Abstract
Pemphigus is a life-threatening autoimmune disease in which antibodies specific for desmogleins (Dsgs) cause loss of keratinocyte cell adhesion and blisters. In order to understand how antibodies cause pathogenicity and whether there are commonalities among antibodies in different patients that could ultimately be used to target specific therapy against these antibodies, we characterized Dsg-specific mAbs cloned by phage display from 3 patients with pemphigus vulgaris and 2 with pemphigus foliaceus. Variable heavy chain gene usage was restricted, but similar genes were used for both pathogenic and nonpathogenic mAbs. However, the heavy chain complementarity-determining region 3 (H-CDR3) of most pathogenic, but not nonpathogenic, mAbs shared an amino acid consensus sequence. Randomization of the H-CDR3 and site-directed mutagenesis indicated that changes in this sequence could block pathogenicity but not necessarily binding. In addition, for 2 antibodies with longer H-CDR3s, a tryptophan was critical for pathogenicity but not binding, a result that is consistent with blocking the tryptophan acceptor site that is thought to be necessary for Dsg-mediated adhesion. These studies indicate that H-CDR3 is critical for pathogenicity of a human autoantibody, that a small region (even 1 amino acid) can mediate pathogenicity, and that pathogenicity can be uncoupled from binding in these antibodies.
Introduction
Pemphigus is a tissue-specific autoimmune disease in which autoantibodies against the keratinocyte cell surface cause skin blisters due to loss of cell-cell adhesion (1). These autoantibodies are directed against desmogleins (Dsgs), which are cell adhesion molecules in the desmosome, which is itself a cell adhesion structure. There are 2 major types of pemphigus, pemphigus vulgaris (PV) and pemphigus foliaceus (PF). In PV, blisters occur because keratinocytes lose cell-cell adhesion just above the basal layer, whereas in PF they lose adhesion in the superficial epidermis. Patients with PV usually have blisters and erosions in mucous membranes, without or with skin involvement. In the former presentation, they have autoantibodies against Dsg3, and with skin involvement, they have additional antibodies against Dsg1. Patients with PF have autoantibodies binding only to Dsg1 and present with scaly and crusted superficial erosions of the skin but do not have mucous membrane involvement. The tissue localization of lesions and the level at which they occur in the epithelium are explained by the distribution of Dsgs in skin and mucous membranes (2).
Autoantibodies in PV and PF have been shown to be directly pathogenic, that is, they cause loss of cell adhesion directly without requiring other inflammatory mediators (3). For example, polyclonal IgGs from PV and PF patients have been shown to be pathogenic in organ culture of normal human skin (4, 5). In addition, the Fab′ fragment of pemphigus IgG as well as single-chain variable fragments (scFv) of pemphigus IgG, both of which lack the effector constant region, can induce typical PF or PV blisters by passive transfer to neonatal mouse and human skin organ culture (6–9).
Current therapies for pemphigus are relatively nonspecific, that is, they are not targeted just to the pathogenic antibodies and do not inhibit the production of only those particular antibodies. Rather, current treatments (e.g., prednisone, azathioprine, mycophenolate mofetil) are aimed mainly at generally suppressing the immune system, with all its attendant potential complications (10). Recent case reports that rituximab, an anti-CD20 monoclonal antibody, is effective in treating pemphigus patients demonstrate that somewhat more targeted therapy is possible (11, 12). However, rituximab eliminates most B cells, not just pathogenic ones. Therefore, more targeted therapy is desirable to treat pemphigus without affecting the patients’ general and beneficial immunity.
To reach this goal at the level of the autoantibodies, we and others (13) have started to pursue studies to characterize at a molecular level the autoantibodies that impart pathogenicity in pemphigus. Genetic analysis of these antibodies may make possible the generation of antiidiotypic reagents that could be clinically useful (13).
To pursue this genetic characterization of pemphigus autoantibodies, we used antibody phage display to molecularly clone anti-Dsg mAbs from 3 PV and 2 PF patients (refs. 7–9 and our unpublished observations). In this report, we analyze the variable heavy chain gene (VH) usage and heavy chain complementarity-determining region 3 (H-CDR3) regions of these cloned mAbs to establish whether there are commonalities among pathogenic pemphigus antibodies. We focused on the heavy chain of the pathogenic antibodies because previous studies have shown that pathogenicity and binding of pemphigus antibodies correlates with heavy chain, rather than light chain, gene usage (7, 8, 13). We focused on the H-CDR3 because, in most antibodies, it is thought to provide the most important variable sequences for antibody specificity (14–16). Our findings suggest that there are specific “hot spots” in areas of homologous amino acid sequences in the H-CDR3s of pathogenic pemphigus antibodies that are necessary for their pathogenicity and that mutations in these areas can uncouple pathogenicity and binding.
Results
Pathogenicity of pemphigus antibodies is not restricted by VH gene usage.
