The nitric oxide donor sodium nitroprusside (SNP) promotes regreening of Fe-deficient maize plants. The effect is not the outcome of increased tissue Fe but of NO-modulation of oxidative changes that may favour conversions of internal Fe to more readily available ferrous iron.
Abstract
Background and aims
Nitric oxide (NO) has been reported to alleviate Fe-deficiency effects, possibly by enhancing the functional Fe status of plants. This study examines changes in tissue Fe status and oxidative metabolism in Fe-deficient maize (Zea mays L.) plants enriched with NO using sodium nitroprusside (SNP) as a source.
Methodology
Measurements included changes in concentrations of H2O2, non-protein thiols, levels of lipid peroxidation and activities of superoxide dismutase (SOD) and of the Fe-requiring antioxidant haem enzymes catalase, peroxidase and ascorbate peroxidases. Internal NO in Fe-deficient maize plants was manipulated with SNP and the NO scavenger, methylene blue (MB). A key control was treatment with sodium ferrocyanide (SF), a non-NO-supplying analogue of SNP.
Principal results
SNP but not SF caused re-greening of leaves in Fe-deficient maize plants over 10–20 days, increased in vivo NO content, raised chlorophyll and carotenoid concentrations, promoted growth in dry weight, increased the activities of H2O2-scavenging haem enzymes and enhanced lipid peroxidation, while decreasing SOD activity and H2O2 concentrations. The NO scavenger, MB, blocked the effects of the SNP. Although SNP and SF each donated Fe and increased active Fe, only SNP increased leaf chlorophyll.
Conclusions
NO plays a role in Fe nutrition, independently of its effect on total or active Fe status. The most probable mechanism of NO involvement is to increase the intracellular availability of Fe by means of modulating redox. This is likely to be achieved by enhancing the chemical reduction of foliar Fe(III) to Fe(II).
Introduction
Iron is an essential element for all forms of life, and its limitation has a profound impact on the productivity of photosynthetic organisms (Martin et al., 1994). It is a component of many proteins required for crucial cellular processes and is involved in numerous vital functions, including respiration, photosynthesis and cell division (Marschner, 1995). Although Fe is abundant in the Earth's crust, much of it exists in insoluble form and is thus not freely available to plants. In some plants (Strategy 1 species), the uptake of Fe is supported by acidification of the rhizosphere and chemical reduction of external Fe(III) to Fe(II) (Guerinot and Yi, 1994; Marschner, 1995). Monocot grasses (Strategy 2 plants) chelate Fe(III) by releasing phytosiderophores [low-molecular-weight Fe(III)-specific ligands, non-proteinogenic amino acids] (Guerinot and Yi, 1994; Marschner, 1995). These mechanisms are expressed in root apices under Fe-deficient conditions and are down-regulated when the Fe supply is replenished (Marschner, 1995).
Since most (80 %) leaf Fe is located in the chloroplast, it must cross membranes to reach this final destination. Fe acquisition from the soil, therefore, is not the only constraint. Others include the chemical reduction of Fe(III) to soluble Fe(II), which is essential for Fe to cross several biological membranes before reaching the chloroplast. This reduction step can be mediated by plasma membrane-bound Fe(III)-chelate reductase (Guerinot and Yi, 1994), but the activity of this enzyme is dependent on apoplastic pH (Kosegarten et al., 1999) and on light (González-Vallejo et al., 2000). In vivo Fe(III) reduction is also aided by superoxide radicals (O2•−) (Brüggemann et al., 1993), indicating that changes in the redox state of the apoplast might be involved in Fe(III) reduction to more useable Fe(II). There is also evidence that Fe could be immobilized and accumulate in inactive forms in the leaf (Morales et al., 1998; Kosegarten et al., 1999), and this explains the total Fe concentration in chlorotic leaves of Fe-deficient plants sometimes being similar to, or even higher than, that in Fe-sufficient plants (Abadía, 1992). Plants exhibit chlorosis of young and emerging leaves, presumably when the physiologically active fraction of tissue Fe is less than the threshold and is insufficient to support Fe requirements. In many cases, total Fe content of plants may not be a dependable index of functional Fe in the tissues (Mehrotra et al., 1976). Attempts to quantify functional Fe have been made by extracting in several different extractants, including mild acids and chelating agents. Some such extracts show a good negative relationship with the degree of chlorosis in plants and have been referred to as ‘active Fe’ by Oserkowsky (1933) and Abadia et al. (1984). Although active Fe extractable in dilute (1 N) HCl was reported to represent functional Fe by Mehrotra et al. (1985), it was found by others to have limited applicability (Manzanares et al., 1990; Mehrotra et al., 1990). The nature and composition of functional Fe in plants thus remain elusive.
Iron is an integral constituent or cofactor of many antioxidant enzymes, such as catalase (CAT), peroxidase (POD), ascorbate peroxidase (APX) and Fe-superoxide dismutase (Fe-SOD), but it may also act as a pro-oxidant. For example, H2O2 in the presence of Fe2+ initiates the Fenton reaction and forms highly deleterious OH• radicals (Halliwell, 2006). However, for Fe-deficient plants, there are reports of decreased lipid peroxidation. These imply that regulatory mechanisms exist (Tewari et al., 2005). Accordingly, it has been suggested that although there is increased production of O2•− and induction of SOD activity in Fe-deficient plants, they are spared damage caused by increases in lipid peroxidation (Iturbe-Ormaetxe et al., 1995; Ranieri et al., 2001; Tewari et al., 2005). However, Sun et al. (2007) has reported an increase in lipid peroxidation in Fe-deficient maize plants.
In recent years, nitric oxide (NO), a biologically active gas, effective in nanomolar concentration (1.0 nmol L−1), has been shown to be ubiquitous in plants and to regulate various physiological and developmental processes. Nitric oxide is involved in germination and induction of lateral roots (Creus et al., 2005; Sarath et al., 2006; Tewari et al., 2008a), delays senescence (Neill et al., 2003), alleviates Cu toxicity (Tewari et al., 2008b), modulates the influx of extracellular Ca2+ and actin filament organization during cell wall construction in Pinus bungeana pollen tubes (Wang et al., 2009), and up-regulates synthesis of secondary metabolites in the adventitious roots of Panax ginseng and Echinacea purpurea (Tewari et al., 2007; Wu et al., 2007). Exogenous application of NO also down-regulates xanthine oxidase-mediated generation of O2•− in Phalaenopsis flowers (Tewari et al., 2009). NO can form complexes with transition metal ions in aqueous media (Stamler et al., 1992). Metal–nitrosyl complexes form under neutral physiological conditions and may act as links between the different redox states of NO (Stamler et al., 1992).
