Abstract
Left ventricular (LV) epicardial pacing acutely reduces wall thickening at the pacing site. Because LV epicardial pacing also reduces transverse shear deformation, which is related to myocardial sheet shear, we hypothesized that impaired end-systolic wall thickening at the pacing site is due to reduction in myocardial sheet shear deformation, resulting in a reduced contribution of sheet shear to wall thickening. We also hypothesized that epicardial pacing would reverse the transmural mechanical activation sequence and thereby mitigate normal transmural deformation. To test these hypotheses, we investigated the effects of LV epicardial pacing on transmural fiber-sheet mechanics by determining three-dimensional finite deformation during normal atrioventricular conduction and LV epicardial pacing in the anterior wall of normal dog hearts in vivo. Our measurements indicate that impaired end-systolic wall thickening at the pacing site was not due to selective reduction of sheet shear, but rather resulted from overall depression of fiber-sheet deformation, and relative contributions of sheet strains to wall thickening were maintained. These findings suggest lack of effective end-systolic myocardial deformation at the pacing site, most likely because the pacing site initiates contraction significantly earlier than the rest of the ventricle. Epicardial pacing also induced reversal of the transmural mechanical activation sequence, which depressed sheet extension and wall thickening early in the cardiac cycle, whereas transverse shear and sheet shear deformation were not affected. These findings suggest that normal sheet extension and wall thickening immediately after activation may require normal transmural activation sequence, whereas sheet shear deformation may be determined by local anatomy.
Keywords: epicardial pacing, transmural deformation, fiber, sheet
Cardiac Resynchronization Therapy (CRT) has proven to be effective in improving cardiac function in moderate-to-severe heart failure associated with an intraventricular conduction delay, most commonly of a left bundle branch block type (3, 5, 6, 15, 16, 24, 29). The advent of CRT has resulted in an increasingly frequent use of left ventricular (LV) epicardial pacing in clinical settings. Despite the clinical benefits of CRT, LV epicardial pacing is associated with detrimental effects on normal transmural function. Systolic wall thickening, which is an important component of regional cardiac function, is significantly reduced at the pacing site (33). Furthermore, LV epicardial pacing reverses the normal activation sequence of transmural depolarization into the epicardial-endocardial propagation (11, 12, 19), which significantly increases QT interval and transmural dispersion of repolarization, and enhances susceptibility to torsade de pointes in a subset of patients (10, 22). To design more effective and safe pacing therapies, it is of critical importance to understand the effects of epicardial pacing on normal transmural function.
Several lines of evidence support the concept that transverse shear deformation is required for normal systolic wall thickening (17, 18, 30). Because the LV myocardium consists of helically woven myofibers (25, 31) that are arranged in transversely oriented myocardial sheets (17), transverse shear deformation is anatomically related to myocardial sheet shear, and sheet shear deformation contributes significantly to normal systolic wall thickening (8). Because LV epicardial pacing significantly reduces transverse shear deformation (33), we hypothesized that impaired end-systolic wall thickening at the epicardial pacing site is due to reduction in myocardial sheet shear deformation, resulting in a reduced contribution of sheet shear to wall thickening. We also hypothesized that LV epicardial pacing would reverse transmural mechanical activation sequence and thereby mitigate normal transmural deformation. To test these hypotheses, we investigated the effects of LV epicardial pacing on transmural fiber-sheet mechanics by determining three-dimensional (3-D) finite deformation during normal atrioventricular (AV) conduction and LV epicardial pacing in the anterior wall of normal dog hearts in vivo. Our measurements indicate that impaired end-systolic wall thickening at the epicardial pacing site was not due to selective reduction of sheet shear, but rather resulted from overall depression of fiber-sheet deformation, and relative contributions of sheet strains to wall thickening were maintained. Epicardial pacing also induced reversal of the transmural mechanical activation sequence, which significantly depressed sheet extension and wall thickening early in the cardiac cycle, whereas transverse shear and sheet shear deformation were not affected.
MATERIALS AND METHODS
All animal studies were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All protocols were approved by the Animal Subjects Committee of the University of California, San Diego, which is accredited by the American Association for Accreditation of Laboratory Animal Care. A subset of data from the five animals included in this study has been presented previously in our report (2), which described local ventricular deformation during early relaxation under normal AV conduction.
