Abstract
Activating transcription factor 3 (ATF3) has been proposed as a marker for injured neurons. Thus, while undetectable normally in sensory, motor, or sympathetic neurons, ATF3--like immunoreactivity (ATF3-IR) is readily detectable in such cells after axotomy. Here we examined ATF3-IR in the superior cervical ganglion (SCG) and the middle and inferior cervical ganglia (MICG) after transection of the predominantly preganglionic cervical sympathetic trunk (CST). The purpose of the study was to determine whether neurons in the SCG would exhibit ATF3-IR after decentralization and, if they did not, whether the induction of ATF3-IR was sensitive enough to identify the small numbers of neurons in the SCG and MICG that project their axons into the CST. Following transection of the CST, the majority of deafferented neurons in the SCG showed no ATF3-IR; however, a small group of neurons in both the SCG and MICG were labeled, and the location of the labeled neurons within these ganglia corresponded to that of neurons axotomized by this procedure. Furthermore, the ATF3-positive neurons in the MICG could be retrogradely labeled from the transected CST. In addition, a large number of smaller cells were labeled in the SCG, though not in the MICG, and some of these cells were double labeled with an antiserum to the glial protein S100. These data indicate that, after transection of the CST, neuronal labeling in the SCG and MICG is restricted to axotomized neurons but that in addition there is extensive labeling of glial cells associated with anterograde degeneration within the SCG.
Keywords: axotomy, middle and inferior cervical ganglia, nerve injury, superior cervical ganglion, sympathetic ganglia
1. Introduction
Axotomy causes changes in neuronal gene expression. Tsujino and colleagues (2000) identified activating transcription factor 3 (ATF3) as a gene that is expressed in both sensory and motor neurons after sciatic nerve injury while being undetectable in these same neurons in the intact animal. Similarly, we have recently found that, while ATF3 is not normally expressed in sympathetic neurons in the superior cervical ganglion (SCG), it is expressed in these neurons after transection of the postganglionic internal and external carotid nerves (Boeshore et al., 2004; Hyatt-Sachs, Schreiber, Shoemaker, Sabe, Reed, and Zigmond submitted). While ATF3 has been proposed as a marker for injured neurons (Tsujino et al., 2000), in a study on the corticospinal tract after axotomy, Mason et al. (2003) found that the expression of ATF3 increased following a proximal lesion (i.e., a lesion 350–500 μm from the cell soma), but not following a more distal lesion.
The cervical sympathetic trunk (CST) is predominantly a preganglionic nerve trunk containing the axons of neurons whose cell bodies are located in the spinal cord and whose terminals are in the SCG. Transection of the CST, often referred to as decentralization, removes the central afferent input to neurons in the SCG. In addition, we previously showed that two populations of postganglionic neurons project into this trunk. One of these is a small group of neurons whose cell bodies are located primarily in the caudal part of the SCG (Bowers and Zigmond, 1979), and the other is a group of neurons whose cell bodies are located primarily in the rostral portions of the middle and inferior cervical ganglia (MICG; Bowers and Zigmond, 1981). Transection of the CST near the SCG, thus, produces a proximal axotomy to certain neurons in the SCG, while producing a distal axotomy to certain neurons in the MICG. In the present study, we sought to determine whether deafferentation leads to a transneuronal change in ATF3-like immunoreactivity (ATF3-IR) throughout the SCG, and, if it did not, whether changes in ATF3-IR could be used as a neuroanatomical technique to identify these minor populations of axotomized neurons in the SCG and MICG.
2. Results
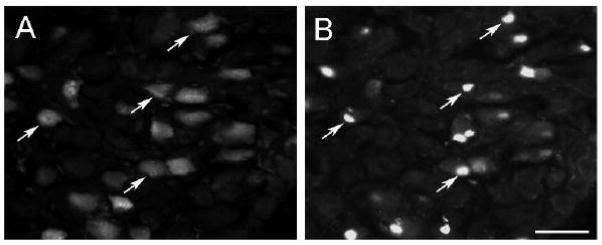
Forty-eight hours after the CST was transected, a small number of labeled neurons were found in the caudal portion of the SCG with none in the rostral portion of the ganglion (Fig 1A, B). The ATF3-IR was heavily concentrated in the large oval nuclei of these neurons (large arrows, Fig. 1A). These nuclei were eccentric as expected of axotomized neurons. In addition, a second population of labeled cells were seen, which was much more abundant, and had considerably smaller and somewhat flattened cell nuclei (small arrows, Fig. 1A, B). This second population of labeled “small cells” was evenly distributed between the rostral and caudal portions of the ganglion. In many cases, these cells were found on the periphery of either labeled or unlabeled neurons, in positions expected of satellite cells. Few, if any, labeled cells were found in sections from sham-operated ganglia (data not shown).
