Abstract
Speed of visual word recognition is an important variable affecting linguistic competence. Although speed of visual word recognition varies widely between individuals, the neural basis of reaction time (RT) differences is poorly understood. Recently, a magnetic resonance technique called diffusion tensor imaging (DTI) has been shown to provide information about white matter (WM) microstructure in vivo. Here, we used DTI to explore whether visual word recognition RT correlates with regional fractional anisotropy (FA) values in the WM of healthy young adults. Participants completed a speeded lexical decision task that involved visual input, linguistic processes, and a motor response output. Results indicated that lexical decision RT was correlated negatively with FA in WM of inferior parietal and frontal language regions rather than in WM of visual or motor regions. Voxels within the inferior parietal and frontal correlation clusters were composed primarily of DTI-based tracts oriented in the anterior-posterior orientation at or near the superior longitudinal fasciculus (SLF) and likely including other smaller association fibers. These results provide new microstructural evidence demonstrating that speed of lexical decision is associated with the degree to which portions of frontal and parietal WM are directionally oriented.
Keywords: word recognition, reading, diffusion tensor imaging, fractional anisotropy
Introduction
Visual word recognition involves the ability to distinguish strings of letters that have a lexical status from those that do not. The ability for rapid visual word recognition contributes to reading ability and comprehension of written language (Perfetti, 1994). By contrast, delayed speed of word recognition contributes to developmental reading disorders (Sigmundsson, 2005), and is a common symptom of language-related degenerative brain disorders (Plaut et al., 1996; Gold et al., 2005). Not surprisingly, then, psycholinguistic variables influencing speed of visual word recognition have been studied for over 100 years (Catell, 1890). In research settings, this skill is usually explored via the speeded visual lexical decision task, in which participants decide as quickly and accurately as possible whether visual letter strings represent words or nonwords. Results from studies using the visual lexical decision task have been influential in the development of models of visual word recognition (McClelland & Rumelhart, 1981; Coltheart et al., 2001), and mapping the neural correlates of visual word recognition processes (Rumsey et al., 1997; Binder et al., 2003; Heim et al., 2005).
The most common dependent variable explored in visual lexical decision is reaction time (RT). Although most young healthy adults score near ceiling on lexical decision tasks, speed of visual word recognition varies widely across different individuals (Balota et al., 2004). There is evidence that cognitive RTs may be in part heritable (Ho et al., 1998; Posthuma et al., 2002). However, the neural basis of RT differences is poorly understood. This may be due in part to the fact that, until recently, no technique existed for the measurement of microstructural properties of cerebral white matter (WM) in vivo. A long-standing hypothesis is that cognitive RT depends on microstructural properties of cerebral WM, such as degree of myelination (Flechsig, 1920). Increased myelination may promote faster nerve conduction velocity which would promote faster RT (Jack et al., 1983).
Recently, a magnetic resonance technique called diffusion tensor imaging (DTI) has been shown to provide information about WM microstructure in vivo. DTI provides a voxel-by-voxel estimate of both the degree and orientation of directionality along which water molecules move preferentially. The degree to which molecular displacements are directionally dependent is referred to as fractional anisotropy (FA). The FA measure ranges from 0, representing diffusion that is equal in all directions, to 1, representing diffusion that occurs exclusively along one direction. The FA measure varies systematically across different compartments of the brain. For example, FA is low in the ventricles, where water movement is relatively unconstrained and thus isotropic. In contrast, FA is relatively high in cerebral WM, because the highly organized structure of WM fiber tracts causes water diffusion to be anisotropic, or unequal across different directions (Basser et al., 1994a, 2000; LeBihan, 2003; Catani et al., 2006).
The biological variables contributing to diffusion anisotropy in WM have not been fully identified but include the degree of myelination, and the density and orientation coherence of axons (reviewed in Beaulieu, 2002). For example, histological studies have demonstrated that myelination of axons increases anisotropy (Wimberger et al., 1995) and demyelination of axons decreases anisotropy (Werring et al., 1999). Consequently, the FA measure is highest in cerebral WM structures that contain the largest numbers of myelinated fibers running in parallel, such as the corpus callosum (Shimony et al., 1999). The established relationship between degree of myelination and speed of nerve conduction velocity (Jack et al., 1983) raises the possibility that increased FA in specific WM regions may be associated with behavioral RT. In fact, several studies have reported a relationship between regional FA and behavioral RT in healthy young adults associated with an oddball task (Madden et al., 2004) and a visuospatial task (Tuch et al., 2005).
The aim of the present study was to determine if speed of visual word recognition correlates with regional FA in WM of young healthy adults. If so, WM correlation clusters may be composed of DTI-based tracts oriented primarily toward adjacent cortex, overlying cortex, or each other. In the present study, these different possibilities were explored by correlating healthy young adults' lexical decision RT with their regional cerebral FA values, and exploring the orientation of axons within RT-FA correlation clusters. Results provide new evidence for a relationship between speed of visual word recognition and FA in left inferior frontal and parietal WM.
Materials and methods
Participants
Sixteen young healthy volunteers (9 females; mean age = 24, SD = 5; mean years of education = 14.4, SD = 2.2) participated. All participants provided written informed consent in a manner approved by the University of Kentucky Institutional Review Board and were paid for participating. All participants were right-handed, as assessed by the Edinburg Handedness Inventory, native English speakers, who reported no neurological disease, and had normal or corrected-to-normal visual acuity.