Although much work has been done on Fe nutrition of plants, several unsolved questions remain regarding sensing, trafficking, homeostasis and delivery of Fe. These are matters of considerable debate (Graziano and Lamattina, 2005). Exogenous supply of NO is reported to prevent interveinal chlorosis (a typical Fe-deficiency symptom) even in plants supplied with very low (10 µM) Fe (Graziano et al., 2002; Graziano and Lamatinna 2005; Graziano and Lamatinna 2007; Sun et al., 2007), and exposure to NO is claimed to increase the labile Fe pool (Jasid et al., 2008). These workers have also suggested possible involvement of endogenous NO in the Fe metabolism of plants. Earlier, NO was shown to protect chlorophyll against losses due to pathogenesis (Laxalt et al., 1997), to increase leaf chlorophyll content by de-etiolation (Beligni and Lamattina, 2000) and to be involved in light-mediated greening of seedlings (Zhang et al., 2006).
We surmised that NO-mediated cellular Fe availability may modulate oxidative status and antioxidant responses of plants under Fe-deficient conditions. There is meagre information on NO-mediated modulation of antioxidant responses and oxidative damage in Fe-deficient plants. Consequently, we investigated the possibility of NO-mediated changes in the functionality of Fe in maize leaf cells brought about by modulation of redox through effects on antioxidant enzymes: SOD, APX, CAT and POD.
Materials and methods
Plant material and treatments
Maize (Zea mays L. ‘GSF-2’) plants were grown in sand culture in a glasshouse. White silica sand was purified in a Keebush sand digester (A.P.V.-Kestner Ltd, Kent, UK) with a steam-heated mixture of HCl (17 %, v/v) and oxalic acid (1 %, w/v). Sand in 5.0-L polyethylene pots was washed with glass-distilled water and leached with 4 mM Ca(NO3)2 until the pH of leachate rose to 6.5–6.8. The composition of the complete nutrient solution (Hewitt, 1966) was: 4.0 mM KNO3, 4.0 mM Ca(NO3)2, 2.0 mM MgSO4, 1.33 mM NaH2PO4, 0.1 mM NaCl, 0.1 mM Fe-K2EDTA, 10.0 µM MnSO4, 1.0 µM CuSO4, 2.0 µM ZnSO4, 33.0 µM H3BO3, 0.2 µM Na2MoO4, 0.1 µM CoSO4 and 0.1 µM NiSO4. All solutions used were first purified against Fe by calcium carbonate–phosphate adsorption (Hewitt, 1966). The pH of the nutrient solution was adjusted to 6.7 ± 0.2 before supplying it to the plants. Plants were grown for 15 days either under Fe-sufficient (100 µM Fe-EDTA; for Lot 1) or Fe-deficient (10 µM Fe-EDTA; for Lots 2–10) conditions. The plants receiving deficient Fe were grouped further into nine lots (Lots 2–10), each having four pots. Plants in Lot 1 continued to receive complete nutrient solution (Fe-sufficient control). Those in Lot 2 were supplied nutrient solution deficient in Fe (10 µM Fe-EDTA). Plants in Lots 3, 4 and 5, respectively, were supplied with 10, 50 or 100 µM sodium nitroprusside (SNP), an exogenous NO donor in Fe-deficient nutrient solution. Plants in Lot 6 received 100 µM SNP plus 100 µM methylene blue (MB) (an NO scavenger) in Fe-deficient nutrient solution. Since the SNP molecule itself contains Fe, respective Fe controls for SNP treatments were maintained in Lots 7, 8 and 9 by supplying 10, 50 and 100 µM sodium ferrocyanide (SF), an analogue of SNP. SF has an amount of Fe equivalent to that of SNP, but is incapable of donating NO. The nutrient solution was re-supplied every morning between 09:00 and 09:30 h, and each week pots were flushed with deionized water to remove root exudates. Measurements were made on fully expanded young leaves of plants 10 and 20 days after initiating differential treatments.
Visual observations and dry matter yield
The visual effects of the treatments were recorded each day. Finally, 30 days after initiating treatments (DAT), the plants were harvested and dried in an oven at 80 °C for 48 h and weighed.
Total and active iron
Total Fe content was estimated in HNO3:HClO4 [10:1 (v/v)] digest of the fourth expanded leaf using atomic absorption spectrophotometry. For determining active Fe, 1 N HCl extracts were prepared by homogenizing fresh leaf material in the extractant in the proportion of 1 g of tissue per 10 mL of 1 N HCl, following the procedure of Mehrotra et al. (1985). The extracts were filtered through Whatman No. 1 filter paper, digested in HNO3:HClO4 (10:1 [v/v]) and Fe was quantified on an atomic absorption spectrophotometer.
NO, chlorophylls and carotenoids
NO content was determined as described by Zhou et al. (2005). Leaves (0.5 g) were ground in a mortar and pestle in 2.0 mL of 50 mM cool acetic acid buffer (pH 3.6, containing 4 % zinc diacetate). The homogenate was centrifuged at 10 000 × g for 15 min at 4 °C. The supernatant was collected. The pellet was washed twice with 0.5 mL of extraction buffer and centrifuged as before. The two supernatants were pooled and 0.05 g of activated charcoal was added. The suspension was vortexed and filtered. A mixture of 0.5 mL of filtrate and 0.5 mL of Greiss reagent was incubated at room temperature for 30 min. Absorbance was determined at 540 nm. NO content was calculated by comparison to a standard curve using NaNO2. Chlorophylls and carotenoids were determined in 80 % (v/v) acetone extract of the young fully expanded leaf using the method of Lichtenthaler (1987). The colour intensity of the cleared extract was measured at 663.2, 646.8 and 470 nm for chlorophyll a, chlorophyll b and total carotenoids, respectively.
Lipid peroxidation, H2O2 and non-protein thiols
Lipid peroxidation in leaf tissue was determined in terms of thiobarbituric acid reactive substances (TBARS), as described by Heath and Packer (1968). Leaf tissue was homogenized in 0.1 % (w/v) trichloroacetic acid (TCA) and aliquots heated for 30 min at 95 °C in a water bath with 0.5 % TBA (w/v) [prepared in 20 % (w/v) TCA] and then cooled quickly in an ice bath. The amount of malondialdehyde equivalent of TBARS was calculated from the difference in absorbance at 532 and 600 nm using its extinction coefficient (155 mM−1 cm−1). Hydrogen peroxide concentration was determined as H2O2–titanium complex by the method of Brennan and Frenkel (1977). The H2O2–titanium complex formed by reaction of tissue H2O2 with titanium tetrachloride was precipitated using concentrated ammonia solution. The precipitate was repeatedly washed with cold acetone to remove the pigments. The precipitate was solubilized in 2 N H2SO4 and absorbance of the solution was read at 415 nm. The concentration of thiols was measured in 5 % TCA extract as described by Tewari et al. (2008b). Fresh leaf tissue (1.0 g) was homogenized in 10.0 mL of 5 % TCA and centrifuged at 10 000 × g for 5 min. Aliquots of 0.5 mL of the homogenates were mixed with Ellman's reagent [0.5 mL of 0.01 M 5,5′-dithiobis-(2-nitrobenzoic acid)] solubilized in 1 M potassium phosphate buffer (pH 7.8). The contents were mixed and absorbance was read within 5 min at 412 nm against a reagent blank. Total sulphydryl groups were calculated from the extinction coefficient of 13.1 mM−1 cm−1.
