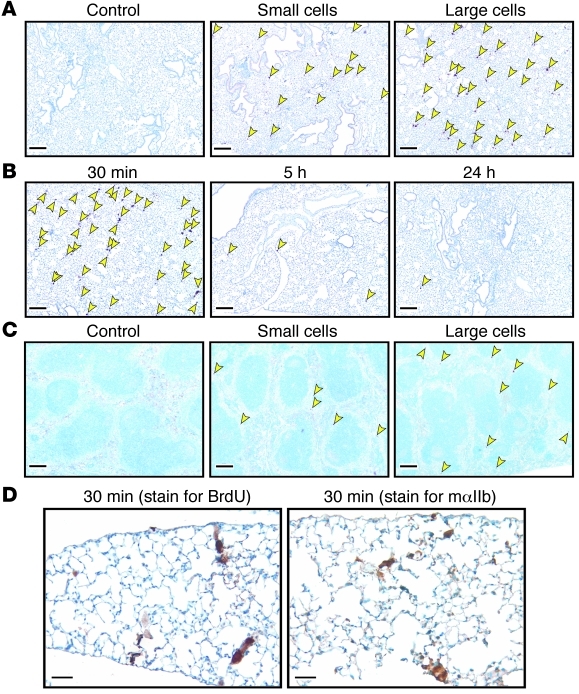
To begin to address where infused megakaryocytes shed platelets, we labeled megakaryocyte nuclei with BrdU. Various organs were isolated up to 36 hours after infusion. Virtually all of the BrdU-stained nuclei were in the lungs (Figure 3A). Stained nuclei were visible for up to 24 hours (Figure 3B and data not shown). A few nuclei were visible in the red pulp of the spleen (Figure 3C). None were present in the liver, heart, brain, or BM (data not shown). The BrdU-labeled large cells appear to retain their cytoplasm for up to 30 minutes after infusion in pulmonary microvessels (Figure 3, B and D), consistent with the delay in peak platelet count after infusion of FL large cells. To confirm this observation, we stained the tissues with an anti–murine CD41 Ab and detected positively stained cytoplasm up to 30 minutes (Figure 3D).
Figure 3. Organ distribution studies of infused cells.
(A) Staining of lung from hαIIb+ mice infused with saline or small or large cells grown in BrdU (arrows point to stained nuclei). (B) Kinetics of BrdU-labeled large cells in the lungs. (C) Same as in A but for spleen. Scale bars (A–C): 200 μm. (D) Large FL cells 30 minutes after infusion in lungs: BrdU-labeled nuclei (left) and mαIIb (right). Scale bars: 50 μm. Data are representative of 3 separate studies.
Our studies and prior histological studies (4) suggest that platelets can be released from circulating, mature, high-ploidy megakaryocytes directly within the vascular bed. Given the recent study showing megakaryocytes shedding large cytoplasmic fragments at the vascular niche in the marrow (3), we propose that both large cytoplasmic fragments and whole megakaryocytes may escape into the vascular system and shed platelets within the pulmonary bed using mechanisms consistent with those revealed by in vitro studies (2), but with concurrent flow and local vascular factors involved as well (15).
One would expect that megakaryocytes lodging in the lungs might pose a cardiovascular challenge. However, based on calculations shown in Supplemental Table 1, we only blocked 0.4%–1.7% of the entire alveolar capillary bed. In addition to the capacity of the pulmonary vasculature to handle an influx of megakaryocytes, another question is whether this bed is specialized for platelet release. Individuals with significant right to left cardiovascular shunts are known to have lower platelet counts, which others have proposed to result from pulmonary bypass by circulating megakaryocytes (16). However, in fetal circulation, where one naturally bypasses the pulmonary bed, platelets are present (17).
We have shown that derived platelets from infused megakaryocytes are normal in size and expression of surface receptors and can be incorporated into growing thrombi; however, why these platelets have a shorter half-life than infused platelets is unclear. We anticipated that newly generated platelets would have a prolonged half-life. Whether the shortened half-life is due to in vitro culture conditions is unknown. Previously, it had been suggested that ADAM17 is present in the media and shortens ex vivo generated platelet half-life (6). Our studies showed no change in derived platelet half-life after megakaryocytes were grown in the presence of two inhibitors of ADAM17, GM6001 and TAP-1 (Supplemental Figure 2, A and B). An alternative explanation for the shortened half-life is that most of our studies were performed with FL megakaryocytes, and fetal platelets have a shorter half-life compared with adult platelets (18). However, cultured infused adult BM megakaryocytes produced a similar delay in appearance and short half-life (Figure 1F). Whether culture conditions affects half-life could not be tested, as there are insufficient primary megakaryocytes in isolated marrow to test this possibility.
