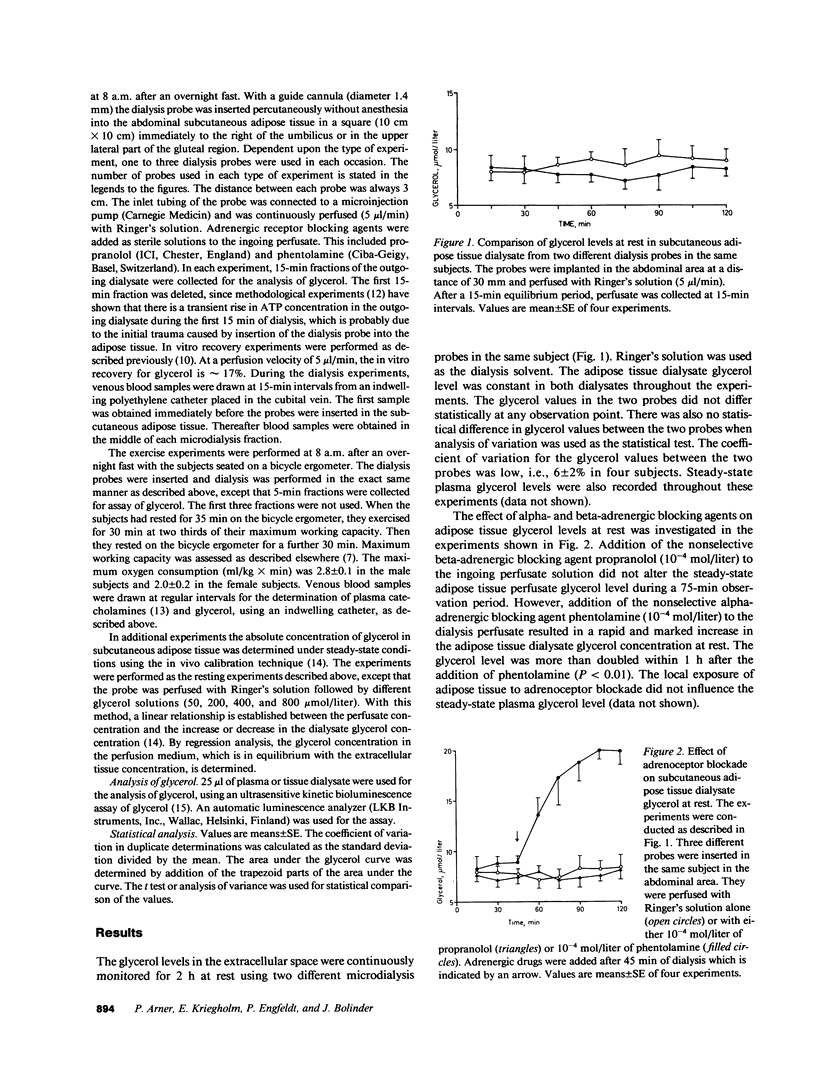
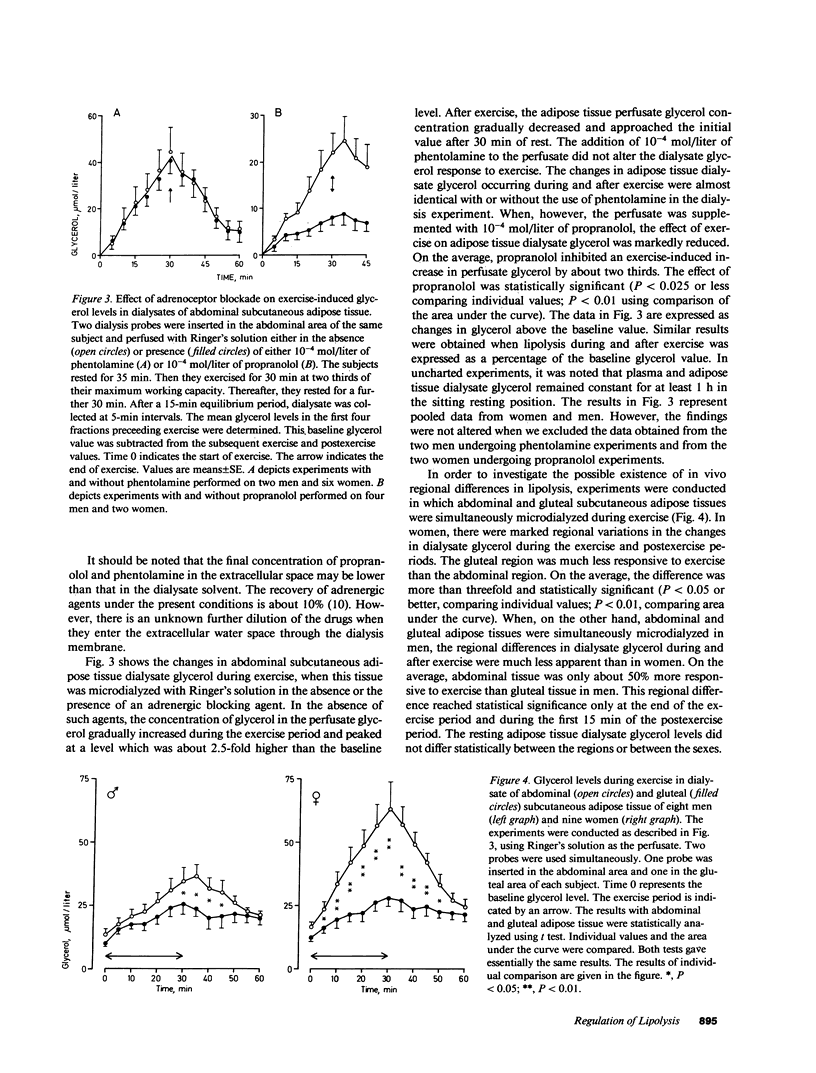
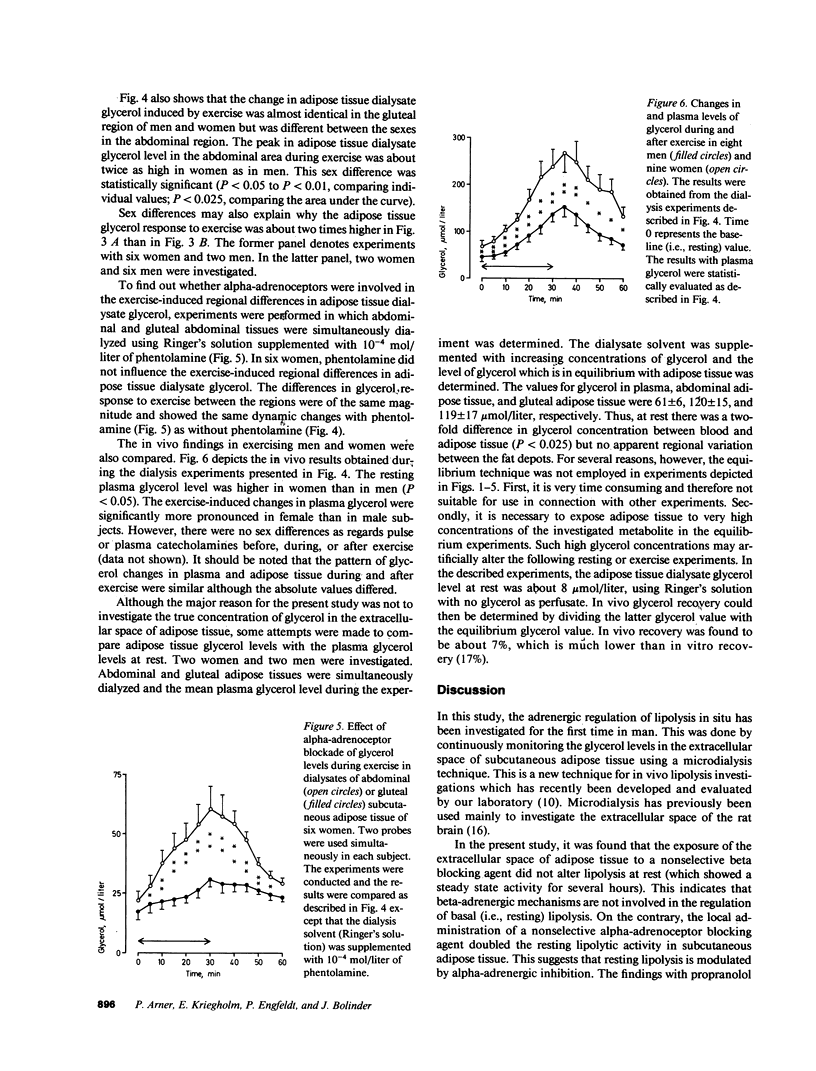
Abstract
The adrenergic regulation of lipolysis was investigated in situ at rest and during standardized bicycle exercise in nonobese healthy subjects, using microdialysis of the extracellular space in subcutaneous adipose tissue. The glycerol concentration was about two times greater in adipose tissue than in venous blood. At rest, the glycerol concentration in adipose tissue was rapidly increased by 100% (P less than 0.01) after the addition of phentolamine to the ingoing perfusate, whereas addition of propranolol did not alter the adipose tissue glycerol level. Glycerol in adipose tissue and plasma increased during exercise and decreased in the postexercise period. Propranolol in the perfusate almost completely inhibited the increase in the tissue dialysate glycerol during the exercise-postexercise period. Phentolamine, however, was completely ineffective in this respect. During exercise, the lipolytic activity was significantly more marked in abdominal than in gluteal adipose tissue; this was much more apparent in women than in men. Thus, in vivo lipolysis in subcutaneous adipose tissue is regulated by different adrenergic mechanisms at rest and during exercise. Alpha-adrenergic inhibitory effects modulate lipolysis at rest, whereas beta-adrenergic stimulatory effects modulate lipolysis during exercise. In addition, regional differences in lipolysis are present in vivo during exercise, which seem governed by factors relating to sex.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arner P., Bolinder J., Eliasson A., Lundin A., Ungerstedt U. Microdialysis of adipose tissue and blood for in vivo lipolysis studies. Am J Physiol. 1988 Nov;255(5 Pt 1):E737–E742. doi: 10.1152/ajpendo.1988.255.5.E737. [DOI] [PubMed] [Google Scholar]
