Abstract
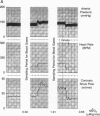
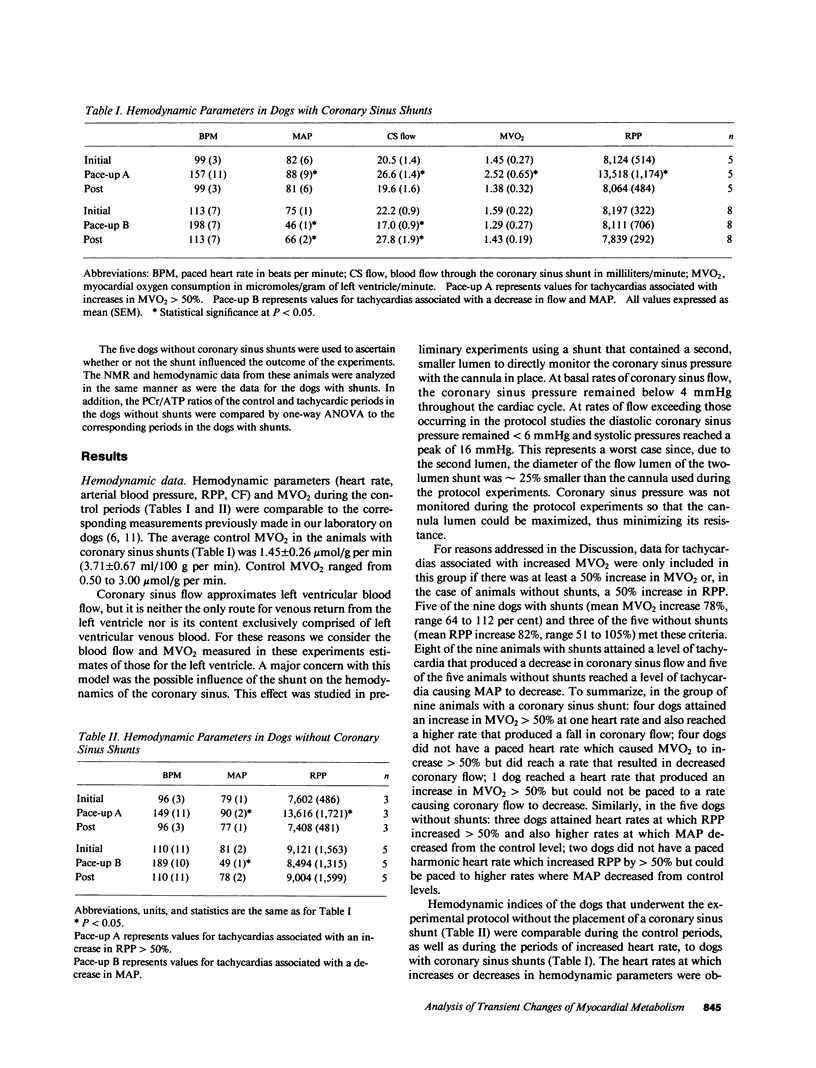
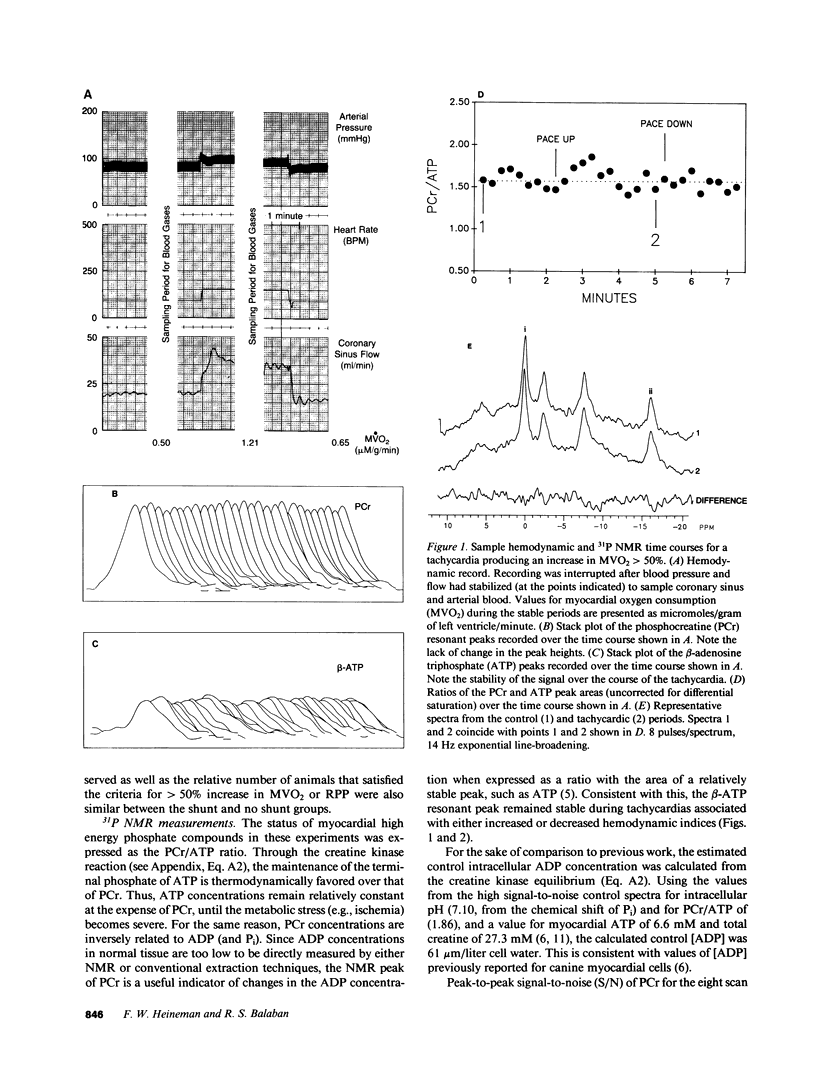
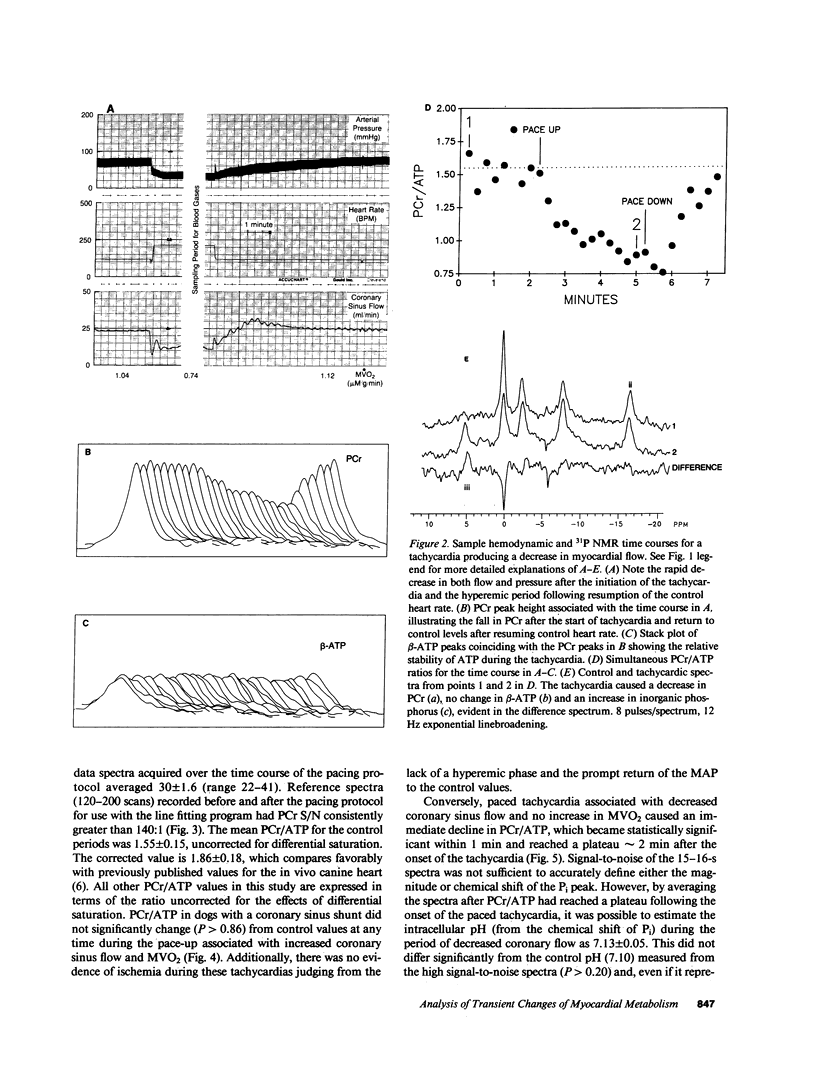
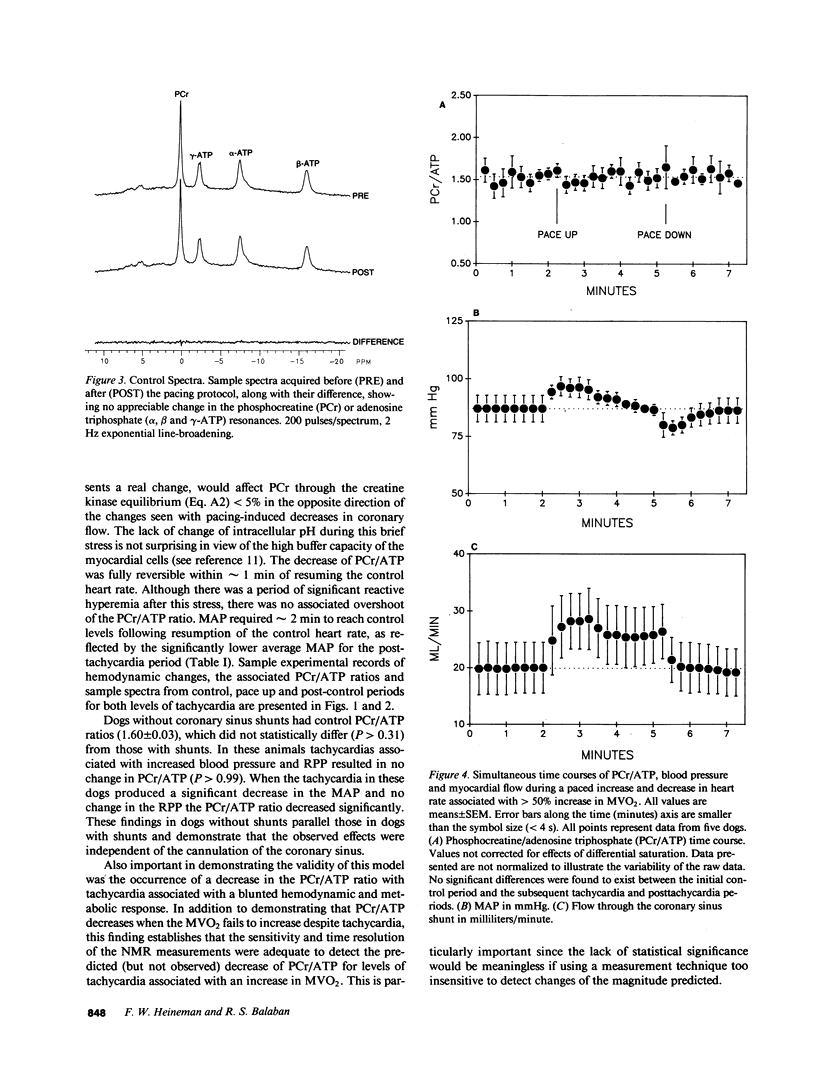
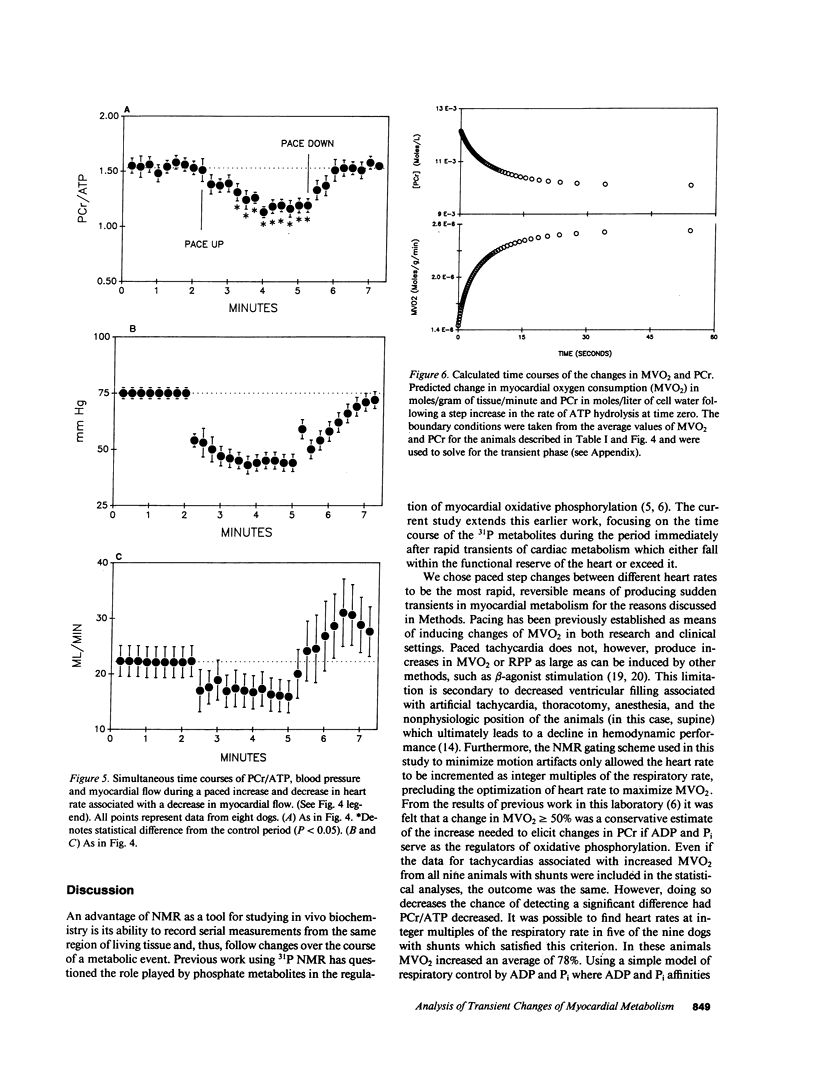
The time course of the relative myocardial phosphocreatine and adenosine triphosphate contents (PCr/ATP) during step changes in heart rate in vivo was studied in 14 dogs using 31P nuclear magnetic resonance (NMR) to determine if transient changes in the high energy phosphates occur with changes in cardiac work. Coronary sinus blood flow (CF), oxygen consumption (MVO2), and NMR data were simultaneously measured during brief (approximately 3 min), paced increases in heart rate in these open chest animals. 31P spectra were collected with a time resolution of 15-16 s (PCr signal to noise 22-41:1). Paced tachycardia associated with increased CF and MVO2 had no significant transient or sustained effect on PCr/ATP. Higher heart rates, associated with decreased CF and blood pressure, caused rapid decreases of PCr/ATP that were reversible upon return to control rates. These data indicate that there are no transient changes in 31P metabolites (on a 15-16-s time base) during step changes in cardiac work associated with increased CF. This lack of change demonstrates that ATP hydrolysis and production are closely matched