Enzyme extraction and protein determination
Fresh leaf tissue (2.5 g) was homogenized in 10.0 mL of chilled 50 mM potassium phosphate buffer (pH 7.0) containing 0.5 % (w/v) insoluble polyvinylpolypyrrolidone and 1.0 mM phenylmethylsulphonyl fluoride and 1.0 mM dithiothreitol in a chilled pestle and mortar kept in an ice bath. The homogenate was filtered through 2-fold muslin cloth and centrifuged at 20 000 × g for 10 min in a refrigerated centrifuge at 2 °C. The supernatant was stored at 2 °C and used for enzyme assays within 4 h. For APX, 5.0 mM ascorbic acid was also included in the extraction buffer. The protein concentration in the homogenate was determined in the TCA-precipitated protein dissolved in 0.5 N NaOH according to Lowry et al. (1951) using bovine serum albumin as standard.
Assays of enzymes
The activities of SOD (EC 1.15.1.1), CAT (EC 1.11.1.6), POD (EC 1.11.1.7) and APX (EC 1.11.1.11) were estimated as described previously (Kumar et al., 2008). Superoxide dismutase activity was estimated by the modified method of Beauchamp and Fridovich (1971), in 5.0 mL of reaction mixture containing 25 mM phosphate buffer (pH 7.8), 65 µM NBT, 2.0 µM riboflavin, suitably diluted enzyme extract and 15 µL N,N,N′,N′-tetramethylethylenediamine. The reaction mixture was exposed to light of 350 µmol m−2 s−1 from fluorescent lamps for 15 min. Corresponding blanks were maintained by keeping the reaction mixtures dark. The change in absorbance was measured at 560 nm. The activity is expressed as units mg−1 protein min−1. Enzyme corresponding to 50 % inhibition of reaction was considered as one enzyme unit.
Activity of APX was assayed according to Nakano and Asada (1981). The reaction mixture contained 50 mM potassium phosphate buffer (pH 7.0), 0.1 mM EDTA, 0.5 mM ascorbate, 0.1 mM H2O2 and suitable aliquots of the enzyme extract in a total volume of 3.0 mL. The reaction was initiated by adding H2O2 and the absorbance decrease was recorded after every 30 s for 3.0 min at 290 nm. The amount of ascorbate oxidized was calculated using an extinction coefficient of 2.8 mM−1 cm−1 and the activity is expressed as μmol ascorbate oxidized mg−1 protein min−1.
The activity of CAT was assayed in a reaction mixture (10 mL) (standardized against 0.1 N KMnO4) containing 500 µmol of H2O2 and 1.0 mmol of potassium phosphate buffer (pH 7.0) was stabilized at 25 °C. The reaction was initiated by adding enzyme extract. The reaction was allowed to proceed for 5 min and was stopped by adding 2.0 mL of 4 N H2SO4. Corresponding blanks in which H2SO4 was added prior to the addition of enzyme extracts were run simultaneously. The final reaction mixture was titrated against 0.1 N KMnO4. The H2O2 decomposed was calculated as the difference in titre value of the respective blanks with the samples. Enzyme activity is expressed as μmol H2O2 reduced mg−1 protein.
Peroxidase was assayed in a reaction mixture (5.0 mL) containing 2.0 mL of 0.1 M potassium phosphate buffer (pH 7.0), 1.0 mL of 0.01 % H2O2 and 1.0 mL of 0.5 % p-phenylenediamine. The reaction was started by adding enzyme extract to the reaction mixture and allowed to proceed for 5.0 min. The reaction was stopped by adding 2.0 mL of 4 N H2SO4. Corresponding blanks were maintained in which H2SO4 was added to the reaction mixture prior to the addition of enzyme extract. The colour intensity was measured at 485 nm after standing the mixture for 30 min at 4 °C. Enzyme activity is expressed as mg−1 protein. The enzyme unit is defined as a ΔOD485 of 0.01 between the blank and the sample per minute.
Statistical analysis
All results are the means of six experimental replicates (n = 6). The data were analysed by analysis of variance and comparisons among the means were tested for significance by Bonferroni tests using Sigma-stat software. Means followed by different letters are statistically significant at P ≤ 0.05.
Results
NO treatment induces re-greening in chlorotic leaves of Fe-deficient maize
Supplying SNP, an NO donor, to the roots of Fe-deficient plants brought about a recovery from interveinal leaf chlorosis (Fig. 1). The effect of raising the supply of SNP was seen clearly as a marked re-greening of previously chlorotic leaves (Fig. 1A and F–H). The re-greening was initiated within 24 h of supplying 50 or 100 µM SNP and the leaves became fully green within 6 days of SNP treatment. Iron-deficient plants supplied with only 10 µM SNP also showed perceptible re-greening of chlorotic leaves. The NO scavenger, MB, blocked the effect of the NO donor (Fig. 1C and D). However, SF, an analogue of SNP having equivalent numbers of Fe atoms per mole as SNP but being incapable of releasing NO, did not cause re-greening (Fig. 1I–L). Treatment with SNP also increased dry matter accumulation by Fe-deficient plants (Table 1) by ∼23 % over 30 days.
Fig. 1.
Visible effects of raising internal NO by treating Fe-deficient maize with SNP. (A) Maize plants showing the effect of graded SNP supply: (1) Fe-sufficient control plants (100 µM Fe-EDTA); (2) Fe-deficient control plants (10 µM Fe-EDTA); (3) Fe-deficient plants treated with 10 µM SNP; (4) Fe-deficient plants treated with 50 µM SNP; (5) Fe-deficient plants treated with 100 µM SNP. (B) Plants showing effects of SNP and SF: (1) Fe-sufficient control plants (100 µM Fe-EDTA); (2) Fe-deficient control plants (10 µM Fe-EDTA); Fe-deficient plants treated with (3) 100 µM SNP; (4) 100 µM SF. (C) SNP-treated Fe-deficient plant supplied with the NO scavenger MB. (D) Close-up of MB-treated, SNP-treated Fe-deficient leaf. Close-ups of (E) Fe-sufficient leaf and leaves of Fe-deficient plants treated with (F) 10 µM, (G) 50 µM and (H) 100 µM SNP. (I) Close-up of Fe-deficient leaf. Close-ups of Fe-deficient leaves of plants treated with SF at (J) 10 µM, (K) 50 µM and (L) 100 µM.
Table 1.