- Arner P. Control of lipolysis and its relevance to development of obesity in man. Diabetes Metab Rev. 1988 Aug;4(5):507–515. [PubMed] [Google Scholar]
- Björkhem I., Arner P., Thore A., Ostman J. Sensitive kinetic bioluminescent assay of glycerol release from human fat cells. J Lipid Res. 1981 Sep;22(7):1142–1147. [PubMed] [Google Scholar]
- Bolinder J., Hagström E., Ungerstedt U., Arner P. Microdialysis of subcutaneous adipose tissue in vivo for continuous glucose monitoring in man. Scand J Clin Lab Invest. 1989 Sep;49(5):465–474. doi: 10.1080/00365518909089123. [DOI] [PubMed] [Google Scholar]
- Burns T. W., Mohs J. M., Langley P. E., Yawn R., Chase G. R. Regulation of human lipolysis. In vivo observations on the role of adrenergic receptors. J Clin Invest. 1974 Jan;53(1):338–341. doi: 10.1172/JCI107556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. I., Souness J. E. The mechanisms of hormone and drug actions on fatty acid release from adipose tissue. Rev Pure Appl Pharmacol Sci. 1981 Jan-Mar;2(1):1–112. [PubMed] [Google Scholar]
- Fain J. N., Garcĩa-Sáinz J. A. Adrenergic regulation of adipocyte metabolism. J Lipid Res. 1983 Aug;24(8):945–966. [PubMed] [Google Scholar]
- Hallman H., Farnebo L. O., Hamberger B., Johnsson G. A sensitive method for the determination of plasma catecholamines using liquid chromatography with electrochemical detection. Life Sci. 1978 Sep 11;23(10):1049–1052. doi: 10.1016/0024-3205(78)90665-3. [DOI] [PubMed] [Google Scholar]
