Abstract
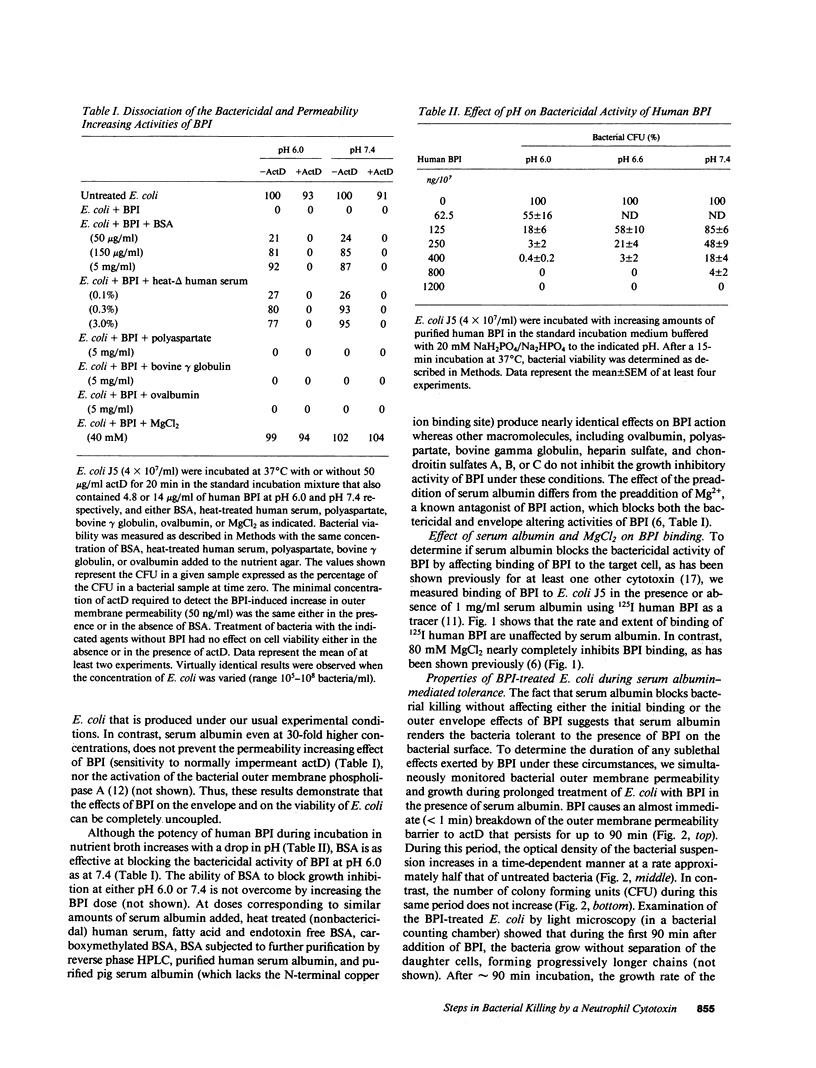
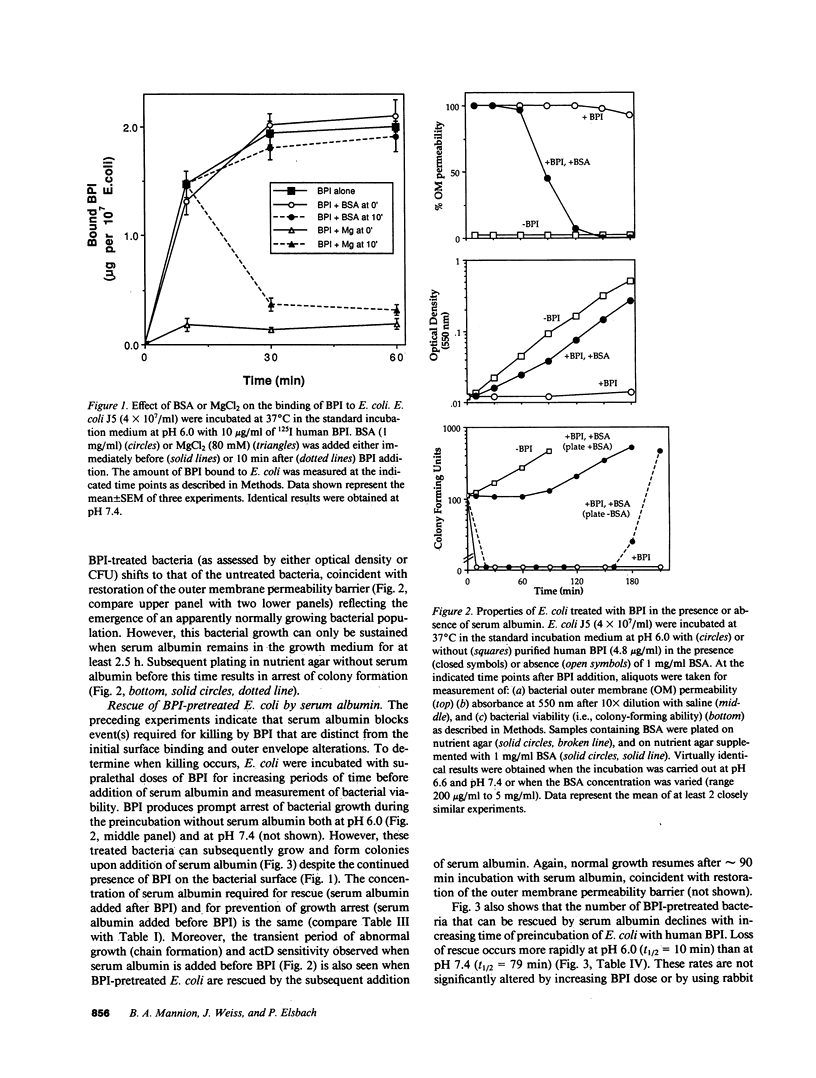
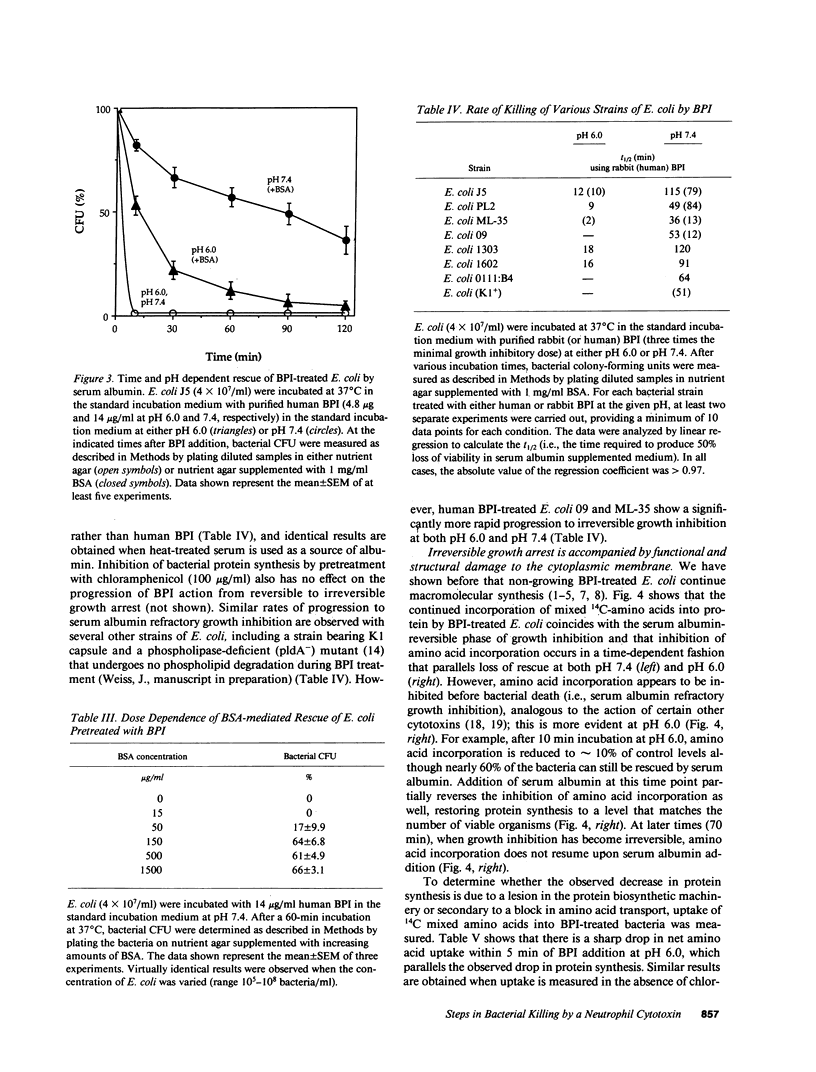
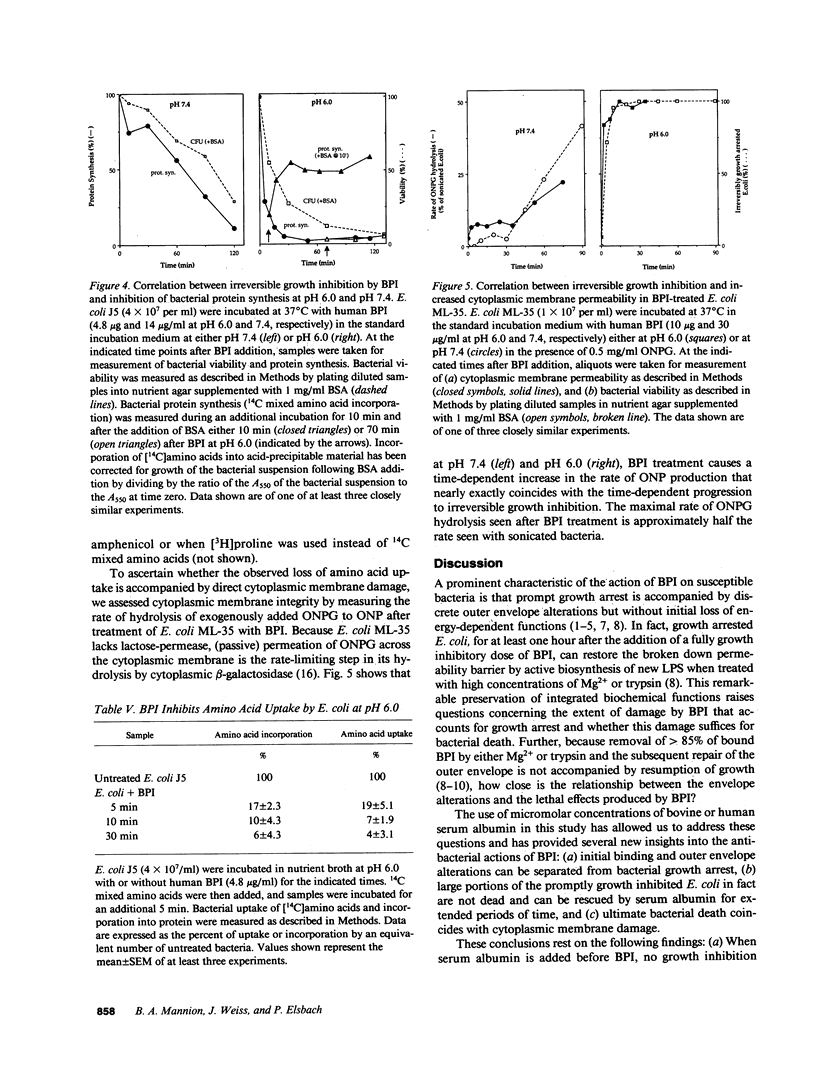
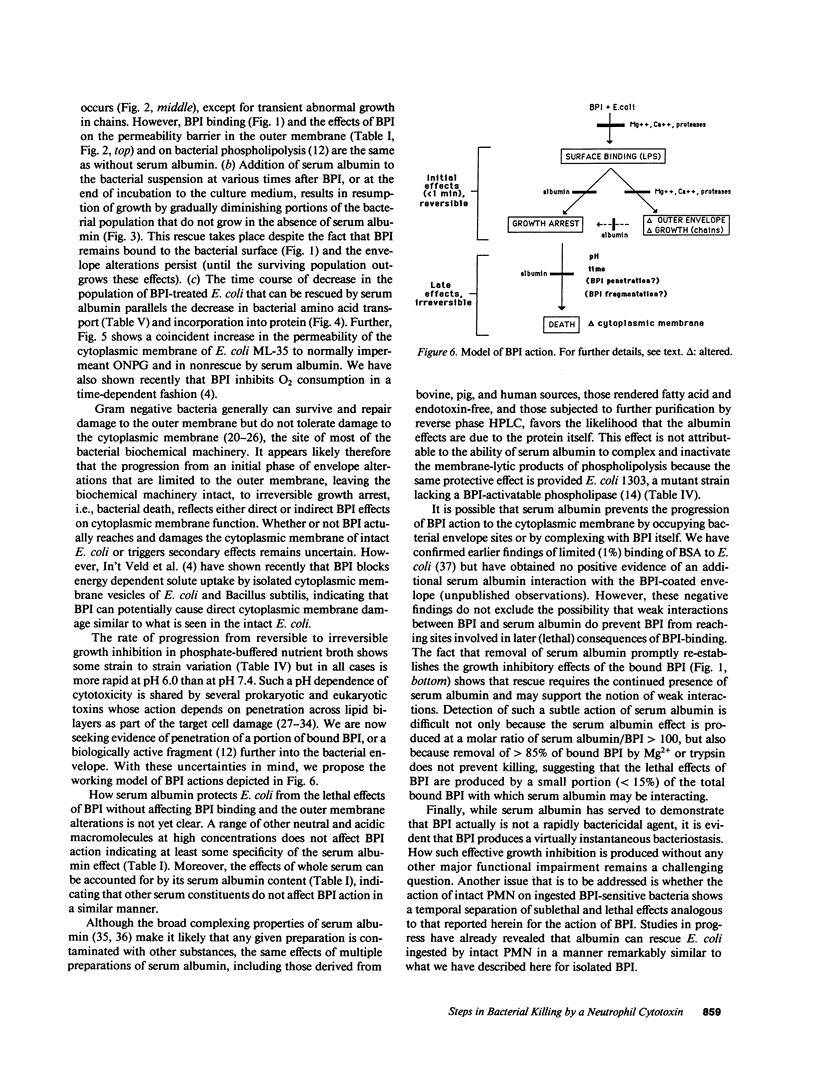
Binding of the bactericidal/permeability increasing protein (BPI) of granulocytes to Escherichia coli promptly produces several discrete outer envelope alterations and growth arrest without major impairment of bacterial structure or biosynthetic capabilities, raising the question whether these early effects of BPI are sufficient to cause bacterial death. In this study, the bactericidal action of BPI was examined more closely. We have found that bovine or human serum albumin blocks bacterial killing without preventing BPI binding or an increase in outer membrane permeability. Moreover, addition of serum albumin after BPI results in growth resumption without displacement of bound BPI and without (early) repair of the envelope alterations. These effects are opposite to those produced by Mg2+ (80 mM), which displaces greater than 85% of bound BPI and rapidly initiates outer envelope repair without restoration of bacterial growth. The extent of rescue by serum albumin depends on the time and pH of preincubation of BPI with E. coli: e.g., for E. coli J5 treated with human BPI, t1/2 = 79 min at pH 7.4 and 10 min at pH 6.0. The serum albumin effects on BPI action are the same in wild-type E. coli and in a mutant strain lacking an activatable phospholipase, indicating that serum albumin does not act by sequestering membrane-damaging products of bacterial phospholipid hydrolysis. The progression from reversible to irreversible growth arrest, revealed by the subsequent addition of serum albumin at different times, is paralleled by a decrease