Behavioral procedures
Participants performed a visual lexical decision task in a behavioral testing room immediately prior to the scanning session. Participants were informed that letter strings would be presented in the center of the computer screen and were instructed to indicate if each letter string was a word or a nonword as quickly and accurately as possible via a button press. Participants received 24 practice trials followed by 4 blocks of 60 trials for the experimental phase. Each block was divided equally between word and nonword trials. A mandatory break of approximately 2 minutes was given between each block. The order of stimulus presentation was randomized for each block for each participant. Trials consisted of a fixation point (+) at the center of the screen for 500 ms, followed immediately by a letter string centered at the fixation point's location. The letter string remained on the screen for 2000 ms. Stimuli were presented in 42-pt Courier uppercase font. Stimulus presentation and recording of responses were implemented with E-Prime software, using a button-box that registers latencies to the nearest ms (Psychology Software Tools Inc., Pittsburgh PA). Behavioral reaction times (RTs) were measured from the onset of target display. Behavioral RTs were analyzed for correct word and nonword trials. Correct trials in which RTs 2.5 standard deviations above or below a participant's mean RT were excluded from analyses to minimize the effect of outliers.
Stimuli
The stimuli consisted of 120 nouns and 120 pronounceable nonwords. The study was concerned with higher linguistic components of visual word recognition. Therefore, words of relatively low-frequency were employed (M = 9, 857 per hundred million observations in The Hyperspace Analogue to Language frequency norms; Burgess and Livesay, 1998). Also, nonword targets were constructed by changing one letter of a group of word stimuli not used in the present experiment to create orthographically legal nonwords (pseudowords). These variables minimize the chances of distinguishing words from nonwords based solely on orthographic familiarity. Words and nonwords were matched for length (M for words 4.72=; M for nonwords = 4.69).
MRI Acquisition
Data were collected on a 3T Siemens Magnetom Trio MRI scanner, using an 8-channel head array coil, at the University of Kentucky's Magnetic Resonance Imaging and Spectroscopy Center. Foam padding was used to limit head motion within the coil. A high-resolution, 3D anatomic image was acquired using a T-1 weighted (MP-RAGE) sequence (TR = 2100ms, TE = 2.93ms, TI = 1100ms, flip angle = 12°, FOV = 224×256×192 mm, 1 mm isotropic voxels, sagittal partitions) for the localization of FA-RT correlation in the stereotactic space of the Montreal Neurological Institute (MNI). Diffusion tensor imaging (DTI) used a fluid attenuated inversion recovery EPI sequence (TR = 13600 ms, TE = 84 ms, TI = 2500 ms, flip angle = 90°, FOV 224 mm, 128 × 128 image matrix, 6 signal averages, acquiring 40 axial slices of 3 mm thick, with no interslice gap, in-plane voxel resolution = 1.75 mm2), covering the whole cerebrum. Diffusion was measured in six directions, and one image with no diffusion weighting. The directions were (x, y, z) = (0, 0, 0), (1, 1, 0), (1, -1, 0), (1, 0, 1), (1, 0, -1), (0, 1, 1), (0, 1, -1), where 1 indicates a gradient applied in that direction (Basser and Pierpaoli, 1996). During the same scanning session, participants also took part in a separate fMRI experiment (in which four gradient-echo EPI runs were also acquired) as part of a primed lexical decision study that has been reported elsewhere (Gold et al., 2006).
Image Pre-Processing
Coregistration of diffusion-weighted images (DWIs) and their subsequent transformation into MNI space was carried out using FSL software (http://www.fmrib.ox.ac.uk/fsl). Both registration and transformation to MNI space were performed using FLIRT and used a 12-df affine transformation, a mutual information cost function and trilinear interpolation. As a first step, the six different direction DWIs were registered to the (0,0,0) direction, no diffusion weighted images, to correct for motion and residual eddy current distortion. Each participant's diffusion tensor, associated eigensystem, and FA metric were then calculated using procedures described previously (Basser et al., 1994b). Briefly, three eigenvectors that define the diffusion ellispoid were calculated in each voxel from the diffusion tensor. These eigenvectors correspond to three eigenvalues, which represent the magnitude of diffusivity in the three principal directions. Based upon the three principal diffusivities and the mean diffusivity, FA and direction of DTI-based tract orientation were calculated in each voxel (Basser and Pierpaoli, 1996). Each participant's motion corrected DWIs were then placed into standard MNI space using parameters derived from the transformation of their high-resolution T1 images. First, T1 images and DWIs were skull-stripped using FSL's BET program. Second, each participant's DWIs were coregistered spatially to their own T1 images. Third, skull-stripped T1 images were normalized anatomically to the skull-stripped version of the MNI 152-participant T1 template. Finally, skull-stripped DWIs were normalized anatomically to MNI space using T1 image transformation parameters.
Whole-Brain Analysis of FA-RT Correlation
Analysis of the relationship between RT and FA was conducted using Statistic Parametric Mapping software (SPM2; http://www.fil.ion.ucl.ac.uk/spm/). FA images were smoothed with a 6-mm FWHM Gaussian filter to increase the statistical sensitivity to correlations equal to or larger than the kernel width. A 6-mm FWHM Gaussian filter was selected based on evidence that three-voxel FWHM provides sufficient smoothing for 3D Gaussian random fields (Nichols and Hayasaka, 2003). However, the optimal size of the smoothing filter is controversial as different filter sizes have been shown to influence the results of DTI analyses (Jones et al., 2005). Consequently, analyses were also conducted on unsmoothed FA volumes in native space (see ROI Analysis section below).
Following Tuck et al. (2005), the correlation between RT and FA was computed using nonparametric Spearman rank regression instead of parametric Pearson regression because of the potential nonnormality of the FA distribution across individuals. For similar reasons, a multiple comparisons correction was applied using the nonparametric Monte Carlo permutation method (Nichols and Holmes, 2002). The goal of the study was to determine if lexical decision RT correlated with any moderate sized WM clusters rather than to localize correlations to peak WM voxels. Therefore, a cluster-level threshold was employed to achieve a corrected significance level of P < 0.05. A significance threshold of P < 0.005 was adopted because it yielded moderate sized clusters of ≥ 20 contiguous voxels. The Monte Carlo calculation used 104 trials. The Spearman correlation coefficient (r) map was visualized as an overlay on the standard MNI T1-template and the mean FA image.