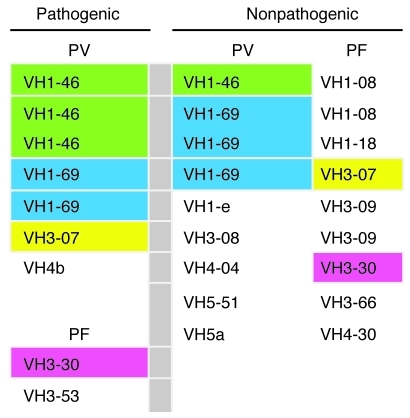
We have cloned a series of mAbs against Dsg3 and Dsg1 in the form of scFv from 3 PV and 2 PF patients (refs. 7–9 and our unpublished observations). We tested these antibodies for pathogenicity by injection into human skin organ culture and passive transfer to neonatal mice (7, 9) and by doing so divided them into pathogenic and nonpathogenic antibodies. In all cases, nonpathogenic antibodies bound the keratinocyte cell surface (or in 1 set of exceptional antibodies bound the precursor of Dsg1 inside the cell; ref. 9) as detected by direct immunofluorescence, even though they did not cause gross or histological blisters. Although the VH gene usage for pathogenic antibodies was restricted, 6 different VH genes were used from VH families 1, 3, and 4 (Figure 1). Interestingly, VH genes that were used for pathogenic antibodies were used, in most cases, for nonpathogenic antibodies as well (Figure 1). We conclude that pathogenic antibodies are not restricted to a specific VH gene usage. These data imply that targeting of conserved regions on specific VH genes might not be feasible for treating pemphigus.
Figure 1. VH gene usage of pathogenic and nonpathogenic anti-Dsg mAbs from PV and PF patients.
Colors indicate which genes are shared by pathogenic and nonpathogenic antibodies.
Shared amino acid sequences in H-CDR3s of pathogenic pemphigus mAbs.
We have previously found that epitopes defined by pathogenic mAbs cloned from a PV and a PF patient are shared by sera from various PV and PF patients, respectively (7, 8). These data suggest that pathogenic antibodies bind similar sites on Dsg and might, therefore, share common idiotypes. To investigate whether these shared idiotypes might be associated with homologous amino acid sequences in various pathogenic antibodies, we sequenced the H-CDR3 regions of the mAbs. We focused on the H-CDR3 because it is thought to provide the most important variable sequences for antibody specificity (14–16).
The H-CDR3 of pathogenic PV and PF mAbs could be sorted into short (7–9 amino acids; Figure 2) and long (10–14 amino acids; Figure 2). In 6 of 9 pathogenic clones, we found a consensus amino acid motif of D/E-X-X-X-W, in which an acidic amino acid (aspartic acid [D] or glutamic acid [E]) was arranged 4 amino acids upstream from a tryptophan (W) (Figure 2). All 21 nonpathogenic anti-Dsg1 or -Dsg3 mAbs cloned from previously published studies of 1 PV and 2 PF patients did not contain this H-CDR3 consensus motif. Furthermore, AK23, a pathogenic anti-Dsg3 mAb isolated by standard mouse hybridoma technology from a mouse with active pemphigus induced by transfer of Dsg3–/– lymphocytes, also had this motif (17, 18). In the clones with the short CDR3, the W was at the end of H-CDR3 in the beginning of framework region 4 (FR4). In the clones with longer H-CDR3, the W of the consensus motif was in the center of H-CDR3. Finally, in the 2 longer H-CDR3s without the consensus motif, there was a nonframework W in the H-CDR3 (Figure 2), which was found to be present in H-CDR3 randomization as well (see below).
Figure 2. H-CDR3 amino acid sequences from cloned pathogenic mAbs from 3 PV and 2 PF patients.
Bold D, E, and W show D/E-x-x-x-W consensus sequence. YYCAR/S and YYCVR indicate end of VH framework 3. WGQ is beginning of framework 4. AK23 is a mouse monoclonal anti-Dsg3 pathogenic mAb. Common names of some mAbs are in parentheses. Nonbolded orange amino acids are homologous in different antibodies.
Randomization of the long H-CDR3 region demonstrates the importance of W for pathogenicity.
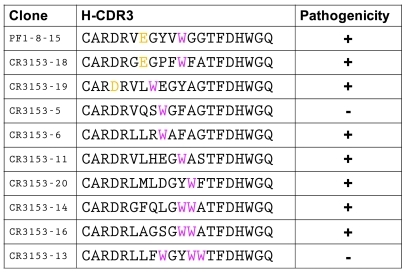
To determine whether the H-CDR3 sequence has to be unique for each mAb to have binding activity and/or pathogenicity or whether we could find additional specific sequences that imparted pathogenicity for a particular mAb, we randomized the H-CDR3 sequence of PF 1-8-15, a pathogenic anti-Dsg1 mAb with a long CDR3 containing the consensus motif (Figure 2). We designed H-CDR3–randomizing PCR primers so that the 9 center amino acids in the H-CDR3 would be randomized. We obtained 9 clones of Dsg1-binding mAbs derived from PF1-8-15 with H-CDR3 randomization after selection for Dsg1-binding activity (Figure 3). Nucleotide sequencing showed that these randomized clones had the same variable heavy chain sequence (VH) as PF1-8-15 except for their H-CDR3.