Fig. 1.
ATF3-IR in the SCG after transection of the CST. Forty eight hours after transection of the CST, ATF3-IR was examined in the caudal (A) and rostral (B) areas of the SCG. In the caudal area, bright staining was found in large oval nuclei of principal neurons (large arrows) and, in addition, in smaller, generally lighter stained and flattened nuclei (small arrows). In the rostral area of the ganglion, the principal neurons were unlabelled but many “small cells” were labeled. Scale bar = 25 μm.
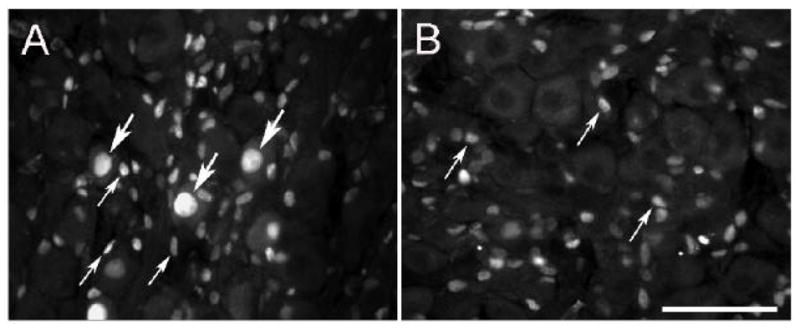
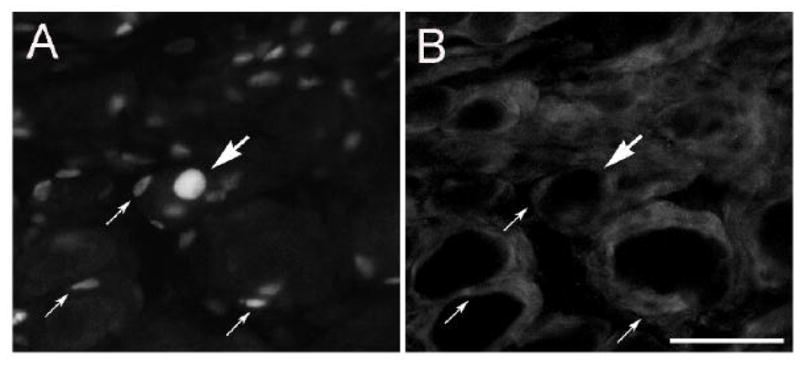
To determine whether the population of labeled cells with small and flattened nuclei represented satellite cells and Schwann cells, sections were double labeled with S-100, a marker for these glial cells. In many cases the ATF3-IR was found to be embedded in a ring of S-100 positive immunoreactivity that surrounded principal neurons (small arrows, Fig. 2A, B). In other cases, a linear array of small cell nuclei were embedded in what appeared to be a fiber track and was immunostained for S-100 (Fig. 3A, B).
Fig. 2.
ATF3-IR in satellite cells in the SCG after decentralization. The CST was cut and 48 h later, sections of the SCG were double immunostained for ATF3 (A) and S100 (B). A labeled principal neuron is indicated by the large arrow, and three labeled satellite cells are indicated by the small arrows. Satellite cells are distinguished from Schwann cells by their apposition to principal neurons. Scale bar = 25 μm.
Fig. 3.
ATF3-IR in Schwann cells in the SCG after decentralization. Forty-eight hours after transection of the CST, sections of the SCG were double immunostained for ATF3 (A) and S100 (B). The arrows point to a fiber pathway within the ganglion with numerous ATF3- and S100-positive Schwann cells. Scale bar = 25 μm.
SCG were also examined 6 weeks after a lesion. In this case, the CST was transected in some animals and frozen in others to see whether the extent of labeling was influenced by whether or not the CST regenerated and reinnervated the SCG. Only a few labeled cells were found at 6 weeks, and no difference was found in ganglia from animals receiving the two types of lesion (Fig. 4C, D).
