Abstract
Several metal ions that are carcinogenic affect cellular iron homeostasis by competing with iron transporters or iron-regulated enzymes. Some metal ions can mimic a hypoxia response in cells under normal oxygen tension, and induce expression of HIF-1α-regulated genes. This study investigated whether 12 metal ions altered iron homeostasis in human lung carcinoma A549 cells as measured by an activation of IRP-1 and ferritin level. We also studied hypoxia signaling by measuring HIF-1α protein levels, hypoxia response element (HRE)-driven luciferase reporter activity, and Cap43 protein level (an HIF-1α responsive gene). Our results show the following: (i) Ni(II), Co(II), V(V), Mn(II), and to a lesser extent As(III) and Cu(II) activated the binding of IRP-1 to IRE after 24 h, while the other metal ions had no effect; (ii) 10 of 12 metal ions induced HIF-1α protein but to strikingly different degrees. Two of these metal ions, Al(III) and Cd(II), did not induce HIF-1α protein; however, as indicated below, only Ni(II), Co (II), and to lesser extent Mn(II) and V(V) activated HIF-1α-dependent transcription. The combined effects of both [Ni(II) + As(III)] and [Ni(II) + Cr(VI)] on HIF-1α protein were synergistic; (iii) Addition of Fe(II) with Ni(II), Co(II), and Cr(VI) attenuated the induction of HIF-1α after 4 h treatment; (iv) Ni(II), Co(II), and Mn(II) significantly decrease ferritin level after 24 h exposure; (v) Ni(II), Co(II), V (V), and Mn(II) activated HRE reporter gene after 20 h treatment; (vi) Ni(II), Co(II), V(V), and Mn(II) increased the HIF-1-dependent Cap43 protein level after 24 h treatment. In conclusion, only Ni (II), Co (II), and to a lesser extent Mn(II) and V(V) significantly stabilized HIF-1α protein, activated IRP, decreased the levels of ferritin, induced the transcription of HIF-dependent reporter, and increased the expression of Cap43 protein levels (HIF-dependent gene). The mechanism for the significant stabilization and elevation of HIF-1α protein which drives these other parameters was previously shown by us and others to involve a loss of cellular Fe as well as inhibition of HIF-1α-dependent prolyl hydroxylases which target the binding of VHL ubiquitin ligase and degrade HIF-1α. Even though there were small effects of some of the other metals on IRP and HIF-1α, downstream effects of HIF-1α activation and therefore robust hypoxia signaling were only observed with Ni(II), Co(II), and to much lesser extents with Mn(II) and V(V) in human A549 lung cells. It is of interest that the metal ions that were most effective in activating hypoxia signaling were the ones that were poor inducers of metallothionein protein and also decreased Ferritin levels, since both of these proteins can bind metal ions and protect the cell against toxicity in human lung cells. It is important to study effects of these metals in human lung cells since this represents a major route of human environmental and occupational exposure to these metal ions.
Keywords: Metal ions, IRP-1, HIF-1α, HRE, Cap43
Introduction
Iron is an essential metal in all living organisms, playing an important role in electron transport and redox reactions, as well as a cofactor for numerous enzymes (van Vliet et al., 2002). Iron is involved in energy production, xenobiotic metabolism, and defense against oxidative stress (Morgan and Oates, 2002). Both iron overload and iron depletion can severely affect physiological processes and cause diseases such as cancer (Rolfs et al., 1997). Cellular iron homeostasis is post-translationally regulated by an iron regulatory protein 1 (IRP-1), which contains a [4Fe–4S] cluster in a cleft between two domains of the protein (Templeton and Liu, 2003). When iron is plentiful, IRP-1 exists in [4Fe–4S] form referred to as cytoplasmic aconitase, which lacks RNA binding activity; when cells are deficient in iron, IRP-1 loses its [4Fe–4S] cluster and becomes an RNA-binding form (Templeton and Liu, 2003). In the latter state, IRP-1 binds to IRE-containing mRNAs with high affinity, inhibits translation of those mRNAs whose IREs are at the 5′ end (e.g., ferritin, succinic dehydrogenase, mitochondrial aconitase), but stabilizes the expression of those mRNAs whose IREs are situated in the 3′ end (e.g., transferrin receptor (TfR) and divalent metal transporter 1 (DMT-1)) (Zheng and Zhao, 2001).