Figure 3. H-CDR3 sequences of PF1-8-15 pathogenic anti-Dsg1 mAb and the same mAb with randomized H-CDR3s cloned by binding to Dsg1.
Note that all mAbs have at least one W in the CDR3 (magenta). The original PF1-8-15 and 2 randomized antibodies return the consensus D/E-x-x-x-W sequence.
We characterized these H-CDR3–randomized mAbs by Dsg ELISA, indirect immunofluorescence (IIF), and injection of normal human skin in organ culture. All of these 9 mAbs had specific binding to Dsg1 on ELISA and showed cell-surface staining of the epidermis on IIF at titers similar to that of the original PF1-8-15 mAb (data not shown). Injection into normal human skin organ culture showed that, of these 9 clones, 7 were pathogenic, and 2, although they bound Dsg1, were nonpathogenic (Figures 3 and 4). These results show that a given H-CDR3 that imparts pathogenicity to an anti-Dsg1 mAb is not necessarily unique for any given mAb. The results also show that antibody binding to Dsg1 can be uncoupled from pathogenicity. Finally, although 2 pathogenic randomized clones had the consensus motif D/E-x-x-x-W, others did not, showing that this consensus motif is not necessary for pathogenicity. However, all randomized clones had at least 1 W in the H-CDR3, compared with only 9 out of 18 unselected randomized clones (P = 0.012), suggesting the importance of W for mAbs that bind to Dsg1 and those that cause pathogenicity. The W in these randomized clones that bind Dsg1 may be necessary (e.g., see Table 1), but is not alone sufficient (see CR3153-5 and CR3153-13), for pathogenicity. It is thought that in desmosomal cadherins, such as Dsgs, W is critical in trans-binding and thus antibodies that interfere with binding may make use of a W in blocking it (see Discussion).
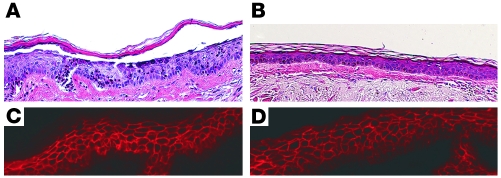
Figure 4. Normal human skin injection with 2 antibodies derived from PF1-8-15 with randomized H-CDR3s.
CR3153-6 (A and C) causes a superficial epidermal blister typical of PF (A, histology, H&E staining) and binds to the cell surface of epidermal cells (C, immunofluorescence), whereas CR3153-5 does not cause a blister (B) but still binds to the epidermis (D). Injections of human skin with randomized antibodies were done in duplicate with the same results. Original magnification, ×20.
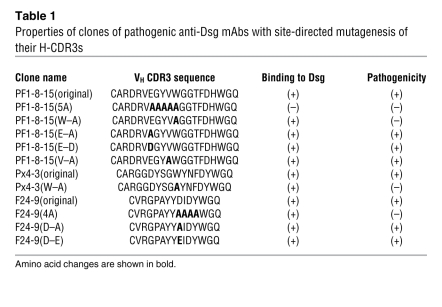
Table 1 .
Properties of clones of pathogenic anti-Dsg mAbs with site-directed mutagenesis of their H-CDR3s
Mutations of H-CDR3 W and consensus sequences cause loss of pathogenicity.
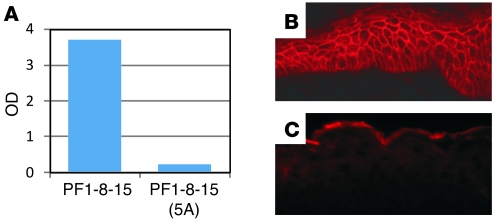
To confirm the importance of W in the H-CDR3 of the pathogenic mAb PF1-8-15, we used site-directed mutagenesis to change it to an A (alanine) (clone called PF1-8-15[W–A]) (Table 1). This change inhibited its pathogenicity but not its binding (Figure 5), confirming the importance of W for pathogenicity. However, point mutations in codons for other amino acids in the E-x-x-x-W consensus sequence (E to D, E to A, V to A) did not inhibit binding or pathogenicity (Figure 5 and Table 1). We noted that replacement of the W (PF1-8-15[W–A]), the E (PF1-8-15[E–D]), or the V (PF1-8-15[V–A]) all resulted in slight decreases of IIF titer to 1:40,000 (Table 2), which is still very high for these assays, but only the PF1-8-15(W–A) lost pathogenicity at this titer; therefore, we do not think loss of pathogenicity was a titer effect. When the entire consensus sequence was replaced by As (PF1-8-15[5A]), binding was completely inhibited (Figure 6). These findings suggest that the W in the H-CDR3 is critical for pathogenicity but not binding, in agreement with the randomization studies above. In PF1-8-15, specific amino acids other than W in the region of the full consensus sequence are necessary for binding.
Figure 5. Normal human skin injection of anti-Dsg mAbs.