Effect of manipulating NO and Fe levels of maize plants on dry weight and internal concentrations of foliar Fe and NO. Dry weight was determined after 30 days treatment. NO and Fe concentrations were estimated after 20 days treatment. Plants were grown in Fe-sufficient (100 µM Fe-EDTA) or Fe-deficient (10 µM Fe-EDTA) nutrient solution and Fe-deficient plants supplied with 0, 10, 50 and 100 µM NO donor SNP. The NO scavenger MB was applied to some plants receiving 100 µM SNP. Iron supply was also varied by adding 10, 50 and 100 µM SF to the growing medium. Data are means of three experimental replicates (n = 6). Values in the same rows carrying different letters are significantly different at P ≤ 0.05 using the Bonferroni t-test.
| Parameters | Fe-EDTA (μM) |
10 µM Fe-EDTA |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| SNP (μM) |
100 µM SNP + 100 µM MB | SF (μM) |
|||||||
| 100 | 10 | 10 | 50 | 100 | 10 | 50 | 100 | ||
| Dry weight (g per plant) | 28.09a | 17.40b | 20.57c | 21.75c | 21.83c | 18.25b | 16.57b | 15.83b | 17.79b |
| Total Fe (µg g−1 DW) | 346.80a | 272.45a | 184.31b | 329.87a | 324.05a | 173.63b | 212.97b | 215.16b | 234.10b |
| Active Fe (µg g−1 DW) | 113.91a | 40.58b | 120.44a | 162.84a | 177.25a | 142.49a | 136.79a | 182.02a | 183.82a |
| NO (nmol g−1 DW) | 53.11a | 14.59b | 36.70c | 48.43a | 55.21a | 12.61b | 15.09b | 16.51b | 14.89b |
SNP supply increases in vivo NO and synthesis of chloroplastic pigments in the chlorotic leaves of Fe-deficient maize
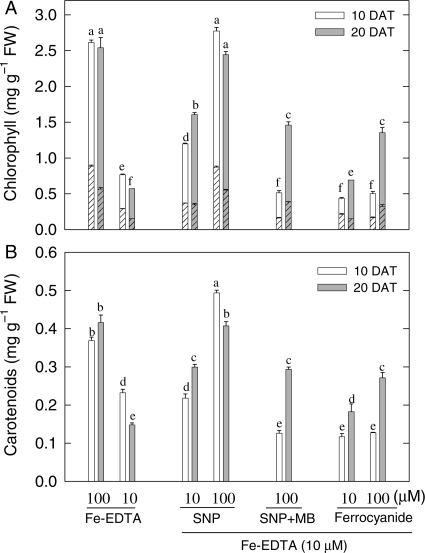
Deficient supply of Fe (10 µM Fe-EDTA) decreased the NO concentration of leaves by over 70 % compared with Fe-sufficient controls. SNP at all levels of supply (10, 50 or 100 µM) overcame this NO depression to a statistically significant extent and internal NO was fully restored by 100 µM SNP (Table 1). Fe-deficient plants given 100 µM SNP also showed large increases in chlorophyll (Fig. 2A) and carotenoid (Fig. 2B) concentrations after 10 or 20 days of treatment, and looked similar to Fe-sufficient (100 µM Fe-EDTA) control plants. In contrast, Fe-deficient plants treated with 100 µM SNP in combination with 100 µM NO scavenger MB, or with 10 or 100 µM SF (a source Fe but not of NO), had only an inconsistent effect on chlorophylls and carotenoids at 20 DAT (Fig. 2A) and failed to stimulate an increase in dry weight (Table 1). Whereas Fe-deficient plants had slow growth (Table 1) and usually failed to complete their life cycle, SNP-treated plants showed normal development. These observations suggested that SNP either enhanced the amounts of Fe under Fe-deficient conditions or diminished the threshold level of internal Fe needed for maize plants to grow normally. The possibility that NO raised internal concentrations of total Fe or of an HCl-extractable fraction of foliar Fe (active Fe) was therefore tested by direct analysis.
Fig. 2.
Effect of manipulating NO and Fe levels on chlorophyll a, b and carotenoids in young expanded leaves of maize. (A) Chlorophyll a (unhatched), chlorophyll b (hatched); (B) carotenoid concentrations. The plants were grown in sand culture with sufficient (100 µM Fe-EDTA) and deficient (10 µM Fe-EDTA) Fe supply. Some Fe-deficient plants were treated with 10 or 100 µM SNP, 100 µM SNP plus 100 µM MB or with 10 or 100 µM SF. Analyses were made 10 days (open bars) and 20 days (grey bars) after treatment (DAT). Bars carrying different letters are significantly different at P ≤ 0.05 after Bonferroni t-tests.
Effects of SNP do not correlate with total or active tissue Fe concentration
Applying 50 or 100 µM SNP gave a statistically non-significant increase in total leaf Fe compared with Fe-deficient controls. In contrast, 10 µM SNP, MB in the presence of SNP, and SF treatments each decreased total Fe concentration significantly (Table 1). However, concentrations of active Fe (i.e. Fe extracted by 1 N HCl) were increased in all plants supplied with SNP and SF compared with Fe-deficient control plants (Table 1). Nevertheless, SF treatment failed to increase concentrations of chlorophylls and carotenoids (Fig. 2A and B) or increase dry matter accumulation (Table 1). Thus, increases in tissue Fe, both total Fe and active Fe, did not necessarily correlate with those in chloroplast pigment concentrations or with other effects such as an increase in dry weight or concentration of NO (Table 2). The re-greening and improved growth of Fe-deficient plants given the NO donor SNP must therefore have some other cause.
Table 2.
Correlations between a range of measurements made on leaves of maize plants in which levels of Fe supply and internal NO were manipulated in various ways. Pearson correlations are shown between (i) concentrations of chlorophyll (Chl), carotenoids (Car), total Fe or active Fe and (ii) concentrations of NO, H2O2, TBARS (a marker of oxidative damage), activities of SOD, CAT, POD and APX. The pair(s) of variables with positive correlation coefficients (r) and P-values <0.05 tend to increase together. For pairs with negative correlation coefficients and P-values <0.05, one variable tends to decrease whereas the other increases. For pairs with P-values >0.05, there is no significant relationship between the two variables.