Metal use is widespread in the workplace and human exposure occurs by inhalation in numerous industrial operations. All metals are potentially toxic at high concentration, even though some are also essential. Metal ions were selected for this study because some of them, including nickel, manganese, and aluminum, have been shown to alter iron homeostasis following exposure in a number of different cell types (Zheng et al., 1999; Ward et al., 2001; Chen et al., 2005), which may contribute to the mechanism by which these metals exert their adverse effects on living organisms.
There are two potential mechanisms for the effects of metals ions on iron homeostasis. One is that the metal ion competes with iron for iron transport proteins such as transferrin or DMT-1 (Ward et al., 2001; Chen et al., 2005); while the other is that the metal ion interferes with iron homeostasis at the level of IRPs by competing with iron for the fourth, labile iron site in [4Fe–4S] cluster and thereby activates IRP binding (Zheng and Zhao, 2001). Since our previous study showed that Ni ions decreased intracellular Fe levels in A549 human lung cells by competing with iron for DMT-1 (Chen et al., 2005), and recently DMT-1 was identified as the major iron transporter in lung epithelia (Wang et al., 2002), it suggests that nickel altered iron homeostasis probably by competing with iron for DMT-1 in A549 cells. Manganese was postulated to alter iron homeostasis by replacing the fourth labile iron in the cubane structure of the aconitase active center in neuronal cells (Zheng and Zhao, 2001). There is little work conducted on the effects of non-iron metals on iron homeostasis, and it is of interest to investigate whether other non-iron metals alter iron homeostasis and whether these metals affect IRP-1 and hypoxia-inducible factor (HIF). However, since Mn is also taken up by the DMT1 system, it also likely competes for Fe uptake in the same way as Ni.
HIF-1 regulates mammalian oxygen homeostasis (Semenza, 1999) by transcriptionally activating the expression of numerous target genes, which are involved in erythropoiesis, iron metabolism, vascular regulation, glucose uptake, and glycolysis, as well as various other biological processes (Wenger, 2002). HIF-1 is a αβ heterodimer, that binds to hypoxia response element (HRE) contained in the promoter of HIF-1 target genes to regulate their expression (Wang et al., 1995). HIF-1α protein usually remains undetectable under normoxic conditions with a half life of about 5 min, but it is stabilized by hypoxia. Stabilization of HIF-1α caused the induction of VEGF which then mediates angiogenesis, but also it also increases glycolysis, pH buffering, and probably other key steps in tumor progression (Wenger, 2002). Four of HIF-1α target genes, which encode transferrin, transferrin receptor, heme oxygenase-1, and ceruloplasmin, coordinately regulate iron metabolism (Wenger, 2002).
One group of enzymes which uses iron is the 2-oxeglutarate-dependent dioxygenase, such as collagen prolyl 4-hydroxylase (C-P4Hs), HIF prolyl 4-hydroxylases (HIF-P4Hs), and factor inhibiting HIF (FIH) (Hirsila et al., 2005). Moreover, iron deficiency is also known to stabilize HIF-1α protein (Wang and Semenza, 1993). Numerous studies have shown that several metals such as As(III), Cr(VI), Cu(II), Zn(II), V(V), Co(II), Mn (II), and Ni(II) can mimic hypoxia by stabilizing HIF-1α protein but to very different extents depending upon the cell line and other conditions (Chun et al., 2000; Salnikow et al., 2000; Gao et al., 2002a, 2002b; Skinner et al., 2004; Martin et al., 2005). However, many of these studies have not examined the impact of HIF-1α stabilization on downstream targets and its relationship to Fe metabolism. In the present study, we examined the time-dependent effects of these metals on hypoxia signaling pathway in A549 cells, including HIF-1α protein level, hypoxia response element (HRE) luciferase reporter activity, and Cap43 protein level (a HIF-1α responsive protein). We have also studied whether the alteration of iron homeostasis as determined by IRP-1 activation by metals had downstream effects on the expression of HIF-1α and its regulated genes since the HIF-1α is closely tied with alterations in Fe homeostasis in A549 cells. Our data demonstrate that Ni(II), Co(II), V(V), and Mn(II) not only induced IRP-1 activity but also stabilized HIF-1α protein, activated HRE reporter, and increased Cap43 level in A549 cells; moreover, the induction of HIF-1α protein by Ni(II), and Co(II), was reduced by coexposure with ferrous iron in A549 cells. The effects of the other metals on each of these parameters in A 549 cells were either not observed or considerably less than Ni (II), Co(II) and Mn (II), or V(V).