Histology (H&E) was used to detect pathogenicity (A, C, E, G, I, K, and M) and immunofluorescence to detect mAb binding to the epidermis (B, D, F, H, J, L, and N). The following mAbs were used: PF1-8-15(W–A) (A and B); PF1-8-15(E–D) (C and D); PF1-8-15(E–A) (E and F); PF1-8-15(V–A) (G and H); F24-9(4A) (I and J); F24-9(D–E) (K and L); F24-9(D–A) (M and N). All mAbs bind to the cell surface of epidermal cells and all are pathogenic except PF1-8-15(W–A) and F24-9(4A). Injection of site-directed mutagenesis scFv into normal human skin was repeated 3 times with the same results. Original magnification, ×20.
Table 2 .
IIF titer on normal human skin of PF1-8-15, Px4-3, and F24-9 anti-Dsg1 antibody and their mutants
Figure 6. Characterization of PF1-8-15 anti-Dsg1 antibody and its mutant, PF1-8-15(5A).
ELISA (A) and IIF (C) show the mutant loses its ability to bind Dsg1 but that the parent mAb binds in both ELISA and IIF (B). Original magnification, ×40.
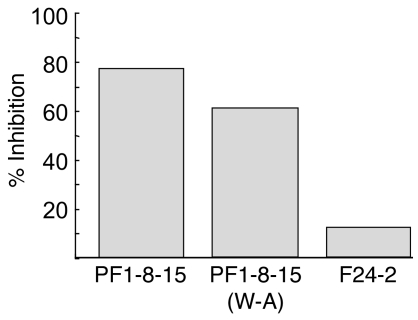
Rather unexpectedly, these data also show that pathogenicity can be uncoupled from binding. When tested by IIF on normal human skin, the pathogenic and nonpathogenic PF1-8-15 antibodies showed high titers (Table 2). This observation again suggests the importance of W for pathogenicity, but not necessarily binding. Finally, although the PF 1-8-15(W–A) is no longer pathogenic, it blocks binding to Dsg1 of the original PF 1-8-15 (Figure 7), suggesting it still binds the same epitope.
Figure 7. PF 1-8-15(W–A) mutant blocks binding of original PF 1-8-15 to Dsg 1 on ELISA.
Dsg1 ELISA plate was incubated first with the scFv indicated below bars, then with phage expressing PF 1-8-15. ELISA was developed with anti-M13 (phage) antibody. F24-2 is a nonpathogenic anti-Dsg 1 antibody.
To determine whether the W in Px4-3, the other pathogenic pemphigus mAb with a long H-CDR3, was also critical for binding or pathogenicity we mutated it to an A (Table 1). In this case, similar to that of PF1-8-15, the mAb lost pathogenicity but not its ability to bind Dsg. However, unlike with PF1-8-15, its IIF titer was markedly decreased (Table 2). These data confirm the importance of the W in these H-CDR3s for pathogenicity.
We tried to mutate the W of the pathogenic anti-Dsg mAbs with the short H-CDR3. However, probably because that W is in the beginning of FR4 of the variable heavy chain, we could not mutate it and produce a stable antibody. Therefore, to investigate whether the consensus sequence (D-x-x-x-W) in the short H-CDR3 was necessary for pathogenicity, we mutated the D in F24-9 to an E or an A (Table 1). In both cases, the antibody maintained its pathogenicity (Table 2 and Figure 5). This result is consistent with that found in PF1-8-15. However, if the entire consensus sequence upstream of the W was mutated with 4 As, then the antibody lost its pathogenicity but, again, not its ability to bind to Dsg, although its IIF titer decreased markedly (Figure 5 and Table 2).
Taken together, these data indicate the importance of the amino acids in a short stretch of the H-CDR3, defined by some homology among various antibodies, for pathogenicity and binding.
Correct context of H-CDR3 in VH is necessary for pathogenicity.
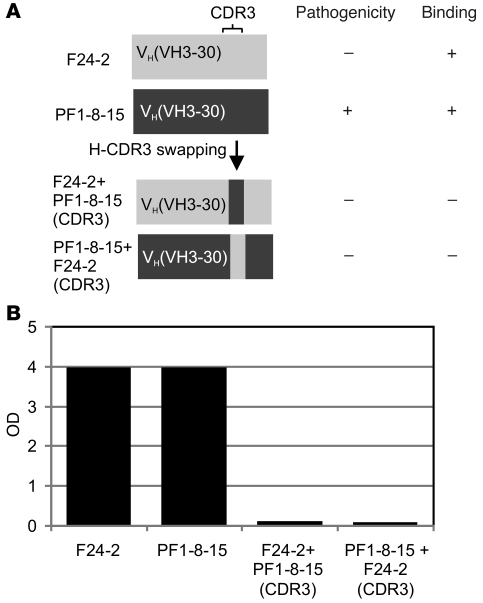
To determine whether H-CDR3 is sufficient for pathogenicity in a particular VH gene, we swapped H-CDR3s between 2 VH3-30 anti-Dsg1 antibodies, 1 pathogenic (PF1-8-15) and 1 nonpathogenic (F24-2) (Figure 8). Both mAbs lost their ability to bind Dsg1 after the swap, showing that the H-CDR3 sequence alone, even in the context of the same VH gene, cannot mediate binding.
