Abstract
The leading joint hypothesis (LJH) offers a novel interpretation of control of human movements that involve multiple joints. The LJH makes control of each multijoint movement transparent. This review highlights effective applications of the LJH to learning of new motor skills and to analysis of movement changes caused by aging and motor disorders.
Keywords: arm movement, intersegmental dynamics, torque analysis, coordination, motor learning, motor disorder
INTRODUCTION
Unraveling the principle used by the brain to organize human movements is one of the primary goals of motor control research. The need for a principle or a control strategy is apparent when the task can be performed via many different movements, yet a single movement needs to be produced. However, an organizing principle is also necessary if the task can be performed with a single motion because the pattern of muscle activation that results in this motion still needs to be determined. A number of theories interpreting control of human movements have been offered. The leading joint hypothesis (LJH) is an alternative interpretation that my colleagues and I have recently proposed (4,6,9). The goal of this review was to demonstrate exclusive opportunities provided by the LJH for analysis of control of human movements. A distinctive advantage of the LJH is that it makes control of limb movements transparent. This approach reveals both the control strategy applied to the entire limb and specific features of control at each participating joint. This advantage opens unique perspectives for unraveling the organization of various types of movements and for analysis of changes in movement control caused by development, motor learning, aging, and motor disorders.
Before describing the LJH, previous approaches are briefly considered.
PREVIOUS INTERPRETATIONS OF CONTROL OF HUMAN MOVEMENTS
The major theories that have been formulated to account for control of human movements are the inverse dynamics approach, generalized motor program theory, equilibrium point hypothesis, and optimal control approach. Although each of these approaches has provided invaluable contribution to development of our knowledge about human movement control, they all have significant limitations.
The inverse dynamics approach suggests that the CNS possesses a detailed biomechanical model of the limbs and manipulated objects. It plans a movement capable to perform the task and uses this kinematic plan as an input to the biomechanical model, the output of which is a set of joint torques that would implement the intended movement (19). The major problem associated with this interpretation is that the biomechanical model needs to be highly detailed. The lack of a component capable to provide adaptation to uncertainties of the model and adjustments to unexpected changes makes this type of control inflexible.
The generalized motor program theory proposes that a set of control commands required for performance of each particular movement is stored in memory (29). The control commands can be retrieved and executed, similar to a computer program. Although the theory suggests that motor programs have parameters that can be modified to provide variations in movement rate and amplitude, flexibility of control still is limited within this interpretation as well. In particular, this theory suggests that a motor program needs to be learned independently for each movement type. This organization of control is in conflict with experimental findings showing that learning of specific movements may generalize to movements that require completely different patterns of muscular control (3,15) and hence different motor programs.
The equilibrium point hypothesis is based on a spring-like approximation of muscle properties (10). It postulates that the resting length of each muscle is centrally manipulated. Changing of the resting length results in changes in elastic force generated by the muscle in a given joint position. This provides a mechanism for creating a difference between elastic forces generated by the two antagonistic muscle groups and thus for a transition of the joint to a new equilibrium, that is, to a position in which the two forces are equal. An attractive feature of this interpretation is its simplicity in application to single-joint movements. However, this simplicity disappears when temporal characteristics of typical single-joint movements are interpreted. Control of movements that involve more than a single joint is even less clear within this approach.
According to the optimal control approach, neural commands to the muscles are a result of the CNS’s solving a problem of optimization of a specific cost function, such as muscle energy expenditure, movement time, accuracy, smoothness, and others (30). Because factors that influence task performance are multiple, it is plausible that the cost function is a task-dependent weighted sum of several criteria. Although this approach has strong theoretical grounds, dominant factors determining movement cost remain unknown.
In addition to the specific shortcomings indicated for each of the previous approaches, a common disadvantage is that it is difficult to use any of them for practical applications, that is, to account for control at each joint during each particular movement, to formulate effective recommendations during motor learning, and to decipher movement changes caused by motor disabilities. Recently, we have proposed an LJH as an alternative interpretation of control of human movements (4,6,9,23). Next, this hypothesis is discussed in detail, and its great potential for diverse practical applications is illustrated with accumulated evidence.
THE LEADING JOINT HYPOTHESIS
Definition and Underlying Principles
The LJH is based on the idea that the CNS exploits the biomechanical properties of the limbs for movement organization. One of the most influential biomechanical properties of human limbs is that they are linkages of several segments. This multijoint structure causes motion-dependent mechanical interactions among the segments represented by passive “interaction” torque (INT) exerted at each joint. Interaction torque has a complex, highly nonlinear nature, making motions at all joints of the limb interdependent. Interaction torque escalates with increases in movement speed, and therefore is highly influential even during moderate movement speed. Although other approaches view INT as a by-product of generated movement, the LJH suggests that this torque is purposefully generated to exploit the multijoint structure of the limbs for movement production.