| TBARS | H2O2 | SOD | CAT | POD | APX | NO | Chl | Car | |
|---|---|---|---|---|---|---|---|---|---|
| Chl | |||||||||
| r | 0.890 | −0.857 | −0.954 | 0.933 | 0.947 | 0.863 | 0.965 | ||
| P | 0.0073 | 0.0137 | 0.0008 | 0.0021 | 0.0012 | 0.012 | 0.0004 | ||
| Car | |||||||||
| r | 0.850 | −0.838 | −0.918 | 0.903 | 0.931 | 0.860 | 0.954 | ||
| P | 0.0154 | 0.0185 | 0.0036 | 0.0054 | 0.0024 | 0.0130 | 0.0009 | ||
| Total Fe | |||||||||
| r | 0.495 | −0.337 | −0.476 | 0.459 | 0.366 | 0.334 | 0.504 | 0.576 | 0.581 |
| P | 0.258 | 0.459 | 0.280 | 0.301 | 0.419 | 0.464 | 0.249 | 0.176 | 0.171 |
| Active Fe | |||||||||
| r | 0.0202 | −0.0016 | −0.0957 | 0.0706 | 0.316 | 0.176 | 0.184 | 0.272 | 0.261 |
| P | 0.966 | 0.997 | 0.838 | 0.880 | 0.490 | 0.706 | 0.693 | 0.555 | 0.572 |
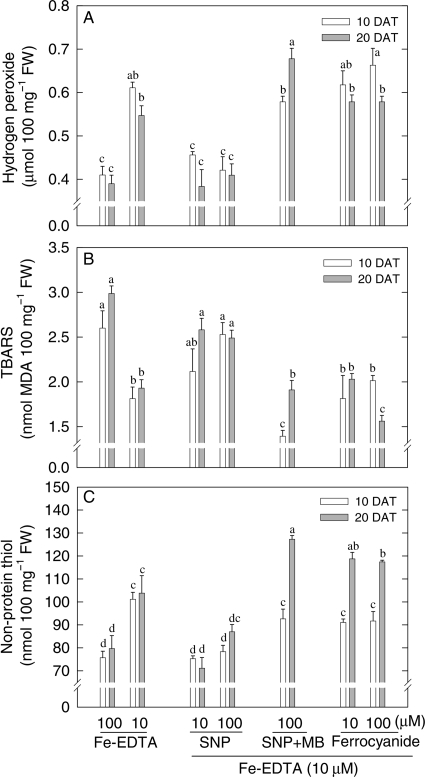
SNP decreases H2O2 and non-protein thiol contents but increases TBARS
Possible mediation by redox adjustment was examined by assaying H2O2 (a potentially damaging oxidizer), non-protein thiol (potential antioxidant) and TBARS (a marker for membrane peroxidation). Deficiency of Fe increased H2O2 concentration by ∼50 % in comparison with Fe sufficiency (Fig. 3A) after 10 or 20 days. SNP treatment largely reversed this effect. However, in the presence of the NO scavenger MB, SNP was no longer effective in reducing H2O2 levels, while the Fe donor SF failed to reproduce the effect of SNP. In contrast to the effect on H2O2, TBARS was decreased by Fe deficiency and increased by NO treatment (Fig. 3B). Moreover, treatment of Fe-deficient plants with SNP + MB or with SF did not increase TBARS and the latter remained comparable to that of untreated Fe-deficient plants. Amounts of non-protein thiol (e.g. glutathione) were also decreased by SNP but remained high in Fe-deficient plants treated with SNP + MB or with SF (Fig. 3C).
Fig. 3.
Effect of manipulating NO and Fe levels on (A) H2O2, (B) TBARS and (C) non-protein thiol in young expanded leaves of maize. Maize plants were grown in the sand culture with sufficient (100 µM Fe-EDTA) and deficient (10 µM Fe-EDTA) Fe supply. Some Fe-deficient plants were treated with 10 or 100 µM SNP, 100 µM SNP plus 100 µM MB or with 10 or 100 µM SF. Analyses were made 10 days (open bars) and 20 days (grey bars) after treatment (DAT). Bars carrying different letters are significantly different at P ≤ 0.05 after Bonferroni t-tests.
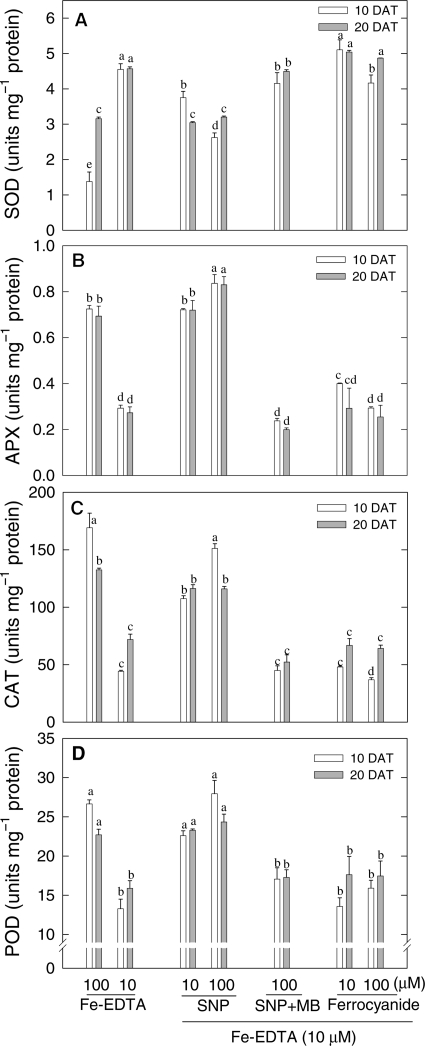
SNP down-regulates SOD activity but up-regulates activities of APX, CAT and POD in Fe-deficient maize
The possibilities that Fe deficiency may down-regulate antioxidant enzymes and that NO may ameliorate the effect were examined. Contrary to this expectation, SOD activity was found to increase in Fe-deficient plants and to remain unaffected by treatments with SNP plus MB or with SF. However, 10 or 100 µM SNP applied alone markedly down-regulated SOD activity in Fe-deficient maize (Fig. 4A) and reduced the values back to those more typical of Fe-sufficient plants. This indicates an NO-dependent effect. The Fe-containing antioxidative enzymes APX (Fig. 4B), CAT (Fig. 4C) and POD (Fig. 4D) responded differently to SOD. Their activities were decreased very strongly by Fe deficiency, but this decrease could be prevented by treatment with the NO-donating SNP given at 10 or 100 µM (Fig. 4). The effect of SNP was negated by the NO scavenger MB and could not be reproduced by treating Fe-deficient plants with SF to raise Fe concentrations independently of NO (Fig. 4). An up-regulation of the activities of H2O2-consuming haem enzymes and a down-regulation of H2O2-generating SOD by NO suggest a mode of action involving redox adjustment and possibly a consequential increase in the activity of the small amounts of the Fe present in Fe-deficient plants.
Fig. 4.
Effect of manipulating NO and Fe levels on activities of antioxidant enzymes in young expanded leaves of maize. (A) SOD, (B) APX, (C) CAT and (D) POD. Maize plants were grown in sand culture with sufficient (100 µM Fe-EDTA) and deficient (10 µM Fe-EDTA) Fe supply. Some Fe-deficient plants were treated with 10 or 100 µM SNP, 100 µM SNP plus 100 µM MB or with 10 or 100 µM SF. Analyses were made 10 days (open bars) and 20 days (grey bars) after treatment (DAT). Bars carrying different letters are significantly different at P ≤ 0.05 after Bonferroni t-tests.
Discussion
Involvement of Fe in chloroplast development and chlorophyll biosynthesis has made chlorophyll content in young leaves the primary index of nutritional Fe status. This index is invaluable because of the absence of a satisfactory direct measure of functional Fe in plants. The observed alleviation of chlorosis and an increase in chlorophyll content by treating Fe-deficient plants with SNP confirm the work of Graziano et al. (2002) and Sun et al. (2007). Failure of Fe-deficient plants treated with 100 µM SNP + 100 µM MB or with 100 µM SF to show any recovery suggests that the recovery induced by SNP applied alone is ascribable to NO supply. Although there are possible differences in the effects of different NO donors, particularly ferritin regulation, cellular redox state, induction of antioxidative enzymes (Murgia et al., 2004; Floryszak-Wieczorek et al., 2006), Graziano et al. (2002) found that the effect of SNP on the recovery of plants from Fe deficiency was comparable to that of NO supplied directly as the gas.