Fig. 4.
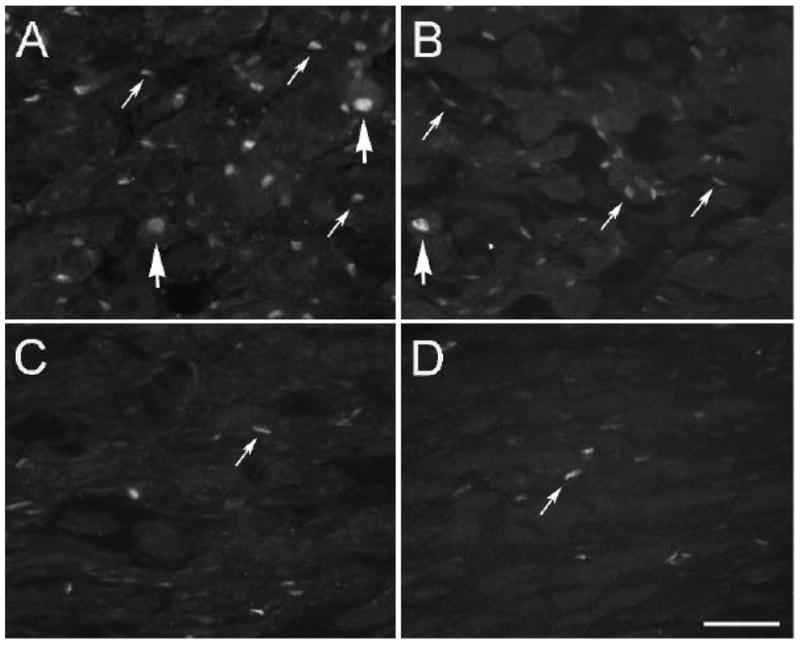
ATF3-IR labeling in the SCG 6 weeks after lesioning of the CST. The CST was lesioned by transection (A, C) or by freezing (B, D), and ATF3-IR was examined 48 h (A, B) and 6 weeks (C, D) later. Labeled neurons are indicated by large arrows and labeled “small cells” by small arrows. Scale bar = 25 μm.
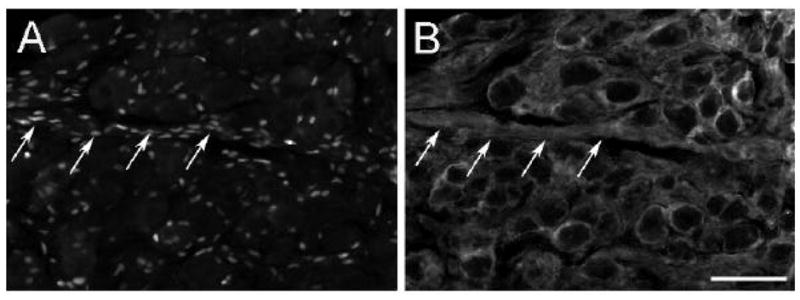
In addition to examining the SCG after transecting the CST, we also looked in the MICG. Because the MICG is further from the site of the lesion than is the SCG, we examined the MICG 7 days after the surgery. Those neurons in the MICG that were axotomized by the lesion were identified by retrograde labeling with fluorogold. Almost every neuron that was labeled with fluorogold also exhibited ATF3-IR, and there was no evidence for non-neuronal cell labeling in this ganglion (Fig. 5A, B).
Fig. 5.
ATF3-IR labeling of axotomized neurons in the MICG identified by retrograde labeling from the transected CST. The CST was cut 2–3 mm caudal to the SCG and the caudal end of the trunk was exposed to fluorogold for 30 min. Seven days later sections of the MICG were immunostained for ATF3-IR and visualized for ATF3-IR and fluorogold. Most fluorogold labeled neurons exhibited ATF3-IR. Scale bar = 25 μm.