The relationship between RT and FA was plotted for significant RT-FA correlation clusters to examine the possible influence of outliers on each correlation. Masks consisted of a three-dimensional area including all voxels (p < 0.001) within 10 mm of the group peak correlation coordinates. Each participant's mean FA values in the region surrounding the peak in each cluster was extracted and plotted against their mean lexical decision RT.
ROI Analysis of FA-RT Correlation
Region of interest (ROI) analyses were conducted to test the validity of the findings from the whole brain analyses. Importantly, ROI analyses were conducted on each individual's FA volume in native space that had not been normalized or smoothed, eliminating the effects of normalization and kernel smoothing on FA values. ROIs were traced using Analyze 6.0 software. The program then computed the mean FA value within each manually traced ROI. Images were first corrected for minor head rotation. ROIs were traced in WM regions near the two significant clusters identified in the whole-brain analysis and in several control regions associated with the visual input and motor output components of the lexical decision task. All ROIs were traced on axial slices and involved relatively small swaths within WM in the x and y directions to minimize partial volume effects. The computation of ROIs resulted in a single FA value for each subject, for each region. These values were then correlated with RT.
The first two ROIs were intended to be situated within left inferior frontal and parietal WM, in the general vicinity of the significant correlation clusters from the whole-brain analysis. ROI boundaries were selected with reference to a human cerebral DTI atlas (Wakana et al., 2004). The inferior frontal ROI was traced from the slice in which the posterior horns of the lateral ventricles assumed their most lateral position until the WM was interrupted by the insular cortex. On average, this ROI spanned 12mm in the axial direction (SD = 0.6). This ROI may have included small portions of WM associated with the middle frontal region. The parietal ROI was traced from the slice in which the lateral ventricles were first visible until the anterior and posterior horns of the lateral ventricles were clearly separated (due to the appearance of the thalamus). On average, this ROI spanned 14mm in the axial direction (SD = 1.1). This ROI may have included small portions of WM associated with the superior temporal region. The control ROIs associated with visual input and motor output were placed in white matter of the optic radiations and posterior limbs of the internal capsule, respectively. The optic radiations (left and right) were defined on a single slice where the posterior horns of the lateral ventricles assumed their most lateral position. The optic radiation ROI was likely to encompass some fibers of the inferior longitudinal fasciculus, inferior-fronto-occipital fasciculus, and posterior thalamic projections in addition to the optic radiations. The posterior limbs (left and right) of the internal capsule were traced on a single slice approximating the center of the genu of the corpus callosum, where the inflection between anterior and posterior limb was greatest.
Diffusion Tensor Map
Diffusion tensor maps were created in order to determine the principal direction (anterior-posterior, inferior-superior or left-right) of fibers in RT-FA correlation clusters emerging from the whole-brain analysis. FA maps were created and then color coded according to the dominant principle diffusion directions as described by Coremans et al. (1994). Once normalized into MNI space (using procedures described above), a mean diffusion tensor volume data set was created using procedures described previously (Jones et al., 2002).
Results
Behavioral
Mean accuracy was near ceiling for both word (M = 96.2%; SD = 2.7) and nonword trials (M = 92.4%; SD = 3.2). The minimal variance found between participants' mean accuracies precluded correlation of accuracy with FA. In contrast, substantial variance was found between participants' mean reaction times (RTs) for correct word (M = 574 msec; SD = 73) and nonword (M = 644 msec; SD = 77) trials.
Imaging
The first voxel-wise, whole-brain analysis sought to determine if lexical decision RT (averaged across correct word and pseudoword trial types) correlated with fractional anisotropy (FA) in any brain voxels. Two significant negative correlations were observed. RT for correct trials correlated negatively with FA in white matter (WM) of the left inferior frontal and inferior parietal regions (Figure 1). The correlation was -0.71 for the inferior frontal region (cluster size = 22 voxels; MNI peak: -28 26 18; ∼BA 45) and -0.82 for the inferior parietal region (cluster size = 44 voxels; MNI peak: -33 -48 32; ∼BA 40).
Fig 1.
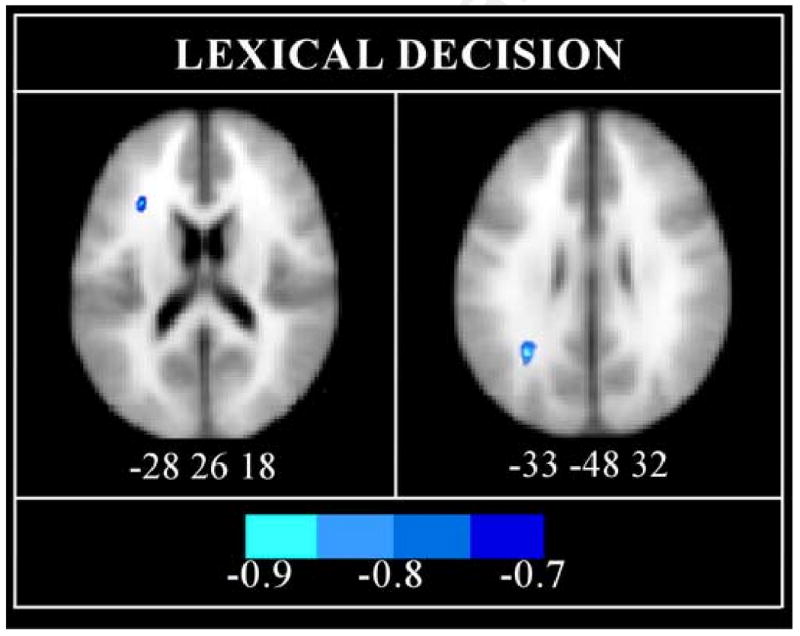
Speed of visual word recognition correlates negatively with fractional anisotropy (FA) in inferior frontal and parietal white matter (WM). The Spearman correlation coefficient (rs) between lexical decision reaction time (RT) and FA is displayed as a color map overlaid on axial slices of the MNI template. The center coordinates of the peak voxels in MNI space are indicated below each slice. The color bar indicates the strength of the correlation.
