This review examines species diversity and structural, physiological and biochemical characteristics associated with survival of annual deep flooding of trees in four contrasting tropical floodplain ecosystems.
Abstract
Background and aims
In the context of the 200th anniversary of Charles Darwin's birth in 1809, this study discusses the variation in structure and adaptation associated with survival and reproductive success in the face of environmental stresses in the trees of tropical floodplains.
Scope
We provide a comparative review on the responses to flooding stress in the trees of freshwater wetlands in tropical environments. The four large wetlands we evaluate are: (i) Central Amazonian floodplains in South America, (ii) the Okavango Delta in Africa, (iii) the Mekong floodplains of Asia and (iv) the floodplains of Northern Australia. They each have a predictable ‘flood pulse’. Although flooding height varies between the ecosystems, the annual pulse is a major driving force influencing all living organisms and a source of stress for which specialized adaptations for survival are required.
Main points
The need for trees to survive an annual flood pulse has given rise to a large variety of adaptations. However, phenological responses to the flood are similar in the four ecosystems. Deciduous and evergreen species respond with leaf shedding, although sap flow remains active for most of the year. Growth depends on adequate carbohydrate supply. Physiological adaptations (anaerobic metabolism, starch accumulation) are also required.
Conclusions
Data concerning the ecophysiology and adaptations of trees in floodplain forests worldwide are extremely scarce. For successful floodplain conservation, more information is needed, ideally through a globally co-ordinated study using reproducible comparative methods. In the light of climatic change, with increasing drought, decreased groundwater availability and flooding periodicities, this knowledge is needed ever more urgently to facilitate fast and appropriate management responses to large-scale environmental change.
Introduction
During the recent Darwin bicentennial year (2009) and throughout the 151 years since the publication of ‘On the Origin of Species’ (Darwin, 1859), discussion on the ‘struggle for survival’ has been topical and controversial. Darwin's theory of ‘survival of the fittest’ is a synonym for ‘natural selection’. Darwin asked ‘Can it be doubted, from the struggle each individual has to obtain subsistence, that any minute variation in structure, habits or instincts, adapting that individual better to new conditions, would tell upon its vigour and health?’ (Darwin, 1842). Accordingly, the present study discusses the struggle for life in the forests of large flood-pulsed wetlands in relation to what is known of variations in structure, physiology and biochemistry that confer resilience. Contrary to the wisdom of Darwin, we cannot, unfortunately, deal with differences within populations because such data are very difficult to obtain for these huge ecosystems. Instead, our aim is to bring together data on responses of trees to flooding in the freshwater wetlands of tropical environments, emphasizing the varying responses to different wetland structures and flooding conditions extant in tropical freshwater floodplains of four continents. Although Gopal et al. (2000) have published a book on the biodiversity of wetlands and Junk (1997) produced a review of comparative biodiversity in floodplains around the world, there is no one publication which focuses on adaptation and survival of trees in tropical wetlands. The present article aims to fill this gap.
The four large wetlands chosen for our analysis are the Central Amazonian floodplains of South America, the Okavango Delta region of Botswana, Africa, the Mekong floodplains of South-east Asia and the tropical wetlands of Northern Australia (Fig. 1; Table 1). These ecosystems, each on a different continent, were chosen largely on the following pragmatic grounds. We looked for very large tropical freshwater floodplains with forest patches (i.e. trees occurring there naturally) where flooding occurs with regularity (the ‘flood pulse’ of Junk et al., 1989), is characterized by high amplitudes and where it is long-lasting (weeks or months). We were careful not to include areas merely prone to flash floods following heavy rain. Our assessments are based on many diverse publications and disparate data concerning the effects of flooding on species richness, ecophysiology and distribution of tropical trees.
Fig. 1.
Map indicating the approximate location of the four chosen floodplain forests.
Table 1.
Characteristics of the four chosen floodplain ecosystems on four continents with a monomodal flood pulse
| Central Amazonia | Okavango Delta | Mekong Tonle Sap | Kakadu Region in Northern Australia | |
|---|---|---|---|---|
| Continent | South America | Africa | Asia | Northern Australia |
| Geographical position | 3°15′S, 59°58′W | 18°30′–20°S, 22°–24°E | 13°N, 104°E | 13°2′S, 133°31′E |
| Latitude | 0 | 19 | 13 | 12 |
| Age of ecosystem (Irion et al., 1997; Junk et al., 2006) | 2.4 million years | 2.5 million years | 7500 years | 4000 years |
| Height asl (m) | 0–50 | 1000 | 0–50 | 0–50 |
| Connected rivers | Major river system | Major river system | Major river system | Smaller rivers |
| Floodplain area (km2) | 300 000 | 2500–8000; 28 000 | 15 000 | 99 000; 2900 |
| Annual precipitation (mm) | 2100 | 460–490 | 1600 | 1300–1450 |
| Predictability of flooding | High | High | High | High |
| Flood amplitude | 15 m | 1.85 m | 8.2 m | 2–5 m |
| Mean/maximum flood height | 8 m | Root level | <2 m | 1 m |
| Flood duration where trees grow | 7 months | Several weeks? | 6–8 months | >6 months |
| Wetland main vegetation | Forest | Mainly grassland | Forest/grassland | Forest/grassland |
| Trophic status | Meso-eutrophic | Mesotrophic | Meso-eutrophic | Oligo-mesotrophic |
| Fire | No | Yes | No? | Yes |
| Salt | No | Yes! | No? | No? |
| Forest cover | Closed forest | Single trees | 10 %, mosaic of stands of large trees and open areas | Open savanna to 70 % forest cover |
| Tree/canopy height | 20–30 m | 5–6 m | 7–15 m | 20 m |
| Woody species (Junk et al., 2006) | >1000 | 180 | 70 | 21 |
| Number of flood-tolerant tree species | >1000 | 10 | 15 | 5 |
| Incidence of endemic tree species | High | Very low | Low | Low? |
| Tree species diversity | High | Very low | Few dominant species | Low? |
| Human pressure | Low | Low? | Very high (wars; fishing) | Minimal |
| Human impacts | Timber extraction; fishing; cattle ranching | Subsistence agriculture; fisheries | Timber; fishing; paddy rice | Cattle grazing; tourism; mining |
| Changes | Increasing incidence and severity of drought | Soil salinization due to tree felling; expansion of agriculture; agrarian-degradation; predicted degeneration of major vegetation types from increased drying (Ringrose et al., 2002) | Dramatic fluctuations in water level of Mekong River; frequent floods and lower water levels in dry season—an increasing problem for farming (IUCN, 1991). | Invasion by alien plants and animals; changed fire regime; water pollution from urban-tourism, mining and salinization; sea level rise (Junk et al., 2006) |
While selecting our four ecosystems, it soon became evident that data are extremely scarce, despite their importance for biodiversity and human resources (Wantzen and Junk, 2000). We were forced to exclude many of the largest wetlands, e.g. the Congo basin in Africa or the Orinoco floodplains in Venezuela, because only basic data on hydrology and climatology are available, with almost no information on plant distribution, tree adaptations and ecophysiology. It is important to bring attention to such poorly researched wetlands, which are often inaccessible for social and political reasons but are threatened by the ever-increasing human population and its need for water, waterways and hydroelectric power. The destruction is so fast that we may never learn of the adaptations underpinning the success of the tree species in these areas.
We are aware that differences between the four ecosystems are large, especially in respect of the influence of fire and salinity. Those which are dominated by grasslands (Okavango and Northern Australian floodplains) are subjected to regular fire (Heinl et al., 2004, 2006, 2007), whereas in the forest-dominated floodplains of Amazonia and Mekong, fire plays no significant role. In the Okavango, the high evapotranspiration causes salinity problems, which are negligible in the remaining three ecosystems. Also, flooding amplitudes vary widely between the ecosystems, with about 2 m in the Okavango and Northern Australian, 8 m in the Mekong and 15 m in the Amazon floodplains (Table 1). This implies that complete submergence of saplings and trees occurs only in the Mekong and Amazon, posing different constraints for plant life than merely waterlogging of roots and stems (Parolin, 2009). However, our review is readily justified because the regular flood pulse is a major influence on all floodplain biology (Junk, 1989; Junk et al., 1989) and a dominating stress which requires a suite of adaptations for its survival.
Throughout the world, wetland ecosystems are under increasing pressure from agriculture, urbanization of catchment areas, tourism and recreational activities, construction of impoundments and changes to hydrology and climate. By comparing diversity and tree responses in four floodplain ecosystems on different continents, we attempt to improve our understanding of the factors influencing the spatial distribution of plants, diversity of species and adaptations, and thus contribute to our knowledge of tropical wetland ecology. In this way, we hope to assist in the successful restoration of degraded floodplains and promote the sustainable use and conservation of these highly valuable ecosystems.
Flooding as a stress factor
Flooding with freshwater, although less harmful than flooding with saltwater, poses a multitude of constraints on growth, survival and reproduction. Trees are basically terrestrial organisms and, in general, die more readily in response to flooding than to desiccation (Larcher, 1994). Flooding involves inundation of part or all of the aboveground structures, whereas waterlogging is restricted mainly to inundation of the soil and rhizosphere (Colmer and Pedersen, 2008). Totally submerged plants have no direct contact with atmospheric oxygen and sunlight is weak or extinguished. Inundated soils become hypoxic or anoxic within a few hours as the combined result of oxygen consumption by respiring roots plus micro-organisms and insufficiently fast diffusion of oxygen through water to replace the amounts consumed (Crawford, 1989, 1992; Armstrong et al., 1994; Visser et al., 2003). Oxygen depletion in soil is accompanied by increased levels of entrapped CO2, anaerobic decomposition of organic matter, increased solubility of mineral substances, notably iron and manganese, and decreased redox potential (Joly and Crawford, 1982; Kozlowski, 1984). The resulting chemically reduced and potentially toxic compounds accumulate, their generation being the result of alterations in the composition of the soil microflora as it responds to the changing conditions (Ponnamperuma, 1984). In some floodplains, e.g. those of the Amazon River, sedimentation rates can be extremely high and the deposition of sediment can decrease soil aeration and thus favour oxygen shortage in the rhizosphere (Wittmann et al., 2004; Wittmann and Parolin, 2005). Elevated decomposition rates of highly productive floating and non-floating macrophytes in floodplains further decrease oxygen concentrations in the floodwater (Armstrong et al., 1994).