Total Fe concentration was not closely related to leaf chlorophyll or carotenoids (Table 2), confirming several other similar reports (Mehrotra et al., 1976; Abadía, 1992; González-Vallejo et al., 2000; Larbi et al., 2001; Graziano et al., 2002). The so-called active Fe (1 N HCl-extractable Fe) was much reduced by Fe deficiency but more than fully restored by supplying SNP (Table 1), even in the presence of MB. This result shows that the effect on restoring active Fe was independent of NO. Only SNP supplied alone promoted re-greening in association with increases in NO titre. This indicates that although SNP can raise active Fe levels directly (presumably because the molecule Na2[Fe(CN)5NO]·2H2O contains Fe), an explanation of the biological effect of SNP lies elsewhere (c.f. Kim and Ponka, 2002; Wang et al., 2006), and is related to NO action. A lack of correlation between tissue Fe and chlorophyll concentration in the leaves (Table 2) is attributable largely to much of the tissue Fe remaining in an insoluble form in the apoplast (Jin et al., 2007).
In our experiments, SNP was applied to the roots while recovery from Fe deficiency was observed in the leaves. This location difference suggests root to shoot signalling by NO by diffusing to the leaves or being carried as a solute in the transpiration stream.
We propose that NO improved Fe functionality in Fe-deficient plants by enhancing the chemical reduction of apoplastic Fe(III) to Fe(II) (Graziano et al., 2002). Since Fe(III) reduction in vivo may be aided by O2•− formation (Brüggemann et al., 1993), the previously reported increased accumulation of O2•− in Fe-deficient plants (Tewari et al., 2005) appears to be a strategy for promoting symplastic acquisition of scarce Fe from the apoplast of Fe-deficient plants.
Suppression, by NO, of the increase in SOD activity caused by Fe deficiency suggests an involvement of NO in improving functional Fe by raising O2•−. The resulting drop in the dismutation of O2•− to H2O2 presumably explains the decrease in H2O2 seen in Fe-deficient plants given SNP (Fig. 3 and Tewari et al., 2005), whereas the undismutated O2•− (Tewari et al., 2005) can be expected to promote apoplastic reduction of Fe(III) to Fe(II) (Stamler et al., 1992; Graziano et al., 2002). The requirement for NO in the SNP effect is clear from the absence of a decline in H2O2 in Fe-deficient plants treated with SNP plus MB or with SF. A excessively damaging build-up of O2•− may be avoided by NO itself scavenging some O2•− to form ONOO− (peroxynitrite) which readily becomes protonated and finally decomposes to harmless H+ and NO3− (Beligni and Lamattina, 2002). Although ONOO− is a strong oxidant and can be highly cytotoxic, and mediates apoptosis in animal cells, it is relatively non-toxic in plants (Romero-Puertas et al., 2004; Delledonne, 2005).
An increased concentration of non-protein thiol (e.g. glutathione), an antioxidant that consumes H2O2 when in its customary chemically reduced form, appears to be an acclimatory response to low functional Fe. In support of this, Fe deficiency was reported to increase glutathione in sugar beet (Zaharieva et al., 2004). A decrease in non-protein thiol concentration by SNP treatment is indicative of a role of thiol in Fe acquisition, probably through the formation of dinitrosyl–iron complexes (DNICs). Decomposition of DNICs has been shown to be regulated by Fe, thiols and NO in cells (Vanin, 1998). Reversible reactions of NO with functional groups such as haem and thiols have been shown to modulate activities of proteins (Graziano et al., 2002; Graziano and Lamattina, 2005).
Contrary to SOD activity, H2O2-scavenging haem enzyme activity was decreased by Fe deficiency and increased by NO. This could help avoid damaging accumulations of H2O2 possibly arising from the associated decrease in thiol levels. Treatment of the Fe-deficient maize plants with SNP + MB or with SF had very little effect on the activities of these enzymes, indicating specific NO effects. Up-regulation of these enzymes by NO reflects improved functional Fe status with alleviation of Fe-deficiency symptoms. Since these enzymes constitutively contain haem-Fe, several workers have considered CAT (Mehrotra et al., 1990; Marschner, 1995) and APX (Iturbe-Ormaetxe et al., 1995; Tewari et al., 2005) as indices of Fe nutritional status. Ascorbate peroxidase expression is reported to be regulated by Fe availability in the tissues (Ishikawa et al., 2003; Fourcroy et al., 2004). Peroxidase, with haem-Fe as cofactor, also showed an expected response similar to that of other haem-Fe enzymes and could also be considered as an index of the functional Fe status of plants. Activities of these enzymes also showed a significant positive correlation with chloroplastic pigments (Table 2). Sun et al. (2007) also observed a similar improvement in the activities of haem-Fe enzymes upon treating Fe-deficient maize plants with SNP.
While increased levels of O2•− that NO may induce in Fe-deficient plant appear to maximize internal utilization of scarce apoplastic Fe in maize leaves and improve plant growth, there is a potentially damaging prospect of oxidative damage by the increased O2•− titre. Our work provides evidence that this is indeed the case by showing that the beneficial effects of NO co-exist with increased TBARS, reflecting increased oxidative damage compared with the characteristically low level in Fe-deficient leaves (Fig. 3B and Iturbe-Ormaetxe et al., 1995; Ranieri et al., 2001; Tewari et al., 2005, but see Sun et al., 2007). These observations suggest that despite having high H2O2, chlorotic leaves are better protected from oxidative damage compared with the NO-treated re-greened leaves. Low TBARS in Fe-deficient plants has also been attributed to decreased catalytic (functional) Fe2+ by Iturbe-Ormaetxe et al. (1995) and Ranieri et al. (2001). Although low functional Fe in Fe-deficient plants is likely to contribute to low OH• generation and oxidative damage, a decrease in chloroplastic pigments is likely to limit absorption of photons and photo-oxidative damage in Fe-deficient plants (Moseley et al., 2002; Larbi et al., 2006).
Conclusions and forward look
This study shows that total or active tissue Fe concentration is poorly correlated with chlorophyll and carotenoid concentrations or other parameters reflecting functional Fe nutritional status. Although SNP, an NO donor, and SF, a non-NO-releasing analogue of SNP, both contributed Fe to the ‘active Fe’ pool, only NO-donating SNP was instrumental in alleviating the Fe-deficiency effects. The results suggest that NO probably ameliorates symptoms and other effects of Fe deficiency by improving the functionality of the small amounts of Fe present in Fe-deficient leaves. The most probable mechanism is an enhanced chemical reduction of apoplastic Fe(III) to Fe(II) by superoxide ions (O2•−). This, in turn, would lead to increased synthesis of chloroplastic pigments and haem–Fe proteins, a decline in H2O2 and faster growth. A downside of the effect is an increased predisposition to oxidative damage. But overall, in Fe-deficient maize plants, the balance lies in favour of improved growth based on increased utilization of existing internal Fe. Our results open up the possibility of ameliorating the effects of Fe-deficient soils by identifying or creating maize lines with enhanced NO production under Fe deficiency. Further understanding of the mechanism of NO amelioration of Fe-deficiency symptoms will enhance the prospects of achieving these goals.
Sources of funding
We thank the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for a Research Associateship to P.K.
Contributions by the authors
All the authors contributed to a similar extent to the experimental work and to writing the paper. R.K.T. analysed the data and prepared the figures.