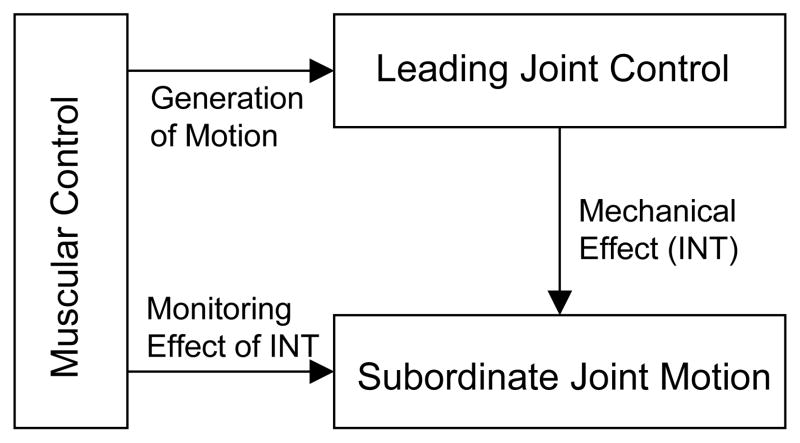
More specifically, the LJH suggests that joints of a multiarticular limb play different roles in movement production according to their mechanical subordination in the joint linkage. There is one (leading) joint that creates a dynamic foundation for motion of the entire limb. Acceleration/deceleration at the leading joint is produced by simple, reciprocal muscle activity in the same way as during single-joint movements, that is, largely disregarding the effect of other joint motions. In contrast, INT generated by leading joint motion produces powerful effect on motion at the other (subordinate) joints. The role of the subordinate joint musculature is to monitor the INT effect and to create net torque (NET) that results in limb motion characteristics required by the task, including movement direction, accuracy, and so on. This organization of joint control is schematically presented in Figure 1.
Figure 1.
Schematic representation of organization of control of multijoint movements suggested by the LJH. The control exploits passive INT generated at the subordinate joint by leading joint motion.
The leading role is endowed to a joint that has mechanical advantage in the limb. Because of relatively high inertia and the increased musculature of the proximal limb segment, the mechanical influence of proximal joint motion on distal joints is much higher than the influence of distal joint motion on proximal joints. For this reason, the leading joint is often the proximal joint that acts similar to a whip handle, a single wave of which can cause complex motion of the cord. However, the choice of the leading joint also depends on the task. If a task requires much smaller range of motion at the proximal than the distal joint, the mechanical effect of the proximal joint would be minor, and therefore, the distal joint may be more suitable for the leading role. In both cases, each movement is performed by exploiting a specific mechanical effect that can be generated through motion of a single joint. This effect creates a dynamic foundation for motion of the entire limb by exerting passive INT at the other (subordinate) joints. Subordinate joint musculature intervenes with passive motion and adjusts it to requirements of the task.
The dependence of the control strategy on the specific properties of the limb is a unique feature of the LJH that differentiates it from all previous interpretations. For instance, whatever the limb’s biomechanical properties are, the optimal control strategy is to optimize a certain cost function. The inverse dynamics control strategy consists in the usage of the limb’s biomechanical model (whatever it is) for computations of joint torques that need to be generated by muscles to produce a required motion. Control developed with these strategies will, of course, depend on limb biomechanics, but the strategies can be applied to any mechanical system. In contrast, the idea underlying the LJH is that the strategies of movement control are highly specific to the biomechanics of the limbs, and they are not applicable for control of objects with substantially different mechanical properties. Strategies of this type are similar to those we use to produce motion of mechanical objects. For instance, a hammer is an object that we use according to its mechanical properties to produce specific movements for specific purposes. Similarly, the mechanical properties of the limbs determine the organization of control and types of movements that can be performed.
Supporting Evidence
The LJH has been tested predominantly with horizontal arm movements (e.g., see [6,8,14]), although other movement types have also been examined (e.g., see [9,13,17]). Typically, the leading and subordinate joints are distinguished through a comparison of the contribution of torque generated by muscles (muscle torque, MUS) and INT (and other passive torques, including gravitational torque, if the movement is not horizontal) to NET. Control of the leading joint is characterized by the dominance of MUS in generation of NET. At the subordinate joint, contribution of passive torques in NET is usually substantial, whereas the role of MUS is to control the effect of passive torque by complimenting or dampening it.
This organization of control can be exemplified by an analysis of horizontal shoulder-elbow movements performed to cyclically draw nine different contours: a circle, four ovals, and four lines of different orientations (6). Torque and EMG data demonstrated that shoulder control was similar during drawing the majority of the contours. Reciprocal activity of antagonistic shoulder muscles generated MUS that accelerated and decelerated this joint, as during single-joint movements. In contrast, elbow control varied across the contours. Elbow MUS interplayed with INT in different ways, resulting in the different shapes of the hand trajectory. The primary role of the shoulder MUS in generation of movement energy (directly at the shoulder and through INT at the elbow) showed that the shoulder was the leading joint. The significant contribution of INT in the production of elbow motion revealed the subordinate function of the elbow joint. Elbow MUS adjusted passive motion at this joint by resisting or assisting INT, depending on the drawn contour.
The leading role of the shoulder and the subordinate role of the elbow were found in drawing eight of the nine contours. An exception was drawing the right-diagonal (RD) line that was performed primarily with cyclical elbow rotation. The small shoulder motion made the dynamic effect of this joint minor, and therefore, the two joints switched the roles. Elbow MUS generated motion, and shoulder MUS compensated for INT to stabilize the upper arm.
Can the differences in control across the joints simply be a consequence of biomechanical properties of the multijoint limbs rather than a result of strategic exploitation of these properties described by the LJH? Multiple evidence rules out this interpretation. For instance, INT contribution to production of elbow (subordinate joint) motion is higher in the dominant arm compared with the nondominant arm (28). Also, deterioration of the leading-subordinate structure of control is typical for motor disorders, as discussed later in the article. These examples show that the leading and subordinate features of joint control are strategic, and they are not dictated by limb biomechanics.