In temperate zones, flooding frequently occurs during winter when plants are dormant and light intensities low. In contrast, the flooding period in tropical floodplains occurs when temperatures and light intensities are high and conditions overall are optimal for plant growth. Therefore, the trees are not dormant and must accommodate shortages of oxygen and, for submerged shoots, shortages of CO2 too at a time when conditions favour fast respiration and depletion of reserves. This implies that extraordinarily efficient adaptations are needed for survival. With all these constraints, imposed by flooding, this stress is clearly life-threatening for higher plants. The struggle for survival of flooding is therefore closely linked to the evolution of physiological, phenological, anatomical and morphological adaptations that confer tolerance and underpin successful and vigorous growth and fecundity despite the intense stress.
Floodplain ecosystems
Here we characterize four extensive floodplain ecosystems present on four continents (Table 1). They include the Central Amazon floodplains (where we have the broadest and deepest knowledge of tree ecophysiology), the Okavango Delta in Africa, the Mekong floodplains in South-east Asia (where relatively little is known about tree ecology) and the Northern Australian floodplains (where much is known about the herbaceous vegetation, but much less about tree responses to freshwater flooding; Table 2).
Table 2.
Characteristics of the forest vegetation (distribution, phenology, physiological adaptations) in the four chosen floodplain ecosystems on four continents
| Central Amazonia | Okavango Delta | Mekong Tonle Sap | Kakadu Region in Northern Australia | |
|---|---|---|---|---|
| Continent | South America | Africa | Asia | Northern Australia |
| Tree distribution | ||||
| Zonation of trees along the flooding gradient | Yes | Yes | Yes | Yes |
| Degree of endemism | Elevated | Low/absent | Low/absent | Low/absent |
| Leaf phenology | ||||
| Deciduous species: leaf shedding at high waters | Yes | No | Yes | ? |
| Evergreen species | Yes | Yes | Yes | ? |
| Reproductive phenology | ||||
| Linked to high water + fish | ? | Linked to high water + fish | ? | |
| Physiological adaptations | ||||
| Reduction of metabolism and growth during high waters | Yes | No | Yes? | ? |
| Morpho-anatomical adaptations | Leaf xeromorphism; hypertrophic lenticels; adventitious roots; aerenchyma | ? | ? | ? |
| Biochemical adaptations | Increased activity of fermentative enzymes; more VOC emission | ? | ? | ? |
South America: Central Amazonian floodplains
There are extensive floodplains along the Amazon River and its large tributaries throughout the Amazon basin. These contain large species-rich and highly adapted floodplain forests that cover more than 300 000 km2 (Irion et al., 1997). The mean annual temperature of 26.6 °C changes little, average rainfall is ∼2100 mm year−1 (Ribeiro and Adis, 1984) and noon light intensities can reach 3000 µmol m−2 s−1 at the water surface (Furch et al., 1985). Seasonal variations in river levels subject trees to periods of up to 210 days of continuous flooding each year. The rate of change in the water level can be fast and reach 10 cm day−1 (Junk, 1989), leading to a total rise of up to 16 m in western Amazonia, 10 m in Central Amazonia and 6 m in eastern Amazonia (Junk, 1989). The ‘flood pulse’ (Junk et al., 1989) is monomodal and the timing is predictable, resulting in well-defined high-water (aquatic phase) and low-water (terrestrial phase) periods each year. The timing of the pulse is predictable, but irregularities occur in the maximum and minimum water levels. This can be of great relevance for seedling establishment (Scarano et al., 1997). At high water levels, tree roots and stems are waterlogged, and small trees and seedlings may be completely submerged for several months by a water column of up to 8 m (Parolin et al., 2004; Parolin, 2009). At low water levels, drought may be a stress factor for several weeks (Junk, 1997; Parolin et al., 2010). Natural fires and salt are absent from this ecosystem. Although large terrestrial mammals play important roles for tree establishment and distribution in the grasslands of other floodplains, they play no significant role in the Amazonian floodplain (Junk and da Silva, 1997).
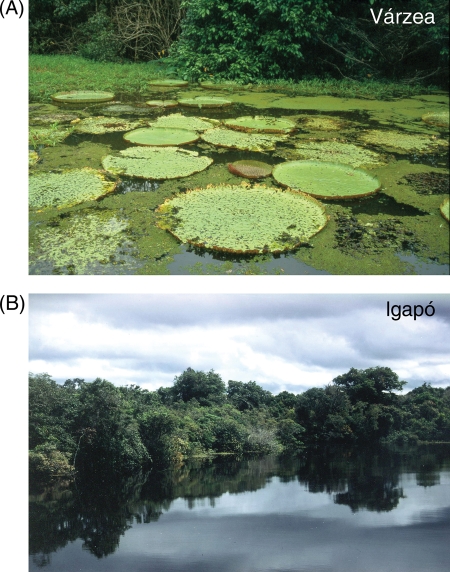
The differing origins of the various tributaries of the Amazonian River system can strongly influence water chemistry, e.g. sources in the western Amazon Andes or the Northern and Southern Amazonian Precambrian shields. The resulting seasonally flooded vegetation can roughly be differentiated into the nutrient-rich and highly productive white-water floodplains (várzea) and the nutrient-poor and less productive black-water or clear-water floodplains (igapó) (Fig. 2; Sioli, 1954; Prance, 1979). In Central Amazonia, both floodplain types undergo seasonal water-level changes of up to 10 m (Fig. 3). Trees establish at mean annual flood levels <7.5 m, corresponding to flooded and/or waterlogged periods of up to 300 days year−1 (Wittmann et al., 2004, 2006).
Fig. 2.
Floodplain forests in Central Amazonia at high water. (A) Nutrient-rich white-water várzea floodplain forest with macrophytes in the foreground (Victoria amazonica; Rio Solimões near Manaus) and forest in the background. (B) Nutrient-poor black-water igapó floodplain forest (Rio Negro near Manaus) (Photographs: Pia Parolin).
Fig. 3.
Várzea floodplain forests in central Amazonia at low water (Photographs: Florian Wittmann, Max-Planck-Institute for Chemistry, Mainz, Germany).
Human impact on Amazonian floodplains is increasing due to agriculture, cattle and buffalo farming, logging, civil construction projects, mining and reservoirs for hydroelectric power (Junk, 2000). Small-scale multiple uses and ecotourism, however, provide sustainable management that partially limits the threats to this ecosystem.
Tree vegetation
Amazonian freshwater floodplains harbour the most species-rich floodplain forests in the world (Wittmann et al., 2006). In the nutrient-rich white-water várzea, there are more than 1000 flood-tolerant tree species (Fig. 3; Wittmann et al., 2006). From igapó, the comparatively low number of inventories still does not allow reliable estimates of overall species richness. However, comparisons from both local and basin-wide scales indicate less species richness than in the várzea (Prance, 1979; Ferreira et al., 2005; Wittmann et al., 2010). Species-poor low-lying forests (low várzea) are remarkably similar throughout the Amazon basin even when separated by long distances. Species-rich high-várzea forests may be more floristically distinct, but share ∼30 % of their tree species with the adjacent uplands (Wittmann et al., 2006). Tree species richness and alpha-diversity of várzea forests are significantly correlated to flood height and length, and to the age of the forest stand (Wittmann et al., 2006). Maximum species richness estimated from trees ≥10 cm in diameter at breast height (cm dbh) recorded in high-várzea forests of Amazonia amounts to 84 species ha−1 in the eastern parts of the basin, 142 species ha−1 in Central Amazonia and 157 species ha−1 in the southern part of western Amazonia (Wittmann et al., 2010). Endemism is highest in highly flooded low-lying forests and was estimated to account for ∼39 % of the 186 most common Central Amazonian várzea tree species (Wittmann et al., 2010). One hundred and twelve (60 %) of the most frequent Central Amazonian várzea tree species are generalists and are also to be found in other neotropical ecosystems. Where flooding does not exceed 210 days year−1 (Junk, 1989), trees are the dominating life form, whereas in longer-flooded environments, grasses and macrophytes take over.
As a result of the different chemical compositions and nutrient inputs of the flooding water, depending on the river catchment, the tree flora of the várzea and igapó differ substantially in species composition and diversity (Prance, 1979; Kubitzki, 1989). Comparisons at local and basin-wide scales suggest floristic similarities between both ecosystems to be <20 % (Wittmann et al., 2010). The main reason for the diverging flora is the contrasting nutrient level. This seems to act as a distribution barrier for many white-water species migrating to the igapó and vice versa. In addition, alluvial dynamism varies, with the white-water floodplains being highly dynamic systems where constant processes of sedimentation and erosion create a large variety of micro-habitats, thereby increasing biodiversity (Salo et al., 1986; Kalliola et al., 1991; Wittmann et al., 2004).
Flooding tolerance and tree distribution
There is a clear zonation of plant communities in the Amazonian várzea along the food-level gradient, which leads to characteristic species associations and forest types. Two main habitats are differentiated (Wittmann et al., 2002): (i) low-várzea forests, influenced by mean inundations with heights between 3.0 and 7.5 m (corresponding to a mean inundation period of 50–230 days year−1) and (ii) high-várzea forests, influenced by mean inundations with heights of <3.0 m (<50 days year−1). However, the distribution of várzea tree families differs considerably between low- and high-várzea forests (Wittmann et al., 2006): Fabaceae, Malvaceae, Salicaceae, Urticaceae and Brassicaceae are more important in low-várzea forests, whereas Euphorbiaceae, Moraceae, Palmae, Annonaceae, Meliaceae and Myristicaceae are more important in high-várzea forests.
The clear zonation of tree species along the flood gradient in both Amazonian igapó and várzea indicates the different levels of acclimation and adaptation that these species have evolved in order to cope with the seasonally hypoxic/anoxic sites. Trees may disperse to higher flooded sites than the parent trees and establish during the terrestrial phase, but they often prove to be intolerant of the peculiar site conditions or quickly lose out competitively to better-adapted species (Wittmann et al., 2010). Many Amazonian floodplain tree species that tolerate high and prolonged inundation show adaptations against a wide range of potentially stressful conditions. For example, they tolerate high sedimentation rates when located near the river channels of white-water rivers and also tolerate poorly aerated soils when located in backwater swamps. Furthermore, they often tolerate full sunlight and drought during the terrestrial phases when low river water levels coincide with seasonally low precipitation. Trees that are successful at highly flooded sites are therefore light-demanding pioneer species which also have a high resprouting capacity (Worbes et al., 1992; Wittmann and Parolin, 2005). They also grow quickly and exhibit relatively short life cycles as, for example, in the white-water pioneers Salix martiana and Cecropia latiloba (Worbes et al., 1992; Parolin et al., 2002; Schöngart, 2003). These successful pioneer trees modify the local site conditions so much that new seedlings of the same species are no longer able to establish at the same site as the parent tree (Wittmann et al., 2010).