Experimental protocol
The protocol for surgical preparation was described in detail previously (2). Briefly, five adult mongrel dogs (19–28 kg) underwent median sternotomy under general anesthesia, with the LV pressure, central aortic pressure, and the surface ECG monitored throughout the study. Low-dose dopamine (2.5–5.0 µg·kg−1·min−1) was administered intravenously to maintain blood pressure in two animals. To measure 3-D myocardial deformation, three transmural columns of four to six 0.8-mm-diameter gold beads and a 1.7-mm-diameter surface gold bead above each column were placed within the anterior wall between the first and the second diagonal branches of the left anterior descending coronary artery (LAD) (Fig. 1A). To provide end points for a LV long axis, 2-mm-diameter gold beads were sutured to the apical dimple (apex bead, Fig. 1A) and on the epicardium at the bifurcation of the LAD and left circumflex coronary arteries (base bead, Fig. 1A). Pacing wire pairs were sutured to the left atrium (LA) and the LV epicardial surface across the triangle of the surface gold beads (Fig. 1A). Atrial pacing was performed by stimulating LA electrodes, and LV epicardial pacing was performed by stimulating both LA and LV electrodes (LA-LV delay = 20–40 ms), via a square-wave, constant-voltage electronic stimulator at a frequency 20% above baseline heart rate to suppress native sinus rhythm. Stimulation parameters (voltage 10% above threshold, duration 8 ms, and frequency) were kept constant in each animal. Each animal was positioned in a biplane radiography system, and synchronous biplane cineradiographic images (125 frames/s) of the bead markers were digitally acquired with mechanical ventilation suspended at end expiration. Image acquisition for the two pacing modes (atrial pacing and LV epicardial pacing) was performed consecutively at the same heart rate to minimize variation in hemodynamic conditions. At the end of the study, the animals were euthanized with pentobarbital sodium and the heart perfusion fixed with 2.5% buffered glutaraldehyde at the end-diastolic pressure measured in the study (2, 39). Because the heart was fixed at end-diastolic pressure, fiber and sheet orientations in the fixed hearts were assumed to represent the fiber-sheet structure in the end-diastolic reference configuration in vivo (2, 8, 32). To avoid the distortional effects of dehydration and shrinkage associated with embedding, histological measurements were obtained using freshly fixed heart tissue. In the transmural block of tissue within the implanted bead set, the mean fiber (α) and sheet angles (β) were determined from epicardium to endocardium at every 1-mm-thick section sliced parallel to the epicardial tangent plane (Fig. 1B) (2, 8). The digital images from the biplane X-ray were spherically corrected (2) to reconstruct the 3-D coordinates (20) of the gold bead markers. Continuous, nonhomogeneous transmural distributions of 3-D finite strains were computed (2). Six independent finite strains [circumferential strain (E11), longitudinal strain (E22), radial strain (E33), circumferential-longitudinal shear (E12), longitudinal-radial shear (E23), and circumferential-radial shear (E13)] were computed in the local cardiac coordinate axis (circumferential, longitudinal, and radial, respectively) system (X1, X2, X3) (23), which were subsequently used to compute another set of six finite strains [fiber strain (Eff), sheet strain (Ess), strain normal to the sheet plane (Enn), shear within the sheet plane (Efs), sheet shear (Esn), and fiber-normal shear (Efn)] in the local fiber-sheet coordinate system (Xf, Xs, Xn) (7) through an orthogonal transformation to convert the strain tensor using α and β at each depth. Finite strains were calculated for each frame (125 frames/s) as a deformed configuration with end diastole as the reference state at three wall depths: 25% (subepicardium), 50% (midwall), and 75% (subendocardium) wall depth from the epicardial surface. End diastole was defined as the time of the peak of the ECG R-wave for atrial pacing and the ventricular pacing artifact (V-spike) for LV epicardial pacing. We chose the V-spike rather than the peak of R-wave as the reference state for LV epicardial pacing because the former reflects the timing of the activation of the pacing site, as opposed to the latter, which represents the timing of activation of the whole ventricle. End systole was derived from the nadir of the dicrotic notch of the central aortic pressure.
Fig. 1.
A: schematic representation of the left ventricle (LV). X1, circumferential axis; X2, longitudinal axis; X3, radial axis; LAD, left anterior descending; LCx, left circumflex; D1, D2, first and second diagonal branch of LAD, respectively. B: schematic representation of local fiber-sheet axes. Fiber angle (α) was measured in the X1–X2 plane at each wall depth with reference to positive X1. Sheet angle (β) was measured in the plane perpendicular to the fiber angle at each wall depth with reference to positive X3. Xf, fiber axis, Xs, sheet axis, Xn, axis oriented normal to the sheet plane. The Xf, Xs, and Xn axes present a Cartesian system (for details, see Ref. 2).