An advantage of the LJH is that it makes organization of joint control during each movement transparent. Revealing the leading and subordinate joints clarifies both the control strategy applied to the entire limb and to each participating joint. For this reason, the LJH opens opportunity to unravel organization of control of various types of movements, including complex movements that involve motion at multiple joints. It also allows studying the process of motor learning and changes in control caused by it. In addition, the LJH approach provides means to decipher changes in movement control responsible for altered movements in special populations. Examples of each of these LJH applications are provided next.
UNRAVELING CONTROL OF MULTIJOINT MOVEMENTS
Control of a number of various multijoint movements has been successfully analyzed with the use of the LJH. An example is a horizontal arm swing that was performed by rotating the trunk, horizontally extending (abducting) the shoulder, and extending the elbow (23). The task was to develop maximal speed of the hand at a target reached when the arm is almost fully extended. This movement is a component of various sports activities such as a backhand in tennis, throwing a Frisbee, and hitting a ball with a baseball bat.
To decipher control of the horizontal arm swing, MUS power and a control index (CI) were computed (Fig. 2). Control index assessed the relative contribution of MUS and INT to motion generation at each joint. The negative values of CI showed that MUS and INT were opposite in sign at all three joints. The values of trunk CI greater than −1.0 signified that MUS was the dominant source of trunk angular acceleration. At the shoulder, CI ≈ −1.0 signified that MUS and INT compensated for each other and stabilized the shoulder. At the elbow, CI of less than −1.0 showed that INT was the dominant source of motion at this joint. These results demonstrated that active trunk rotation was the primary cause of the entire movement. Energy generated by this motion was transferred through the shoulder to the elbow. This caused swift passive acceleration of the elbow that resulted in high velocity of the hand at the target. This organization of joint control was confirmed with MUS power analysis (Fig. 2B). Trunk MUS generated large, positive power; shoulder MUS power was relatively small; and elbow MUS power was near zero at the beginning of movement and then became negative, showing that MUS slightly dampened passive elbow motion.
Figure 2.
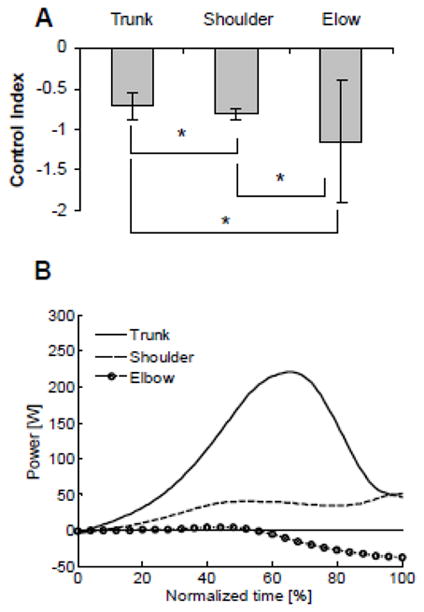
Characteristics of control of trunk rotation and shoulder and elbow extension during horizontal arm swing. A, Mean values of the CI. *Significant differences between levels. B, Power of trunk, shoulder, and elbow MUS. Both characteristics demonstrate that trunk MUS played the dominant role in the production of motion, the shoulder served primarily to transfer the effect of trunk motion to the elbow, and the elbow moved largely passively. (Reprinted from Kim, Y.K., R.N. Hinrichs, and N. Dounskaia. Multicomponent control strategy underlying production of maximal hand velocity during horizontal arm swing. J. Neurophysiol. 2009;102(5):2889–99. Copyright © 2009 The American Physiological Society. Used with permission.)
In addition to the investigation of the general structure of movement organization, a detailed analysis of control at each joint was performed by dividing the entire movement time in 10 successive periods and computing CI in each period for each joint. This analysis revealed that the control structure changed for the last 20% of movement. During this movement portion, the dynamic advantage of the trunk was exhausted, and the shoulder took the leading role, adding to increases in hand velocity. Thus, the analyses revealed both the gross strategy of exploiting the mechanical effect of trunk rotation for performance of the arm swing and the microstructure of control at each joint.
Among other reported studies of control of multijoint movements, two deserve specific attention, an analysis of baseball throwing (17) and of arm movements during piano keystroke (e.g., see [16]). The baseball-throwing study is remarkable because it included analysis of a complex, unconstrained movement of seven degrees of freedom of the upper body. The findings were consistent with the LJH, revealing a hierarchical control strategy in which MUS generated by proximal musculature caused INT beneficial for distal joint rotations. Analyses of the piano keystroke demonstrated the effectiveness of the LJH approach for examination of control of fine movements that require high skill and accuracy.
MOTOR LEARNING
The LJH promotes the idea that motor learning is a process of discovering a biomechanical property of the body that can be used for achieving a desired goal. The LJH predicts that motor learning includes two major stages. First, the motion of a leading joint, which results in movement of the entire limb that approximates the desired movement, needs to be discovered. Second, learning how to control and modify passive motion at the other (subordinate) joints is needed.