and that the feedback mechanism linking these processes occurs rapidly with no detectable transient change in the phosphate metabolites. In contrast, when the CF response to tachycardia is insufficient PCr is quickly depleted. This latter result suggests that the PCr/ATP ratio may be a sensitive, rapidly responding indicator of coronary supply/demand mismatching in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achs M. J., Garfinkel D., Opie L. H. Computer simulation of metabolism of glucose-perfused rat heart in a work-jump. Am J Physiol. 1982 Sep;243(3):R389–R399. doi: 10.1152/ajpregu.1982.243.3.R389. [DOI] [PubMed] [Google Scholar]
- Ackerman J. J., Grove T. H., Wong G. G., Gadian D. G., Radda G. K. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980 Jan 10;283(5743):167–170. doi: 10.1038/283167a0. [DOI] [PubMed] [Google Scholar]
- Balaban R. S., Heineman F. W. Control of mitochondrial respiration in the heart in vivo. Mol Cell Biochem. 1989 Sep 7;89(2):191–197. doi: 10.1007/BF00220775. [DOI] [PubMed] [Google Scholar]
- Balaban R. S., Kantor H. L., Katz L. A., Briggs R. W. Relation between work and phosphate metabolite in the in vivo paced mammalian heart. Science. 1986 May 30;232(4754):1121–1123. doi: 10.1126/science.3704638. [DOI] [PubMed] [Google Scholar]