Tree responses to flooding
The terrestrial phase is the main growth period for trees in the Amazonian floodplains. In contrast, in the flooded period, growth decreases, metabolic activity slows and even complete dormancy is induced in many species. However, none of these responses lasts for the entire flooding period. Limited growth lasts for only a few weeks and is often followed by new leaf flush, flowering, fruiting and wood increment while the tree is still flooded (Worbes, 1997; Schöngart et al., 2002). After fruit maturation, which usually occurs at high water levels (Kubitzki and Ziburski, 1994), seeds fall into the water and may float and/or are submerged for several weeks without losing their viability. Seed germination starts only when the flood recedes, although some may protrude a radicle (Scarano et al., 2003) or even produce a complete seedling while floating (Oliveira-Wittmann et al., 2007; Parolin, 2009). In most species, overall growth in height and new leaf production are not severely inhibited merely by waterlogging of the soil, and elongation may even be enhanced, as in Senna reticulata. Here, waterlogging is reported to accelerate seedling shoot growth considerably (Parolin, 2001).
Submergence of part of or the entire shoot is a more severe stress. Most tree species tolerate this in a state of rest and sprout new leaves soon after the water recedes. In species with leaves without a thick cuticle or thick outer epidermis walls, leaves rot fast when submerged and are shed after only a few days (Waldhoff and Furch, 2002). Other species may retain their leaves in a healthy state below water for several months. Leaf shedding during the aquatic phase has been documented not only in deciduous species but also in evergreen trees, which tend to produce new leaves only slowly at high water levels (Parolin et al., 2002). Whether deciduous or evergreen, and regardless of whether leaves are kept or shed under water, the leaves of Amazonian floodplain trees exhibit traits that are generally considered as xeromorphic (Medina, 1983; Waldhoff, 2003).
Physiological responses to waterlogging of the soil include reductions of mean CO2 uptake in aerial leaves ranging from 10 to 50 % slower than in the terrestrial phase (Parolin et al., 2004). CO2 uptake rises again before the end of the flooded phase and remains high throughout the terrestrial phase (Parolin, 2000).
Morphological adaptations of the root system include hypertrophy of lenticels, formation of adventitious roots, development of aerenchyma, and the deposition of cell wall biopolymers such as suberin and lignin in peripheral cell layers (Schlüter and Furch, 1992; Schlüter et al., 1993; De Simone et al., 2002a, b). Different types of above-ground roots, e.g. plank-buttressing and adventitious roots, are closely related to flooding duration and habitat dynamics (Wittmann and Parolin, 2005). The development of adventitious roots in the oxygenated layer at the surface of the floodwater table and hypertrophy of lenticels on the surface of stems just above water level are thought to improve the internal oxygen status by facilitating the entry of oxygen into the root and the stem by the shortest possible pathway (Crawford, 1992). Pneumatophores are also familiar adaptations in mangroves but are absent in várzea trees (Junk, 1984) except in palms found in headwater regions and swamps (e.g. Mauritia, Mauritiella), where flood amplitudes are small. Stem nodulation and nodulated adventitious roots have been observed in various species, and are understood to be adaptations that allow legumes to fix nitrogen in a flooded environment (James et al., 2001). The frequency of such nodulation among genera can be higher in flooded than in non-flooded sites in both várzea and igapó, indicating that nodulation may be favoured in flooded areas (Moreira et al., 1992).
Increased activity of fermentative enzymes such as alcohol dehydrogenase (ADH), lactate dehydrogenase (LDH), glutamate–pyruvate transaminase (GPT) and malate dehydrogenase (MDH) has been observed under anaerobic soil conditions in the roots of several tree species (Schlüter and Furch, 1992; Schlüter et al., 1993; De Simone et al., 2002b). In addition, larger amounts of volatile organic compounds are emitted to the atmosphere by terrestrial vegetation when flooded (Kesselmeier and Staudt, 1999). Acetaldehyde and ethanol may be emitted in larger amounts by flooded trees and under other stress conditions such as sulphur dioxide and ozone exposure, water deficit, freezing and fast-changing light conditions (Kimmerer and Macdonald, 1987; Kesselmeier et al., 1997). Acetaldehyde (and formaldehyde) is exchanged bi-directionally between the vegetation and the atmosphere, i.e. they are emitted or taken up, depending on environmental and atmospheric conditions (Kesselmeier et al., 1997). Recent measurements in the terra firme Amazonian rain forest provide evidence that more short-chain aldehydes and the corresponding organic acids were taken up from the air than produced, although release was observed when ambient concentrations were below a specific level (Rottenberger et al., 2008).
Africa: Okavango Delta
The Okavango Delta (Fig. 4) is the world's largest inland delta. It is located in northwestern Botswana and fed by the Okavango River, which originates in Angola's western highlands. The floodwaters take ∼9 months to flow from the source to the delta due to the extremely gentle gradient. The river discharges about 10 km3 of water onto the delta fan each year, augmented by about 6 km3 of rainfall, which sustains about 2500 km2 of permanent wetland and up to 8000 km2 of seasonal wetland. Interaction between this surface water and the groundwater strongly influences the structure and function of the wetland ecosystem. The climate is semi-arid, and only 2 % of the water leaves as surface flow and probably very little as groundwater flow. The bulk of the water is lost to the atmosphere. The Okavango River also delivers about 170 000 tonnes of bedload sediment and about 360 000 tonnes of solutes to the delta each year, most of which is deposited on the fan. Local rainfall is low, averaging 490 mm year−1. This is greatly exceeded by the rate of evapotranspiration (1580 mm year−1; Ellery et al., 1993). Temperatures range from maxima of 33.7 °C in summer to 28.7 °C in winter, with a mean relative humidity of 60–78 % in summer and 43–63 % in winter (Bonyongo et al., 2000). Precipitation data show <10 mm of rainfall per month from May through October, whereas between January and March, it lies between 120 and 320 mm. The Okavango Delta experiences seasonal flooding starting towards the north between October and April, and ending in May, June or July towards the southern part of the delta. Natural fluctuations in water level result from variations in annual rainfall in the catchment area and rainfall within the delta itself (Bonyongo, 1999). The delta is almost permanently flooded in the north, but only seasonally flooded in the south. The rain falls during the summer and first seeps into the parched ground before the rivers start flowing. As it is the dry season, the floodwaters gradually evaporate over the subsequent months, leaving their valuable salts and minerals in the ground.
Fig. 4.
Forested savanna in the Okavango Delta with mopane trees (C. mopane) at high and low water (Photographs: Michael Heinl, University of Innsbruck, Austria).
Fire plays a role in this ecosystem (Heinl et al., 2004, 2006, 2007). It is more frequent in the floodplains than on the drylands because of greater biomass and fuel load. The incidence of fire on the drylands correlates with annual rainfall events, while the frequency of fires on floodplains is determined mostly by flooding frequency. The greatest burn potential is found on floodplains that become flooded every second year. Temporal variations in flooding cause accumulation and sudden mobilization of nutrients which are readily utilized by well-adapted plants. As a consequence, locally high biological productivity occurs, which, in turn, supports many grazing mammals (Heinl et al., 2004, 2006, 2007; Tacheba et al., 2009).
Changes in the types of vegetation cover, due to both human and natural causes, have taken place since the first vegetation map was produced in 1971 (Ringrose et al., 2002). In the south-west, shifts to thorn trees prevail, whereas in the eastern part of the country, widespread bush encroachment takes place. An increased human population density suggests that these are anthropogenic (agrarian-degradation) effects. Wherever broadleaved evergreen trees are cleared, widespread salinity occurs (Ellery et al., 2000). In the sparsely settled central Kalahari region, changes from tree savanna to shrubs may indicate the influence of climate change with the associated effects of fires and local adaptations. Projection of future vegetation changes to about 2050 indicates degeneration of the major vegetation types due to expected drying of the local climate (Ringrose et al., 2002).
Tree vegetation
The floodplains consist mostly of grasslands with 1250 species (Ellery and Tacheba, 2003). Woody plants are found in the riverine forests (e.g. species of Ficus). On the higher, often salt-rich islands which are flooded less frequently, acacias, mopane (Colophospermum mopane; Fig. 4) and the woody shrub Pechuel-loeschea leubnitziae (a weed in many ecosystems) predominate. Ellery and Tacheba (2003) reported 43 woody species in total in the dryland riverine woodland. None of these is endemic since most of them also occur in South Africa and Namibia (M. Heinl, University of Innsbruck, Austria, pers. comm.). Despite an overall high plant species diversity in the delta, only 18 % of the vegetation is phanerophytes (trees), compared with 56 % hemicryptophytes and 8 % true aquatic species (Ellery and Tacheba, 2003).
Although seldom flooded, the riparian woodland trees have their roots in the water table in permanent and seasonal swamps (Ellery and Tacheba, 2003). Acacias and mopane are less flood tolerant with pechuel showing greater tolerance of flooding, and also of fire. Riparian woodlands are responsible for much of the water lost from the ecosystem and deplete groundwater by transpiration (Ringrose, 2003). This leads to the uptake of toxic solutes by the transpiring trees, which results in exceptionally good quality surface water. The trees therefore ensure that islands of vegetation function as ‘kidneys’ within the landscape—a reason why riparian woodlands are considered particularly important habitats in this ecosystem (Ellery and Tacheba, 2003).