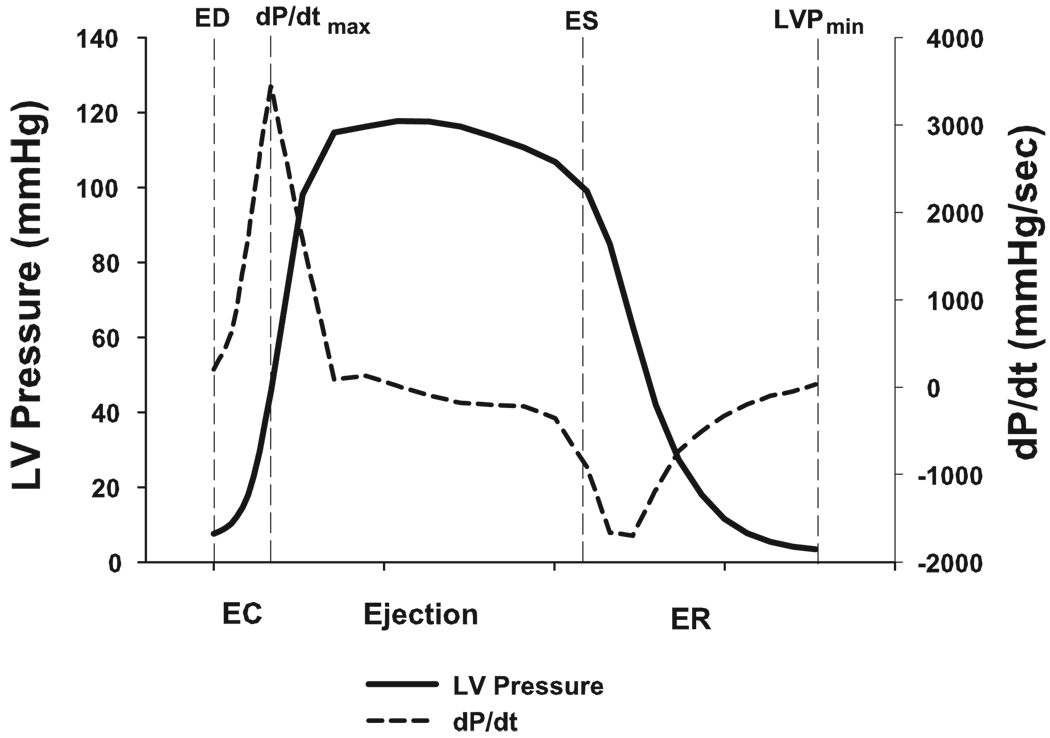
Three phases were defined in the cardiac cycle: early contraction phase [beginning at end diastole and ending at the peak positive (dP/dtmax)], ejection phase (beginning at dP/dtmax and ending at end systole), and early relaxation phase (beginning at end systole and ending at minimum LV pressure) (2, 13) (Fig. 2). The QT interval was defined as the time interval between the initial deflection of the QRS complex and the point at which a tangent drawn to the steepest portion of the terminal part of the T wave crossed the isoelectric line (22). Corrected QT was calculated using Bazett’s formula (4). Transmural dispersion of repolarization was defined as the interval between the peak to the end of the T wave on the surface ECG (1, 37).
Fig. 2.
Three cardiac phases are shown. dP/dtmax, LV peak positive developed pressure over time; EC, early contraction phase; Ejection, ejection phase; ER, early relaxation phase; ED, end diastole; ES, end systole; LVPmin, minimum LV pressure.
To assess the fiber-sheet mechanics of impaired wall thickening (E33) during epicardial pacing, the contribution of each term on the right-hand side of the following equation was investigated (8)
| (1) |
It is evident from Eq. 1 that in terms of fiber-sheet coordinates, E33 depends only on the sheet angle (β) and the fiber-sheet strain components in the (Xs, Xn) plane normal to the local fiber axis, namely Esn, Ess, and Enn (8).
To assess transmural mechanical activation sequence with both pacing modes, mechanical activation time (tm) was defined as the time to reach 10% of the maximum fiber shortening (Eff) at each transmural depth. To evaluate the effect of reversed transmural mechanical activation sequence on transmural deformation, transmural mechanical activation strains (E′) were defined in both the local cardiac and fiber-sheet coordinate system. With atrial pacing, the reference configuration was tm at subendocardium and the deformed configuration was mechanical activation time at subepicardium. Conversely, with epicardial pacing, the reference configuration was tm at subepicardium and the deformed configuration was tm at the subendocardium. Because transmural mechanical activation sequence propagates from subendocardium to subepicardium with arial pacing, and from subepicardium to subendocardium with epicardial pacing (see RESULTS), E′ strains describe mean transmural myocardial deformation when mechanical activation travels between subendocardium and subepicardium.
Statistical analysis
Values are means ± SE unless otherwise specified. A paired t-test was used to compare atrial pacing and LV epicardial pacing for global hemodynamic parameters, time intervals, and each strain component. Two-factor repeated-measures ANOVA was used for the time course analysis, with the effects of pacing mode (atrial vs. LV epicardial pacing) and time on each strain component determined at three depths (subepicardium, midwall, and subendocardium) individually. Statistics were performed using SigmaStat version 3.0 (SPSS; Chicago, IL). Statistical significance was accepted at P < 0.05.
RESULTS
Anatomic measurements for these five animals were described previously (2). Briefly, the mean fiber angle ranged approximately from −60° (epicardium) to +60° (endocardium). The mean sheet angle was predominantly negative with smaller variations across the wall (−36° to −2°). The centroid of the bead set was 65 ± 1% of the distance from base bead to apex bead, in the anterior LV free wall 1~1.5 cm lateral from the LAD. Mean wall thickness at the bead set location was 10 ± 1 mm, and the deepest bead was located at 91 ± 2% wall depth. Global parameters are summarized in Table 1.
Table 1.
Hemodynamic parameters and time intervals
| Atrial Pace | Epicardial Pace | |
|---|---|---|
| Heart rate, beats/min | 133 ± 12 | 133 ± 12 |
| Peak LV pressure, mmHg | 119 ± 9 | 109 ± 10* |
| dP/dtmax, mmHg/s | 3,436 ± 858 | 2,754 ± 791* |
| dP/dtmin, mmHg/s | −2,105 ± 325 | −1,680 ± 219 |
| LVEDP, mmHg | 8 ± 2 | 5 ± 1 |
| LVESP, mmHg | 99 ± 6 | 89 ± 7 |
| τ, ms | 23 ± 5 | 25 ± 5* |
| Early contraction, ms | 34 ± 5 | 102 ± 14* |
| Ejection, ms | 186 ± 34 | 174 ± 23 |
| Early relaxation, ms | 134 ± 15 | 138 ± 15 |
| ED-ES, ms | 219 ± 35 | 277 ± 36* |
| QT, ms | 204 ± 16 | 248 ± 16* |
| QTc, ms | 446 ± 36 | 539 ± 21* |
| TDR, ms | 30 ± 3 | 63 ± 10* |
Values are means ± SE; n = 5 dogs.