A second analysis was carried out to assess the possibility that the correlation between speed of lexical decision and FA may be influenced by lexical status. Mean word and pseudoword RTs were each correlated with voxel-based FA. Results indicated that, like overall lexical decision RT, both word RT and pseudoword RT correlated negatively with FA in left inferior frontal and parietal WM (results not shown). These results indicate that FA in these regions correlated with speed of both correct acceptance of words and correct rejection of word-like nonwords. Consequently, subsequent analyses described below were performed on lexical decision RT averaged across correct word and pseudoword trial types.
Figure 2 display regression plots of overall lexical decision RT and FA in the frontal (panel A) and parietal (panel B) regions. In order to explore the influence of outliers on the observed correlations, standardized z-scores were computed for RT, and for FA in both the parietal and frontal clusters. All participants' mean RTs fell within 2 standard deviations of the overall group mean. All participants' mean FA scores in the inferior frontal cluster were within 2 standard deviations of the group mean. Two participants' mean FA scores in the inferior parietal cluster fell just outside 2 standard deviations of the group mean (both SD = 2.1). However, removing these two participants' data had a minimal effect on the new overall correlation in the inferior parietal WM region (r = -0.81 p < .001). These data indicate that the observed RT-FA correlations in inferior frontal and parietal regions are not the result of extreme RT or FA values.
Fig 2.
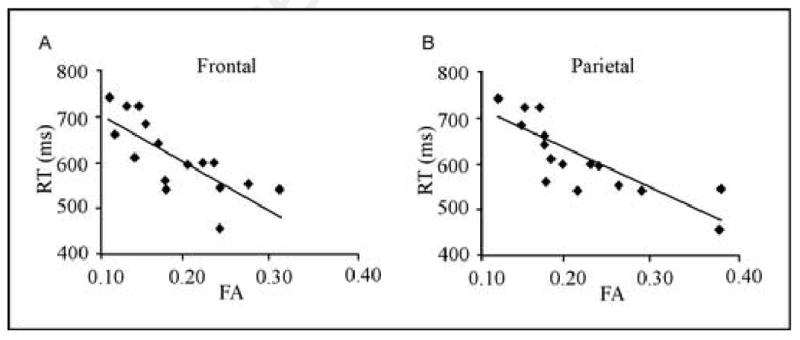
Correlation between visual word recognition RT and FA in inferior frontal and parietal WM. Scattergrams plot participant's mean RT in msec against their mean FA in clusters from Figure 1 for (A) left inferior frontal region and (B) left inferior parietal region. Each point represents one of the 16 participants.
Results from the ROI analyses confirmed those from the whole-brain analysis. Figure 3 presents a representation of the 6 ROIs on a single, representative subject's FA images. The relationship between RT and FA was significant in the inferior frontal ROI [r (16) = -0.74, p < .001] and the parietal ROI [r (16) = -0.72, p < .001].
Fig 3.
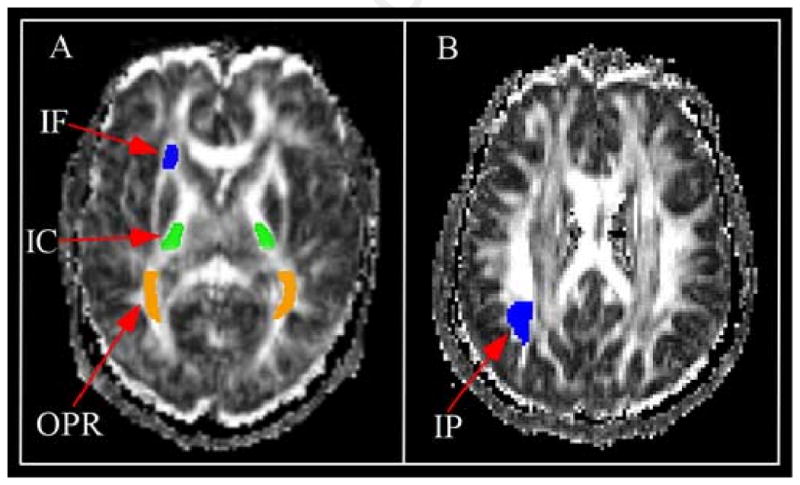
Example of regions of interest (ROIs). ROIs are shown on a single, representative subject's FA images. ROI analyses were conducted on FA images in native space, prior to normalization and smoothing. Panel A displays the IF = inferior frontal (IF), internal capsule (IC; posterior limb), and the optic radiation (OPR) ROIs. Panel B displays the inferior parietal (IP) ROI.
Figure 4 presents the regression plots of lexical decision RT and FA in the frontal (panel A) and parietal (panel B) regions derived from the ROI analyses. The relationship between RT and FA did not approach significance (p < .05) in any of the control ROIs. In order to explore the influence of outliers on the observed correlations, standardized z-scores were computed for FA in each of the 6 ROIs. All participants' mean FA scores in five of the ROIs were within 2 standard deviations of the group mean. One participant's mean FA scores in the right optic radiation ROI cluster fell outside 2 standard deviations of the group mean (both SD = 2.4). However, removing this participants' data did not change the non-significant correlation in this region (r = -0.19 p = .23).
Fig 4.
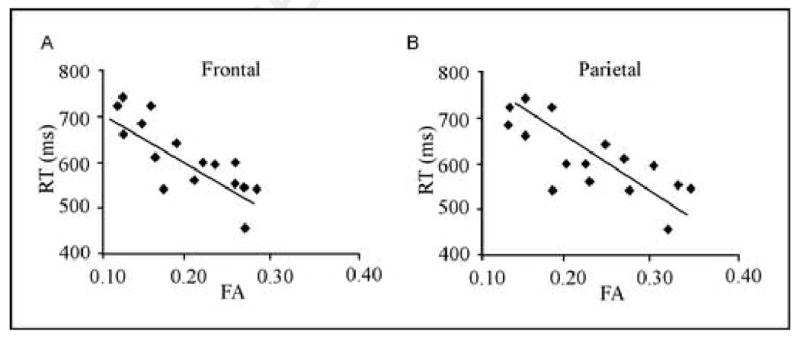
Correlation between visual word recognition RT and FA in inferior frontal and parietal WM regions derived from the ROI analyses (see Figure 3). Scattergrams plot participant's mean RT in msec against their mean FA in clusters from (A) left inferior frontal region and (B) left inferior parietal region. Each point represents one of the 16 participants.