Flooding tolerance and tree distribution
Vegetation on islands in the perennial swamps of the Okavango Delta exhibits a marked zonation (Ellery et al., 1993). This is related primarily to aspects of the hydrological regime such as depth, duration and timing of inundation, but mainly to soil and groundwater salinity (Ellery and Tacheba, 2003). Also, processes associated with nutrient and sediment supply and sediment deposition, and with the nature of the substratum, play a role (Ellery and Tacheba, 2003). Trees, which are almost exclusively confined to islands, are particularly important as they lower the water table beneath islands relative to the surrounding wetlands and cause a net inward flow of groundwater (McCarthy, 2006). Island fringes are generally characterized by a broadleaf evergreen riparian community of Syzigium cordatum, Ficus verruculosa, F. natalensis, F. sycamorus, Phoenix reclinata, Garcinia livingstonei and Diospyros mespiliformis. This gives way to interiors dominated by Acacia nigrescens, Croton megalobotrys and Hyphaene ventricosa. The most central regions are characterized either by short, sparse grassland dominated by Sporobolus spicatus or are completely devoid of vegetation with sodium carbonate (trona)-encrusted soil surrounding a central pan of extremely high conductivity (Ellery et al., 1993). Soil pH and mineral content (especially sodium) and groundwater chemistry (conductivity and pH) play a major role in the spatial distribution of plant communities. However, Bonyongo et al. (2000) state that the timing and duration of the seasonal flooding are the most important factors determining the species composition of the vegetation.
Tree responses to flooding
The riparian trees remain green all year and partly sustain their growth as a result of groundwater uptake in the dry periods. Riverine forests in savanna areas depend on the river for their water supply (Hughes, 1988). Flooding and lateral groundwater flow stimulate growth (Ringrose, 2003). Renewal of leaf growth, however, is primarily related to rainfall, not to flood events in the distal delta (Ringrose, 2003). Regenerative phenology has not yet been described for the trees of the Okavango Delta. In general, however, riverine forests in African savannas show a high percentage of even-aged stands of trees, indicating that hydrological factors are important for tree regeneration because they provide spasmodically favourable circumstances for establishment (Hughes, 1988). Although there are some data on the phenology, growth rhythms, physiological responses and morphological adaptations to flooding in the non-woody vegetation (Ellery et al., 1992; Mantlana, 2008), almost no published data were found for trees in the Okavango Delta. An exception is a recent study of leaf gas exchange of C. mopane in northwest Botswana (Veenendaal et al., 2008). Here, differences in physiological and morphological traits between tall and short forms of mopane [C. mopane (Kirk ex Benth.) Kirk ex J. Léonard] trees were compared. The tall form had a smaller leaf:fine-root biomass ratio, higher leaf nitrogen concentrations and less negative leaf water potentials. These differences appeared to be attributable to differences in root depth and density between the physiognomic types, and thus to different ways that the two growth forms exploit available soil water, the tall form having a consistently more conservative water-use strategy as the dry season progressed than the short form (Veenendaal et al., 2008).
Asia: Mekong floodplains
The Mekong is the world's eighth-longest river (Sarkkula et al., 2005). The lower Mekong basin in Cambodia and Vietnam includes floodplains (Fig. 5) which are among the few remaining global examples of relatively intact and functioning floodplains in a large river basin. This is the case despite its high-density human population of 54.8 million. It is widely accepted that this is one of the explanations for the highly productive fisheries of the Mekong and its very high biological diversity. The floodplains cover 795 000 km2 (Sarkkula et al., 2005). Since the only available data on tree ecophysiology come from Tonle Sap Lake, which is fed by the Mekong River, we concentrate on the 15 000 km2 of floodplain forest of this lake.
Fig. 5.
Asia: Mekong floodplains in Giang Province at high water (Photographs: Manfred Niekisch, Zoological Garden Frankfurt, Germany).
At the peak of the wet season, the Tonle Sap can expand to 250 km long and up to 100 km wide in places. The lake is shallow, measuring only 1–2 m at its deepest in the dry season, rising to more than 10 m in the wet season. As a result, when it floods, the total inundated area increases 4-fold. Mean annual rainfall is 1600 mm. Much of this eco-region is flooded for at least 6 months—from August to January or February (Wikramanayake and Rundel, 2002).
Most of the delta's human inhabitants fish, farm and live at subsistence levels. Although the annual flood cycle of the Mekong provides resources for these people, it is a fragile balance. The floodplains of the Tonle Sap have been strongly affected by human activity and little of the original forest cover remains pristine. Throughout the dry season, burning is common, with fires used to clear land before ploughing or to facilitate access. Flooding has recently damaged the infrastructure and caused extensive loss of property and livelihood. At the same time, roads and their associated developments have had a considerable impact on flooding by fragmenting the wetlands and interrupting the natural flow of water, sediments, nutrients and aquatic life. These impacts negate the beneficial effects normally brought by the natural flood cycle. The most significant threat comes from infrastructure development, particularly 149 planned large hydroelectric dams (Wikramanayake and Rundel, 2002). An additional threat is the invasion of the giant mimosa (Mimosa pigra). This aggressive shrubby species becomes established in fallow fields and disturbed shrub land and swamp forest area after clearance or burning. Once established, giant mimosa forms dense, impenetrable thickets of spiny growth that choke out other native species and have little value as wildlife habitat.
Tree vegetation
The swamp shrub lands and forest of the Tonle Sap Freshwater Swamp Forests eco-region include two forest associations that have been described for the extensive floodplain area of Tonle Sap. This is a short-tree shrub land covering much of the area and comprises a stunted swamp forest around the lake itself. Similar swamp forests are also present along the floodplains of the Mekong and other major rivers in Cambodia (Wikramanayake and Rundel, 2002). Swamp forest originally dominated the dry-season shoreline of Tonle Sap, covering about 10 % of the floodplain. It occurred in a mosaic of patch stands rather than as continuous forest stands (Wikramanayake and Rundel, 2002). Typically, these forests are flooded for 6–8 months each year and most species lose their leaves during this time. A continuous canopy 4 m high is formed by the dominant deciduous woody species. The most common species belong to the Euphorbiaceae, Fabaceae and Combretaceae together with Barringtonia acutangula and Diospyros cambodiana. Terminalia cambodiana is an important local endemic. The forest vegetation is dominated by the flood-tolerant tree Melaleuca cajuputi ssp. cumingiana (Safford et al., 2009). The swamp forest is 7–15 m high. Although M. cajuputi may reach 40 m in Australasia, in the Mekong floodplain trees are no taller than 22 m even when 100 years old (Safford et al., 2009) and most commonly form bushes 3–6 m tall.
Flooding tolerance and tree distribution
Little information is available on plant distribution and zonation along the Mekong River floodplains. In the Tonle Sap floodplain, the structure and composition of woody vegetation appear to be largely a function of the micro-heterogeneity of soil moisture and seasonal flood dynamics. Tree height is related to soil moisture conditions, with the tallest trees growing closer to the permanent lake basin and shorter ones at the periphery of the floodplain. Several species with shrubby growth forms in this peripheral community reach tree size in swamp forest habitats (Wikramanayake and Rundel, 2002).
Tree responses to flooding
In the Mekong floodplains, the terrestrial phase is the main growth period for trees. Most woody species of the floodplain of Tonle Sap are deciduous, a probable adaptation to the periodic flood pulse (Safford et al., 2009). Rather than lose their leaves in the dry season, however, these species lose their leaves when submerged as the lake deepens and the plants become partially or totally submerged. However, there are several woody species that remain evergreen (Lamberts and Koponen, 2008), despite leaves being submerged for 6–8 months each year. With only a few exceptions, flowering and fruit production in the floodplain trees and shrubs are delayed for several months after the flush of new leaves. Fruits reach maturity at the time of submergence, suggesting that fish may be important dispersal agents (Safford et al., 2009). Unfortunately, no data on physiological responses to flooding and morphological adaptations of the Mekong floodplain tree species were found. There are some publications on the ecophysiology of non-flooded environments, mainly dealing with drought-prone deciduous and dry evergreen tropical forests (Tanaka et al., 2004; Ishida et al., 2006; Huete et al., 2008). Few, if any, studies describe the responses of trees typical of the Mekong floodplain.
Australia's tropical floodplain wetlands
Floodplain wetlands are uncommon in the mostly arid continent of Australia. However, an important partly forested wetland, the Kakadu National Park in Northern Australia, extends over 99 000 km2 (Lowry and Finlayson, 2004). These floodplains are in an area broadly known as the ‘wet–dry tropics’. These have been defined as areas with an annual rainfall of 600–1600 mm spread over 4–7 months. The size of forested wetlands comprises 2900 km2. There is a wet season characterized by thunderstorms, tropical cyclones and rain depressions. These commence late in the year (November–December) and last for 3–4 months (Taylor and Tulloch, 1985). The hydrological cycle has been identified as being important in shaping the pattern of the vegetation in the freshwater wetlands (Finlayson et al., 1989). Water flows on a seasonal basis, starting early in the wet season and lasting until after the end of the rains. Flooding occurs once the catchment is saturated; heavy falls of rain later in the season generate more widespread flooding. Freshwater flow in the creeks and rivers ceases within a few months of the end of the rains, and the creeks and floodplains dry out except for a few permanent swamps and billabongs (Finlayson et al., 1990). Some creeks or river reaches are fed by springs or groundwater seeps. Analyses of the water quality within thick stands of submerged herbs and emergent grasses late in the wet season reveal that, in addition to variations in dissolved O2 and CO2 concentrations, the water becomes alkaline in the late afternoon when CO2 concentrations are at their lowest.
Fire and invasive plants and animal species have a significant impact on the extent and distribution of plant species and of the land cover (Finlayson et al., 1990). Damage to the natural levees that separate freshwater and saline wetland communities caused by climate change and by feral animals (especially water buffalo) may also change the vegetation. Notable responses by floodplain vegetation have already occurred following the removal of feral buffalo (Skeat et al., 1996).
Tree vegetation
Around 55 % of the terrestrial vegetation in the Kakadu Region is tropical tall grass savanna, composed of eucalypt-dominated open forest and woodland with a 1- to 2-m-tall grassy understorey (Finlayson, 2005). A further 30 % of the region is covered by heaths, and open woodlands with a sparse grass understorey. Closed-canopy monsoon rainforests are restricted to floodplains, besides lowland springs, rock outcrops and beach levees. The seasonally inundated floodplains include fringing woodland and forests, and billabongs (seasonally or permanently inundated lagoons associated with the floodplain or river channels) (Finlayson, 2005). The forests are inundated by up to 1 m of water during the wet season but are dry at other times.