LV, left ventricle; dP/dtmax, peak positive developed pressure over time; dP/dtmin, peak negative dP/dt; EDP, end-diastolic pressure; ESP, end-systolic pressure; τ, the time constant of LV isovolumic pressure decay (logarithmic method) (26, 34); ED-ES, time interval from end diastole (ED) to end systole (ES); QT, QT interval on surface ECG; QTc, corrected QT interval; TDR, transmural dispersion of repolarization.
P < 0.05 vs. atrial pacing.
End-systolic wall thickening and fiber sheet strains during epicardial pacing
Epicardial pacing significantly reduced wall thickening (E33) and transverse shear (E23), and E13 (P < 0.05; see Table 2). Epicardial pacing also depressed all the fiber-sheet strains, and all the terms on the right-hand side of Eq. 1 (2Esn sin βcos β, Ess cos2 β, and Enn sin2 β). As a result, the relative contribution of each term to wall thickening (2Esn sin β cos β/E33, Ess cos2 β/E33, and Enn sin2 β/E33) was not significantly affected by epicardial pacing (P = NS). With either atrial or epicardial pacing, the relative contribution of the sheet shear term (2Esn sin β cos β), sheet extension term (Ess cos2 β), and sheet-thickening term (Enn sin2 β) was fixed at 48–63%, 33–44%, and 4–8%, respectively.
Table 2.
End-systolic strains
| Atrial Pace | Epicardial Pace | ||
|---|---|---|---|
| E11 | −0.154 ± 0.019 | −0.060 ± 0.016* | |
| E22 | −0.043 ± 0.009 | −0.027 ± 0.012 | |
| E33 | 0.235 ± 0.032 | 0.085 ± 0.023* | |
| E12 | 0.030 ± 0.006 | 0.027 ± 0.005 | |
| E23 | 0.066 ± 0.014 | 0.018 ± 0.012* | |
| E13 | 0.067 ± 0.009 | 0.040 ± 0.009* | |
| Eff | −0.118 ± 0.010 | −0.040 ± 0.015* | |
| Ess | 0.121 ± 0.024 | 0.034 ± 0.014* | |
| Enn | 0.036 ± 0.027 | 0.004 ± 0.015* | |
| Efs | 0.065 ± 0.015 | 0.028 ± 0.012* | |
| Esn | −0.078 ± 0.038 | −0.026 ± 0.022* | |
| Efn | −0.055 ± 0.013 | −0.031 ± 0.010* | |
| Ess cos2 β | 0.096 ± 0.021 | 0.028 ± 0.011* | |
| Enn sin2 β | 0.020 ± 0.010 | 0.008 ± 0.006* | |
| 2Esn sin β cos β | 0.119 ± 0.020 | 0.049 ± 0.016* | |
| 0.438 ± 0.070 | 0.333 ± 0.091 | ||
| 0.082 ± 0.038 | 0.043 ± 0.063 | ||
| 0.481 ± 0.047 | 0.631 ± 0.077 |
Values are transmural means (subepicardium, midwall, and subendocardium) ± SE (n = 5).
E11, circumferential strain; E22, longitudinal strain; E33 radial strain; E12, circumferential-longitudinal shear means; E23, longitudinal-radial shear means; E13, circumferential-radial shear means; Eff, fiber strain; Ess, sheet strain; Enn, strain normal to the sheet plane; Efs; shear means within the sheet plane; Esn, sheet shear means; Efn, fiber-normal shear means; β, sheet angle.
P < 0.05 vs. atrial pace.
Time course of finite strains at the pacing site
With atrial pacing, most strain components underwent a simple contraction-relaxation pattern with the peak deformation near end systole, reflecting synchronous contraction within the LV (see Fig. 3). Epicardial pacing markedly changed the time course of all strain components in both cardiac and fiber-sheet coordinates, indicated by a significant interaction between the effects of pacing mode and time (P < 0.05). The hallmark effect of epicardial pacing was multiple phases of deformation, which are exemplified by Eff. The myofibers started to shorten immediately after the epicardial pacing pulse, whereas the LV was still in diastole. This early shortening occurred before ventricular ejection and was at its maximum before dP/dtmax. The myofibers subsequently underwent elongation during the ejection phase, followed by shortening, which reached the next peak near end systole. The myofibers then underwent stretch and shortening again, followed by another peak shortening during the early relaxation period and subsequent stretch. Although the early peak shortening near dP/dtmax (Eff = −0.091 ± 0.018) was comparable to the end-systolic shortening with atrial pacing [Eff = −0.124 ± 0.009, P = not significant (NS)], it clearly preceded aortic valve opening and did not contribute to ejection. The wall thickening that occurred at this time point also did not contribute to ejection. The subsequent peak shortening near end systole (Eff = −0.055 ± 0.031) was significantly decreased (P < 0.05). The peak shortening during early relaxation (Eff = −0.053 ± 0.016) was significantly decreased compared with end-systolic shortening with atrial pacing (P < 0.05). Most strains exhibited multiple peaks of deformation similar to Eff.