- Baller D., Hoeft A., Korb H., Wolpers H. G., Zipfel J., Hellige G. Basic physiological studies on cardiac pacing with special reference to the optimal mode and rate after cardiac surgery. Thorac Cardiovasc Surg. 1981 Jun;29(3):168–173. doi: 10.1055/s-2007-1023469. [DOI] [PubMed] [Google Scholar]
- Bittl J. A., Ingwall J. S. Reaction rates of creatine kinase and ATP synthesis in the isolated rat heart. A 31P NMR magnetization transfer study. J Biol Chem. 1985 Mar 25;260(6):3512–3517. [PubMed] [Google Scholar]
- Clarke K., Willis R. J. Energy metabolism and contractile function in rat heart during graded, isovolumic perfusion using 31P nuclear magnetic resonance spectroscopy. J Mol Cell Cardiol. 1987 Dec;19(12):1153–1160. doi: 10.1016/s0022-2828(87)80525-4. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Wilson D. F. Regulation of cellular energy metabolism. J Membr Biol. 1982;70(1):1–14. doi: 10.1007/BF01871584. [DOI] [PubMed] [Google Scholar]
- Groen A. K., Wanders R. J., Westerhoff H. V., van der Meer R., Tager J. M. Quantification of the contribution of various steps to the control of mitochondrial respiration. J Biol Chem. 1982 Mar 25;257(6):2754–2757. [PubMed] [Google Scholar]
- Heineman F. W., Eng J., Berkowitz B. A., Balaban R. S. NMR spectral analysis of kinetic data using natural lineshapes. Magn Reson Med. 1990 Mar;13(3):490–497. doi: 10.1002/mrm.1910130316. [DOI] [PubMed] [Google Scholar]