Gallery and floodplain forests in monsoonal Northern Australia are mostly sclerophyllous and dominated by five closely related species of Melaleuca (Myrtaceae), among which niche differentiation is unclear (Franklin et al., 2007). The most important tree communities (Finlayson, 2005) include Melaleuca open forest and woodland with a tree canopy cover of 10–70 %. These are dominated by one or more Melaleuca species (M. viridiflora and M. cajaputi around the edges and at the northern end of the floodplain). The dominant species in the backswamps that are inundated for 6–8 months every year is M. leucadendra. There is also open woodland providing canopy cover of <10 % dominated by M. leucadendra. There are 12 terrestrial tree species, including Eucalyptus spp., Pandanus spiralis, Lophostemon lactifluus and Syzygium suborbiculare. Paperbark swamp forest is dominated by trees including M. viridiflora, M. cajaputi and M. leucadendra, and to a lesser extent B. acutangula and Pandanus spp.
The productivity of the floodplain vegetation changes with the annual cycle. This is indicated by periodic litterfall from Melaleuca trees. In an intensively sampled Melaleuca forest on the Magela floodplain, total litterfall was ∼0.7 kg m−2 year−1, whereas at a second site on the floodplain, less intensively investigated, a value of ∼1.5 kg m−2 year−1 was recorded (Finlayson, 1988). Comparative data for Melaleuca forests are limited to a small number of studies of different species found in the wetlands in southern Australia. These deposit an annual litterfall of 0.39–0.43 kg m−2 year−1 (Finlayson et al., 1993). The distribution and density of trees on at least part of the floodplain were seen to change considerably between 1975 and 1990 (Finlayson, 2005), indicating the dynamic nature of the wetland environment.
Flooding tolerance and tree distribution
The duration of flooding, depth of water and the velocity of water flow are major determinants of the vegetation composition of the floodplain (Finlayson et al., 1989). The changing pattern is a function of both the flooding and drying phases of the hydrological cycle (Finlayson et al., 1989, 1990). The vegetation of the floodplain billabongs is much influenced by adjacent plant communities on the seasonally inundated floodplain (e.g. grass mats extending across the floodplain and into the billabongs). Franklin et al. (2007) propose that Melaleuca forests occur where disturbance by fire and/or floodwater is too great for rain forest to persist, making them the wetland analogue of the eucalypt species that dominate the better-drained parts of north Australia.
Tree responses to flooding
Little information is available on physiological responses and adaptations of trees in Australian floodplains. Cowie et al. (2000) have reviewed adaptations in wetlands by herbs and macrophytic vegetation, and summarized the nature of the floodplain environment. They noted that variability due to changes in the hydrological cycle has resulted in many specific adaptations that enable the plants to establish and grow (Finlayson et al., 1989). The few details given include that trees of the floodplains often have modified bark structures such as the corky bark of Sesbania formosa and B. acutangula, and the distinctive papery bark of some melaleucas which possesses internal, longitudinal, gas-filled passages. It is also said that the majority of seed-dispersal mechanisms involve water, even though many parts of the floodplains are drier for a longer period than they are wet. However, it is not clear if this also applies to trees. Nothing seems to be known of the physiological and morphological adaptations of trees of the Kakadu National Park. Responses to flooding by melaleucas of North Queensland have, however, been documented and these may be relevant to the Kakadu since this is an environment with a median duration of inundation of 75 days (McJannet, 2008). Using a heat-pulse method, McJannet found a strong relationship between tree size and tree water use, and showed that transpiration by M. quinquenervia was unaffected by inundation. This ability to transpire during flooding may be due to physiological adaptations of this species, and to dynamic root systems that can quickly respond to rising and falling water tables and dense networks of fine ageotropic roots, which grow on and within the papery bark. Waterlogged M. quinquenervia also develops negatively gravitropic roots (Sena Gomes and Kozlowski, 1980).
A study by O'Grady et al. (2006) of Corymbia bella and M. argentea in riparian zones of the Northern Territory, along the Daly River, showed that throughout the dry season predawn leaf water potentials were above −0.5 MPa, indicating that neither species suffered significant unrelieved water-deficit stress during the dry season. This was despite low soil matric potentials in the top 1 m of soil. There were also no seasonal differences in tree water use in either species. Xylem sap deuterium concentrations indicated that M. argentea trees along the riverbank relied principally on river water or shallow groundwater, whereas C. bella growing along the levee was reliant on deep soil water reserves (O'Grady et al., 2006). This study demonstrates strong gradients of tree water use within tropical riparian communities in Northern Australia which probably can be extrapolated to the Kakadu Region.
Discussion
We have demonstrated that in all tropical continents, highly adapted tree species populate the floodplain. There is little taxonomic overlap except for the Asian and Australian floodplain where the genus Melaleuca dominates, but Barringtonia also occurs in both ecosystems. Forest types range from highly diverse dense forests (Amazonia, Mekong) to gallery forests or small tree stands scattered in savanna-dominated environments (Okavango, Kakadu Region). In each floodplain, the aquatic phase occurs when temperature and light conditions are optimal for plant growth and development, implying the need for developmental and biochemical adaptations rather than relying on avoidance through dormancy (Parolin et al., 2004). Flooding patterns vary, with durations ranging from more than half a year (Amazonia, Mekong, partly Kakadu) to only a few weeks (Okavango). The most important characteristic is the predictability of the time of the annual flood (the flood pulse concept of Junk et al., 1989). This predictability has allowed the trees to form morphological and physiological adaptations against flooding. On the other hand, as a consequence of the differing flood intensities and different flooding tolerance of the plants along the flooding gradient, tree distribution presents clear zonations in all four ecosystems. However, our wish to compare the underlying physiological and morphological reasons for this zonation was frustrated by a lack of relevant published data. A small number of species from the Asian, African and Australian floodplains have been analysed to date, and the dearth in our knowledge is alarming in the face of the speed at which the floodplains are being damaged or destroyed and the pressing need for well-informed recovery programmes.
Clearly, it is difficult to be sure of the extent to which trees in the four ecosystems share similar underlying adaptations to flooding stress. Phenological data indicate that this may be the case. For example, many deciduous species respond to flooding with leaf shedding, presumably as a means of reducing transpiration and water loss at a time when hypoxic or anaerobic roots may offer large resistances to water uptake. However, evergreen species are common in each of the floodplains that we examined with the exception of the Australian wetlands. This indicates the possibility that other mechanisms exist to reduce transpirational losses, although here too a proportion of the leaves of evergreen trees is also lost during flooding.
Physiological adaptations similar to those well known in temperate and Amazonian species can also be expected in the tree species of the Mekong, Okavango and Kakadu floodplains. Among the most important will be root system adaptations to anoxia (Crawford, 2003; Gibbs and Greenway, 2003). These will include the accumulation of adequate carbohydrate reserves and the ability to switch to alcoholic fermentation as an alternative, albeit inefficient, alternative source of energy to Krebs cycle-based aerobic respiration (Crawford, 2003). Induction of the activity of fermentative enzymes such as ADH, LDH, GPT and MDH has been observed under anaerobic growth conditions in Amazonian tree species (Schlüter and Furch, 1992; Schlüter et al., 1993; De Simone et al., 2002a; Ferreira, 2002), and this probably also applies to species of the Okavango, Mekong or Kakadu regions.
Underwater photosynthesis is common in temperate herbs (Mommer and Visser, 2005) and postulated for some tree species in Amazonian floodplains (Schlüter et al., 1993; Waldhoff et al., 2002; Parolin, 2009). It is possible that underwater photosynthesis, which both increases internal oxygen concentrations and raises energy supply, could partially alleviate the adverse effects of submersion. This may well be of adaptive significance in the trees of the Mekong floodplains where flood heights can exceed 2 m and might be associated with periods of complete submergence.
In the four floodplains that we examined, the terrestrial non-flooded phase is when tree growth is most vigorous. However, many tree species retain actively flowing phloem sap even during flooding (Waldhoff et al., 2002; Visser et al., 2003), indicating that active sources and sinks for respirable substrates operate under these conditions. A set of metabolic adaptations is inevitably required to achieve this. We suggest that in most tree species of the tropical floodplains, the primary morphological strategies in response to flooding are similar to those of temperate species (Jackson and Armstrong, 1999) or in the well-analysed tropical Amazonian floodplains (Parolin et al., 2004, 2010). In particular, there must be a development of gas-filled spaces in the roots and stems to allow diffusion of oxygen from the aerial portions of the plant into the roots. Morphological adaptations that favour this are hypertrophy of lenticels, formation of adventitious roots, plank-buttressing and stilt rooting, development of aerenchyma, and the deposition of cell wall biopolymers such as suberin and lignin in the root peripheral cell layers. The formation of aerial roots may compensate for losses of respiration and function by roots affected by lack of oxygen in the soil. Under experimental conditions with stable water levels, most species show the potential to produce adventitious roots. But, in the field, they are seldom found, probably because their formation is hampered by a rapidly changing water level. Leaves of tropical forests in general, and the Amazonian floodplain forests in particular, commonly have xeromorphic structures (Waldhoff, 2003). This attribute contributes to suppressing water loss at times of low water supply. This can apply to tree crowns during the aquatic phase and to periods of drought in the terrestrial phase.
Strict comparability between the four ecosystems we studied is limited by region-specific constraints such as the influence of groundwater quality and the incidence of fire and/or salt. These constraints might also be responsible for the large differences in tree diversity encountered in the four ecosystems. It is also true that the two forested floodplains also exhibit striking differences in their diversity. Tree species richness in the Amazonian white-water floodplains (várzea) is ∼10 times greater than that in the forests of Cambodia (Tonle Sap; Campbell et al., 2006) and about three times above that in the Brazilian Pantanal, another extensive wetland dominated by savannah (Wittmann et al., 2006). One reason for the comparatively high tree species richness in várzea forests of Amazonia is the coexistence of species well adapted to flooding and generalist species that also occur in the uplands (Wittmann et al., 2006). Very little is known about the number of generalists vs. specialists among floodplain trees. In Amazonia, only a few tree species occur along the whole flooding gradient, with most being restricted to very small topographic amplitudes (Wittmann et al., 2002). Only ∼20 % of all várzea tree species occur in both low- and high-land habitats, demonstrating the striking difference in ecophysiological constraints for tree survival and growth along the flooding gradient. In the other floodplains of the world, generalists may dominate, as in savanna ecosystems. However, we have too little information to speculate about the ecological amplitude of trees in floodplains.