Fig. 3.
Temporal sequence of each strain component. Values are means ± SE (n = 5). Subepicardium, midwall, and subendocardium represents 25%, 50%, and 75% wall depth from the epicardial surface, respectively. The reference state for strain calculation was end diastole for both atrial pacing (peak of the ECG R-wave) and LV epicardial pacing (V-spike). Note different scales for shear and normal strains. A: Cardiac strains; B: fiber-sheet strains.
Transmural mechanical activation sequence at the pacing site
Epicardial pacing reversed the normal transmural sequence of fiber shortening at the pacing site (see Fig. 4 and Table 3). With atrial pacing, tm was 30 ± 8 ms earlier in subendocardium than in subepicardium (P < 0.05), reflecting the normal electrical activation sequence in the endocardial-epicardial direction. Epicardial pacing significantly reduced tm in subepicardium (P < 0.05), whereas subendocardium was unaffected (P = NS). The net result was reversal of the transmural sequence of fiber shortening; subepicardial fiber shortening occurred 19 ± 5 ms earlier than subendocardial counterpart (P < 0.05; Fig. 4).
Fig. 4.
Transmural delay in mechanical activation time (tm). Values are means ± SE (n = 5). Subepicardium and subendocardium refer to 25% and 75% wall depth, respectively. End diastole was used as the reference state (time 0) for both atrial pacing and LV epicardial pacing. Maximum fiber shortening in atrial pacing and LV epicardial pacing was found near end systole and dP/dtmax, respectively. *P < 0.05 vs. subepicardium of the same pacing mode; †P < 0.05 vs. subepicardium of atrial pacing.
Table 3.
Transmural mechanical activation strains
| Atrial Pace | Epicardial Pace | ||
|---|---|---|---|
| −0.042 ± 0.009 | −0.024 ± 0.004* | ||
| −0.023 ± 0.007 | −0.002 ± 0.002* | ||
| 0.045 ± 0.008 | 0.016 ± 0.003* | ||
| 0.019 ± 0.004 | −0.002 ± 0.003* | ||
| −0.004 ± 0.006 | 0.007 ± 0.003 | ||
| 0.022 ± 0.005 | 0.003 ± 0.002* | ||
| −0.024 ± 0.003 | −0.020 ± 0.003 | ||
| 0.029 ± 0.006 | 0.006 ± 0.004* | ||
| −0.026 ± 0.009 | 0.003 ± 0.004* | ||
| 0.014 ± 0.005 | 0.004 ± 0.003* | ||
| −0.017 ± 0.011 | −0.006 ± 0.003 | ||
|
|
−0.022 ± 0.005 | −0.005 ± 0.003* |
Values are transmural average (subepicardium, midwall, and subendocardium) ± SE; n = 5 dogs.
E′, transmural mechanical activation strains.
P < 0.05 vs. atrial pace.
Because the reference and deformed configurations of E′ strains were defined by transmural changes of fiber strains, fiber shortening was not affected by reversal of transmural mechanical activation sequence (P = NS). Despite normal fiber shortening, most E′ strains were significantly reduced (P < 0.05), including wall thickening , sheet extension , and sheet thickening . However, transverse shear and sheet shear were not significantly altered by reversal of transmural mechanical activation sequence (P = NS).
DISCUSSION
Although the effect of LV epicardial pacing on fiber strain has been described at single selected layers at the pacing site, including the subepicardium (9, 27) and midwall (21, 28, 35, 36), the present study is the first to examine the transmural gradient of fiber-sheet strains at the pacing site.
Mechanism of impaired endocardial wall thickening at the epicardial pacing site
On the basis of reduced transverse shear deformation at the epicardial pacing site, we hypothesized that impaired end-systolic wall thickening results from reduction in myocardial sheet shear. However, our results indicate that impaired end-systolic wall thickening at the epicardial pacing site was not due to selective reduction of sheet shear but rather resulted from overall depression of sheet deformation. In fact, all end-systolic fiber-sheet strains were significantly depressed by epicardial pacing (Table 2). Relative contributions of sheet shear, sheet extension or sheet thickening to wall thickening were consistent with the results of Costa et al. (8) and were not altered significantly by epicardial pacing. These findings imply that impaired wall thickening results from lack of effective myocardial deformation, most likely due to insufficient calcium levels at end systole at the pacing site. Intracellular calcium levels at the pacing site may be depleted by the time LV reaches end systole because the pacing site initiates contraction earlier than the rest of the ventricle, and the time interval from electrical activation to end systole is significantly increased (ED-ES duration, Table 1). Moreover, early myofiber shortening at a pacing site is unloaded (14, 28), and unloaded shortening is associated with increased rates of decay of intracellular calcium transient (38).
