Abstract
Adaptation to hypoxia is an essential cellular response controlled by the oxygen-sensitive master transcription factor hypoxia-inducible factor 1 (HIF-1). HIF-1 expression is also controlled by specific microRNAs and, in turn, controls the expression of other microRNAs, which fine-tune adaptation to low oxygen tension. In this issue of the JCI, Ghosh and colleagues identify a unique microRNA in hypoxic endothelial cells, miR424, that promotes HIF-1 stabilization and angiogenesis. The actions of this microRNA are considered in the context of the complex interactions that act to ensure optimal endothelial adaptation to this critical environmental condition.
Adaptation to hypoxia is an essential homeostatic mechanism in mammalian cells. Hypoxia can occur not only as a global consequence of low atmospheric oxygen tension, but also locally at sites of inflammation, tissue ischemia and injury, and solid tumor growth. Fundamental mechanisms have, therefore, evolved by which cells adapt to hypoxic conditions and their potentially adverse consequences, both acutely and chronically, in health and disease.
Among the key responses to acute hypoxia is the upregulation of hypoxia-inducible factors (HIFs), master transcription factors that induce the highly conserved expression of many genes (likely more than 100) and are responsible for the majority of the hypoxic program in metazoan cells (1). The prototypical HIF is a heterodimer of α and β subunits. Under normoxic conditions, the α subunit is rapidly hydroxylated at specific prolyl residues by prolyl hydroxylase domain (PHD) proteins. Upon hydroxylation, the α subunit is recognized by the von Hippel–Lindau protein and consequently undergoes rapid proteasomal degradation. Under hypoxic conditions, however, the α subunit does not undergo prolyl hydroxylation and, as a result, forms a stable complex with the β subunit, which binds as a heterodimer to hypoxia response elements (HREs) in the promoter regions of hypoxia-sensitive genes to induce gene transcription. HIF-dependent transcriptional changes lead to a broad range of cellular adaptations, including metabolic, proliferative, apoptotic, and angiogenic. In higher metazoans, three different HIF-α proteins have been identified: HIF-1α and HIF-2α have some transcriptional targets in common and some that are unique to each subunit, while splice variants of HIF-3α have dominant negative effects on HIF-dependent gene transcription (2, 3).
MicroRNAs and transcriptional tuning
MicroRNA molecules (miRNAs) are essential, noncanonical RNA species of 18–23 nucleotides in length that regulate gene expression. There are believed to be more than 1,000 miRNA genes in the human genome, which are estimated to regulate more than one-third of all mRNA transcripts (4). As part of the RNA-induced silencing complex (RISC), miRNAs negatively regulate gene transcription by annealing to the 3′ untranslated region of specific mRNA targets to repress translation, enhance mRNA degradation, or both. In contrast to the promoter-based catalytic amplification of mRNA synthesis, miRNAs are viewed as weak modulators of the transcriptional response, fine-tuning gene expression largely by negative regulation in a process that requires stoichiometric binding to mRNA targets (5).
Hypoxamirs: microRNAs induced by hypoxia
Hypoxia has recently been shown to induce the expression of a number of miRNAs, which have been termed “hypoxamirs” (6). Owing to the central importance of HIFs to the hypoxic response, its potent regulation of many genes whose expression is regulated by oxygen tension, and the significant number of hypoxamirs, it is logical to explore the relationships among HIFs and hypoxamirs in order to understand better the complex regulation of the hypoxic program in metazoans.
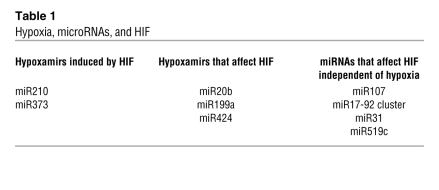
From the perspective of the miRNA response to hypoxia, miRNAs can be viewed as comprising three groups (Table 1): first, those induced by HIFs under hypoxic conditions (HIF-dependent hypoxamirs); second, those induced by hypoxia that, in turn, affect expression of HIF; third, those whose expression is not dependent on hypoxia but that affect HIF expression. The number of hypoxamirs that have been identified in each of these groups is expanding rapidly, illustrating the extraordinary regulatory complexity of the hypoxic program and the importance of considering that program in a holistic, system-based context. In this issue of the JCI, Ghosh and colleagues (7) identify a new hypoxamir, miR424, that affects HIF expression. This report is unique for three reasons: it is the first demonstration of the hypoxic induction of this microRNA, as a prior study showed that hypoxia suppressed its expression in trophoblasts (8); it is the first demonstration of a hypoxamir unique to the endothelial cell; and it enhances HIF stability in a novel way (see below).