These results cross-validate the negative correlations between RT and FA in left inferior frontal and parietal white matter observed in the whole-brain analyses. Thus, the relationship between RT and FA in these two regions is not attributable to normalization or smoothing of FA volumes.
Figure 5 shows the location of the peak of correlation clusters derived from the whole-brain analysis on both the mean FA volume (left panel) and mean diffusion tensor map (right panel) for the frontal (panel A; MNI peak: -28 26 18; ∼BA 45) and parietal (panel B; MNI peak: -33 -48 32; ∼BA 40) regions. Voxels within each of the regions were composed primarily of axons oriented in the anterior-posterior orientation. This information, along with comparison with a human cerebral DTI atlas (Wakana et al., 2004) suggested that the parietal and frontal correlation clusters were at or near the superior longitudinal fasciculus (SLF), and likely to also contain other, smaller association fibers. In addition, the parietal cluster may contain some thalamic projection and commissural fibers and the frontal region may contain some intra-lobar and commissural fibers.
Fig 5.
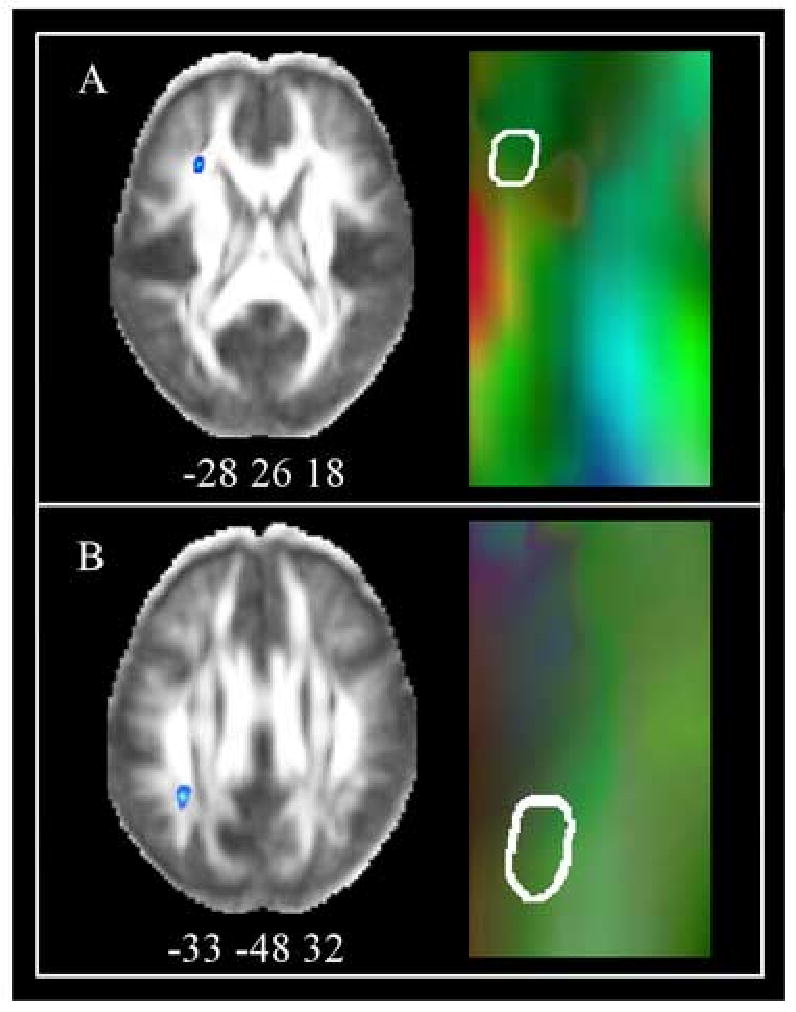
Principal direction of diffusion in RT-FA correlation clusters. The left side of each panel presents the lexical decision RT correlation clusters on axial slices of the mean FA map. The center coordinates of the peak voxels in MNI space are indicated below each slice. The right side of each panel displays a magnified portion of the mean fiber orientation map at the same level as the correlation clusters. The colors green, red, and blue indicate anterior-posterior, left-right, and superior-inferior diffusion directions, respectively. The contour of the correlation clusters are outlined in white for the (A) left inferior frontal lobe and (B) left inferior parietal lobule. Both correlation clusters were composed primarily of tracts running in an anterior-posterior direction.
Discussion
The present study sought to determine if speed of visual word recognition correlates with microstructural properties of cerebral white matter (WM). Participants performed a visual lexical decision task and diffusion tensor images were acquired subsequently to correlate reaction time (RT) with fractional anisotropy (FA). Voxel-wise, whole-brain analyses indicated a negative correlation between lexical decision RT and FA in two left hemisphere WM regions. Individuals with relatively fast lexical decision times tended to have relatively high FA in WM of the left inferior frontal (medial to ∼BA 45) and parietal (medial to ∼BA 40) regions bordering perisylivian language cortex. Importantly, these results were confirmed with independent ROI analyses conducted on FA volumes that had not been normalized or smoothed.
The lexical decision task also involved more basic early visual processes and a motor output response. However, no correlations were observed between lexical decision RT and FA in WM regions associated with early visual processing (optic radiations) or voluntary motor output (corticospinal tracts), possibly because of restricted between-participant RT variance associated with these components of the lexical decision task. The localization of RT-FA correlations to WM regions situated medial to cortical areas involved in language processing suggests a relationship between FA in these regions and speed of linguistic or metalinguistic components of lexical decision, as opposed to visual or motor components.