Findings of developmental studies are consistent with this interpretation of motor learning. For instance, the adult-like pattern of shoulder motion is observed already at relatively early stages of reaching movements in infants, whereas the development of elbow motion is much more prolonged (26) and is still different from the adult-like pattern even at 3 years of age (24). In particular, early reaching is characterized by restricted elbow motion (1), which may be a “freezing of degrees-of-freedom” strategy used to decrease the effect of INT before the individual masters its exploitation and control. The strategic nature of the elbow movement is supported by an observation that during prereaching period, the elbow is restricted during toy-oriented movements but not during spontaneous arm movements (2).
Studies of skillful movements in adults also provide support for the LJH interpretation of motor learning. For example, comparison of piano keystroke performance between expert pianists and novices demonstrated that expert pianists decrease muscular contribution to control of elbow and wrist motions, delegating this function to INT generated by shoulder motion (e.g., see [12]). This strategy was hypothesized to decrease muscle activity at the distal joints that are especially prone to fatigue during piano playing. Furthermore, during learning of a novel arm movement (21), shoulder (leading joint) motion gradually stabilized, but motion variability at the elbow and wrist (subordinate joints) did not decrease with practice because these joints were used to adjust motion of the arm to the target in each trial. The latter finding is consistent with the prediction of the LJH that the subordinate joints, and not the leading joint, provide accuracy of task performance.
Understanding of the leading/subordinate function of each joint helps to generate instructions on how to improve movement performance. This was demonstrated for the task requiring generation of maximal hand velocity at the target during the horizontal arm swing (23). Comparison between the fastest and slowest movements performed by each participant showed that slower movements were characterized by deterioration in control at both the leading and subordinate joint. During these trials, the dominance of trunk (leading joint) MUS in the production of the entire limb motion decreased. Also, INT was not efficiently exploited at the elbow (subordinate joint) as was revealed by a tendency to increase the contribution of MUS in the production of elbow motion. These findings suggested that to increase hand velocity during the horizontal arm swing, first, leading joint MUS had to strongly suppress passive influence of distal segments, and second, elbow MUS had to minimize INT dampening. This example demonstrates practical benefits of motor learning analysis from the LJH perspective.
A theoretical inference from the LJH interpretation of motor learning is that learning is specific for each movement type. Each set of movements that can be built on a biomechanical effect of a leading joint motion through variations of control at the other joints is learned independently from movements built on another biomechanical effect. The proposed specificity of motor learning is consistent with highly limited transfer of learning (e.g., see [15]).
DECIPHERING CHANGES IN MOVEMENT CONTROL IN SPECIAL POPULATIONS
The LJH promotes a view that movement control is manipulation of the limbs as biomechanical objects that allow us to achieve goals of everyday life. Based on this view, two sources of altered human movements can be distinguished, central and peripheral. The central source is represented by disrupted neural control, that is, by decreased ability to use proper control strategies and to generate proper commands to the muscles. The peripheral source is associated with disturbances in the musculoskeletal system that prevent normal response of the limbs to the control commands. Research suggests that the effect of central and peripheral deficits on movement control is principally different. Whereas central deficits cause decreased ability to manipulate the biomechanical structures of the limbs, peripheral limitations instigate reorganization of central control that compensates for the musculoskeletal impairment by exploiting biomechanical properties of the limbs.
Central Limitations on Movement Control
The LJH provides two specific predictions for deterioration of control due to declined neural processes. First, control at the leading and subordinate joint will be differentially disrupted, depending on the function of each joint in movement production. In a way dependent on the movement disorder, the leading joint would not provide sufficient speed and range of motion upon which to build the required limb movements. The subordinate joint will fail in fine regulation of INT, resulting in changes such as deformation of the hand path and loss of accuracy. Second, the degree of movement disruption will vary across movement types, depending on the demands for timing and magnitude of MUS required for INT control.
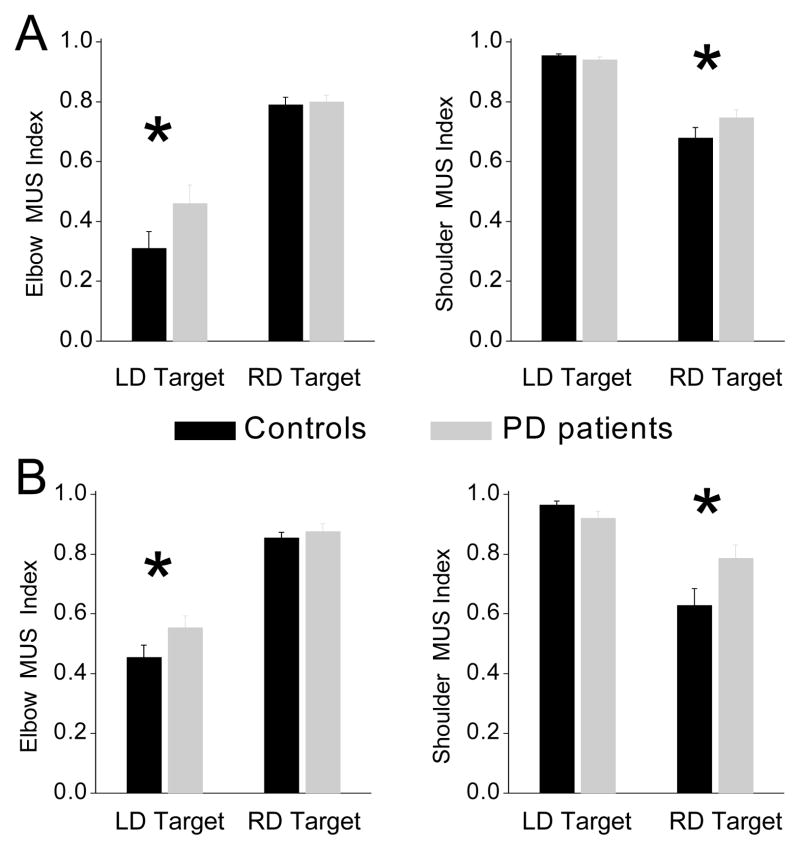
Our studies of movements in Parkinson disease (PD) have provided support for both predictions. During drawing and pointing arm movements performed in the horizontal plane, PD effect on shoulder and elbow control depended on the leading/subordinate function of the joint (5,11). At the leading joint responsible for generation of movement energy, PD predominantly caused underproduction of MUS magnitude. A characteristic feature of subordinate joint control in PD was impaired INT regulation. This effect of PD was found, for instance, with analysis of cyclic and discrete pointing movements to two targets performed by PD patients and control subjects (11). The targets required movements in the left-diagonal (LD) and RD direction. The LD target was reached with the shoulder serving as the leading joint and the elbow serving as the subordinate joint. The RD target required elbow extension and minimal motion at the shoulder, and therefore, the elbow was the leading joint. The role of shoulder MUS during this movement was to interplay with INT and stabilize the upper arm, and therefore, the shoulder was the subordinate joint.