in amino acid uptake and an increase in the permeability of the cytoplasmic membrane to o-nitrophenyl-beta-D-galactoside. These findings demonstrate at least two stages in the action of BPI: (a) an early, reversible, sublethal stage in which BPI has effects on the outer envelope and causes growth arrest, and (b) time- and pH-dependent progression to a lethal stage, apparently involving cytoplasmic membrane damage, possibly caused by penetration of a small subpopulation of BPI.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. J., Wilson J. M., Oxender D. L. Defective transport and other phenotypes of a periplasmic "leaky" mutant of Escherichia coli K-12. J Bacteriol. 1979 Nov;140(2):351–358. doi: 10.1128/jb.140.2.351-358.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerdite S., Mooney C., Weiss J., Franson R., Elsbach P. Early and discrete changes in permeability of Escherichia coli and certain other gram-negative bacteria during killing by granulocytes. J Exp Med. 1974 Aug 1;140(2):396–409. doi: 10.1084/jem.140.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Kuller G., Muhly M., Fromm S., Seibert G., Parrisius J. Formation of transmural complement pores in serum-sensitive Escherichia coli. Infect Immun. 1987 Jan;55(1):206–210. doi: 10.1128/iai.55.1.206-210.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankert J. R., Esser A. F. Complement-mediated killing of Escherichia coli: dissipation of membrane potential by a C9-derived peptide. Biochemistry. 1986 Mar 11;25(5):1094–1100. doi: 10.1021/bi00353a023. [DOI] [PubMed] [Google Scholar]