Figure 8. Correct context of H-CDR3 in VH is necessary for binding.
Schematic diagram of H-CDR3 swapping between F24-2, a nonpathogenic anti-Dsg1 mAb, and PF1-8-15, a pathogenic mAb (A). ELISA shows that such swapping results in loss of binding to Dsg1 of both mAbs (B).
Discussion
Patients with PV and PF develop autoantibodies that bind to Dsg3 and Dsg1, respectively, and cause loss of keratinocyte cell adhesion (1). Because these antibodies bind near presumed Dsg adhesion sites (19), Dsgs are in the cadherin cell adhesion family (20–22), Dsgs are in cell adhesion structures (20, 22), and pathogenicity does not require the effector region of IgG and does not require a bivalent antibody (i.e., monovalent Fab′ or scFv antibody fragments are pathogenic) (6–9), it is thought that pathogenicity is, at least in part, due to direct antibody interference with Dsg adhesion. In addition, individual mAbs cloned by phage display from PV and PF patients define epitopes on Dsgs that are shared by IgG from various pemphigus patients (7, 8). All these observations show that pathogenic autoantibodies from pemphigus patients bind to closely related functional epitopes on 2 homologous molecules and therefore, may share molecular similarities. In this study, we searched for these similarities among mAbs cloned by antibody phage display from 3 PV and 2 PF patients.
Although these cloned antibodies bound Dsg1, Dsg3, or both, some were pathogenic (i.e., they could cause blisters typical of pemphigus in organ culture of normal skin or by passive transfer to neonatal mice) and some were nonpathogenic. First, we determined whether pathogenicity strictly correlated with VH gene usage. We noted that such usage was restricted to a few VH genes and that pathogenic and nonpathogenic anti-Dsg antibodies used similar genes. A recent study using the heterohybridoma technique to clone anti-Dsg3 and anti-Dsg1 mAbs from PV also showed VH restriction, although whether clonal mAbs were pathogenic or nonpathogenic antibodies was not determined (13, 23). Some of these antibodies also used VH1-69 and VH3-30, as found in our clones (Figure 1). Furthermore, as in our previous studies (7–9), the VH gene usage of the heterohybridoma clones was rather restricted, especially of the IgG mAb clones, and the VL gene usage was much more promiscuous. A recent study of heterohybridoma cloning of anti-Dsg mAbs from PF patients (23) also showed somewhat restricted VH usage (especially in IgG mAbs) and usage of VH3-07, VH1-46, VH1-69, and VH3-30 genes used in our cloned mAbs. However, pathogenicity of antibodies could not be determined.
Next, we sought similarities in the H-CDR3s of the pathogenic compared with the nonpathogenic mAbs. We found a consensus sequence of D/E-x-x-x-W in 6 of 9 pathogenic antibodies and, in addition, a pathogenic mouse anti-Dsg3 mAb. Finding a similar sequence in pathogenic anti-Dsg3 and anti-Dsg1 mAbs may at first seem unexpected. However, Dsg1 and Dsg3 are very similar at the DNA and protein levels and, as desmosomal cadherins, presumably use similar adhesion sites. Therefore, antibodies that block that adhesion might also share pathogenic sequences. Furthermore, it is interesting to note that in heterohybridoma cloning of mAbs from PV and PF antibodies, many mAbs bound both Dsg3 and Dsg1 (13, 23), again showing that the same antibody sequences can bind both Dsgs.
Site-directed mutagenesis, replacing the 4 amino acids upstream of the W in this consensus sequence, demonstrated its importance for pathogenicity and showed that pathogenicity could be uncoupled from binding, although binding was weaker, as indicated by decreased IIF titer. Similar results have recently been shown with neutralizing mAbs to HIV in which some H-CDR3 sequences can be used for binding and others for pathogenicity (24). In the case of these anti-HIV antibodies, some sequences in the H-CDR3 permit weak binding to the protein coat, allowing the antibody to be in place to bind an HIV conformational antigen when it appears transiently during internalization. By analogy, it may be that some sequences in pathogenic anti-Dsg mAbs cause binding (which could be weak) but enough to position the antibody so that other sequences can cause pathology.
Interestingly, although the consensus sequence showed an acidic amino acid (D or E) 4 amino acids upstream of the W, this acidic amino acid could be replaced with an A without the antibody losing pathogenicity, showing that the D/E in the consensus sequence is not necessary for pathogenicity. This result is consistent with the finding of 3 of our originally cloned pathogenic antibodies (Figure 1) and some of our pathogenic mAbs with randomized H-CDR3s (Figure 3) without a D or E 4 amino acids upstream of a W.