Table 1 .
Hypoxia, microRNAs, and HIF
Among the HIF-dependent hypoxamirs are miR210 and miR373 (7, 8). While miR373 has not been studied in great detail (9), miR210 has been extensively studied in its role as a hypoxamir (8–13). The most robustly induced hypoxamir, miR210, is induced by HIF-1α (10) and suppresses expression of the cell-cycle regulator E2F transcription factor 3 (E2F3) (11), the receptor tyrosine kinase ligand ephrin A3 (12), and the DNA repair protein RAD52 (13). miR210 was recently also shown to have the important role of suppressing mitochondrial metabolism in hypoxic states by decreasing expression of the iron-sulfur cluster assembly proteins ISCU1/2, thereby limiting cytochrome assembly and ROS generation from inefficient mitochondrial electron transport under low oxygen tensions (14, 15). This observation adds to the other known effects of HIF-1 on mitochondrial metabolism. These include suppression of ROS generation under hypoxic conditions by switching expression of the regulatory subunit of cytochrome c oxidase from COX4 to COX2, which increases the efficiency of complex IV; induction of pyruvate dehydrogenase 1, which shunts pyruvate from the mitochondrion; and induction of BCL2/adenovirus E1B 19-kDa interacting protein 3 (BNIP3), which triggers mitophagy (16).
Three hypoxamirs have been shown to affect HIF expression: miR20b, miR199a, and, most recently, miR424. miR20b targets HIF-1α to suppress its expression (in MCF-7 breast cancer cells) (17), and downregulation of miR199a derepresses HIF-1α (in cardiomyocytes) (18). Ghosh and colleagues show that miR424 regulates HIF-α isoforms in endothelial cells by targeting cullin 2 (CUL2), the scaffolding protein on which the ubiquitin ligase system assembles, thereby stabilizing HIF-α isoforms by impairing their prolyl hydroxylation (7). miR424 itself is induced by hypoxia via PU.1 transactivation, levels of which are increased in hypoxic endothelial cells by runt-related transcription factor 1 (RUNX-1) and CCAAT/enhancer binding protein α (C/EBPα) (7).
In addition to these hypoxamir-mediated responses, at least four miRNAs have been shown to influence HIF expression independent of hypoxia. Induced by p53, miR107 decreases expression of HIF-β (19); induced by c-MYC, the miR17-92 cluster suppresses expression of HIF-1α (20); and suppressed by hepatocyte growth factor, miR519c suppresses expression of HIF-1α (21). In contrast, miR31, by decreasing expression of the HIF regulatory factor factor-inhibiting HIF (FIH), increases expression of HIF-1α (22).
Hypoxamir expression and cellular context
It is important to consider the cellular context within which hypoxamirs are expressed. The upregulation of miR210 is robust and has been observed in all hypoxic cell types in which it has been studied (14). In contrast, the upregulation of miR424 reported by Ghosh and colleagues (7) is unique to the endothelial cell. The reason for this distinction is not entirely clear, but may reflect the central importance of the endothelium at the interface of flowing blood and metabolizing tissue. Poised at this interface, the endothelium is uniquely situated to sense hypoxia upon blood flow reduction and to initiate the reparative angiogenic response required for its restoration. The endothelium has great metabolic plasticity, acquiring a significant fraction of its ATP needs from glycolysis; HIF-1α regulates this shift from oxidative phosphorylation to anaerobic glycolysis under hypoxic conditions and does so in conjunction with reducing mitochondrial number (23) and function (14). The induction of miR424 in endothelial cells prolongs the action of HIF-1α, which would be expected to promote further glycolytic metabolism and limit ROS-mediated cytotoxicity. In this way, miR424 is an important regulator of the durability of the hypoxic HIF program. In addition and importantly, miR424 is induced by another potent transcription factor, PU.1, levels of which increase in hypoxic endothelium (7). This transcription factor working in concert with miR424 has been shown to regulate monocyte differentiation (24); miR424 has also been shown to regulate monocyte differentiation by combinatorial interactions with miR155, miR222, and miR503 (25). Whether miR424 also regulates endothelial cell differentiation from precursor cells is an unanswered question that would add another dimension to the action of this pleiotropic miRNA and would clearly complement its indirect, HIF-1α–dependent effects on angiogenesis so essential for restoring tissue perfusion and oxygen delivery.