- Isselhard W., Lauterjung K. L., Witte J., Ban T., Hübner G., Giersberg O., Heugel E., Hirt H. J. Metabolic and structural recovery of left ventricular canine myocardium from regional complete ischemia. Eur Surg Res. 1975;7(3):136–155. doi: 10.1159/000127800. [DOI] [PubMed] [Google Scholar]
- Jacobus W. E., Moreadith R. W., Vandegaer K. M. Mitochondrial respiratory control. Evidence against the regulation of respiration by extramitochondrial phosphorylation potentials or by [ATP]/[ADP] ratios. J Biol Chem. 1982 Mar 10;257(5):2397–2402. [PubMed] [Google Scholar]
- Kantor H. L., Briggs R. W., Metz K. R., Balaban R. S. Gated in vivo examination of cardiac metabolites with 31P nuclear magnetic resonance. Am J Physiol. 1986 Jul;251(1 Pt 2):H171–H175. doi: 10.1152/ajpheart.1986.251.1.H171. [DOI] [PubMed] [Google Scholar]
- Katz L. A., Koretsky A. P., Balaban R. S. Respiratory control in the glucose perfused heart. A 31P NMR and NADH fluorescence study. FEBS Lett. 1987 Sep 14;221(2):270–276. doi: 10.1016/0014-5793(87)80939-0. [DOI] [PubMed] [Google Scholar]
- Katz L. A., Swain J. A., Portman M. A., Balaban R. S. Relation between phosphate metabolites and oxygen consumption of heart in vivo. Am J Physiol. 1989 Jan;256(1 Pt 2):H265–H274. doi: 10.1152/ajpheart.1989.256.1.H265. [DOI] [PubMed] [Google Scholar]