- Dankert J., Hammond S. M., Cramer W. A. Reversal by trypsin of the inhibition of active transport by colicin E1. J Bacteriol. 1980 Aug;143(2):594–602. doi: 10.1128/jb.143.2.594-602.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson V. L., Brunden K. R., Cramer W. A. Acidic pH requirement for insertion of colicin E1 into artificial membrane vesicles: relevance to the mechanism of action of colicins and certain toxins. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1386–1390. doi: 10.1073/pnas.82.5.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper R. K., O'Keefe D. O., Stookey M., Graves J. Identification of a cold-sensitive step in the mechanism of modeccin action. J Biol Chem. 1984 Apr 10;259(7):4083–4088. [PubMed] [Google Scholar]
- Elsbach P., Beckerdite S., Pettis P., Franson R. Persistence of regulation of macromolecular synthesis by Escherichia coli during killing by disrupted rabbit granulocytes. Infect Immun. 1974 Apr;9(4):663–668. doi: 10.1128/iai.9.4.663-668.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsbach P., Weiss J., Franson R. C., Beckerdite-Quagliata S., Schneider A., Harris L. Separation and purification of a potent bactericidal/permeability-increasing protein and a closely associated phospholipase A2 from rabbit polymorphonuclear leukocytes. Observations on their relationship. J Biol Chem. 1979 Nov 10;254(21):11000–11009. [PubMed] [Google Scholar]
- Escuyer V., Boquet P., Perrin D., Montecucco C., Mock M. A pH-induced increase in hydrophobicity as a possible step in the penetration of colicin E3 through bacterial membranes. J Biol Chem. 1986 Aug 15;261(23):10891–10898. [PubMed] [Google Scholar]
- Feingold D. S., Goldman J. N., Kuritz H. M. Locus of the lethal event in the serum bactericidal reaction. J Bacteriol. 1968 Dec;96(6):2127–2131. doi: 10.1128/jb.96.6.2127-2131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kragh-Hansen U. Molecular aspects of ligand binding to serum albumin. Pharmacol Rev. 1981 Mar;33(1):17–53. [PubMed] [Google Scholar]