The lexical decision task can involve varying degrees orthographic, phonologic and semantic processing. When participants are asked to complete lexical decisions as quickly and accurately as possible, as in the present experiment, they tend to engage the least time consuming set of processes that can reliably discriminate words and nonwords (Balota et al., 2004). This set of processes is influenced by the characteristics of stimuli used in the experiment. Words tend to have more familiar orthographic, phonologic and semantic patterns than nonwords. When nonwords are orthographically illegal, containing uncommon bigrams (e.g., CPTOA), and/or word stimuli are high frequency (e.g., TABLE), discriminations can be made on the basis of orthographic information alone (James, 1975; Allen et al., 1995). By contrast, when orthographically legal nonwords (e.g., PLASS) are included, or word stimuli are low frequency (e.g., KITE), orthographic analysis alone cannot discriminate reliably words from nonwords. Under these conditions, in which words and nonwords look but do not sound equally familiar, a phonological process may be applied in which an attempt is made to identify the word by sounding it out (Gordon, 1983; Perfetti, 1999). Finally, when nonwords are pseudohomones (e.g., BRAIV), which look and sound equally familiar as words, only semantics can discriminate reliably words from nonwords (Plaut et al., 1996).
The present study employed relatively low-frequency words and orthographically legal nonwords, suggesting that participants may have engaged a phonological sounding out process (Perfetti, 1999) or consulted the semantic system (Plaut et al., 1996) to aid word recognition. Interestingly, RT-FA correlations were in WM close to cortical regions implicated in the transformation of visual language symbols into auditory codes (orthographic-to-phonological transformation). Based on several cases of alexia-with-agraphia, Dejerine (1891) suggested that the inferior parietal lobule was a multimodal language region involved in both reading and writing, both of which require phonologically-based transformations between visual and auditory linguistic codes. Confirmation of a role for the inferior parietal lobule, and demonstration of a role for left inferior frontal cortex, in phonology has come from modern functional neuroimaging studies (Pugh et al., 1996; Gold and Buckner, 2002; Sharp et al., 2005). In addition, left inferior frontal cortex has been reported to show strong activation associated with phonological components of visual word recognition (Rumsey et al., 1997; Heim et al., 2005). Although we looked for a possible lexicality effect, we found that both the speed of word acceptance and nonword rejection correlated negatively with FA in the same regions of inferior parietal and frontal WM. This suggests that high FA in these WM regions may correlate with speed of phonological components of lexical decision, as opposed to speed of lexical retrieval per se (although FA in these regions may also correlate with metalinguistic processes; discussed below).
Results from our RT-FA correlation analysis add to a growing body of data suggesting that, in young healthy adults, high performance on a particular cognitive task appears to be associated with increased FA in WM bordering cortical regions implicated in that task. For example, Tuch et al. (2004) reported a correlation between visuospatial RT and FA in the right optic radiation and other regions underlying the visuospatial network, whereas Madden et al. (2004) reported a correlation between RT and FA in the splenium of the corpus callosum during a visual oddball task thought to require inter-hemispheric transfer of visual information. The present visual word recognition RT-FA correlations did not overlap with those from these two recent studies. However, one of the present RT-FA correlations (in inferior parietal WM), was close to that reported by Klingberg et al. (2000), who observed a positive correlation between reading accuracy and FA in left temporoparietal WM. This study involved a different task (reading), output modality (speech), and performance measure (accuracy) than the present study. Still, the present lexical decision task shares more cognitive features with the reading task than the oddball or visuospatial tasks (which used the same motor output and RT performance measure as the present study). Visual word recognition is thought to represent the most central set of linguistic processes underlying skilled reading (McClelland & Rumelhart, 1981).
Examination of the mean diffusion tensor map indicated that the present correlation clusters were composed primarily of axons oriented in the anterior-posterior orientation. This information, along with comparison with a human cerebral DTI atlas (Wakana et al., 2004) suggested that the parietal and frontal correlation clusters were part of or very close to the superior longitudinal fasciculus (SLF) and likely to also include other, smaller association fibers. The SLF is one of the major cortical association fiber pathways in the human brain, interconnecting perisylvian frontal, parietal and temporal association areas (Dejerine, 1895; Catani et al., 2002). The present results demonstrate that speed of visual word recognition is associated with the extent to which a portion of inferior frontal and parietal WM association fibers are directionally oriented. These findings raise the possibility that speed of lexical decision may be in part dependent upon the strength of connection of WM association tracts running between inferior frontal and parietal regions. However, our data do not allow for an optimal test of whether the present correlation clusters are connected by a DTI-based fiber tract, because 6 directions may not be sufficient to robustly compute DTI-based fiber tracking (Jones, et al., 1999).
Future research exploring this question may be able to provide a more detailed account of the relationship between FA in inferior parietal and frontal WM and speed of lexical decision. For example, a finding that the present parietal and frontal correlation clusters are connected by a WM tract may suggest that these regions play a coordinated role in lexical decision. On this account, larger FA in these regions could provide more direct, or ‘stronger’, connections between anterior and posterior language regions, speeding lexical decision. In contrast, smaller FA in these regions could provide less direct, or ‘weaker’, connections between anterior and posterior language regions, slowing lexical decision. Such a view would be consistent with results from previous research demonstrating a correlation in functional activation between inferior parietal and inferior frontal cortices during phonological processing in young healthy adults (Bokde et al., 2001).
On the other hand, if the present frontal WM and parietal WM clusters were not connected this may suggest separate contributions of these regions to speed of lexical decision. In particular, such a finding may raise the possibility that the inferior frontal WM cluster could contribute to metalinguistic processes thought to be involved in the binary lexical decision task. Some models of visual word recognition view the lexical decision task as an evidence building process during which metalinguistic, executive processes operate until such a time when a lexical representation's threshold is reached and a response is executed (Balota et al., 2004). Thus, speed of lexical decision may be in part predicated on the speed of metalinguistic, executive processes that enable the reader to shift between and/or integrate information from different linguistic domains in order to reach a binary decision. This view gains some credence from the results of a previous study in which a more naturalistic phonology task of reading (albeit exploring accuracy rather than RT) was found to correlate with FA in a temporoparietal WM region but not a frontal WM region (Klingberg, 2000). Future research correlating RT in both a naturalistic phonology task such as reading, and a metalinguistic task such as lexical decision, with FA in a single group of subjects, may shed light on the more precise relationship between FA in inferior frontal WM and speed of linguistic or metalinguistic processing.