The degree of flood tolerance depends, in large part, on the time taken to colonize the floodplains. Morphological adaptations may be remnants of pre-adaptations from non-flooded upland tree species (Kubitzki, 1989) which evolved further, leading over time to highly adapted species. Thus, the phylogenetic development of adaptations depends on the age of the ecosystem, and also on the dominating plant families that colonized these ecosystems originally. In each of the four ecosystems, there are large differences in developmental age (Table 1). This implies different stages of adaptations among the organisms living there. Amazonian and African floodplains are extremely old, dating at least to the Pleistocene (>12 million years old), or even earlier. Such ancient landscapes will have experienced several changes in climate and hydrology, i.e. during the glacial and interglacial periods. In contrast, the Mekong River basin and the Australian floodplains are much younger, and are thought to be no older than 7500 and 4000 years, respectively (Junk et al., 2006).
Conclusions and forward look
Our comparative review on the adaptive responses of trees to flooding in four tropical freshwater floodplains of different continents demonstrates that substantial data about the floodplain tree flora, its ecology and functioning are lacking. Despite many physio-ecologically motivated studies on trees of the Central Amazonian floodplains, and a few studies on other tropical freshwater floodplains, we are still only at the start of our understanding of how terrestrial plants, especially trees, cope with extended periods of flooding. However, this knowledge is fundamental to understanding the evolution of flooded landscapes and their organisms, as well as their interaction with non-flooded ecosystems. The range of plant responses recorded in the different tropical floodplains leads us to the following conclusions:
Regular flooding is a severe stress to trees, resulting in reduced species richness compared with non-flooded uplands.
The regular flood pulse has given rise to a large diversity of growth forms and adaptations by trees. These seem to increase in variety with the age of the respective floodplains and the climatic stability to which they were exposed.
Most floodplain tree species are restricted to small topographic amplitudes along the flooding gradients, leading to a distinct zonation of tree species. This implies different mechanisms or combinations of mechanisms conferring zone-specific tolerance to flood stress. This zonation is not dependent on the height of the flooding amplitude. Most highly flooded (>2 m) tree species react to high-water periods with leaf shedding, which is associated with decreased metabolism and growth.
The occurrence of evergreen tree species does not depend on the height and magnitude of flooding, since evergreen species are found in all three floodplains.
Although the responses of trees to flooding seem to be manifold, variability within an ecosystem is greater than that between ecosystems despite widely differing species/genera/families dominating the respective floodplains.
Endemic tree species are rare, except in Amazonian floodplains. This may be the outcome of a markedly stable climate over geological time.
Future research must address the current paucity of published data on the ecophysiology and adaptations and requirements of trees in the major floodplain forests.
Attention also needs to be given to small wetlands along small rivers or in remote places. These areas have been almost totally neglected.
Where possible, future studies should adopt methods which will allow comparisons to be made with confidence. Climatic change, increasing prevalence of droughts, alterations to groundwater availability and flooding periodicities make such work increasingly urgent since freshwater floodplain forests are a vital human resource that is under threat. They harbour many fish and mammal species, help moderate widespread flooding of inhabited areas, regulate river levels, improve water quality, act as substantial sinks for carbon and provide timber and non-timber forest products. Improving our understanding of their workings will underpin their preservation and effective future management.
Contributions by the authors
Both authors contributed to a similar extent in the preparation of this article.
Conflict of interest statement
None declared.
Acknowledgements
We thank the Association of Tropical Biology and Conservation (ATBC) and the Society of Tropical Ecology (gtö) for support. We also thank Michael Heinl (University of Innsbruck, Germany) for much information on the Okavango Delta.
References
- Armstrong W, Brändle R, Jackson MB. Mechanisms of flood tolerance in plants. Acta Botanica Neerlandica. 1994;43:307–358. [Google Scholar]
- Bonyongo MC. Vegetation ecology of the seasonal floodplains in the Okavango Delta, Botswana. University of Pretoria; 1999. Unpublished Thesis (MSc) [Google Scholar]
- Bonyongo MC, Veenendaal E, Bredenkamp G. Floodplain vegetation in the Nxaraga Lagoon area, Okavango Delta, Botswana. South African Journal of Botany. 2000;66:15–21. [Google Scholar]
- Campbell I, Poole C, Giesen W, Valbo-Jorgensen J. Species diversity and ecology of Tonle Sap Great Lake, Cambodia. Aquatic Sciences. 2006;68:355–373. doi:10.1007/s00027-006-0855-0. [Google Scholar]
- Colmer TD, Pedersen O. Underwater photosynthesis and respiration in leaves of submerged wetland plants: gas films improve CO2 and O2 exchange. New Phytologist. 2008;177:918–926. doi: 10.1111/j.1469-8137.2007.02318.x. doi:10.1111/j.1469-8137.2007.02318.x. [DOI] [PubMed] [Google Scholar]
- Cowie ID, Armstrong MD, Woinarski JCZ, Brocklehurs PS, Short PS, Dunlop CR. An overview of the floodplains. In: Cowie ID, Short PS, Osterkamp P, Madsen M, editors. Floodplain flora: a flora of the coastal floodplains of the Northern Territory, Australia. Canberra, Australia: ABRS; 2000. pp. 1–33. [Google Scholar]
- Crawford RMM. The anaerobic retreat. In: Crawford R.M.M., editor. Studies in plant survival. Ecological case histories of plant adaptation to adversity. Studies in Ecology 11. Oxford: Blackwell Scientific Publications; 1989. pp. 105–129. [Google Scholar]
- Crawford RMM. Oxygen availability as an ecological limit to plant distribution. Advances in Ecological Research. 1992;23:93–185. doi:10.1016/S0065-2504(08)60147-6. [Google Scholar]
- Crawford RMM. Seasonal differences in plant responses to flooding and anoxia. Canadian Journal of Botany. 2003;81:1224–1246. doi:10.1139/b03-127. [Google Scholar]
- Darwin C. Pencil sketch of 1842. In: Darwin F, editor. The foundations of the origin of species: two essays written in 1842 and 1844. Cambridge: Cambridge University Press; 1842. (published 1909) [Google Scholar]
- Darwin C. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. Cambridge, MA: Harvard University Press; 1859. London: John Murray [Facsimile of 1st 1964. [PMC free article] [PubMed] [Google Scholar]
- De Simone O, Haase K, Müller E, Junk WJ, Schmidt W. Adaptations of Central Amazon tree species to prolonged flooding: root morphology and leaf longevity. Plant Biology. 2002a;2:515–522. doi:10.1055/s-2002-34127. [Google Scholar]
- De Simone O, Haase K, Müller E, Junk WJ, Gonsior GA, Schmidt W. Impact of root morphology on metabolism and oxygen distribution in roots and rhizosphere from two Central Amazon floodplain tree species. Functional Plant Biology. 2002b;29:1025–1035. doi: 10.1071/PP01239. doi:10.1071/PP01239. [DOI] [PubMed] [Google Scholar]
- Ellery WN, Tacheba B. Floristic diversity of the Okavango Delta, Botswana. In: Alonso LE, Nordin LA, editors. A rapid biological assessment of the aquatic ecosystems of the Okavango Delta, Botswana. 2003. Chapter 5. High Water Survey: RAP Bulletin of Biological Assessment. [Google Scholar]
- Ellery K, Ellery WN, Verhagen BT. The distribution of C3 and C4 plants in a successional sequence in the Okavango Delta. South African Journal of Botany. 1992;58:400–402. [Google Scholar]
- Ellery WN, Ellery K, McCarthy TS. Plant distribution in islands of the Okavango Delta, Botswana: determinants and feedback interactions. African Journal of Ecology. 1993;31:118–134. doi:10.1111/j.1365-2028.1993.tb00526.x. [Google Scholar]
- Ellery WN, McCarthy TS, Dangerfield JM. Floristic diversity in the Okavango Delta, Botswana as an endogenous product of biological activity. In: Gopal B, Junk WJ, Davis JA, editors. Biodiversity in wetlands: assessment, function and conservation. I. Leiden: Backhuis; 2000. [Google Scholar]
- Ferreira CS. Germinação e adaptações metabólicas e morfo-anatômicas em plântulas de Himatanthus succuuba (Spruce) Wood., de ambientes de várzea e terra firme na Amazônia Central. 2002 MSc Thesis, Universidade do Amazonas (UA), Instituto Nacional de Pesquisas da Amazônia (INPA), Manaus, 95. [Google Scholar]
- Ferreira LV, Venticinque E, Almeida S. O desmatamento na Amazônia e a importância das áreas protegidas. Estudos Avançados. 2005;19:1–10. doi:10.1590/S0103-40142005000100010. [Google Scholar]
- Finlayson CM. Productivity and nutrient dynamics of seasonally inundated flood plains in the Northern Territory. In: Wade-Marshall D, Loveday P, editors. North Australia: progress and prospects, Vol. 2 floodplain research. Darwin: ANU Press; 1988. pp. 58–83. [Google Scholar]
- Finlayson CM. Plant ecology of Australia's tropical floodplain wetlands: a review. Annals of Botany. 2005;96:541–555. doi: 10.1093/aob/mci209. doi:10.1093/aob/mci209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson CM, Bailey BJ, Cowie ID. Macrophytic vegetation of the Magela flood plain, Northern Australia. Research report no. 5. Sydney: Office of the Supervising Scientist; 1989. [Google Scholar]