However, the W in the H-CDR3 may play a key role in pathogenicity, as indicated by randomization and site-directed mutagenesis of the 2 antibodies with a longer CDR3. When the W is mutated to an A, the antibodies still bind to Dsg but are not pathogenic. By analogy to classical cadherins, W at position 2 (W2) in Dsg is thought to be important for transadhesion because it fits into a hydrophobic acceptor pocket in the opposing cadherins (25–27). We speculate that W in the H-CDR3s of at least some of our pathogenic anti-Dsg mAbs might bind this W2 acceptor pocket, thus interfering with adhesion. Interestingly, epitope mapping shows that PF1-8-15 binds to amino acids 89–92 of Dsg1, where that pocket is located (our unpublished observations). Other amino acid residues presumably moderate binding to Dsg to permit the W to be in the correct position to block the acceptor pocket. Thus, when W is mutated, pathogenicity is lost, but not binding. It is interesting in this regard that 1 pathogenic antibody that we considered negative for the D/E-x-x-x-W consensus actually has it in the reverse direction (PV2, VH1-69). However, exactly whether, and how, these amino acid sequences might position the W will not be known until the crystal structures are solved.
Two recent papers analyzed the H-CDR3 of PF and PV antibodies isolated by heterohybridomas (13, 23). Of the H-CDR3s analyzed in IgG antibodies from PF patients, 2 of 23 had the consensus DFDYW (the W being in the framework) and 5 of 23 had a W inside the CDR3. Of the PV patients, 0 of 20 had the consensus sequence but 4 had internal Ws in the H-CDR3. In these studies, pathogenicity was not determined; therefore, a correlation between the consensus sequence and pathogenicity could not be evaluated.
There are several implications of our findings. Our data show that in a human tissue-specific autoantibody response, the H-CDR3 is critical in antibody functional and antigen specificity (16). That pathogenic mAbs to Dsgs from different pemphigus patients bind to similar epitopes with common adhesive functions and that they share some sequence homology in small linear regions of their H-CDR3 suggests that it might be possible to block pathogenic antibodies with agents (such as small molecules or peptides) interfering with these areas that we think of as “hot spots” of antibody pathogenicity. Mutation of single or a few amino acids in these hot spots prevents pathogenicity, suggesting that targeting therapy to these areas could decrease pathogenicity. Finally, the fact that pathogenicity may be uncoupled from binding suggests that such targeted therapy might be able to decrease pathogenicity even of bound antibodies.
Methods
Anti-Dsg mAbs cloned from phage libraries.
Using previously described phage display methods (7, 28), we isolated anti-Dsg mAbs from the peripheral blood of pemphigus patients (3 PV and 1 PF) with clinically active and typical clinical and histological disease. Recombinant phagemids were purified with a plasmid preparation system (QIAGEN), and the VH and VL inserts were sequenced using pComb3X-specific primers. The nucleotide sequences were compared with the germline sequences in the V Base sequence directory ( http://vbase.mrc-cpe.cam.ac.uk/) to determine their germline gene origins and interrelatedness. The Top10 F′ nonsuppressor strain of E. coli (Invitrogen) was infected with an individual phage clone, and soluble scFvs were purified from the bacterial periplasmic space using FastBreak (Promega) and Talon metal affinity resin (Clontech Laboratories Inc.) as previously described (9).
H-CDR3 randomization of anti-Dsg scFvs.
H-CDR3 randomization was performed as reported previously (29). cDNA for PF1-8-15 in the pComb3X vector was used as a template for the PCR. Two PCR reactions were performed. The mixture for reaction 1 consisted of 10 ng of template plasmid, primers corresponding from VL to before H-CDR3, and Taq polymerase in a final volume of 100 μl. Reaction 2 used a 5′ primer corresponding to the end of VH and then had random nucleotides in place of the CDR3 sequence (5′ end of VH sequence–NNSNNSNNSNNSNNSNNSNNSNNSNNS-3′) in which N is A, C, G, or T (equimolar) and S is G or C (equimolar). The 3′ primer of reaction 2 corresponded to the end of the VH framework. The PCR was performed by denaturing for 1 minute at 94°, annealing for 1 minute at 50°, and extending at 72° for 2 minutes for 35 cycles. The resulting PCR products were gel purified. Finally, in PCR mixture 3, 100 ng of product 1 from reaction 1 and 100 ng of product 2 from reaction 2 were added as templates for overlap extension, with the addition of primers corresponding to the beginning of VL and the end of VH in a 100 μl reaction mixture as described above. The resulting fragment was gel purified, digested with the restriction enzymes AvaI and SpeI, and gel purified again to yield product 3, cDNAs encoding scFvs with randomized H-CDR3s. The plasmid of PF1-8-15 constructed in the pComb3X vector was digested with AvaI and SpeI and gel purified. Ligation of product 3 with the prepared vector was performed at 16° overnight by the addition of 1400 ng of vector and 700 ng of product 3 with 20 units of T4 DNA ligase in a total volume of 400 μl. The DNA was precipitated and introduced by electroporation into E. coli XL1-Blue as described.
We selected monoclonal phage from the H-CDR3 randomized library by panning on ELISA plates coated with recombinant Dsg1 as previously described (9). We sequenced selected (after panning) and unselected (before panning) monoclonal phage using pComb3X-specific primers. Monoclonal scFvs were produced and purified from cloned phage as previously described (9).