Conclusion
Clearly, the response to hypoxia is complex and driven by a network-based system with various levels of interaction and feedback. Hypoxamirs interacting with HIF-driven responses add a combinatorial richness to this system that defines the tissue context of the hypoxic program and provides a method for tuning the responses of transcription factors under the wide range of conditions that contribute to the genesis of hypoxic stress. The paper by Ghosh and colleagues (7) adds yet another dimension to this response by demonstrating the HIF-independent hypoxic induction of a hypoxamir unique to the endothelium, which prolongs HIF expression and thereby promotes the hypoxic induction of the angiogenic response required to restore tissue perfusion and attenuate tissue hypoxia (Figure 1). It is an elegant system of extraordinary complexity that is essential for homeostatic control under variable oxygen tensions.
Figure 1. The endothelial cell response to hypoxia.
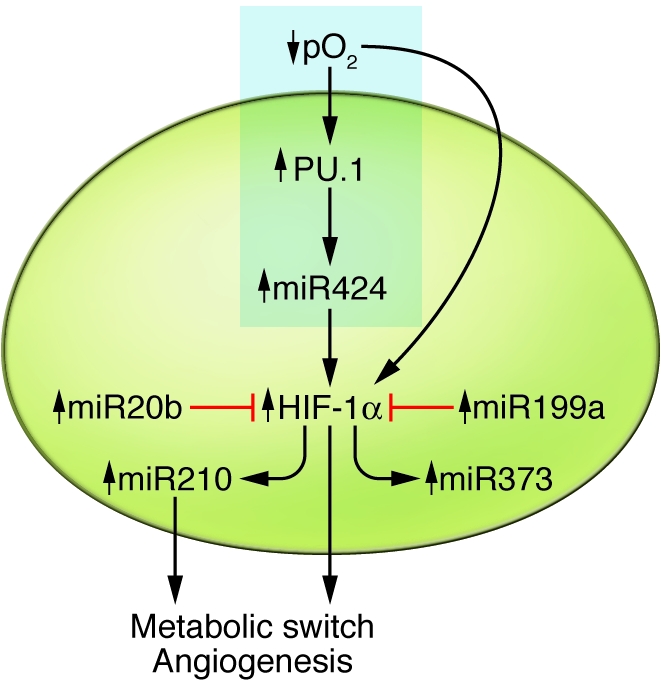
The endothelium is poised between flowing blood and metabolizing tissue. Under the hypoxic (ischemic) conditions accompanying reductions in blood flow, the endothelial response is governed by HIF, as well as hypoxamirs, which are themselves either induced by HIF or affect HIF expression and action. The net effect is a metabolic switch to glycolysis and endothelial proliferation and differentiation, actions that facilitate durable adaptation to hypoxia sufficient to promote angiogenesis and restore blood flow. The text highlighted in the blue rectangle indicates how the work of Ghosh and colleagues integrates with current understanding of hypoxamirs.
Acknowledgments
This work was supported in part by NIH grants HL61795, HL81587, HL70819, and HL48743. The author wishes to thank Stephanie Tribuna for expert technical assistance.
Footnotes
Conflict of interest: The author has declared that no conflict of interest exists.
Citation for this article: J Clin Invest. 2010;120(11):3815–3817. doi:10.1172/JCI45105.
See the related article beginning on page 4141.