3. Discussion
The data indicate that ATF3 is not induced by a transneuronal process following decentralization of the SCG. On the other hand, the induction of ATF3-IR following a lesion is a sensitive retrograde method for identifying minor populations of neurons that project into a particular nerve tract. Using retrograde labeling with horseradish peroxidase, we previously estimated that the population of neurons in the SCG that project into the CST was rarely more than 1% of the neurons in the ganglion (Bowers and Zigmond, 1979); however, these neurons were readily detectable in the current study by means of ATF3-IR. Again using horseradish peroxidase, we previously found neurons in the MICG that project to the SCG and exit that ganglion via the external carotid nerve (Bowers and Zigmond, 1981). In the current study, almost every neuron retrogradely labeled in the MICG with fluorogold also contained detectable ATF3-IR. Since the site of the transection of the CST was approximately 2 mm from the SCG and about 20 mm from the MICG, these data also demonstrate that, unlike in the corticospinal tract (Mason et al., 2003), ATF3 induction in the sympathetic nervous system is not sensitive to the distance from the cell body the lesion is made.
While ATF3 was proposed as a marker for injured neurons (Tsujino et al., 2000), it also can be induced in glial cells. This was first reported by Hunt et al. (2004) in Schwann cells in the sciatic nerve distal to a lesion. We also found ATF3-IR in both satellite cells and Schwann cells in the SCG after transection of the CST. No such labeling was seen in the MICG, suggesting that the stimulus for this labeling was the degeneration of the preganglionic nerve terminals, which only occurs in the SCG after this lesion. This idea is further supported by the fact that glial cell labeling in the SCG was uniformly found throughout the ganglion, whereas neuronal labeling was concentrated in the caudal region of the ganglion. To determine whether glial labeling in the SCG remained elevated until reinnervation occurred, ganglia were examined 6 weeks after a lesion, under conditions in which reinnervation was facilitated or inhibited. Glial labeling within the SCG was greatly reduced whether or not regeneration occurred. These data indicate that the glial labeling was a transitory response to Wallerian degeneration rather than a permanent feature of glial cells that are no longer in contact with axons.
ATF3 is a transcription factor with a basic region/leucine zipper (or bZip) domain that can form both homodimers and heterodimers with other bZip transcription factors (Hai et al., 1999). Heterodimerization of ATF3 with members of the Jun family have been emphasized (e.g., Hai and Curran, 1991; Tsujino et al., 2000; Nakagomi et al., 2003). In addition to increasing ATF3, axotomy increases immunoreactivity to c-Jun, Jun B and Jun D in the SCG. Since c-Jun is increased in both neurons and non-neuronal cells and Jun B and Jun D are elevated only in non-neuronal cells (Koistinaho et al., 1993), ATF3 might dimerize with different partners in different cell types within the ganglion and might regulate the expression of different genes depending on its dimerization partner. Overexpression of ATF3 in neonatal SCG explants increased fiber outgrowth and decreased apoptosis in the absence of NGF (Nakagomi et al., 2003). The latter effect has been hypothesized to result from the induction of heat shock protein 27 expression and Akt activation within axotomized sympathetic neurons (Nakagomi et al., 2003). Possible functional consequences of ATF3 induction in satellite and Schwann cells remain to be determined.
4. Experimental Procedure
Adult male Sprague-Dawley rats (125–150 g) were anesthetized with an intraperitoneal injection of ketamine (43 mg/kg), xylazine (8.6 mg/kg), and acepromazine (1.4 mg/kg). Bilateral decentralization of the SCG was performed by transecting the CST 2–3 mm caudal to the ganglion. A small piece of the nerve trunk 1–2 mm was removed to minimize any chance for regeneration to occur. In sham-operated animals, the CST was exposed but not transected. Animals were sacrificed 48 h or 6 weeks following surgery. Forty eight hours was chosen as the main time point examined as this appeared to be the peak of ATF3-IR following transection of the postganglionic internal and external carotid nerves (Hyatt-Sachs, Schreiber, Shoemaker, Sabe, Reed, and Zigmond submitted). In a group of six animals, the CST was not transected but was frozen three times by touching it with a small piece of dry ice for 10 sec each time. This procedure lesions the axons in the CST and facilitates regeneration (Raisman et al., 1974). Half of these animals were examined at 48 h and half at six weeks following surgery.