In vitro generation of a large number of biologically responsive platelets has remained problematic. Our studies open the possibility of establishing a human cell line that can be expanded and differentiated into megakaryocytes, leading to a non-donor-dependent source of platelets. Such a line exists from mice: G1ME cells, which are derived from GATA-1–deficient embryonic stem cells and form approximately 50% megakaryocytes after reexpression of GATA-1 (19). Modification of such lines to ectopically express a protein of interest during megakaryopoiesis might then be useful for targeted delivery of ectopic protein to sites of injury (20). This additional benefit of generating modified platelets in vivo from infused modified megakaryocytes is supported by studies showing efficacious, targeted urokinase delivery by platelets derived from mUK mouse megakaryocytes (Table 1).
In summary, we have addressed whether infused megakaryocytes can release platelets within the pulmonary bed and demonstrated that biologically active platelets can be formed in vivo with characteristics similar to those of normal platelets. The process is vigorous, and enough platelets are formed so that platelet count can be boosted and hemostasis can be improved and modified by targeted delivery of ectopic proteins. Based on our estimate of 100–200 platelets generated per infused megakaryocyte, the challenge is to generate 109 mature megakaryocytes to achieve a 10% rise in platelet count in an average 70-kg patient.
Methods
Characterization of the mice studied.
Donor cells and platelets were derived from C57BL/6 WT mice (The Jackson Laboratory) or mUK-transgenic mice, which ectopically express murine urokinase within megakaryocytes (14). Recipient mice were homozygously transgenic for hαIIb and null for the expression of platelet mouse αIIb (mαIIb–/–) (8), designated hαIIb+ mice, and expressed 20% of the level of CD41 seen on human platelets (12). All animal studies were done with approval of the Institutional Animal Utilization Committee at the Children’s Hospital of Philadelphia.
Isolation of platelets and megakaryocytes ex vivo.
FL megakaryocytes were obtained from E14 FL cells homogenized and cultured as previously described (7). Adult BM cells were obtained from femurs and tibiae of C57BL/6 mice (21). Mature megakaryocytes were isolated using a 2-step density gradient (21). Washed platelets derived from the inferior vena cava of C57BL/6 mice in acid-citrate-dextrose were prepared as previously described (22). Platelet counts were determined using a HemaVet counter (Triad Associates). Platelets and/or megakaryocytes were infused into recipient mice retro-orbitally or by tail vein; the two approaches gave similar outcomes (Supplemental Figure 3).
Characterization of the megakaryocytes.
Megakaryocytes to proplatelet number was determined visually with a hemocytometer. DNA ploidy was assessed by flow cytometry after staining with propidium iodide using a FACScan (BD) as described previously (23).
Flow cytometric studies in infused hαIIb+ mice.
Retro-orbital blood samples from recipient mice were double stained with monoclonal FITC-conjugated mouse anti–human CD41 Ab (eBioscience) and monoclonal phycoerythrin-conjugated (PE-conjugated) rat anti–mouse CD41 Ab (BD Biosciences) for 30 minutes and analyzed by flow cytometry.
Activation of infused platelets was assessed by 3-color whole blood flow cytometry, using monoclonal mouse anti–human CD41 (PerCP-Cy5.5), PE–rat anti–mouse CD41, and monoclonal FITC–rat anti–mouse P-selectin Abs (all from BD Biosciences). To examine the relative expression of membrane receptors in recipient versus donor platelets, whole blood was stained with mouse anti–human CD41 (PerCP-Cy5.5), PE–rat anti–mouse CD41, and either monoclonal FITC-labeled rat anti–mouse GPIbα or rat anti–mouse GPIX Ab (Emfret Analytics).
Infusion studies in thrombocytopenic hαIIb+ mice.