Parkinson disease caused decreases in MUS magnitude. However, this effect was more pronounced at the leading than at the subordinate joint, that is, at the shoulder during LD movements and at the elbow during RD movements. To assess the role of MUS in the production of motion at each joint, an MUS index was computed that varied between 0.0 and 1.0. Values near 1.0 signified dominance of MUS in generation of NET, which is a sign of leading joint control. Values substantially lower than 1.0 pointed to significant contribution of INT to NET and thus to production of subordinate joint motion. Figure 3 demonstrates that during both cyclic and discrete movements, the MUS index was increased in patients compared with control subjects, but only at the subordinate joint, that is, at the elbow during LD movements and at the shoulder during RD movements. The increased MUS index indicated that PD patients exploited INT for production of subordinate joint movement less effectively than did control subjects.
Figure 3.
The shoulder and elbow MUS index in PD patients and controls during (A) cyclic and (B) discrete pointing movements to the LD and RD targets. The asterisks indicate that there was a trend in patients to increase the MUS index at the elbow for the LD target and at the shoulder for the RD target. Data were compiled from Fradet et al. (23).
The obtained results suggested that disruptions in control of each, the leading and subordinate joint, are associated with a specific feature of parkinsonian movements. Bradykinesia (a cardinal PD motor symptom represented by shortened amplitude and movement slowness) predominantly stems from abnormal control of the leading joint, whereas impaired control of subordinate joints is a locus of dyscoordination of joint motions, which is another typical dysfunction in PD. The localization of impairments in joint control that underlie bradykinesia and dyscoordination in PD opens opportunities for the development of new rehabilitation approaches.
The second LJH prediction that movement disruptions depend on demands for control of biomechanical effects emerging during limb motion was supported in a study of handwriting-like movements in PD (7). The movements included cyclic motions of the wrist and fingers during drawing small lines and a circle. The lines were drawn with simultaneous flexion and extension of the wrist and fingers (“equivalent” pattern resulting in a right-tilted line) and with wrist flexion/extension accompanied with finger extension/flexion (“nonequivalent” pattern resulting in a left-tilted line). Circle drawing required a specific phase difference between wrist and finger motions. Consistent deformations of the circle into right-tilted ovals and lower variability of the equivalent compared with nonequivalent lines revealed a tendency to produce specific coordination of wrist and finger movements that resulted in right-tilted shapes. This tendency was accounted for by compliance with the biomechanical interactions of wrist and finger motions, as was supported by comparison of performance between right- and left-handed subjects in our previous study. Although this tendency was apparent in both groups, it was amplified in PD patients, reaching a level of a deficit during movements other than drawing right-tilted shapes. These results support the LJH prediction that the effect of abnormal neural control is more apparent for multijoint movements that require resistance to biomechanical properties of the limb compared with movements that conform to these properties.
The altered features of joint control revealed in movements of PD patients correspond to motor impairments specific for this disease, such as bradykinesia, dyscoordination, and handwriting deficit. It can be expected that application of the LJH to movements in other motor disorders, for example, cerebellar ataxia and stroke, would help to better understand the effect of each disorder on movement control and to develop novel rehabilitative approaches.
Peripheral Deficits and Compensatory Control Strategies
Research provides a number of examples of the leading-subordinate control structure being reorganized to compensate for a musculoskeletal deficit. For instance, a C6–C7 spinal lesion causes paralysis of triceps, which is the main elbow extensor. Nevertheless, patients with this impairment can produce arm movements that require large elbow extension. Analysis revealed that these patients extended their elbow via INT generated by skillful motion at the shoulder complex (18). Thus, patients exploited the dynamic effect of shoulder (leading joint) motion on elbow (subordinate joint) motion to compensate for the reduced ability to activate triceps.