Animals were sacrificed by C02 inhalation. The SCG and/or MICG were removed, desheathed and immersion fixed in 4% paraformaldehyde for 1 h on ice. Following fixation, ganglia were cryoprotected in sucrose, embedded in Tissue Tek O.C.T. compound (Ted Pella, Redding, CA), and 10 μm frozen sections were cut in a cryostat. Sections were pre-incubated in 5% normal donkey serum and 0.3% Triton X-100 in phosphate buffered saline (PBS) and then incubated with rabbit anti-ATF3 (1:250; sc-188, Santa Cruz Biotechnology, Santa Cruz, CA). The sections were then rinsed thoroughly with PBS and incubated with CY3-donkey anti-rabbit IgG F(ab′)2 (1:250 Jackson ImmunoResearch Laboratories Inc., West Grove, PA). All incubations were done at room temperature for 1 h. For double labeling experiments, some sections were also incubated overnight with mouse anti-S100 (1:1000 Sigma-Aldrich, St. Louis, MO) followed by donkey anti-mouse F(ab′)2 CY2 (1:50).
In a few animals, the portion of the transected CST proximal to the MICG was placed on a small piece of parafilm and exposed to a solution of 5% fluorogold (Molecular Probes, Eugene, OR) in saline for 30 min. The nerve was then washed with saline, and the animal was allowed to survive for seven days. The MICG were removed and processed for ATF3-IR as described above.
Acknowledgments
This research was supported by grant NS17512 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boeshore KL, Schreiber RC, Vaccariello SA, Sachs HH, Salazar R, Lee J, Ratan RR, Leahy P, Zigmond RE. Novel changes in gene expression following axotomy of a sympathetic ganglion: a microarray analysis. J Neurobiol. 2004;59:216–235. doi: 10.1002/neu.10308. [DOI] [PubMed] [Google Scholar]
- Bowers CW, Zigmond RE. Localization of neurons in the rat superior cervical ganglion that project into different postganglionic trunks. J Comp Neurol. 1979;185:381–391. doi: 10.1002/cne.901850211. [DOI] [PubMed] [Google Scholar]
- Bowers CW, Zigmond RE. Sympathetic neurons in lower cervical ganglia send axons through the superior cervical ganglion. Neuroscience. 1981;6:1783–1791. doi: 10.1016/0306-4522(81)90213-x. [DOI] [PubMed] [Google Scholar]
- Hai T, Curran T. Cross-family dimerization of transcription factors Fos/Jun and ATF/CREB alters DNA binding specificity. Proc Natl Acad Sci U S A. 1991;88:3720–3724. doi: 10.1073/pnas.88.9.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai T, Wolfgang CD, Marsee DK, Allen AE, Sivaprasad U. ATF3 and stress responses. Gene Expr. 1999;7:321–335. [PMC free article] [PubMed] [Google Scholar]
- Hunt D, Hossain-Ibrahim K, Mason MR, Coffin RS, Lieberman AR, Winterbottom J, Anderson PN. ATF3 upregulation in glia during Wallerian degeneration: differential expression in peripheral nerves and CNS white matter. BMC Neurosci. 2004;5:9. doi: 10.1186/1471-2202-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinaho J, Pelto-Huikko M, Sagar SM, Dagerlind A, Roivainen R, Hokfelt T. Injury-induced long-term expression of immediate early genes in the rat superior cervical ganglion. Neuroreport. 1993;4:37–40. doi: 10.1097/00001756-199301000-00009. [DOI] [PubMed] [Google Scholar]
- Mason MR, Lieberman AR, Anderson PN. Corticospinal neurons up-regulate a range of growth-associated genes following intracortical, but not spinal, axotomy. Eur J Neurosci. 2003;18:789–802. doi: 10.1046/j.1460-9568.2003.02809.x. [DOI] [PubMed] [Google Scholar]
- Nakagomi S, Suzuki Y, Namikawa K, Kiryu-Seo S, Kiyama H. Expression of the activating transcription factor 3 prevents c-Jun N-terminal kinase-induced neuronal death by promoting heat shock protein 27 expression and Akt activation. J Neurosci. 2003;23:5187–5196. doi: 10.1523/JNEUROSCI.23-12-05187.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisman G, Field PM, Ostberg AJ, Iversen LL, Zigmond RE. A quantitative ultrastructural and biochemical analysis of the process of reinnervation of the superior cervical ganglion in the adult rat. Brain Res. 1974;71:1–16. doi: 10.1016/0006-8993(74)90187-5. [DOI] [PubMed] [Google Scholar]
- Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15:170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]