- Håkanson E., Rutberg H., Jorfeldt L., Wiklund L. Endocrine and metabolic responses after standardized moderate surgical trauma: influence of age and sex. Clin Physiol. 1984 Dec;4(6):461–473. doi: 10.1111/j.1475-097x.1984.tb00132.x. [DOI] [PubMed] [Google Scholar]
- Jansson P. A., Fowelin J., Smith U., Lönnroth P. Characterization by microdialysis of intracellular glucose level in subcutaneous tissue in humans. Am J Physiol. 1988 Aug;255(2 Pt 1):E218–E220. doi: 10.1152/ajpendo.1988.255.2.E218. [DOI] [PubMed] [Google Scholar]
- Kather H., Bieger W., Michel G., Aktories K., Jakobs K. H. Human fat cell lipolysis is primarily regulated by inhibitory modulators acting through distinct mechanisms. J Clin Invest. 1985 Oct;76(4):1559–1565. doi: 10.1172/JCI112137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönnroth P., Jansson P. A., Smith U. A microdialysis method allowing characterization of intercellular water space in humans. Am J Physiol. 1987 Aug;253(2 Pt 1):E228–E231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- Marcus C., Ehrén H., Bolme P., Arner P. Regulation of lipolysis during the neonatal period. Importance of thyrotropin. J Clin Invest. 1988 Nov;82(5):1793–1797. doi: 10.1172/JCI113793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tossman U., Ungerstedt U. Microdialysis in the study of extracellular levels of amino acids in the rat brain. Acta Physiol Scand. 1986 Sep;128(1):9–14. doi: 10.1111/j.1748-1716.1986.tb07943.x. [DOI] [PubMed] [Google Scholar]
- Wahrenberg H., Engfeldt P., Bolinder J., Arner P. Acute adaptation in adrenergic control of lipolysis during physical exercise in humans. Am J Physiol. 1987 Oct;253(4 Pt 1):E383–E390. doi: 10.1152/ajpendo.1987.253.4.E383. [DOI] [PubMed] [Google Scholar]