- LEIVE L. A NONSPECIFIC INCREASE IN PERMEABILITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Proc Natl Acad Sci U S A. 1965 Apr;53:745–750. doi: 10.1073/pnas.53.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Barton A., Ganz T. Concurrent assessment of inner and outer membrane permeabilization and bacteriolysis in E. coli by multiple-wavelength spectrophotometry. J Immunol Methods. 1988 Apr 6;108(1-2):153–158. doi: 10.1016/0022-1759(88)90414-0. [DOI] [PubMed] [Google Scholar]
- Lichtenstein A. K., Ganz T., Nguyen T. M., Selsted M. E., Lehrer R. I. Mechanism of target cytolysis by peptide defensins. Target cell metabolic activities, possibly involving endocytosis, are crucial for expression of cytotoxicity. J Immunol. 1988 Apr 15;140(8):2686–2694. [PubMed] [Google Scholar]
- Lugtenberg B., Peters R., Bernheimer H., Berendsen W. Influence of cultural conditions and mutations on the composition of the outer membrane proteins of Escherichia coli. Mol Gen Genet. 1976 Sep 23;147(3):251–262. doi: 10.1007/BF00582876. [DOI] [PubMed] [Google Scholar]
- Mannion B. A., Kalatzis E. S., Weiss J., Elsbach P. Preferential binding of the neutrophil cytoplasmic granule-derived bactericidal/permeability increasing protein to target bacteria. Implications and use as a means of purification. J Immunol. 1989 Apr 15;142(8):2807–2812. [PubMed] [Google Scholar]
- Marnell M. H., Shia S. P., Stookey M., Draper R. K. Evidence for penetration of diphtheria toxin to the cytosol through a prelysosomal membrane. Infect Immun. 1984 Apr;44(1):145–150. doi: 10.1128/iai.44.1.145-150.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. E., Saelinger C. B. Reduced temperature alters Pseudomonas exotoxin A entry into the mouse LM cell. Infect Immun. 