Materials and methods
Chemicals
K2CrO4, NaAsO2, CdCl2, ZnCl2, HgCl2, Pb3(C2H3O2)2·3H2O, CuCl2, NiCl2·6H2O, AlCl3·6H2O, MnCl2·4H2O, CoCl2, NaVO3, and FeSO4·7H2O were obtained from Sigma.
Cell culture
Human lung carcinoma A549 cells were grown in Ham’s F-12 K medium (GIBCO BRL, Grand Island, NY) containing 10% fetal bovine serum and 1% penicillin–streptomycin. Cells were cultured at 37 °C in an incubator with a humidified 5% CO2 atmosphere. Cells were treated with metal ions when the cell density was approximately 90% confluent.
Electrophoretic mobility shift assay (EMSA)
The 32P-labeled IRE probe was prepared and the EMSA assay was performed as previously described (Chen et al., 2005).
Enzyme-linked immunosorbent assay (ELISA)
After exposure to various metal ions for 24 h, A549 cells in 6-well culture plates were washed twice with ice-cold phosphate-buffered saline (PBS), lysed by 150 μl ice-cold M-PER mammalian protein extract reagent (PIERCE) at 4 °C for 30 min, then moved into eppendorf tube by scraping with a rubber policeman. After centrifugation at 4000 rpm at 4 °C for 10 min, the supernatants were collected and stored at −70 °C for the ferritin assay. Levels of ferritin in A549 cell lysates were determined according to a previously published protocol (Fang and Aust, 1997). An antibody to a mixture of human spleen and liver ferritin was used as the capture antibody to coat an ELISA plate. Human liver ferritin was used as the standard. The conjugate of peroxidase and antibody to human spleen and liver ferritin was then added to serve as the detector to determine the amount of ferritin bound to the capture antibody. Tetramethylbenzidine (TMB) was then added as the substrate for the peroxidase, and the absorbance of the oxidation product of TMB was determined at 450 nm using a microplate reader (SpectroMax Plus, Molecular Devices, Sunnyvale, CA). Total protein in the cell lysates was determined by using Coomassie Plus-The Better Bradford™ Assay Kit (PIERCE), and the results were expressed as microgram of ferritin per milligram protein.
Western blots
Approximately 5 × 105 A549 cells were cultured in 60 mm dishes to ~90% confluency. Cells were then treated with different metal ions for 4 h, 8 h, and 24 h. After treatment, the medium was removed, the cells were washed twice with ice-cold PBS, and proteins were extracted using lysis buffer (1% SDS, 1 mM sodium orthovanadate, and 10 mM Tris pH 7.4), quickly scraped and placed into Eppendorf tubes, followed by boiling for another 5 min. The scraped cells were collected to Eppendorf tubes and boiled for an additional 5 min. To reduce viscosity, the samples were sonicated using a Branson Sonifier 450 and centrifuged at 14,000 × g for 5 min; the supernatant was collected for Western blotting. The protein concentration was determined by using the Bio-Rad DC protein assay. Forty micrograms of protein was separated by electrophoresis in a 7.5% SDS-PAGE gel for HIF-1α and 15 micrograms of protein was run on a 12.5% SDS-PAGE gel for Cap43. The proteins were then transferred to a PVDF membrane at constant 40 V overnight. Immunoblotting was performed with 1:500 diluted HIF-1α antibodies (BD Biosciences) or 1:2000 diluted anti-NDRG-1/Cap43 antibodies (Piquemal et al., 1999). The primary antibody was detected by chemical fluorescence following a Western blotting protocol (Amersham).