Acknowledgments
This research was supported by National Institutes of Health grants DC007315 and P50 AG05144-21. The authors thank Drs. Anders Andersen and Charles Smith for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen PA, Wallace B, Weber TA. Influence of case type, word frequency, and exposure duration on visual word recognition. JEP: Hum Percep and Perform. 1995;21:14–934. doi: 10.1037//0096-1523.21.4.914. [DOI] [PubMed] [Google Scholar]
- Balota DA, Yap MJ. Attentional control and flexible lexical processing: Explorations of the magic moment of word recognition. In: Andrews S, editor. From inkmarks to ideas: Current issues in lexical processing. Psychology Press; in press. [Google Scholar]
- Balota DA, Cortese MJ, Sergent-Marshall SD, Spieler DH, Yap M. Visual word recognition of single-syllable words. JEP: Gen. 2004;133:283–316. doi: 10.1037/0096-3445.133.2.283. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, Le Bihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994a;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBinhan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994b;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In Vivo Fiber-Tractography in Human Brain Using Diffusion Tensor MRI (DT-MRI) Data. Magn Reson Med. 2000;44:625–632. doi: 10.1002/1522-2594(200010)44:4<625::aid-mrm17>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system—a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Breznitz Z, Misra M. Speed of processing of the visual-orthographic and auditory-phonological systems in adult dyslexics: the contribution of “asynchrony” to word recognition deficits. Brain Lang. 2003;85:486–502. doi: 10.1016/s0093-934x(03)00071-3. [DOI] [PubMed] [Google Scholar]
- Binder JR, McKiernan KA, Parsons ME, Westbury CF, Possing ET, Kaufman JN, Buchanan L. Neural correlates of lexical access during visual word recognition. J Cog Neurosci. 2003;15:372–393. doi: 10.1162/089892903321593108. [DOI] [PubMed] [Google Scholar]
- Bokde ALW, Tagamets MA, Friedman RB, Horwitz B. Functional interactions of the inferior frontal cortex during the processing of words and word-like stimuli. Neuron. 2001;30:609–617. doi: 10.1016/s0896-6273(01)00288-4. [DOI] [PubMed] [Google Scholar]
- Burgess C, Livesay K. The effect of corpus size in predicting reaction time in a basic word recognition task: Moving on from Kucera and Francis. Behav Res Methods Instrum Comput. 1998;30:272–277. [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M. Diffusion tensor magnetic resonance imaging tractography in cognitive disorders. Curr Opin Neurol. 2006;19:599–606. doi: 10.1097/01.wco.0000247610.44106.3f. [DOI] [PubMed] [Google Scholar]
- Catell JM. Mental tests and measurements. Mind. 1890;15:373–381. [Google Scholar]
- Coltheart M, Rastle K, Perry C, Langdon R, Ziegler J. DRC: a dual route cascaded model of visual word recognition and reading aloud. Psych Rev. 2001;108:204–256. doi: 10.1037/0033-295x.108.1.204. [DOI] [PubMed] [Google Scholar]
- Coremans J, Luypaert R, Verhelle F, Stadnik T, Osteaux M. A method for myelin fiber orientation mapping using diffusion-weighted MR images. Magnetic Resonance Imaging. 1994;12:443–454. doi: 10.1016/0730-725x(94)92538-0. [DOI] [PubMed] [Google Scholar]
- Dejerine J. Anatomie des centres nerveux. Vol. 1. Paris: Rueff et Cie; 1895. [Google Scholar]
- Fiebach CJ, Friederici AD, Muller K, von Cramon DY. fMRI evidence for dual routes to the mental lexicon in visual word recognition. Journal of Cognitive Neuroscience. 2002;14:11–23. doi: 10.1162/089892902317205285. [DOI] [PubMed] [Google Scholar]
- Flechsig P. Anatomie des Menschlichen Gehirns und Rückenmarks auf Myelogenetischer Grundlage. Leipzig: Thieme; 1920. [Google Scholar]
- Geshwind N. Disconnection syndromes in animals and man. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions co-activate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35:803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Cortese MJ, Sergent-Marshall SD, Snyder AZ, Salat DH, Fischl B, Dale AM, Morris JC, Buckner RL. Differing neuropsychological and neuroanatomical correlates of abnormal reading in early-stage semantic dementia and dementia of the Alzheimer type. Neuropsychologia. 2005;43:833–846. doi: 10.1016/j.neuropsychologia.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Gold BT, Balota DA, Jones SJ, Powell DK, Smith CD, Andersen AH. Dissociation of automatic and strategic lexical-semantics: Functional magnetic resonance imaging evidence for differing roles of multiple frontotemporal regions. The Journal of Neuroscience. 2006;26:6523–6532. doi: 10.1523/JNEUROSCI.0808-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon B. Lexical access and lexical decision: Mechanisms of frequency sensitivity. Journal of Verbal Learning and Verbal Behavioral. 1983;22:146–160. [Google Scholar]
- Heim S, Alter K, Ischebeck AK, Amunts K, Eickhoff SB, Mohlberg H, Zilles K, von Cramon DY, Friederici AD. The role of the left Brodmann's areas 44 and 45 in reading words and pseudowords. Brain Res Cogn Brain Res. 2005;25:982–993. doi: 10.1016/j.cogbrainres.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Ho HZ, Baker LA, Decker SN. Covariation between intelligence and speed of cognitive processing: genetic and environmental influences. Behavioral Genetics. 1988;8:247–261. doi: 10.1007/BF01067845. [DOI] [PubMed] [Google Scholar]