Reversal of transmural mechanical activation
Our measurements demonstrate that epicardial pacing reversed the transmural mechanical activation sequence, resulting in an earlier onset of myofiber shortening in the epicardium. Notably, this is the first study to show that the onset of fiber shortening in the subendocardium leads that of the subepicardium and that this mechanical sequence is reversed when the transmural sequence of activation is reversed with epicardial pacing. To assess the effect of reversed transmural mechanical activation sequence on transmural deformation, we defined E′, which describe mean transmural myocardial deformation when mechanical activation travels between subendocardium and subepicardium. Because and were not affected by epicardial pacing, these shear deformations do not depend on the transmural sequence of mechanical activation but depend on the magnitudes of fiber shortening. This finding implies that the direction and the magnitude of sheet shear deformation may be determined by local anatomy and supports the concept that sheet shear deformation is required for normal systolic wall thickening (8, 17, 18, 30). In contrast, other E′ strain components were significantly depressed by epicardial pacing. For example, and were significantly reduced when “after-load” was low. These results suggest that a normal transmural mechanical activation sequence may be required for normal sheet extension and therefore wall thickening. Normal endocardial-epicardial activation sequence may allow optimal co-ordination of sequential fiber shortening and sheet mechanics across the wall to maximize wall thickening.
Limitations
Our data describe transmural mechanics in the LV midanterior wall from the epicardium to 90% wall depth. Regional and transmural variations should be taken into consideration when these results are extrapolated to other regions of the LV, such as the lateral, posterior wall and the septum. In addition, the 3-D finite strains that we measured in open-chest, anesthetized dogs may not accurately reflect the transmural mechanics in closed-chest, conscious animals. Finally, the magnitude of baseline dP/dtmax and dP/dtmin was relatively high for open-chest dogs in this study, most likely due to dopamine use in two of five animals during surgical procedure, which may have affected the normal transmural mechanics. To minimize the confounding effects of surgery, anesthesia, and dopamine between normal AV conduction and epicardial pacing, two pacing modes were studied consecutively in a short period of time.
In conclusion, we demonstrate that impaired end-systolic wall thickening at the epicardial pacing site was not due to selective reduction of sheet shear but rather resulted from overall depression of fiber-sheet deformation, and relative contributions of sheet strains to wall thickening were maintained. These findings suggest lack of effective end-systolic myocardial deformation at the pacing site, most likely because the pacing site initiates contraction significantly earlier than the rest of the ventricle. Epicardial pacing also induced reversal of the transmural mechanical activation sequence, which significantly depressed sheet extension and wall thickening early in the cardiac cycle, whereas transverse shear and sheet shear deformation were not affected. These findings suggest that normal sheet extension and wall thickening immediately after activation may require normal transmural activation sequence, whereas sheet shear deformation may be determined by local anatomy.
ACKNOWLEDGMENTS
We thank Rish Pavelec and Rachel Alexander for excellent managerial and surgical assistance. We also acknowledge outstanding technical assistance of Satoko Nagato for data analysis.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants HL-29589 (to N. B. Ingels, Jr.) and HL-32583 (to J. W. Covell). H. Ashikaga is a recipient of the American Heart Association Postdoctoral Fellowship (Western States Affiliate).
REFERENCES
- 1.Antzelevitch C, Shimizu W, Yan GX, Sicouri S, Weissenburger J, Nesterenko VV, Burashnikov A, Di Diego J, Saffitz J, Thomas GP. The M cell: its contribution to the ECG and to normal and abnormal electrical function of the heart. J Cardiovasc Electrophysiol. 1999;10:1124–1152. doi: 10.1111/j.1540-8167.1999.tb00287.x. [DOI] [PubMed] [Google Scholar]
- 2.Ashikaga H, Criscione JC, Omens JH, Covell JW, Ingels NB., Jr Transmural left ventricular mechanics underlying torsional recoil during relaxation. Am J Physiol Heart Circ Physiol. 2004;286:H640–H647. doi: 10.1152/ajpheart.00575.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auricchio A, Stellbrink C, Block M, Sack S, Vogt J, Bakker P, Klein H, Kramer A, Ding J, Salo R, Tockman B, Pochet T, Spinelli J. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. The Pacing Therapies for Congestive Heart Failure Study Group. The Guidant Congestive Heart Failure Research Group. Circulation. 1999;99:2993–3001. doi: 10.1161/01.cir.99.23.2993. [DOI] [PubMed] [Google Scholar]