Site-directed mutagenesis of scFv.
Site-directed mutagenesis was done with QuikChange II (Stratagene), following the manufacturer’s instructions.
H-CDR3 substitution of mAbs.
Two regions of cDNA encoding scFvs in pComb3X were amplified by PCR using specific primers of the corresponding sequences: (a) from the start of VL to just before H-CDR3 and (b) H-CDR3. H-CDR3 substitution was completed by overlapping PCR using the products of those fragments as templates. After confirming the nucleotide sequences, we cloned the products into pComb3X and transformed those plasmids into TOP10 competent cells to make mAbs in the form of scFvs.
Dsg1 scFv ELISA.
The reactivity of scFv against human Dsg1 was measured by Dsg1 ELISA (MBL) using HRP-conjugated anti-HA mAb (clone 3F10, 1:1000 dilution; Roche Diagnostics Corp.) as a secondary antibody as described (7, 8). To increase the ratio of mature form on standard Dsg1 ELISA plate, the plates were pretreated with 10 U/well of furin (New England Biolabs) in 20 mM Tris, 500 mM sodium chloride, pH 7.5 (TBS), with 1 mM CaCl2 at room temperature overnight.
Inhibition ELISAs were performed by first incubating Dsg1 ELISA plates with 0.5 μg of scFv, then adding phages expressing PF1-8-15 scFv and developing with anti–M13-horseradish peroxidase antibody (1:5000 dilution; GE Healthcare). We calculated inhibition with the following formula: % inhibition = (OD P – OD B)/OD P × 100, where P is the phage being tested without blocking and B is the phage blocked with the scFv.
Direct and IIF.
Immunofluorescence for scFvs at 1 mg/ml was performed on human skin and mouse tail as previously described (9). Binding was detected with rat monoclonal anti-HA antibody (3F10, 1:100 dilution, Roche Diagnostics) followed by Alexa Fluor 568–conjugated anti-rat IgG (1:200 dilution; Invitrogen).
Human skin organ culture injection of scFvs to test for pathogenicity.
Specimens were obtained from excess normal skin after excisional surgery. The specimens were trimmed by removing fat tissue and then cut into 5-mm diameter pieces. After intradermal injection of 50 μl of purified scFv (1 mg/ml) using an insulin syringe, skin specimens were put on the insert of Transwells (Corning) with defined keratinocyte SFM medium (Invitrogen) containing 1.2 mM CaCl2 in the outer compartment. At 24 hours, the skin was harvested for direct immunofluorescence and histology.
Animals and human subjects.
Approval for animal protocols used herein and for human subjects were obtained from appropriate review boards at the University of Pennsylvania.
Statistics.
All parameters were compared by Fisher’s exact test, as appropriate, with P < 0.05 considered significant.
Acknowledgments
This work was supported by grants from the National Institutes of Arthritis, Musculoskeletal, and Skin Diseases (R01-AR052672 and P30AR057217 to J.R. Stanley; K08-AR53505 to A.S. Payne).
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2010;120(11):4111–4117. doi:10.1172/JCI44425.
References
- 1.Stanley JR, Amagai M. Pemphigus, bullous impetigo, and staphylococcal scalded skin syndrome. N Engl J Med. 2006;355(17):1800–1810. doi: 10.1056/NEJMra061111. [DOI] [PubMed] [Google Scholar]
- 2.Mahoney MG, Wang Z, Rothenberger K, Koch PJ, Amagai M, Stanley JR. Explanation for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris. J Clin Invest. 1999;103(4):461–468. doi: 10.1172/JCI5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanley JR. Pemphigus and pemphigoid as paradigms of organ-specific, autoantibody-mediated diseases. J Clin Invest. 1989;83(5):1443–1448. doi: 10.1172/JCI114036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schiltz JR, Michel B. Production of epidermal acantholysis in normal human skin in vitro by the IgG fraction from pemphigus serum. J Invest Dermatol. 1976;67(2):254–260. doi: 10.1111/1523-1747.ep12513454. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto K, Shafran KM, Webber PS, Lazarus GS, Singer KH. Anti-cell surface pemphigus autoantibody stimulates plasminogen activator activity of human epidermal cells. J Exp Med. 1983;157(1):259–272. doi: 10.1084/jem.157.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rock B, Labib RS, Diaz LA. Monovalent Fab’ immunoglobulin fragments from endemic pemphigus foliaceus autoantibodies reproduce the human disease in neonatal Balb/c mice. J Clin Invest. 1990;85(1):296–299. doi: 10.1172/JCI114426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Payne AS, et al. Genetic and functional characterization of human pemphigus vulgaris monoclonal autoantibodies isolated by phage display. J Clin Invest. 2005;115(4):888–899. doi: 10.1172/JCI24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishii K, Lin C, Siegel DL, Stanley JR. Isolation of pathogenic monoclonal anti-desmoglein 1 human antibodies by phage display of pemphigus foliaceus autoantibodies. J Invest Dermatol. 2008;128(4):939–948. doi: 10.1038/sj.jid.5701132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamagami J, Kacir S, Ishii K, Payne AS, Siegel DL, Stanley JR. Antibodies to the desmoglein 1 precursor proprotein but not to the mature cell surface protein cloned from individuals without pemphigus. J Immunol. 2009;183(9):5615–5621. doi: 10.4049/jimmunol.0901691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stanley JR. Pemphigus. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, eds.Fitzpatrick’s Dermatology in General Medicine . New York, New York, USA: McGraw–Hill; 2008:459–468. [Google Scholar]