Muscle fatigue has also been shown to cause reorganization of joint control of multijoint movements (20). In this study, throwing movements performed with extension of the elbow and wrist in the horizontal plane were compared before and after fatigue of either the wrist or elbow extensor. In response to the wrist muscle fatigue, elbow motion was modified to decrease INT at the wrist. When the elbow muscle was fatigued, contribution of the wrist in movement generation increased. In both cases, the control modifications can be understood, taking into account the leading role (INT generation) of the elbow and the subordinate role (INT control) of the wrist in this movement. During fatigue of the wrist muscle, elbow motion was changed to decrease INT, which resulted in decreased demands for wrist muscle activity required for INT regulation. The elbow muscle fatigue decreased the ability of this joint to generate INT, which was compensated by increased contribution of subordinate joint musculature in movement production.
Compensatory reorganization of multijoint control has also been found in normal aging. Horizontal shoulder and elbow movements during drawing of ovals and lines at three levels of cycling frequency were compared between young and older adults (22,25). Interaction torque escalates with increases in movement speed, requiring higher MUS magnitude for its control. The cycling frequency manipulations therefore emphasized declined muscle force, which is one of the primary deficits caused by aging. Analysis of the leading and subordinate features of joint control resulted in a surprising finding that older adults exploited INT better than young adults, which enabled them to decrease MUS magnitude with no loss in movement accuracy.
The provided examples show that application of the LJH allows deep understanding of changes in movement control caused by each deficit, thus opening new opportunities for the development of effective rehabilitation approaches.
BIOMECHANICAL FACTORS SHAPE HUMAN MOVEMENT REPERTOIRE
Although the LJH suggests that the exploitation of biomechanical properties of the limbs for movement control increases movement efficiency, it also implies a limited movement repertoire. Movements that require suppression of INT with MUS may be difficult to perform, specifically at high speed, that is, when INT is high. This was demonstrated by analysis of three patterns of cyclic elbow-wrist movements (9). A unidirectional pattern required simultaneous flexion or extension at both joints. A bidirectional pattern required combination of flexion at one joint with extension at the other joint. A free-wrist pattern included rhythmic flexion/extension at the elbow and relaxation of the wrist muscles.
A specific feature of the studied movements was that they were performed at the limits of the wrist’s range of motion, which caused passive elastic torque when the wrist was near its extreme positions. Thus, passive torque at the wrist included both INT and the elastic torque. Analysis revealed that elbow (leading joint) motion was similar in all movements, and the different patterns were produced because of different control of passive torque at the wrist (the subordinate joint). Only minor intervention of muscular activity into passive wrist motion was performed during unidirectional pattern. The bidirectional pattern required more intensive suppression of the passive torque at the wrist. Wrist motion was predominantly passive during the free-wrist pattern. Results of EMG analysis were consistent with this interpretation of control. Across the three patterns, the levels of muscle activity were similar at the elbow and different at the wrist, being the highest during the bidirectional pattern and the lowest during the free-wrist pattern. Furthermore, gradual increases in cyclic frequency resulted in deterioration of the unidirectional and bidirectional patterns, although the former started to deteriorate at a higher frequency level than the latter. At the highest frequency (3.05 Hz), both these patterns were lost and became similar to the free-wrist pattern.
The destructive effect of stringent requirements for INT control has also been demonstrated for horizontal arm movements (8). During line drawing, increases in cyclic frequency caused trajectory deviations from the mediolateral and anteroposterior directions and not from the two diagonal directions. Torque analysis demonstrated that the lines, orientation of which was distorted, were also characterized by relatively high MUS generated at the subordinate joint to intervene with INT.
These examples show that INT resists performance of some multijoint movements, decreasing their accuracy and stability. Because INT escalates with movement speed, fast performance of these movements may be extremely difficult or even impossible. This observation predicts that if a task allows choice of movement type, performers would avoid movements that require opposition of MUS to INT and prefer movements during which INT is assistive. Supportive evidence has been obtained with the use of a free-stroke drawing task (16). Participants produced straight strokes from a center to the perimeter of a circle with the index finger, choosing movement directions in a random order. Movements were performed with horizontal shoulder and elbow rotations.
Although participants were encouraged to distribute strokes uniformly across all directions, they demonstrated consistent preferences to move the hand in the two diagonal directions. This tendency was apparent from a histogram of stroke orientations produced by all subjects (Fig. 4). The strokes in the preferred directions were characterized by a tendency to produce passive motion at one of the two joints, the elbow in the LD direction and the shoulder in the RD direction. Apparently, participants avoided intervention with INT at the subordinate joint. It is plausible that movements demanding in terms of INT control are also avoided during activities of daily living by adjusting the trunk position with respect to the target and/or by arranging objects in space in a way that supports performance of preferred movements. Thus, the leading-subordinate control strategy facilitates movement performance, allowing exploitation of INT, but it also narrows the movement repertoire, discouraging performance of movements that require extensive effort for INT control.
Figure 4.