- 4.Bazett JC. An analysis of time relation of electrocardiograms. Heart. 1920;7:353–367. [Google Scholar]
- 5.Blanc JJ, Etienne Y, Gilard M, Mansourati J, Munier S, Boschat J, Benditt DG, Lurie KG. Evaluation of different ventricular pacing sites in patients with severe heart failure: results of an acute hemodynamic study. Circulation. 1997;96:3273–3277. doi: 10.1161/01.cir.96.10.3273. [DOI] [PubMed] [Google Scholar]
- 6.Cazeau S, Ritter P, Lazarus A, Gras D, Backdach H, Mundler O, Mugica J. Multisite pacing for end-stage heart failure: early experience. Pacing Clin Electrophysiol. 1996;19:1748–1757. doi: 10.1111/j.1540-8159.1996.tb03218.x. [DOI] [PubMed] [Google Scholar]
- 7.Costa KD, May-Newman K, Farr D, O’Dell WG, McCulloch AD, Omens JH. Three-dimensional residual strain in midanterior canine left ventricle. Am J Physiol Heart Circ Physiol. 1997;273:H1968–H1976. doi: 10.1152/ajpheart.1997.273.4.h1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa KD, Takayama Y, McCulloch AD, Covell JW. Laminar fiber architecture and three-dimensional systolic mechanics in canine ventricular myocardium. Am J Physiol Heart Circ Physiol. 1999;276:H595–H607. doi: 10.1152/ajpheart.1999.276.2.H595. [DOI] [PubMed] [Google Scholar]
- 9.Delhaas T, Arts T, Prinzen FW, Reneman RS. Relation between regional electrical activation time and subepicardial fiber strain in the canine left ventricle. Pflügers Arch. 1993;423:78–87. doi: 10.1007/BF00374964. [DOI] [PubMed] [Google Scholar]
- 10.Di Diego JM, Belardinelli L, Antzelevitch C. Cisapride-induced transmural dispersion of repolarization and torsade de pointes in the canine left ventricular wedge preparation during epicardial stimulation. Circulation. 2003;108:1027–1033. doi: 10.1161/01.CIR.0000085066.05180.40. [DOI] [PubMed] [Google Scholar]
- 11.Faris OP, Evans FJ, Dick AJ, Raman VK, Ennis DB, Kass DA, McVeigh ER. Endocardial versus epicardial electrical synchrony during LV free-wall pacing. Am J Physiol Heart Circ Physiol. 2003;285:H1864–H1870. doi: 10.1152/ajpheart.00282.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frazier DW, Krassowska W, Chen PS, Wolf PD, Danieley ND, Smith WM, Ideker RE. Transmural activations and stimulus potentials in three-dimensional anisotropic canine myocardium. Circ Res. 1988;63:135–146. doi: 10.1161/01.res.63.1.135. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson MO, Glasson JR, Bolger AF, Daughters GT, Komeda M, Foppiano LE, Miller DC, Ingels NB., Jr Mitral valve opening in the ovine heart. Am J Physiol Heart Circ Physiol. 1998;274:H552–H563. doi: 10.1152/ajpheart.1998.274.2.H552. [DOI] [PubMed] [Google Scholar]
- 14.Kass DA. Ventricular resynchronization: pathophysiology and identification of responders. Rev Cardiovasc Med. 2003;4 Suppl 2:S3–S13. [PubMed] [Google Scholar]
- 15.Kass DA, Chen CH, Curry C, Talbot M, Berger R, Fetics B, Nevo E. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999;99:1567–1573. doi: 10.1161/01.cir.99.12.1567. [DOI] [PubMed] [Google Scholar]
- 16.Leclercq C, Cazeau S, Le Breton H, Ritter P, Mabo P, Gras D, Pavin D, Lazarus A, Daubert JC. Acute hemodynamic effects of biventricular DDD pacing in patients with end-stage heart failure. J Am Coll Cardiol. 1998;32:1825–1831. doi: 10.1016/s0735-1097(98)00492-6. [DOI] [PubMed] [Google Scholar]
- 17.LeGrice IJ, Smaill BH, Chai LZ, Edgar SG, Gavin JB, Hunter PJ. Laminar structure of the heart: ventricular myocyte arrangement and connective tissue architecture in the dog. Am J Physiol Heart Circ Physiol. 1995;269:H571–H582. doi: 10.1152/ajpheart.1995.269.2.H571. [DOI] [PubMed] [Google Scholar]
- 18.LeGrice IJ, Takayama Y, Covell JW. Transverse shear along myocardial cleavage planes provides a mechanism for normal systolic wall thickening. Circ Res. 1995;77:182–193. doi: 10.1161/01.res.77.1.182. [DOI] [PubMed] [Google Scholar]
- 19.Libbus I, Rosenbaum DS. Transmural action potential changes underlying ventricular electrical remodeling. J Cardiovasc Electrophysiol. 2003;14:394–402. doi: 10.1046/j.1540-8167.2003.02436.x. [DOI] [PubMed] [Google Scholar]
- 20.MacKay SA, Potel MJ, Rubin JM. Graphics methods for tracking three-dimensional heart wall motion. Comput Biomed Res. 1982;15:455–473. doi: 10.1016/0010-4809(82)90027-1. [DOI] [PubMed] [Google Scholar]
- 21.McVeigh ER, Prinzen FW, Wyman BT, Tsitlik JE, Halperin HR, Hunter WC. Imaging asynchronous mechanical activation of the paced heart with tagged MRI. Magn Reson Med. 1998;39:507–513. doi: 10.1002/mrm.1910390402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medina-Ravell VA, Lankipalli RS, Yan GX, Antzelevitch C, Medina-Malpica NA, Medina-Malpica OA, Droogan C, Kowey PR. Effect of epicardial or biventricular pacing to prolong QT interval and increase transmural dispersion of repolarization: does resynchronization therapy pose a risk for patients predisposed to long QT or torsade de pointes? Circulation. 2003;107:740–746. doi: 10.1161/01.cir.0000048126.07819.37. [DOI] [PubMed] [Google Scholar]