Mice were subjected to a high dose of irradiation (1,000 centigrays total; two sessions, 24 hours apart). Platelet counts were initially monitored daily to determine a temporal platelet profile. Animals included in these studies had platelet counts between 1 × 108 to 2 × 108/ml on day 7 after irradiation and immediately before infusion of the cells in 200 μl CATCH buffer. Additional counts were done at 4, 24, and 48 hours after infusion.
Cremaster laser injury functional studies.
One hour after cell infusion, recipient hαIIb+ male mice were studied in the cremaster laser injury model (24). Anti–mouse CD41 Fab fragments labeled with Alexa Fluor 488 (Pierce Biotechnology) were injected intravenously 5 minutes before injury. Data were collected over a course of 2.5 minutes at 5 frames per second.
FeCl3 carotid artery injury functional studies.
FeCl3-induced arterial injury was induced in hαIIb+ mice 1 hour after infusion. Studies were done as previously described (20), but the 20% FeCl3 injury was for 3 minutes. Total flow was recorded for 30 minutes.
Infused megakaryocyte fate studies.
FL-derived cells were cultured for 5 days and then exposed to 10 μM BrdU (Sigma-Aldrich) for 48 hours prior to infusion. Following infusions, recipient mice were sacrificed and organs isolated, then fixed in formalin at predetermined time points. Detection of BrdU-labeled nuclei was performed with a rat polyclonal anti-BrdU Ab and a biotinylated rabbit polyclonal anti-rat IgG as the secondary Ab (Abcam), followed by a DAKO detection kit (EnVision). Megakaryocyte cytoplasm in the lungs was similarly studied using a goat polyclonal Ab against murine αIIb (Santa Cruz Biotechnology Inc.) and a secondary biotinylated rabbit polyclonal anti-goat IgG Ab (Abcam).
Statistics.
Differences between groups were compared using 2-tailed Student’s t test. Statistical analyses were performed using Microsoft Excel. Differences were considered significant when P values were 0.05 or less.
Supplementary Material
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute grants T32HL007971 (to R. Fuentes) and U01HL99656 (to M. Poncz).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2010;120(11):3917–3922. doi:10.1172/JCI43326.
References
- 1.Sullivan MT, Cotten R, Read EJ, Wallace EL. Blood collection and transfusion in the United States in 2001. Transfusion. 2007;47(3):385–394. doi: 10.1111/j.1537-2995.2007.01128.x. [DOI] [PubMed] [Google Scholar]
- 2.Italiano JE, Jr, Lecine P, Shivdasani RA, Hartwig JH. Blood platelets are assembled principally at the ends of proplatelet processes produced by differentiated megakaryocytes. J Cell Biol. 1999;147(6):1299–1312. doi: 10.1083/jcb.147.6.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Junt T, et al. Dynamic visualization of thrombopoiesis within bone marrow. Science. 2007;317(5845):1767–1770. doi: 10.1126/science.1146304. [DOI] [PubMed] [Google Scholar]
- 4.Zucker-Franklin D, Philipp CS. Platelet production in the pulmonary capillary bed: new ultrastructural evidence for an old concept. Am J Pathol. 2000;157(1):69–74. doi: 10.1016/S0002-9440(10)64518-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi ES, Nichol JL, Hokom MM, Hornkohl AC, Hunt P. Platelets generated in vitro from proplatelet-displaying human megakaryocytes are functional. Blood. 1995;85(2):402–413. [PubMed] [Google Scholar]
- 6.Nishikii H, et al. Metalloproteinase regulation improves in vitro generation of efficacious platelets from mouse embryonic stem cells. J Exp Med. 2008;205(8):1917–1927. doi: 10.1084/jem.20071482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lecine P, Blank V, Shivdasani R. Characterization of the hematopoietic transcription factor NF-E2 in primary murine megakaryocytes. J Biol Chem. 1998;273(13):7572–7578. doi: 10.1074/jbc.273.13.7572. [DOI] [PubMed] [Google Scholar]