Conflict of interest statement
None declared.
References
- Abadía J. Leaf responses to Fe deficiency: a review. Journal of Plant Nutrition. 1992;15:1699–1713. [Google Scholar]
- Abadia J, Monge E, Montanes L, Heras L. Extraction of iron from plant leaves by Fe(II) chelators. Journal of Plant Nutrition. 1984;7:777–784. [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and assays applicable to acrylamide gels. Analytical Biochemistry. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L. Nitric oxide stimulates seed germination and e-etiolation, and inhibits hypocotyl elongation, three light inducible responses in plants. Planta. 2000;210:215–221. doi: 10.1007/PL00008128. [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L. Nitric oxide interferes with plant photo-oxidative stress by detoxifying reactive oxygen species. Plant, Cell and Environment. 2002;25:737–748. [Google Scholar]
- Brennan T, Frenkel C. Involvement of hydrogen peroxide in regulation of senescence in pear. Plant Physiology. 1977;59:411–416. doi: 10.1104/pp.59.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüggemann W, Maas-Kantel K, Moog PR. Iron uptake by leaf mesophyll cells: the role of the plasma membrane-bound ferric-chelate reductase. Planta. 1993;190:151–155. [Google Scholar]
- Creus CM, Graziano M, Casanovas EM, Pereyra MA, Simontacchi M, Puntarulo S, Barassi CA, Lamattina L. Nitric oxide is involved in the Azospirillum brasilense-induced lateral root formation in tomato. Planta. 2005;221:297–303. doi: 10.1007/s00425-005-1523-7. [DOI] [PubMed] [Google Scholar]
- Delledonne M. NO news is good news for plants. Current Opinion in Plant Biology. 2005;8:390–396. doi: 10.1016/j.pbi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Floryszak-Wieczorek J, Milczarek G, Arasimowicz M, Ciszewski A. Do nitric oxide donors mimic endogenous NO-related response in plants? Planta. 2006;224:1363–1372. doi: 10.1007/s00425-006-0321-1. [DOI] [PubMed] [Google Scholar]
- Fourcroy P, Vansuyt G, Kushnir S, Inzé D, Briat JF. Iron-regulated expression of a cytosolic ascorbate peroxidase encoded by the APX1 gene in Arabidopsis seedlings. Plant Physiology. 2004;134:605–613. doi: 10.1104/pp.103.029876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Vallejo EB, Morales F, Cistué L, Cistué L, Abadía A, Abadía J. Iron deficiency decreases the Fe(III)-chelate reducing activity of leaf protoplasts. Plant Physiology. 2000;122:337–344. doi: 10.1104/pp.122.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano M, Lamattina L. Nitric oxide and iron in plants: an emerging and converging story. Trends in Plant Science. 2005;10:4–8. doi: 10.1016/j.tplants.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Graziano M, Lamattina L. Nitric oxide accumulation is required for molecular and physiological responses to iron deficiency in tomato roots. The Plant Journal. 2007;52:949–960. doi: 10.1111/j.1365-313X.2007.03283.x. [DOI] [PubMed] [Google Scholar]
- Graziano M, Beligni MV, Lamattina L. Nitric oxide improves internal iron availability in plants. Plant Physiology. 2002;130:1852–1859. doi: 10.1104/pp.009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerinot ML, Yi Y. Iron: nutritious, noxious, and not readily available. Plant Physiology. 1994;104:815–820. doi: 10.1104/pp.104.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Reactive species and antioxidants. Redox biology is a fundamental theme of life aerobic life. Plant Physiology. 2006;141:312–322. doi: 10.1104/pp.106.077073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplast, I. Kinetics and stoichiometry of fatty acid peroxidation. Archives of Biochemistry and Biophysics. 1968;125:180–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hewitt EJ. Sand and water culture methods used in the study of plant nutrition. Farnham Royal, Buckinghamshire: Commonwealth Agricultural Bureaux; 1966. [Google Scholar]
- Ishikawa T, Madhusudhan R, Shigeoka S. Effect of iron on the expression of ascorbate peroxidase in Euglena gracilis. Plant Science. 2003;165:1363–1367. [Google Scholar]
- Iturbe-Ormaetxe I, Moran JF, Arrese-Igor C, Gogorcena Y, Klucas RV, Becana M. Activated oxygen and antioxidant defences in iron-deficient pea plants. Plant, Cell and Environment. 1995;18:421–429. [Google Scholar]
- Jasid S, Simontacchi M, Puntarulo S. Exposure to nitric oxide protects against oxidative damage but increases the labile iron pool in sorghum embryonic axes. Journal of Experimental Botany. 2008;59:3953–3962. doi: 10.1093/jxb/ern235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CW, You GY, He YF, Tang C, Wu P, Zheng SJ. Iron-deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover (Trifolium pratense L.) Plant Physiology. 2007;144:278–285. doi: 10.1104/pp.107.095794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Ponka P. Nitrogen monoxide-mediated control of ferritin synthesis: implications for macrophage iron homeostasis. Proceedings of the National Academy of Sciences of the USA. 2002;99:12214–12219. doi: 10.1073/pnas.192316099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosegarten HU, Hoffmann B, Mengel K. Apoplastic pH and Fe3+ reduction in intact sunflower leaves. Plant Physiology. 1999;121:1069–1079. doi: 10.1104/pp.121.4.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Tewari RK, Sharma PN. Modulation of copper toxicity induced oxidative damage by excess supply of iron in maize plants. Plant Cell Reports. 2008;27:399–409. doi: 10.1007/s00299-007-0453-1. [DOI] [PubMed] [Google Scholar]
- Larbi A, Morales F, López-Millán AF, Gogorcena Y, Abadía A, Moog PR, Abadía J. Technical advance: reduction of Fe(III)-chelates by mesophyll leaf disks of sugar beet. Multi-component origin and effects of Fe deficiency. Plant and Cell Physiology. 2001;42:94–105. doi: 10.1093/pcp/pce012. [DOI] [PubMed] [Google Scholar]
- Larbi A, Abadia A, Abadia J, Morales F. Down co-regulation of light absorption, photochemistry, and carboxylation in Fe-deficient plants growing in different environments. Photosynthesis Research. 2006;89:113–126. doi: 10.1007/s11120-006-9089-1. [DOI] [PubMed] [Google Scholar]
- Laxalt A, Beligni MV, Lamattina L. Nitric oxide preserves the level of chlorophyll in potato leaves infected by Phytophthora infestans. European Journal of Plant Pathology. 1997;73:643–651. [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods in Enzymology. 1987;148:350–382. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Manzanares M, Lucena JJ, Garate A. Iron (II) determination in leaves of strawberry (Fragaria vesca). In: van Beusichem ML, editor. Plant nutrition—physiology and application. Dordrecht: Kluwer; 1990. pp. 805–808. In. [Google Scholar]