- 23.Meier GD, Ziskin MC, Santamore WP, Bove AA. Kinematics of the beating heart. IEEE Trans Biomed Eng. 1980;27:319–329. doi: 10.1109/TBME.1980.326740. [DOI] [PubMed] [Google Scholar]
- 24.Nelson GS, Curry CW, Wyman BT, Kramer A, Declerck J, Talbot M, Douglas MR, Berger RD, McVeigh ER, Kass DA. Predictors of systolic augmentation from left ventricular preexcitation in patients with dilated cardiomyopathy and intraventricular conduction delay. Circulation. 2000;101:2703–2709. doi: 10.1161/01.cir.101.23.2703. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen PM, Le Grice IJ, Smaill BH, Hunter PJ. Mathematical model of geometry and fibrous structure of the heart. Am J Physiol Heart Circ Physiol. 1991;260:H1365–H1378. doi: 10.1152/ajpheart.1991.260.4.H1365. [DOI] [PubMed] [Google Scholar]
- 26.Pouleur H, Rousseau MF, van Eyll C, Brasseur LA, Charlier AA. Force-velocity-length relations in hypertrophic cardiomyopathy: evidence of normal or depressed myocardial contractility. Am J Cardiol. 1983;52:813–817. doi: 10.1016/0002-9149(83)90420-4. [DOI] [PubMed] [Google Scholar]
- 27.Prinzen FW, Augustijn CH, Arts T, Allessie MA, Reneman RS. Redistribution of myocardial fiber strain and blood flow by asynchronous activation. Am J Physiol Heart Circ Physiol. 1990;259:H300–H308. doi: 10.1152/ajpheart.1990.259.2.H300. [DOI] [PubMed] [Google Scholar]
- 28.Prinzen FW, Hunter WC, Wyman BT, McVeigh ER. Mapping of regional myocardial strain and work during ventricular pacing: experimental study using magnetic resonance imaging tagging. J Am Coll Cardiol. 1999;33:1735–1742. doi: 10.1016/s0735-1097(99)00068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saxon LA, Kerwin WF, Cahalan MK, Kalman JM, Olgin JE, Foster E, Schiller NB, Shinbane JS, Lesh MD, Merrick SH. Acute effects of intraoperative multisite ventricular pacing on left ventricular function and activation/contraction sequence in patients with depressed ventricular function. J Cardiovasc Electrophysiol. 1998;9:13–21. doi: 10.1111/j.1540-8167.1998.tb00862.x. [DOI] [PubMed] [Google Scholar]
- 30.Spotnitz HM, Spotnitz WD, Cottrell TS, Spiro D, Sonnenblick EH. Cellular basis for volume related wall thickness changes in the rat left ventricle. J Mol Cell Cardiol. 1974;6:317–331. doi: 10.1016/0022-2828(74)90074-1. [DOI] [PubMed] [Google Scholar]
- 31.Streeter DD, Jr, Spotnitz HM, Patel DP, Ross J, Jr, Sonnenblick EH. Fiber orientation in the canine left ventricle during diastole and systole. Circ Res. 1969;24:339–347. doi: 10.1161/01.res.24.3.339. [DOI] [PubMed] [Google Scholar]
- 32.Takayama Y, Costa KD, Covell JW. Contribution of laminar myofiber architecture to load-dependent changes in mechanics of LV myocardium. Am J Physiol Heart Circ Physiol. 2002;282:H1510–H1520. doi: 10.1152/ajpheart.00261.2001. [DOI] [PubMed] [Google Scholar]
- 33.Waldman LK, Covell JW. Effects of ventricular pacing on finite deformation in canine left ventricles. Am J Physiol Heart Circ Physiol. 1987;252:H1023–H1030. doi: 10.1152/ajpheart.1987.252.5.H1023. [DOI] [PubMed] [Google Scholar]
- 34.Weiss JL, Frederiksen JW, Weisfeldt ML. Hemodynamic determinants of the time-course of fall in canine left ventricular pressure. J Clin Invest. 1976;58:751–760. doi: 10.1172/JCI108522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wyman BT, Hunter WC, Prinzen FW, Faris OP, McVeigh ER. Effects of single- and biventricular pacing on temporal and spatial dynamics of ventricular contraction. Am J Physiol Heart Circ Physiol. 2002;282:H372–H379. doi: 10.1152/ajpheart.2002.282.1.H372. [DOI] [PubMed] [Google Scholar]
- 36.Wyman BT, Hunter WC, Prinzen FW, McVeigh ER. Mapping propagation of mechanical activation in the paced heart with MRI tagging. Am J Physiol Heart Circ Physiol. 1999;276:H881–H891. doi: 10.1152/ajpheart.1999.276.3.H881. [DOI] [PubMed] [Google Scholar]
- 37.Yan GX, Antzelevitch C. Cellular basis for the normal T wave and the electrocardiographic manifestations of the long-QT syndrome. Circulation. 1998;98:1928–1936. doi: 10.1161/01.cir.98.18.1928. [DOI] [PubMed] [Google Scholar]
- 38.Yasuda S, Sugiura S, Yamashita H, Nishimura S, Saeki Y, Momomura S, Katoh K, Nagai R, Sugi H. Unloaded shortening increases peak of Ca2+ transients but accelerates their decay in rat single cardiac myocytes. Am J Physiol Heart Circ Physiol. 2003;285:H470–H475. doi: 10.1152/ajpheart.00012.2003. [DOI] [PubMed] [Google Scholar]
- 39.Yoran C, Covell JW, Ross J., Jr Rapid fixation of the left ventricle: continuous angiographic and dynamic recordings. J Appl Physiol. 1973;35:155–157. doi: 10.1152/jappl.1973.35.1.155. [DOI] [PubMed] [Google Scholar]