- Kusachi S., Nishiyama O., Yasuhara K., Saito D., Haraoka S., Nagashima H. Right and left ventricular oxygen metabolism in open-chest dogs. Am J Physiol. 1982 Nov;243(5):H761–H766. doi: 10.1152/ajpheart.1982.243.5.H761. [DOI] [PubMed] [Google Scholar]
- Lawson J. W., Veech R. L. Effects of pH and free Mg2+ on the Keq of the creatine kinase reaction and other phosphate hydrolyses and phosphate transfer reactions. J Biol Chem. 1979 Jul 25;254(14):6528–6537. [PubMed] [Google Scholar]
- Lemasters J. J., Grunwald R., Emaus R. K. Thermodynamic limits to the ATP/site stoichiometries of oxidative phosphorylation by rat liver mitochondria. J Biol Chem. 1984 Mar 10;259(5):3058–3063. [PubMed] [Google Scholar]
- Portman M. A., Heineman F. W., Balaban R. S. Developmental changes in the relation between phosphate metabolites and oxygen consumption in the sheep heart in vivo. J Clin Invest. 1989 Feb;83(2):456–464. doi: 10.1172/JCI113904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portman M. A., James S., Heineman F. W., Balaban R. S. Simultaneous monitoring of coronary blood flow and 31P NMR detected myocardial metabolites. Magn Reson Med. 1988 Jun;7(2):243–247. doi: 10.1002/mrm.1910070213. [DOI] [PubMed] [Google Scholar]
- Saito D., Yasuhara K., Nishiyama O., Kusachi S., Haraoka S. Comparative effects of heart rate and aortic blood pressure on MVO in the anesthetized open-chest dog. Jpn Heart J. 1981 Sep;22(5):833–837. doi: 10.1536/ihj.22.833. [DOI] [PubMed] [Google Scholar]