- Horwitz B, Rumsey JM, Donhue BC. Functional connectivity of the angular gyrus in normal reading and dyslexia. PNAS USA. 1998;95:8939–8944. doi: 10.1073/pnas.95.15.8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack JJB, Noble D, Tsien RW. Electrical Current Flow in Excitable Cells. Oxford University Press; Oxford: 1983. [Google Scholar]
- James CT. JEP: Hum Percep and Perform. 1975;1:130–136. [Google Scholar]
- Jones DK, Horsfield MA, Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magnetic Resonance in Medicine. 1999;42:515–525. [PubMed] [Google Scholar]
- Jones DK, Griffin LD, Alexander DC, Catani M, Horsfield MA, Howard R, Williams SCR. Spatial normalization and averaging of diffusion tensor MRI data sets. NeuroImage. 2002;17:592–617. [PubMed] [Google Scholar]
- Jones DK, Symms MR, Cercingnani M, Howard RJ. The effect of filter size on VBM analyses of DT-MRI data. NeuroImage. 2005;26:546–554. doi: 10.1016/j.neuroimage.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Hedehus M, Temple E, Salz T, Gabrieli JDE, Moseley ME, Poldrack RA. Microstructure of temporo-parietal white matter as a basis for reading ability: Evidence from diffusion tensor magnetic resonance imaging. Neuron. 2000;25:493–500. doi: 10.1016/s0896-6273(00)80911-3. [DOI] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–80. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Lieberman IY, Shankweiler D. Phonology and the problems of learning to read and write. Remedial and Special Education. 1985;6:8–17. [Google Scholar]
- Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM. Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. NeuroImage. 2004;21:1174–1181. doi: 10.1016/j.neuroimage.2003.11.004. [DOI] [PubMed] [Google Scholar]
- McClelland JL, Rumelhart DE. An interactive activation model of context effects in letter perception: Part 1. An account of basic findings. Psych Rev. 1981;88:375–407. [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonpaametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T, Hayasaka S. Controlling the familywise error rate in functional neuroimaging: a comparative review. Statistical Methods in Medical Research. 2003;12:419–446. doi: 10.1191/0962280203sm341ra. [DOI] [PubMed] [Google Scholar]
- Paulesu E, Frith U, Snowling M, Gallagher A, Morton J, Frackowiak RS, Frith CD. Is developmental dyslexia a disconnection syndrome? Evidence from PET scanning. Brain. 1996;119:143–157. doi: 10.1093/brain/119.1.143. [DOI] [PubMed] [Google Scholar]
- Perfetti CA. Psycholinguistics and reading ability. In: Gernsbacher MA, editor. Handbook of psycholinguistics. San Diego, CA: Academic Press; 1994. pp. 849–894. [Google Scholar]
- Perfetti CA. Comprehending written language: A blueprint of the reader. In: Brown CM, Hagoort P, editors. The neurocognition of language. Oxford, UK: Oxford University Press; 1999. pp. 167–208. [Google Scholar]
- Plaut DC, McClelland JL, Seidenberg MS, Patterson K. Understanding normal and impaired word reading: Computational principles in quasi-regular domains. Psych Rev. 1996;103:56–115. doi: 10.1037/0033-295x.103.1.56. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Mulder EJ, Boomsma DI, de Geus EJC. Genetic analysis of IQ, processing speed and stimulus-response incongruency effects. Biological Psychology. 2002;61:157–182. doi: 10.1016/s0301-0511(02)00057-1. [DOI] [PubMed] [Google Scholar]
- Pugh KR, Shaywitz BA, Constable RT, Shaywitz SA, Skudlarski P, Fulbright RK, et al. Cerebral organization of component processes in reading. Brain. 1996;119:1221–1238. doi: 10.1093/brain/119.4.1221. [DOI] [PubMed] [Google Scholar]
- Rumsey JM, Horwitz B, Donohue BC, Nace K, Maisog JM, Andreason P. Phonological and orthographic components of word recognition. Brain. 1997;120:739–759. doi: 10.1093/brain/120.5.739. [DOI] [PubMed] [Google Scholar]
- Sharp DJ, Scott SK, Cutler A, Wise RJ. Lexical retrieval constrained by sound structure: the role of the left inferior frontal gyrus. Brain Lang. 2005;92:309–19. doi: 10.1016/j.bandl.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Shimony JS, McKinstry RC, Akbudak E, Aronovitz JA, Snyder AZ, Lori NF, Cull TS, Conturo TE. Quantitative diffusion-tensor anisotropy brain MR imaging: normative human data and anatomic analysis. Radiology. 1999;212:770–784. doi: 10.1148/radiology.212.3.r99au51770. [DOI] [PubMed] [Google Scholar]
- Sigmundsson H. Do visual processing deficits cause problems on response time task for dyslexics? Brain Cog. 2005;58:213–216. doi: 10.1016/j.bandc.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Stein J, Walsh V. To see but not to read; the magnocellular theory of dyslexia. Trends in Neuroscience. 1997;20:147–152. doi: 10.1016/s0166-2236(96)01005-3. [DOI] [PubMed] [Google Scholar]
- Tuch DS, Salat DH, Wisco JL, Zaleta AK, Hevelone ND, Rosas DH. Choice reaction time performance correlates with diffusion anisotropy in white matter pathways supporting visuospatial attention. PNAS. 2005;102:12212–12217. doi: 10.1073/pnas.0407259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PCM, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- Werring DJ, Clark CA, Barker GJ, Thomson AJ, Miller DH. Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology. 1999;52:1626–1632. doi: 10.1212/wnl.52.8.1626. [DOI] [PubMed] [Google Scholar]
- Wimberger DM, Roberts TP, Barkovich AJ, Prayer LM, Moseley ME, Kucharczyk J. Identification of “premyelination” by diffusion-weighted MRI. J Comp Assit Tomography. 1995;19:28–33. doi: 10.1097/00004728-199501000-00005. [DOI] [PubMed] [Google Scholar]