- 11.Joly P, et al. A single cycle of rituximab for the treatment of severe pemphigus. N Engl J Med. 2007;357(6):545–552. doi: 10.1056/NEJMoa067752. [DOI] [PubMed] [Google Scholar]
- 12.Mouquet H, et al. B-cell depletion immunotherapy in pemphigus: effects on cellular and humoral immune responses. J Invest Dermatol. 2008;128(12):2859–2869. doi: 10.1038/jid.2008.178. [DOI] [PubMed] [Google Scholar]
- 13.Qian Y, Diaz LA, Ye J, Clarke SH. Dissecting the anti-desmoglein autoreactive B cell repertoire in pemphigus vulgaris patients. J Immunol. 2007;178(9):5982–5990. doi: 10.4049/jimmunol.178.9.5982. [DOI] [PubMed] [Google Scholar]
- 14.Brooks CL, et al. The role of CDR H3 in antibody recognition of a synthetic analog of a lipopolysaccharide antigen. Glycobiology. 2010;20(2):138–147. doi: 10.1093/glycob/cwp150. [DOI] [PubMed] [Google Scholar]
- 15.Schelonka RL, et al. Preferential use of DH reading frame 2 alters B cell development and antigen-specific antibody production. J Immunol. 2008;181(12):8409–8415. doi: 10.4049/jimmunol.181.12.8409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu JL, Davis MM. Diversity in the CDR3 region of V(H) is sufficient for most antibody specificities. Immunity. 2000;13(1):37–45. doi: 10.1016/S1074-7613(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 17.Tsunoda K, et al. Induction of pemphigus phenotype by a mouse monoclonal antibody against the amino-terminal adhesive interface of desmoglein 3. J Immunol. 2003;170(4):2170–2178. doi: 10.4049/jimmunol.170.4.2170. [DOI] [PubMed] [Google Scholar]
- 18.Ota T, Aoki-Ota M, Tsunoda K, Nishikawa T, Koyasu S, Amagai M. Autoreactive B-cell elimination by pathogenic IgG specific for the same antigen: implications for peripheral tolerance. Int Immunol. 2008;20(10):1351–1360. doi: 10.1093/intimm/dxn095. [DOI] [PubMed] [Google Scholar]
- 19.Sekiguchi M, Futei Y, Fujii Y, Iwasaki T, Nishikawa T, Amagai M. Dominant autoimmune epitopes recognized by pemphigus antibodies map to the N-terminal adhesive region of desmogleins. J Immunol. 2001;167(9):5439–5448. doi: 10.4049/jimmunol.167.9.5439. [DOI] [PubMed] [Google Scholar]
- 20.Kottke MD, Delva E, Kowalczyk AP. The desmosome: cell science lessons from human diseases. J Cell Sci. 2006;119(pt 5):797–806. doi: 10.1242/jcs.02888. [DOI] [PubMed] [Google Scholar]
- 21.Yin T, Green KJ. Regulation of desmosome assembly and adhesion. Semin Cell Dev Biol. 2004;15(6):665–677. doi: 10.1016/j.semcdb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Waschke J. The desmosome and pemphigus. Histochem Cell Biol. 2008;130(1):21–54. doi: 10.1007/s00418-008-0420-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian Y, Clarke SH, Aoki V, Hans-Filhio G, Rivitti EA, Diaz LA. Antigen selection of anti-DSG1 autoantibodies during and before the onset of endemic pemphigus foliaceus. J Invest Dermatol. 2009;129(12):2823–2834. doi: 10.1038/jid.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alam SM, et al. Role of HIV membrane in neutralization by two broadly neutralizing antibodies. Proc Natl Acad Sci U S A. 2009;106(48):20234–20239. doi: 10.1073/pnas.0908713106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro L, et al. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374(6520):327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- 26.Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296(5571):1308–1313. doi: 10.1126/science.1071559. [DOI] [PubMed] [Google Scholar]
- 27.Nagar B, Overduin M, Ikura M, Rini JM. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 1996;380(6572):360–364. doi: 10.1038/380360a0. [DOI] [PubMed] [Google Scholar]
- 28. Barbas CFI, Burton DR, Scott JK, Silverman GJ.Phage Display: A Laboratory Manual . Cold Spring Harbor, New York, USA: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- 29.Barbas CF, III, Bain JD, Hoekstra DM, Lerner RA. Semisynthetic combinatorial antibody libraries: a chemical solution to the diversity problem. Proc Natl Acad Sci U S A. 1992;89(1):4457–4461. doi: 10.1073/pnas.89.10.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]