References
- 1.Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. . Mol Cell. 2008;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Wang V, Davis DA, Haque M, Huang LE, Yarchoan R. Differential gene up-regulation by hypoxia-inducible factor-1alpha and hypoxia-inducible factor-2alpha in HEK293T cells. Cancer Res. 2005;65(8):3299–3306. doi: 10.1158/0008-5472.CAN-04-4130. [DOI] [PubMed] [Google Scholar]
- 3.Maynard M, Evans JA, Hosomi T, Hara S, Jewett MA, Ohh M. Human HIF-3alpha4 is a dominant-negative regulator of HIF-1 and is downregulated in renal cell carcinoma. FASEB J. 2005;19(11):1396–1406. doi: 10.1096/fj.05-3788com. [DOI] [PubMed] [Google Scholar]
- 4.Berezikov E, Furyev V, van de Belt J, Wienholds E, Plasterk RH, Cuppen E. Phylogenetic shadowing and computational identification of human microRNA genes. Cell. 2005;120(1):21–24. doi: 10.1016/j.cell.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 5.Sun W, Julie Li YS, Huang HD, Shyy JY, Chien S. microRNA: a master regulator of cellular processes for bioengineering systems. Annu Rev Biomed Eng. 2010;12:1–27. doi: 10.1146/annurev-bioeng-070909-105314. [DOI] [PubMed] [Google Scholar]
- 6.Chan SY, Loscalzo J. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle. 2010;9(6):1072–1083. doi: 10.4161/cc.9.6.11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosh G, et al. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-a isoforms and promotes angiogenesis. J Clin Invest. 2010;120(11):4141–4154. doi: 10.1172/JCI42980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mouillet JF, Chu T, Nelson DM, Mishima T, Sadovsky Y. MiR-205 silences MED1 in hypoxic primary chain trophoblasts. FASEB J. 2010;24(6):2030–2039. doi: 10.1096/fj.09-149724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crosby ME, Devlin CM, Glazer PM, Calin GA, Ivan M. Emerging roles of microRNAs in the molecular responses to hypoxia. Curr Pharm Des. 2009;15(33):3861–3866. doi: 10.2174/138161209789649367. [DOI] [PubMed] [Google Scholar]
- 10.Kulshreshtha R, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27(5):1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giannakakis A, et al. miR-210 links hypoxia with cell cycle regulation and is deleted in human epithelial ovarian cancer. Cancer Biol Ther. 2008;7(2):255–264. doi: 10.4161/cbt.7.2.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fasanaro P, et al. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. . J Biol Chem. 2008;283(23):15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crosby ME, Kulshreshtha R, Ivan M, Glazer PM. MicroRNA regulation of DNA repair gene expression in hypoxic stress. Cancer Res. 2009;69(3):1221–1229. doi: 10.1158/0008-5472.CAN-08-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10(4):273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Favaro E, et al. MicroRNA-210 regulates mitochondrial free radical response to hypoxia and krebs cycle in cancer cells by targeting iron-sulfur cluster protein ISCU. PLoS One. 2010;5(4):e10345. doi: 10.1371/journal.pone.0010345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semenza GL. Hypoxia-inducible factor 1: Regulator of mitochondrial metabolism and mediator of ischemic preconditioning [published online ahead of print August 21, 2010]. Biochim Biophys Acta. doi: 10.1016/j.bbamcr.2010.08.006. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cascio S, et al. miR20b modulates VEGF expression by targeting HIF-1alpha and STAT3 in MCF-7 breast cancer cells. J Cell Physiol. 2010;224(1):242–249. doi: 10.1002/jcp.22126. [DOI] [PubMed] [Google Scholar]
- 18.Rane S, et al. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxic preconditioning in cardiac myocytes. Circ Res. 2009;104(7):879–886. doi: 10.1161/CIRCRESAHA.108.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamakuchi M, et al. P-53-induced microRNA-107 inhibits HIF-1 and tumor angiogenesis. Proc Natl Acad Sci U S A. 2010;107(14):6334–6339. doi: 10.1073/pnas.0911082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taguchi A, et al. Identification of hypoxia-inducible factor-1alpha as a novel target for miR-17-92 microRNA cluster. Cancer Res. 2008;68(14):5540–5545. doi: 10.1158/0008-5472.CAN-07-6460. [DOI] [PubMed] [Google Scholar]
- 21.Cha ST, et al. MicroRNA-519c suppresses hypoxia-inducible factor-1alpha expression and tumor angiogenesis. Cancer Res. 2010;70(7):2675–2685. doi: 10.1158/0008-5472.CAN-09-2448. [DOI] [PubMed] [Google Scholar]
- 22.Liu CJ, et al. miR31 ablates expression of the HIF regulatory factor FIH to activate the HIF pathway in head and neck carcinoma. Cancer Res. 2010;70(4):1635–1644. doi: 10.1158/0008-5472.CAN-09-2291. [DOI] [PubMed] [Google Scholar]
- 23.Fijalkowska I, et al. Hypoxia-inducible-factor-1alpha regulates the metabolic shift of pulmonary hypertensive endothelial cells. Am J Path. 2010;176(3):1130–1138. doi: 10.2353/ajpath.2010.090832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosa A, et al. The interplay between the master transcription factor PU.1 and miR424 regulates human monocyte/macrophage differentiation. Proc Natl Acad Sci U S A. 2007;104(50):19849–19854. doi: 10.1073/pnas.0706963104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forrest AR, et al. Induction of microRNAs, miR-155, miR-222, miR-424, and miR-503, promotes monocytic differentiation through combinatorial regulation. . Leukemia. 2010;24(2):460–466. doi: 10.1038/leu.2009.246. [DOI] [PubMed] [Google Scholar]