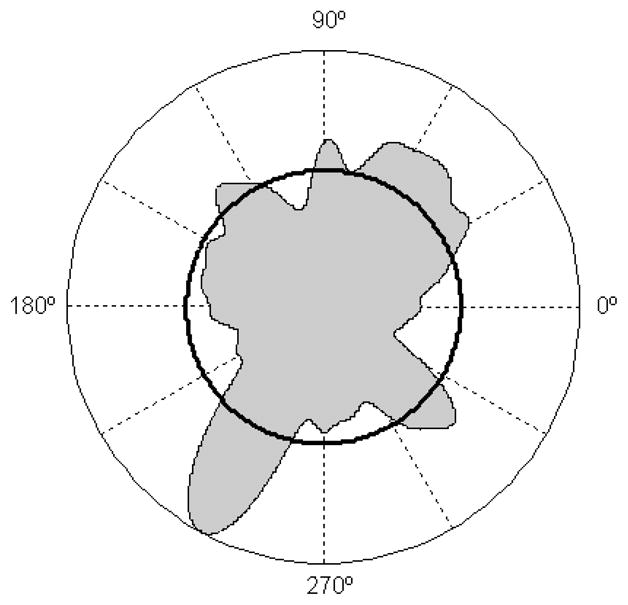
Smoothed polar histogram showing distribution of stroke orientations for 15 participants who performed the free-stroke drawing task (29). The black circle denotes the uniform distribution that could be achieved with the same number of strokes. The deviations of the histogram curve from the black circle reveal biases to produce movements in the two diagonal directions. Data were compiled from Goble et al. (29).
CONCLUSIONS AND FUTURE PERSPECTIVES
The reviewed studies demonstrate the practical applications of the LJH. This approach allows reconstruction of the global strategy of control, primarily distinguishing the leading joint and recognizing the biomechanical effect produced by its motion. This knowledge may facilitate learning of complex sport movements because leading joint motion is a core component that shapes the entire movement. The LJH also enables detection of subtle variations in control of each joint, which is valuable for many applications, such as assessing the progression of disease in special populations and the efficacy of rehabilitation.
The LJH is still at the beginning of its development. Future research may link the LJH with one or more other motor control theories. For instance, the leading-joint control strategy may be a result of an optimization process. However, the underlying cost function may be complex (16). The optimization process may also be complex and may include a number of components performed in different time scales, such as tuning of movement parameters during performance, development of the control strategy in ontogenesis, and adaptation of biomechanical properties of the limbs in phylogenesis. The LJH would also need to be reconciled with the idea of hierarchical organization of movement control. For example, the LJH describes control at the joint level, whereas organization of muscle activations that result in the required torques remains unclear.
Finally, future research may extend the scope of the LJH beyond the single joint as the leading component of the limb. Although the multijoint structure of the limbs favors exploitation of INT exerted by motion of a single joint for movement production, other anatomical units may also serve as leading structures. For instance, activation of biarticular muscles spanning the hip and knee was found responsible for production of leg motion during some phases of the pedaling cycle (27). The role of the ankle muscles during these phases was to position the feet properly to transfer energy generated by the proximal joints to the crank. This organization of control points to the leading role of the hip-knee linkage and subordinate role of the ankle. Future research may reveal movements that are performed by exploiting specific musculoskeletal properties of other linkages in the human body. Exciting discoveries may be awaiting researchers with respect to biomechanical effects that can be generated within the human body and how they are used to produce diversity of human movements.
Acknowledgments
The author is grateful to Dr. Berta Leis for useful comments on the article.
This work was supported by the National Institutes of Health (grant NS 43502).
References
- 1.Berthier NE, Clifton RK, McCall DD, Robin DJ. Proximodistal structure of early reaching in human infants. Exp Brain Res. 1999;127(3):259–69. doi: 10.1007/s002210050795. [DOI] [PubMed] [Google Scholar]
- 2.Bhat AN, Galloway JC. Toy-oriented changes in early arm movements III: constraints on joint kinematics. Infant Behav Dev. 2007;30(3):515–22. doi: 10.1016/j.infbeh.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Conditt MA, Gandolfo F, Mussa-Ivaldi FA. The motor system does not learn the dynamics of the arm by rote memorization of past experience. J Neurophysiol. 1997;78(1):554–60. doi: 10.1152/jn.1997.78.1.554. [DOI] [PubMed] [Google Scholar]
- 4.Dounskaia N. The internal model and the leading joint hypothesis: implications for control of multi-joint movements. Exp Brain Res. 2005;166(1):1–16. doi: 10.1007/s00221-005-2339-1. [DOI] [PubMed] [Google Scholar]
- 5.Dounskaia N, Ketcham CJ, Leis BC, Stelmach GE. Disruptions in joint control during drawing arm movements in Parkinson’s disease. Exp Brain Res. 2005;164(3):311–22. doi: 10.1007/s00221-005-2251-8. [DOI] [PubMed] [Google Scholar]
- 6.Dounskaia N, Ketcham CJ, Stelmach GE. Commonalities and differences in control of various drawing movements. Exp Brain Res. 2002;146(1):11–25. doi: 10.1007/s00221-002-1144-3. [DOI] [PubMed] [Google Scholar]
- 7.Dounskaia N, Van Gemmert AW, Leis BC, Stelmach GE. Biased wrist and finger coordination in parkinsonian patients during performance of graphical tasks. Neuropsychologia. 2009;47(12):2504–14. doi: 10.1016/j.neuropsychologia.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dounskaia NV, Ketcham C, Stelmach GE. Influence of biomechanical constraints on horizontal arm movements. Motor Control. 2002;6(4):366–87. doi: 10.1123/mcj.6.4.366. [DOI] [PubMed] [Google Scholar]