- 8.Thornton MA, Zhang C, Kowalska MA, Poncz M. Identification of distal regulatory regions in the human alpha IIb gene locus necessary for consistent, high-level megakaryocyte expression. Blood. 2002;100(10):3588–3596. doi: 10.1182/blood-2002-05-1307. [DOI] [PubMed] [Google Scholar]
- 9.Hsu-Lin S, Berman CL, Furie BC, August D, Furie B. A platelet membrane protein expressed during platelet activation and secretion. Studies using a monoclonal antibody specific for thrombin-activated platelets. J Biol Chem. 1984;259(14):9121–9126. [PubMed] [Google Scholar]
- 10.Bergmeier W, et al. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates GPIbalpha shedding from platelets in vitro and in vivo. Circ Res. 2004;95(7):677–683. doi: 10.1161/01.RES.0000143899.73453.11. [DOI] [PubMed] [Google Scholar]
- 11.Bergmeier W, et al. Metalloproteinase inhibitors improve the recovery and hemostatic function of in vitro-aged or -injured mouse platelets. Blood. 2003;102(12):4229–4235. doi: 10.1182/blood-2003-04-1305. [DOI] [PubMed] [Google Scholar]
- 12.Wagner CL, Mascelli MA, Neblock DS, Weisman HF, Coller BS, Jordan RE. Analysis of GPIIb/IIIa receptor number by quantification of 7E3 binding to human platelets. Blood. 1996;88(3):907–914. [PubMed] [Google Scholar]
- 13.Kahr WH, et al. Platelets from patients with the Quebec platelet disorder contain and secrete abnormal amounts of urokinase-type plasminogen activator. Blood. 2001;98(2):257–265. doi: 10.1182/blood.V98.2.257. [DOI] [PubMed] [Google Scholar]
- 14.Kufrin D, et al. Antithrombotic thrombocytes: ectopic expression of urokinase-type plasminogen activator in platelets. Blood. 2003;102(3):926–933. doi: 10.1182/blood-2003-01-0054. [DOI] [PubMed] [Google Scholar]
- 15.Dunois-Larde C, Capron C, Fichelson S, Bauer T, Cramer-Borde E, Baruch D. Exposure of human megakaryocytes to high shear rates accelerates platelet production. Blood. 2009;114(9):1875–1883. doi: 10.1182/blood-2009-03-209205. [DOI] [PubMed] [Google Scholar]
- 16.Lill MC, Perloff JK, Child JS. Pathogenesis of thrombocytopenia in cyanotic congenital heart disease. Am J Cardiol. 2006;98(2):254–258. doi: 10.1016/j.amjcard.2006.01.083. [DOI] [PubMed] [Google Scholar]
- 17.Van den Hof MC, Nicolaides KH. Platelet count in normal, small, and anemic fetuses. Am J Obstet Gynecol. 1990;162(3):735–739. doi: 10.1016/0002-9378(90)90997-l. [DOI] [PubMed] [Google Scholar]
- 18.Jilma-Stohlawetz P, et al. High levels of reticulated platelets and thrombopoietin characterize fetal thrombopoiesis. Br J Haematol. 2001;112(2):466–468. doi: 10.1046/j.1365-2141.2001.02524.x. [DOI] [PubMed] [Google Scholar]
- 19.Stachura DL, Chou ST, Weiss MJ. Early block to erythromegakaryocytic development conferred by loss of transcription factor GATA-1. Blood. 2006;107(1):87–97. doi: 10.1182/blood-2005-07-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yarovoi HV, et al. Factor VIII ectopically expressed in platelets: efficacy in hemophilia A treatment. Blood. 2003;102(12):4006–4013. doi: 10.1182/blood-2003-05-1519. [DOI] [PubMed] [Google Scholar]
- 21.Shivdasani RA, Schulze H. Culture, expansion, and differentiation of murine megakaryocytes. Curr Protoc Immunol. 2005;Chapter 22:Unit 22F.6. doi: 10.1002/0471142735.im22f06s67. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C, et al. Localization of distal regulatory domains in the megakaryocyte-specific platelet basic protein/platelet factor 4 gene locus. Blood. 2001;98(3):610–617. doi: 10.1182/blood.V98.3.610. [DOI] [PubMed] [Google Scholar]
- 23.Kanaji T, Russell S, Cunningham J, Izuhara K, Fox JE, Ware J. Megakaryocyte proliferation and ploidy regulated by the cytoplasmic tail of glycoprotein Ibalpha. Blood. 2004;104(10):3161–3168. doi: 10.1182/blood-2004-03-0893. [DOI] [PubMed] [Google Scholar]
- 24.Neyman M, Gewirtz J, Poncz M. Analysis of the spatial and temporal characteristics of platelet-delivered factor VIII-based clots. Blood. 2008;112(4):1101–1108. doi: 10.1182/blood-2008-04-152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.