- Marschner H. Mineral nutrition of higher plants. 2nd edn. London: Academic Press; 1995. [Google Scholar]
- Martin JH, Coale KH, Johnson KS, Fitzwater SE, Gordon RM, Tanner SJ, Hunter CN, Elrod VA, Nowicki JL, Coley TL, Barber RT, Lindley S, Watson AJ, Van Scoy K, Law CS, Liddicoat MI, Ling R, Stanton T, Stockel J, Collins C, Anderson A, Bidigare R, Ondrusek M, Latasa M, Millero FJ, Lee K, Yao W, Zhang JZ, Friederich G, Sakamoto C, Chavez F, Buck K, Kolber Z, Greene R, Falkowski P, Chisholm SW, Hoge F, Swift R, Yungel J, Turner S, Nightingale P, Hatton A, Liss P, Tindale NW. Testing the iron hypothesis in ecosystems of the equatorial Pacific Ocean. Nature. 1994;371:123–129. [Google Scholar]
- Mehrotra SC, Mehrotra NK, Bisht SS, Sharma CP. Resolution of iron chlorosis. Geophytology. 1976;6:282–295. [Google Scholar]
- Mehrotra SC, Sharma CP, Agarwala SC. A search for extractants to evaluate the iron status of plants. Soil Science and Plant Nutrition. 1985;31:155–162. [Google Scholar]
- Mehrotra SC, Gupta P, Chaturvedi K, Bisht SS. Active Fe in relation to chlorophyll and activities of some Fe-enzymes in maize. Indian Journal of Experimental Biology. 1990;28:349–351. [Google Scholar]
- Morales F, Grasa R, Abadía A, Abadía J. Iron chlorosis paradox in fruit trees. Journal of Plant Nutrition. 1998;21:815–825. [Google Scholar]
- Moseley JL, Allinger T, Herzog S, Hoerth P, Wehinger E, Merchant S, Hippler M. Adaptation to Fe-deficiency requires remodeling of the photosynthetic apparatus. EMBO Journal. 2002;21:6709–6720. doi: 10.1093/emboj/cdf666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia I, de Pinto MC, Delledonne M, Soave C, Gara LD. Comparative effects of various nitric oxide donors on ferritin regulation, programmed cell death and cell redox state in plant cells. Journal of Plant Physiology. 2004;161:777–783. doi: 10.1016/j.jplph.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant and Cell Physiology. 1981;22:867–880. [Google Scholar]
- Neill SJ, Desikan R, Hancock JT. Nitric oxide signalling in plants. New Phytologist. 2003;159:11–35. doi: 10.1046/j.1469-8137.2003.00804.x. [DOI] [PubMed] [Google Scholar]
- Oserkowsky J. Quantitative relation between chlorophyll and iron in green and chlorotic pear leaves. Plant Physiology. 1933;8:449–468. doi: 10.1104/pp.8.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranieri A, Castagna A, Baldan B, Soldatini GF. Iron deficiency differently affects peroxidase isoforms in sunflower. Journal of Experimental Botany. 2001;52:25–35. [PubMed] [Google Scholar]
- Romero-Puertas MC, Perazzolli M, Zago ED, Delledonne M. Nitric oxide signaling functions in plant–pathogen interactions. Cellular Microbiology. 2004;6:795–803. doi: 10.1111/j.1462-5822.2004.00428.x. [DOI] [PubMed] [Google Scholar]
- Sarath G, Bethke PC, Jones R, Baird LM, Hou G, Mitchell RB. Nitric oxide accelerates seed germination in warm-season grasses. Planta. 2006;223:1154–1164. doi: 10.1007/s00425-005-0162-3. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Singel DJ, Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- Sun B, Jing Y, Chen K, Song L, Chen F, Zhang L. Protective effect of nitric oxide on iron deficiency-induced oxidative stress in maize (Zea mays) Journal of Plant Physiology. 2007;164:536–543. doi: 10.1016/j.jplph.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Tewari RK, Kumar P, Neetu, Sharma PN. Signs of oxidative stress in the chlorotic leaves of iron starved plants. Plant Science. 2005;169:1037–1045. [Google Scholar]
- Tewari RK, Lee S-Y, Hahn E-J, Paek K-Y. Temporal changes in the growth, saponin content, and antioxidant defense in the adventitious roots of Panax ginseng subjected to nitric oxide elicitation. Plant Biotechnology Reports. 2007;1:227–235. [Google Scholar]
- Tewari RK, Hahn E-J, Paek K-Y. Function of nitric oxide and superoxide anion in the adventitious root development and antioxidant defence in Panax ginseng. Plant Cell Reports. 2008a;27:563–573. doi: 10.1007/s00299-007-0448-y. [DOI] [PubMed] [Google Scholar]
- Tewari RK, Hahn E-J, Paek K-Y. Modulation of copper toxicity-induced oxidative damage by nitric oxide supply in the adventitious roots of Panax ginseng. Plant Cell Reports. 2008b;27:171–181. doi: 10.1007/s00299-007-0423-7. [DOI] [PubMed] [Google Scholar]
- Tewari RK, Kumar P, Kim S, Hahn EJ, Paek K-Y. Nitric oxide retards xanthine oxidase-mediated superoxide anion generation in Phalaenopsis flower: an implication of NO in the senescence and oxidative stress regulation. Plant Cell Reports. 2009;28:267–279. doi: 10.1007/s00299-008-0632-8. [DOI] [PubMed] [Google Scholar]
- Vanin AF. Dinitrosyl iron complexes and S-nitrosothiols are two possible forms for stabilization and transport of nitric oxide in biological systems. Biochemistry. 1998;63:782–793. [PubMed] [Google Scholar]
- Wang J, Fillebeen C, Chen G, Andriopoulos B, Pantopoulos K. Sodium nitroprusside promotes IRP2 degradation via an increase in intracellular iron and in the absence of S-nitrosylation at C178. Molecular and Cellular Biology. 2006;26:1948–1954. doi: 10.1128/MCB.26.5.1948-1954.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chen T, Zhang C, Hao H, Liu P, Zheng M, Baluška F, Šamaj J, Lin J. Nitric oxide modulates the influx of extracellular Ca2+ and actin filament organization during cell wall construction in Pinus bungeana pollen tubes. New Phytologist. 2009;182:851–862. doi: 10.1111/j.1469-8137.2009.02820.x. [DOI] [PubMed] [Google Scholar]
- Wu CH, Tewari RK, Hahn E-J, Paek K-Y. Nitric oxide elicitation induces accumulation of secondary metabolites and antioxidant defence in the adventitious roots of Echinacea purpurea. Journal of Plant Biology. 2007;50:636–643. [Google Scholar]
- Zaharieva TB, Gogorcena Y, Abadia J. Dynamics of metabolic responses to iron deficiency in sugar beet roots. Plant Science. 2004;166:1045–1050. [Google Scholar]
- Zhang L, Wang Y, Zhao L, Shi S, Zhang L. Involvement of nitric oxide in light-mediated greening of barley seedlings. Journal of Plant Physiology. 2006;163:818–826. doi: 10.1016/j.jplph.2005.07.011. [DOI] [PubMed] [Google Scholar]
- Zhou B, Guo Z, Xing J, Huang B. Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. Journal of Experimental Botany. 2005;56:3223–3228. doi: 10.1093/jxb/eri319. [DOI] [PubMed] [Google Scholar]