- 9.Dounskaia NV, Swinnen SP, Walter CB, Spaepen AJ, Verschueren SMP. Hierarchical control of different elbow-wrist coordination patterns. Exp Brain Res. 1998;121(3):239–54. doi: 10.1007/s002210050457. [DOI] [PubMed] [Google Scholar]
- 10.Feldman AG. Once more on the equilibrium-point hypothesis (lambda model) for motor control. J Mot Behav. 1986;18(1):17–54. doi: 10.1080/00222895.1986.10735369. [DOI] [PubMed] [Google Scholar]
- 11.Fradet L, Lee G, Stelmach G, Dounskaia N. Joint-specific disruption of control during arm movements in Parkinson’s disease. Exp Brain Res. 2009;195(1):73–87. doi: 10.1007/s00221-009-1752-2. [DOI] [PubMed] [Google Scholar]
- 12.Furuya S, Kinoshita H. Expertise-dependent modulation of muscular and non-muscular torques in multi-joint arm movements during piano keystroke. Neuroscience. 2008;156(2):390–402. doi: 10.1016/j.neuroscience.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 13.Galloway JC, Bhat A, Heathcock JC, Manal K. Shoulder and elbow joint power differ as a general feature of vertical arm movements. Exp Brain Res. 2004;157(3):391–6. doi: 10.1007/s00221-004-1955-5. [DOI] [PubMed] [Google Scholar]
- 14.Galloway JC, Koshland GF. General coordination of shoulder, elbow and wrist dynamics during multijoint arm movements. Exp Brain Res. 2002;142(2):163–80. doi: 10.1007/s002210100882. [DOI] [PubMed] [Google Scholar]
- 15.Gandolfo F, Mussa-Ivaldi FA, Bizzi E. Motor learning by field approximation. Proc Natl Acad Sci U S A. 1996;93(9):3843–6. doi: 10.1073/pnas.93.9.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goble JA, Zhang Y, Shimansky Y, Sharma S, Dounskaia NV. Directional biases reveal utilization of arm’s biomechanical properties for optimization of motor behavior. J Neurophysiol. 2007;98(3):1240–52. doi: 10.1152/jn.00582.2007. [DOI] [PubMed] [Google Scholar]
- 17.Hirashima M, Kudo K, Watarai K, Ohtsuki T. Control of 3D limb dynamics in unconstrained overarm throws of different speeds performed by skilled baseball players. J Neurophysiol. 2007;97(1):680–91. doi: 10.1152/jn.00348.2006. [DOI] [PubMed] [Google Scholar]
- 18.Hoffmann G, Laffont I, Hanneton S, Roby-Brami A. How to extend the elbow with a weak or paralyzed triceps: control of arm kinematics for aiming in C6-C7 quadriplegic patients. Neuroscience. 2006;139(2):749–65. doi: 10.1016/j.neuroscience.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Hollerbach JM. Computers, brains and the control of movement. Trends Neurosci. 1982;5(6):189–92. [Google Scholar]
- 20.Huffenus AF, Amarantini D, Forestier N. Effects of distal and proximal arm muscles fatigue on multi-joint movement organization. Exp Brain Res. 2006;170(4):438–47. doi: 10.1007/s00221-005-0227-3. [DOI] [PubMed] [Google Scholar]
- 21.Ikegami T, Taga G. Decrease in cortical activation during learning of a multi-joint discrete motor task. Exp Brain Res. 2008;191(2):221–36. doi: 10.1007/s00221-008-1518-2. [DOI] [PubMed] [Google Scholar]
- 22.Ketcham CJ, Dounskaia NV, Stelmach GE. Age-related differences in the control of multijoint movements. Motor Control. 2004;8(4):422–36. doi: 10.1123/mcj.8.4.422. [DOI] [PubMed] [Google Scholar]
- 23.Kim YK, Hinrichs RN, Dounskaia N. Multicomponent control strategy underlying production of maximal hand velocity during horizontal arm swing. J Neurophysiol. 2009;102(5):2889–99. doi: 10.1152/jn.00579.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konczak J, Dichgans J. The development toward stereotypic arm kinematics during reaching in the first 3 years of life. Exp Brain Res. 1997;117(2):346–54. doi: 10.1007/s002210050228. [DOI] [PubMed] [Google Scholar]
- 25.Lee G, Fradet L, Ketcham CJ, Dounskaia N. Efficient control of arm movements in advanced age. Exp Brain Res. 2007;177(1):78–94. doi: 10.1007/s00221-006-0648-7. [DOI] [PubMed] [Google Scholar]
- 26.Lee HM, Bhat A, Scholz JP, Galloway JC. Toy-oriented changes during early arm movements IV: shoulder-elbow coordination. Infant Behav Dev. 2008;31(3):447–69. doi: 10.1016/j.infbeh.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 27.Raasch CC, Zajac FE. Locomotor strategy for pedaling: muscle groups and biomechanical functions. J Neurophysiol. 1999;82(2):515–25. doi: 10.1152/jn.1999.82.2.515. [DOI] [PubMed] [Google Scholar]
- 28.Sainburg RL, Kalakanis D. Differences in control of limb dynamics during dominant and nondominant arm reaching. J Neurophysiol. 2000;83(5):2661–75. doi: 10.1152/jn.2000.83.5.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt RA. Schema theory of discrete motor skill learning. Psychol Rev. 1975;82(4):225–60. [Google Scholar]
- 30.Todorov E. Optimality principles in sensorimotor control. Nat Neurosci. 2004;7(9):907–15. doi: 10.1038/nn1309. [DOI] [PMC free article] [PubMed] [Google Scholar]