- Finlayson CM, Cowie ID, Bailey BJ. Characteristics of a seasonally flooded freshwater system in monsoonal Australia. In: Whigham DF, Good RE, Kvet J, editors. Wetland ecology and management: case studies. Dordrecht: Kluwer Academic; 1990. pp. 141–162. [Google Scholar]
- Finlayson CM, Cowie ID, Bailey BJ. Litterfall in a Melaleuca forest on a seasonally inundated flood plain in tropical northern Australia. Wetlands Ecology and Management. 1993;2:177–188. [Google Scholar]
- Franklin DC, Brocklehurst PS, Lynch D, Bowman DMJS. Niche differentiation and regeneration in the seasonally flooded Melaleuca forests of northern Australia. Journal of Tropical Ecology. 2007;23:457–468. doi:10.1017/S0266467407004130. [Google Scholar]
- Furch B, Corrêa AFF, Nunes JASM, Otto K-R. Lichtklimadaten in drei aquatischen Ökosystemen verschiedener physikalisch-chemischer Beschaffenheit. I. Abschwächung, Rückstreuung und Vergleich zwischen Einstrahlung, Rückstrahlung und sphärisch gemessener Quantenstromdichte (PAR) Amazoniana. 1985;9:411–430. [Google Scholar]
- Gibbs J, Greenway H. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology. 2003;30:1–47. doi: 10.1071/PP98095. doi:10.1071/PP98095. [DOI] [PubMed] [Google Scholar]
- Gopal B, Junk WJ, Davis JA. Biodiversity in wetlands: assessment, function and conservation. Vol. 1. Leiden: Backhuis; 2000. [Google Scholar]
- Heinl M, Sliva J, Tacheba B. Vegetation changes after single fire-events in the Okavango Delta wetland, Botswana. South African Journal of Botany. 2004;70:695–704. [Google Scholar]
- Heinl M, Neuenschwander A, Sliva J, Vanderpost C. Interactions between fire and flooding in the Okavango Delta floodplains, Botswana. Landscape Ecology. 2006;21:699–709. doi:10.1007/s10980-005-5243-y. [Google Scholar]
- Heinl M, Frost P, Vanderpost C, Sliva J. Fire activity on drylands and floodplains in the southern Okavango Delta, Botswana. Journal of Arid Environments. 2007;68:77–87. doi:10.1016/j.jaridenv.2005.10.023. [Google Scholar]
- Huete AR, Restrepo-Coupe N, Ratana P, Didan K, Saleska SR, Ichii K, Panuthai S, Gamo M. Multiple site tower flux and remote sensing comparisons of tropical forest dynamics in monsoon Asia. Agricultural and Forest Meteorology. 2008;148:748–760. doi:10.1016/j.agrformet.2008.01.012. [Google Scholar]
- Hughes FMR. The ecology of African floodplain forests in semi-arid and arid zones: a review. Journal of Biogeography. 1988;15:127–140. doi:10.2307/2845053. [Google Scholar]
- Irion G, Junk WJ, Mello JASN. The large central Amazonian river floodplains near Manaus: geological, climatological, hydrological and geomorphological aspects. In: Junk WJ, editor. The Central Amazon floodplain: ecology of a pulsing system. Ecological Studies 126. Heidelberg: Springer; 1997. pp. 23–46. [Google Scholar]
- Ishida A, Diloksumpun S, Ladpala P, Staporn D, Panuthai S, Gamo M, Yazaki K, Ishizuka M, Puangchit L. Contrasting seasonal leaf habits of canopy trees between tropical dry-deciduous and evergreen forests in Thailand. Tree Physiology. 2006;26:643–656. doi: 10.1093/treephys/26.5.643. [DOI] [PubMed] [Google Scholar]
- IUCN. 1991 http://cmsdata.iucn.org/downloads/mrwd_nwg_regional_meeting_december_2009_final_2.pdf . [Google Scholar]
- Jackson MB, Armstrong W. Formation of aerenchyma and the processes of plant ventilation in relation to soil flooding and submergence. Plant Biology. 1999;1:274–287. doi:10.1111/j.1438-8677.1999.tb00253.x. [Google Scholar]
- James EK, Loureiro MD, Pott A, Pott VJ, Martins CM, Franco AA, Sprent JI. Flooding-tolerant legume symbioses from the Brazilian Pantanal. New Phytologist. 2001;150:723–738. doi:10.1046/j.1469-8137.2001.00126.x. [Google Scholar]
- Joly CA, Crawford RMM. Variation in tolerance and metabolic responses to flooding in some tropical trees. Journal of Experimental Botany. 1982;33:799–809. doi:10.1093/jxb/33.4.799. [Google Scholar]
- Junk WJ. Ecology of the várzea, floodplain of Amazonian whitewater rivers. In: Sioli H, editor. The Amazon. Limnology and landscape ecology of a mighty tropical river and its basin. Junk. 1984. pp. 215–243. [Google Scholar]
- Junk WJ. The central Amazonian floodplain: ecology of a pulsing system. Ecological Studies 126. Heidelberg: Springer; 1997. [Google Scholar]
- Junk WJ. Mechanisms for development and maintenance of biodiversity in neotropical floodplains. In: Gopal B, Junk WJ, Davis JA, editors. Biodiversity in wetlands: assessment, function and conservation. Vol. 1. Leiden: Backhuis; 2000. pp. 119–139. [Google Scholar]
- Junk WJ, da Silva VMF. Mammals, reptiles and amphibians. In: Junk WJ, editor. The central Amazon floodplain: ecology of a pulsing system. Ecological studies, 126. Berlin, Heidelberg: Springer; 1997. pp. 409–418. [Google Scholar]
- Junk WJ, Bayley PB, Sparks RE. The flood pulse concept in river-floodplain systems. In: Dodge DP. Proceedings of the International Large River Symposium. Canadian Special Publication of Fisheries and Aquatic Sciences. 1989;106:110–127. [Google Scholar]
- Junk WJ, Brown M, Campbell IC, Finlayson M, Gopal B, Ramberg L, Warner BG. The comparative biodiversity of seven globally important wetlands: a synthesis. Aquatic Sciences. 2006;68:400–414. doi:10.1007/s00027-006-0856-z. [Google Scholar]
- Kalliola R, Salo J, Puhakka M, Rajasilta M. New site formation and colonizing vegetation in primary succession on the Western Amazon floodplains. Journal of Ecology. 1991;79:877–901. doi:10.2307/2261087. [Google Scholar]
- Kesselmeier J, Staudt M. Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. Journal Atmospheric Chemistry. 1999;33:23–88. doi:10.1023/A:1006127516791. [Google Scholar]
- Kesselmeier J, Bode K, Hofmann U, Müller H, Schäfer L, Wolf A, Ciccioli P, Brancaleoni E, Cecinato A, Frattoni M, Foster P, Ferrari C, Jacob V, Fugit JL, Dutaur L, Simon V, Torres L. Emission of short chained organic acids, aldehydes and monoterpenes from Quercus ilex L., Pinus pinea L. in relation to physiological activities, carbon budget and emission algorithms. Atmospheric Environment. 1997;31:119–133. doi:10.1016/S1352-2310(97)00079-4. [Google Scholar]
- Kimmerer TW, Macdonald RC. Acetaldehyde and ethanol biosynthesis in leaves of plants. Plant Physiology. 1987;84:1204–1209. doi: 10.1104/pp.84.4.1204. doi:10.1104/pp.84.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski TT. Responses of woody plants to flooding. In: Kozlowski TT, editor. Flooding and plant growth. Orlando: Academic Press; 1984. pp. 129–163. [Google Scholar]
- Kubitzki K. The ecogeographical differentiation of Amazonian inundation forests. Plant Systematics and Evolution. 1989;162:285–304. doi:10.1007/BF00936922. [Google Scholar]
- Kubitzki K, Ziburski A. Seed dispersal in flood plain forests of Amazonia. Biotropica. 1994;26:30–43. doi:10.2307/2389108. [Google Scholar]
- Lamberts D, Koponen J. Flood pulse alterations and productivity of the Tonle Sap ecosystem: a model for impact assessment. Ambio. 2008;37:178–184. doi: 10.1579/0044-7447(2008)37[178:fpaapo]2.0.co;2. doi:10.1579/0044-7447(2008)37[178:FPAAPO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Larcher W. Ökophysiologie der Pflanzen: Leben, Leistung und Streßbewältigung der Pflanzen in ihrer Umwelt. 1994 5th Edn. Ulmer Stuttgart, UTB für Wissenschaft. [Google Scholar]
- Lowry J, Finlayson CM. Supervising scientist report 178. Darwin.: 2004. A review of spatial data sets for wetland inventory in northern Australia. [Google Scholar]
- Mantlana KB. Seasonal and inter-annual variations of leaf-level photosynthesis and soil respiration in the representative ecosystems of the Okavango Delta, Botswana. Wageningen University: The Netherlands; 2008. PhD Thesis. [Google Scholar]
- McCarthy TS. Groundwater in the wetlands of the Okavango Delta, Botswana, and its contribution to the structure and function of the ecosystem. Journal of Hydrology. 2006;320:264–282. doi:10.1016/j.jhydrol.2005.07.045. [Google Scholar]
- McJannet D. Water table and transpiration dynamics in a seasonally inundated Melaleuca quinquenervia forest, north Queensland, Australia. Hydrological Processes. 2008;22:3079–3090. doi:10.1002/hyp.6894. [Google Scholar]
- Medina E. Adaptations of tropical trees to moisture stress. In: Golley FB, editor. Ecosystems of the world: tropical rain forest ecosystems. Amsterdam: Elsevier; 1983. pp. 225–237. [Google Scholar]
- Mommer L, Visser EJW. Underwater photosynthesis in flooded terrestrial plants: a matter of leaf plasticity. Annals of Botany. 2005;96:581–589. doi: 10.1093/aob/mci212. doi:10.1093/aob/mci212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira FMDS, Silva MFD, Faria SMD. Occurrence of nodulation in legume species in the Amazon region of Brazil. New Phytologist. 1992;121:563–570. doi:10.1111/j.1469-8137.1992.tb01126.x. [Google Scholar]
- O'Grady AP, Eamus D, Cook PG, Lamontagne S. Comparative water use by the riparian trees Melaleuca argentea and Corymbia bella in the wet–dry tropics of northern Australia. Tree Physiology. 2006;26:219–228. doi: 10.1093/treephys/26.2.219. doi:10.1093/treephys/26.2.219. [DOI] [PubMed] [Google Scholar]
- Oliveira-Wittmann A, Piedade MTF, Wittmann F, Parolin P. Germination in four low-várzea tree species of Central Amazonia. Aquatic Botany. 2007;86:197–203. doi:10.1016/j.aquabot.2006.10.001. [Google Scholar]
- Parolin P. Phenology and CO2-assimilation of trees in Central Amazonian floodplains. Journal of Tropical Ecology. 2000;16:465–473. doi:10.1017/S0266467400001516. [Google Scholar]