Transient transfection assay
A549 cells were transiently transfected with pGL3-HRE-Leu by the calcium phosphate coprecipitation method. Aliquots of 2.5 × 105 cells/well were seeded into 6-well plates. On the next day, cells were cotransfected with 2.2 μg of pGL3-HRE-Leu together with 2.2 μg of transfection control construct pβ-gal-Control. 24 h later, the medium was changed and cells were allowed to recover for another 24 h. Freshly prepared metal ion solution was added into the medium and the cells were incubated for 24 h at 37 °C humidified 5% CO2 atmosphere. The cells were lysed and the luciferase activities were determined using the Luciferase Assay Kit (Promega). Briefly, cells were washed with PBS twice and covered by 200 μl 1× reporter lysis buffer (RLB) (Promega), followed by a single freeze-thaw. Plates containing cells were rocked several times and the attached cells were scraped from the plates. The sample was transferred to a 1.5 ml-Eppendorf tube and the tubes were vortexed for 10–15 s, and then centrifuged at 14,000 × g for 2 min at 4 °C. The supernatant was transferred to a new tube and cell lysates were assayed by Microplate Luminometer LB 96v for luciferase activity, and the efficiency of transfection was determined using β-gal activity which was measured with a spectrophotometer.
Results
Effects of 12 metal ions on IRP-1 activity in A549 cells after 24 h treatment
Previous studies showed that Ni(II) treatment depleted cellular Fe and significantly induced IRP-1 activity in A549 cells (Chen et al., 2005). Here, we have addressed whether other metal ions could induce IRP-1 activity in A549 cells, as well as to study additional downstream parameters of hypoxia signaling. As shown in Fig. 1, Ni(II), Co(II), V(V), and Mn (II) induced IRP-1 activity in a dose-dependent manner (Figs. 1A and B), and the increase of IRP-1 activity was more remarkable with these metal ions as compared to As(III) and Cu (II), which at higher toxic doses (10 μM and 20 μM for As(III) and Cu(II) at 0.1 mM) also resulted in noticeable but less significant activation of IRP-1 activity (Figs. 1C and D). Other metal ions, including Cr(VI), Zn(II), Pb(II), Cd(II), Hg(II), and Al(III), failed to activate IRP-1 at the levels tested which included both toxic and non-toxic exposure conditions. The stabilization and activation of IRP-1 are indicative of the depletion of cellular Fe levels which can create a hypoxic like state in cells under normal oxygen tension.
Fig. 1.
Ni(II), Co(II), V(V), Mn(II), As(III), and Cu(II) induced IRP-1 activity in A549 cells. A549 cells were treated with (A–D) 12 metal ions at different concentrations (indicated in the figures) for 24 h, with Ni(II) as a positive control in panels B–D as well as untreated cells as a control in panels A–D. Two micrograms of cytosolic extract was used for EMSA as described in the Materials and methods section. Two percent 2-mercaptoethanol (2-ME) included in another lane was used to assess the loading of each lane. Quantitation of panels A–D, is given in panel E as a densitometric analysis normalized to 2-ME. Densitometric data are given as fold-control.
Effects of 12 metal ions on HIF-1α protein in A549 cells
To study the relation between IRP-1 activation and hypoxia signaling, we determined the level of HIF-1α protein in A549 cells after 4, 8, and 24 h treatment using Western blotting. A number of studies have previously shown that cobalt and nickel ions can mimic hypoxia by stabilizing HIF-1α transcription factor (Salnikow et al., 2003; Hirsila et al., 2005), and these results were also supported by our data. As shown in Figs. 2A and B, 1 mM Ni(II) and 0.2 mM Co(II) treatment dramatically induced HIF-1α protein in A549 cells after 4 h exposure and this stabilization persisted at 24 h. These exposure conditions were not toxic to the cells as measured by colony formation and by a vital dye assay. A 4 h exposure to 20 μM Cr(VI) induced HIF-1α; however, the increased stabilization of HIF-1α by Cr (VI) was short lived, and the HIF-1α protein level was decreased and undetectable after 24 h, even in the presence of Cr(VI). These results suggested that Cr(VI) poorly and transiently stabilized HIF-1α protein in A549 compared to Ni and Co for example and it is also likely stabilized this protein by a different mechanism. The data also showed that 0.1 mM V(V) and 0.1 mM Mn(II) induced HIF-1α protein after 24 h exposure (Figs. 2A and C), but the extent was much less remarkable than Ni(II) and Co(II) at all dose ranges. Figs. 2A–C shows that 10 of 12 metals increased HIF-1α protein in A549 cells to different extents. Only two of these metals, Al(III) and Cd(II), had no effect on HIF-1α protein levels. In the final analysis, only Ni (II), Co (II), and Mn (II) had significant effects on HIF-1α directly and on its downstream effectors. V(V), Mn(II), As(III), Pb(II), Zn(II), and Hg(II) all increased HIF-1α protein in a time-dependent manner but only Mn(II) along with Ni(II) and Co(II) activated this protein to an extent that exhibited downstream effects in A549 cells. However, cotreatment with different metals for 4 h, such as [Ni(II) + As(III)] and [Ni(II) + Cr(VI)], had synergistic effects on HIF-1α protein (Fig. 2E).