1986 May;52(2):445–453. doi: 10.1128/iai.52.2.445-453.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myhre E. B., Kronvall G. Demonstration of specific binding sites for human serum albumin in group C and G streptococci. Infect Immun. 1980 Jan;27(1):6–14. doi: 10.1128/iai.27.1.6-14.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe D., Collier R. J. Cloned diphtheria toxin within the periplasm of Escherichia coli causes lethal membrane damage at low pH. Proc Natl Acad Sci U S A. 1989 Jan;86(1):343–346. doi: 10.1073/pnas.86.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi C. E., Weiss J., Elsbach P., Frangione B., Mannion B. A 25-kDa NH2-terminal fragment carries all the antibacterial activities of the human neutrophil 60-kDa bactericidal/permeability-increasing protein. J Biol Chem. 1987 Nov 5;262(31):14891–14894. [PubMed] [Google Scholar]
- Peters T., Jr Serum albumin. Adv Protein Chem. 1985;37:161–245. doi: 10.1016/s0065-3233(08)60065-0. [DOI] [PubMed] [Google Scholar]
- SIMON E. J., VANPRAAG D. INHIBITION OF RNA SYNTHESIS IN ESCHERICHIA COLI BY LEVORPHANOL. Proc Natl Acad Sci U S A. 1964 May;51:877–883. doi: 10.1073/pnas.51.5.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S. Diphtheria toxin entry into cells is facilitated by low pH. J Cell Biol. 1980 Dec;87(3 Pt 1):828–832. doi: 10.1083/jcb.87.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaara M., Vaara T. Sensitization of Gram-negative bacteria to antibiotics and complement by a nontoxic oligopeptide. Nature. 1983 Jun 9;303(5917):526–528. doi: 10.1038/303526a0. [DOI] [PubMed] [Google Scholar]
- Weiss J., Elsbach P., Olsson I., Odeberg H. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J Biol Chem. 1978 Apr 25;253(8):2664–2672. [PubMed] [Google Scholar]