- Parolin P. Senna reticulata, a pioneer tree from Amazonian várzea floodplains. The Botanical Review. 2001;67:239–254. doi:10.1007/BF02858077. [Google Scholar]
- Parolin P. Submerged in darkness: adaptations to prolonged submergence by woody species of the Amazonian Floodplains. Annals of Botany. 2009;103:359–376. doi: 10.1093/aob/mcn216. doi:10.1093/aob/mcn216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolin P, Armbrüster N, Wittmann F, Ferreira LV, Piedade MTF, Junk WJ. A review of tree phenology in central Amazonian floodplains. Pesquisas, Botânica. 2002;52:195–222. [Google Scholar]
- Parolin P, De Simone O, Haase K, Waldhoff D, Rottenberger S, Kuhn U, Kesselmeier J, Schmidt W, Piedade MTF, Junk WJ. Central Amazon floodplain forests: tree survival in a pulsing system. The Botanical Review. 2004;70:357–380. doi:10.1663/0006-8101(2004)070[0357:CAFFTA]2.0.CO;2. [Google Scholar]
- Parolin P, Lucas C, Piedade MTF, Wittmann F. Drought responses of flood-tolerant trees in Amazonian floodplains. Annals of Botany. 2010;105:129–139. doi: 10.1093/aob/mcp258. doi:10.1093/aob/mcp258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnamperuma FN. Effects of flooding on soils. In: Kozlowski TT, editor. Flooding and plant growth. Orlando: Academic Press; 1984. pp. 9–45. [Google Scholar]
- Prance GT. Notes on the vegetation of Amazonia. III. Terminology of Amazonian forest types subjected to inundation. Brittonia. 1979;31:26–38. doi:10.2307/2806669. [Google Scholar]
- Ribeiro MNG, Adis J. Local rainfall variability—a potential bias for bioecological studies in the Central Amazon. Acta Amazonica. 1984;14:159–174. [Google Scholar]
- Ringrose S. Characterisation of riparian woodlands and their potential water loss in the distal Okavango Delta, Botswana. Applied Geography. 2003;23:281–302. doi:10.1016/j.apgeog.2003.08.006. [Google Scholar]
- Ringrose S, Chipanshi AC, Matheson W, Chanda R, Motoma L, Magole I, Jellema A. Climate- and human-induced woody vegetation changes in Botswana and their implications for human adaptation. Environmental Management. 2002;30:98–109. doi: 10.1007/s00267-002-2486-0. doi:10.1007/s00267-002-2486-0. [DOI] [PubMed] [Google Scholar]
- Rottenberger S, Kleiss B, Kuhn U, Wolf A, Piedade MTF, Junk W, Kesselmeier J. The effect of flooding on the exchange of the volatile C2-compounds ethanol, acetaldehyde and acetic acid between leaves of Amazonian floodplain tree species and the atmosphere. Biogeosciences. 2008;5:1085–1100. [Google Scholar]
- Safford RJ, Maltby E, Duong Van Ni , Branch NP. Melaleuca wetlands and sustainable development in the Mekong Delta, Vietnam. In: Maltby E, Barker T, editors. The wetlands handbook. Oxford: Blackwells; 2009. pp. 829–849. [Google Scholar]
- Salo J, Kalliola R, Häkkinen I, Mäkinen Y, Niemelä P, Puhakka M, Coley PD. River dynamics and the diversity of Amazon lowland forest. Nature. 1986;322:254–258. doi:10.1038/322254a0. [Google Scholar]
- Sarkkula J, Koponen J, Kummu M. Tools for integrated basin flow management at Lower Mekong basin floodplains. In: Zerger A, Argent RM, editors. MODSIM 2005 International Congress on Modelling and Simulation. Canberra: Modelling and Simulation Society of Australia and New Zealand; 2005. pp. 2153–2159. [Google Scholar]
- Scarano FR, Ribeiro KT, Moraes LFD, Lima HC. Plant establishment on flooded and unflooded patches of a freshwater swamp forest in southeastern Brazil. Journal of Tropical Ecology. 1997;14:793–803. doi:10.1017/S0266467400011007. [Google Scholar]
- Scarano FR, Pereira TS, Rocas G. Seed germination during floatation and seedling growth of Carapa guianensis, a tree from flood-prone forests of the Amazon. Plant Ecology. 2003;168:291–296. doi:10.1023/A:1024486715690. [Google Scholar]
- Schlüter U-B, Furch B. Morphologische, anatomische und physiologische Untersuchungen zur Überflutungstoleranz des Baumes Macrolobium acaciaefolium, charakteristisch für die Weiß- und Schwarzwasserüberschwemmungswälder bei Manaus, Amazonas. Amazoniana. 1992;12:51–69. [Google Scholar]
- Schlüter U-B, Furch B, Joly CA. Physiological and anatomical adaptations by young Astrocaryum jauari Mart. (Arecaceae) in periodically inundated biotopes of Central Amazonia. Biotropica. 1993;25:384–396. doi:10.2307/2388862. [Google Scholar]
- Schöngart J. Dendrochronological and dendroclimatic studies in Central Amazonian Forests. 2003:1–13. Research Program May 2003–April 2005. [Google Scholar]
- Schöngart J, Piedade MFT, Ludwigshausen S, Horna V, Worbes M. Phenology and stem-growth periodicity of tree species in Amazonian floodplain forests. Journal of Tropical Ecology. 2002;18:581–597. [Google Scholar]
- Sena Gomes AR, Kozlowski TT. Responses of Melaleuca quinquenervia seedlings to flooding. Physiologia Plantarum. 1980;49:373–377. doi:10.1111/j.1399-3054.1980.tb03319.x. [Google Scholar]
- Sioli H. Betrachtungen über den Begriff ‘Fruchtbarkeit’ eines Gebiets anhand der Verhältnisse in Böden und Gewässern Amazoniens. Forschung Fortschritt. 1954;28:65–72. [Google Scholar]
- Skeat AJ, East TJ, Corbett LK. Impact of feral water buffalo. In: Finlayson CM, von Oertzen I, editors. Landscape and vegetation ecology of the Kakadu Region, Northern Australia. Dordrecht: Kluwer Academic; 1996. pp. 155–177. [Google Scholar]
- Tacheba B, Segosebe E, Vanderpost C, Sebego R. Assessing the impacts of fire on the vegetation resources that are available to the local communities of the seasonal wetlands of the Okavango, Botswana, in the context of different land uses and key government policies. African Journal of Ecology. 2009;47:71–77. doi:10.1111/j.1365-2028.2008.01052.x. [Google Scholar]
- Tanaka K, Takizawa H, Kume T, Xu J, Tantasirin C, Suzuki M. Impact of rooting depth and soil hydraulic properties on the transpiration peak of an evergreen forest in northern Thailand in the late dry season. Journal of Geophysical Research. 2004;109 [Google Scholar]
- Taylor JA, Tulloch D. Rainfall in the wet–dry tropics: extreme events at Darwin and similarities between years during the period 1870–1983. Australian Journal of Ecology. 1985;10:281–295. doi:10.1111/j.1442-9993.1985.tb00890.x. [Google Scholar]
- Veenendaal EM, Mantlana KB, Pammenter NW, Weber P, Huntsman-Mapila P, Lloyd J. Growth form and seasonal variation in leaf gas exchange of Colophospermum mopane savanna trees in northwest Botswana. Tree Physiology. 2008;28 doi: 10.1093/treephys/28.3.417. [DOI] [PubMed] [Google Scholar]
- Visser EJW, Voesenek LACJ, Vartapetian BB, Jackson MB. Flooding and plant growth. Annals of Botany. 2003;91:107–109. doi:10.1093/aob/mcg014. [Google Scholar]
- Waldhoff D. Leaf structure in trees of Central Amazonian floodplain forests (Brazil) Amazoniana. 2003;17:451–469. [Google Scholar]
- Waldhoff D, Furch B. Leaf morphology and anatomy in eleven tree species from Central Amazonian floodplains (Brazil) Amazoniana. 2002;17:79–94. [Google Scholar]
- Waldhoff D, Junk WJ, Furch B. Fluorescence parameters, chlorophyll concentration, and anatomical features as indicators for flood adaptation of an abundant tree species in Central Amazonia: Symmeria paniculata. Environmental and Experimental Botany. 2002;48:225–235. doi:10.1016/S0098-8472(02)00037-0. [Google Scholar]
- Wantzen KM, Junk WJ. The importance of stream-wetland-systems for biodiversity: a tropical perspective. In: Gopal B, Junk WJ, Davis JA, editors. Biodiversity in wetlands: assessment, function and conservation. Vol. 1. Leiden: Backhuys; 2000. pp. 11–34. [Google Scholar]
- Wikramanayake E, Rundel P. Tonle sap freshwater swamp forests. In: Wikramanayake E, Dinerstein E, Loucks C, editors. Terrestrial ecoregions of the Indo-Pacific: a conservation assessment. Washington, DC: Island Press; 2002. p. 824. [Google Scholar]
- Wittmann F, Parolin P. Aboveground roots in Amazonian floodplain trees. Biotropica. 2005;37:609–619. doi:10.1111/j.1744-7429.2005.00078.x. [Google Scholar]
- Wittmann F, Anhuf D, Junk WJ. Tree species distribution and community structure of Central Amazonian várzea forests by remote sensing techniques. Journal of Tropical Ecology. 2002;18:805–820. doi:10.1017/S0266467402002523. [Google Scholar]
- Wittmann F, Junk WJ, Piedade MTF. The várzea forests in Amazonia: flooding and the highly dynamic geomorphology interact with natural forest succession. Forest Ecology and Management. 2004;196:199–212. doi:10.1016/j.foreco.2004.02.060. [Google Scholar]
- Wittmann F, Schöngart J, Montero JC, Motzer T, Junk WJ, Piedade MTF, Queiroz HL, Worbes M. Tree species composition and diversity gradients in white-water forests across the Amazon Basin. Journal of Biogeography. 2006;33:1334–1347. doi:10.1111/j.1365-2699.2006.01495.x. [Google Scholar]
- Wittmann F, Junk WJ, Schöngart J. Phytogeography, species diversity, community structure and dynamics of central Amazonian floodplain forests. In: Junk WJ, Piedade MTF, Parolin P, Wittmann F, Schöngart J, editors. Central Amazonian Floodplain forests: ecophysiology, biodiversity and sustainable management. Ecological Studies. Heidelberg: Springer.; 2010. [Google Scholar]
- Worbes M. The forest ecosystem of the floodplains. In: Junk WJ, editor. The Central Amazon floodplain: ecology of a pulsing system. Ecological Studies 126. Heidelberg: Springer; 1997. pp. 223–266. [Google Scholar]
- Worbes M, Klinge H, Revilla JD, Martius C. On the dynamics, floristic subdivision and geographical distribution of várzea forests in Central Amazonia. Journal of Vegetation Science. 1992;3:553–564. doi:10.2307/3235812. [Google Scholar]