- Weiss J., Franson C., Schmeidler K., Elsbach P. Reversible envelope effects during and after killing of Escherichia coli w by a highly-purified rabbit polymorpho-nuclear leukocyte fraction. Biochim Biophys Acta. 1976 Jun 4;436(1):154–169. doi: 10.1016/0005-2736(76)90227-3. [DOI] [PubMed] [Google Scholar]
- Weiss J., Franson R. C., Beckerdite S., Schmeidler K., Elsbach P. Partial characterization and purification of a rabbit granulocyte factor that increases permeability of Escherichia coli. J Clin Invest. 1975 Jan;55(1):33–42. doi: 10.1172/JCI107915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Muello K., Victor M., Elsbach P. The role of lipopolysaccharides in the action of the bactericidal/permeability-increasing neutrophil protein on the bacterial envelope. J Immunol. 1984 Jun;132(6):3109–3115. [PubMed] [Google Scholar]
- Weiss J., Victor M., Elsbach P. Role of charge and hydrophobic interactions in the action of the bactericidal/permeability-increasing protein of neutrophils on gram-negative bacteria. J Clin Invest. 1983 Mar;71(3):540–549. doi: 10.1172/JCI110798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Geus P., van Die I., Bergmans H., Tommassen J., de Haas G. Molecular cloning of pldA, the structural gene for outer membrane phospholipase of E. coli K12. Mol Gen Genet. 1983;190(1):150–155. doi: 10.1007/BF00330338. [DOI] [PubMed] [Google Scholar]
- in't Veld G., Mannion B., Weiss J., Elsbach P. Effects of the bactericidal/permeability-increasing protein of polymorphonuclear leukocytes on isolated bacterial cytoplasmic membrane vesicles. Infect Immun. 1988 May;56(5):1203–1208. doi: 10.1128/iai.56.5.1203-1208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