Fig. 2.
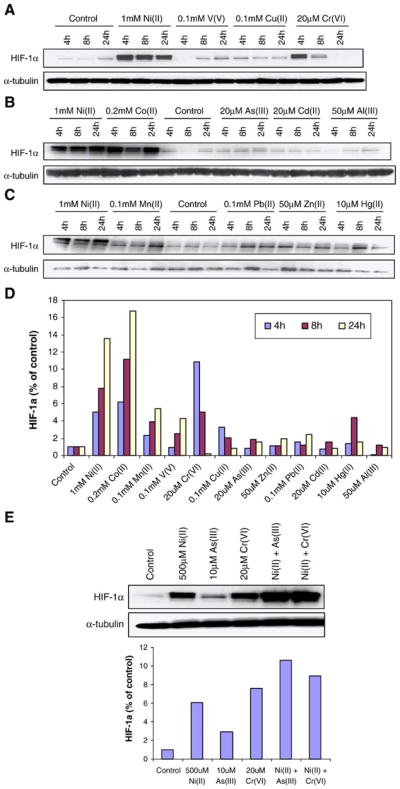
Effects of 12 metal ions on HIF-1α protein in A549 cells. A549 cells were treated with 12 metal ions (indicated respectively in panels A–C) for 4 h, 8 h, and 24 h. After treatment, cells were lysed and analyzed by 7.5% SDS-PAGE gel followed by Western blotting with HIF-1α or α-tubulin (to control for loading). In panel E, A549 cells were exposed for 4 h to no metal (lane 1), 500 μM Ni(II) (lane 2), 10 μM As(III) (lane 3), 20 μM Cr(VI) (lane 4), 500 μM Ni(II) + 10 μM As(III) (lane 5), and 500 μM Ni(II) + 20 μM Cr(VI) (lane 6). Forty micrograms of total protein was loaded on the gel. Similar data were obtained in at least 2 other independent experiments. Panel D is a quantitative summary of panels A–C, using densitometric analysis normalized to α-tubulin. In panel E, the upper panel is a representative blot while the lower panel is the densitometric analysis normalized to α-tubulin. Densitometric data are given as fold-control.
Effects of non-iron metals and iron co-exposure on HIF-1α protein in A549 cells
To investigate whether iron depletion contributed to HIF-1α induction, we studied the effects of metal ions and ferrous iron coexposure on HIF-1α protein levels in A549 cells after a 4 h treatment. As shown in Fig. 3, 0.5 mM Fe (II) decreased HIF-1α compared to control; moreover, the coexposure of 0.5 mM Fe(II) with 1 mM Ni(II), 0.2 mM Co (II), or 20 μM Cr(VI) reversed the induction of HIF-1α protein in A549 cells.
Fig. 3.
Iron coexposure reversed the induction of HIF-1α in A549 cells. In panel A, A549 cells were treated with no metal, 0.5 mM Fe(II), 1 mM Ni(II), 0.5 mM Fe(II) + 1 mM Ni(II). In panel B, A549 cells were treated with no metal, 0.5 mM Fe(II), 0.2 mM Co(II), 0.5 mM Fe(II) + 0.2 mM Co(II), 20 μM Cr(VI), and 0.5 mM Fe(II) + 20 μM Cr(VI). 4 h later, the cells were harvested, lysed, and analyzed by 7.5% SDS-PAGE gel followed by Western blotting with HIF-1α or α-tubulin antibody. Forty micrograms of total protein for each sample was loaded on the gel. Similar data were obtained in at least 2 other independent experiments. The upper panel is a representative blot while the lower panel is the densitometric analysis normalized to α-tubulin. Densitometric data are given as fold-control.
Ni(II), Co(II), and Mn(II) decrease ferritin level in A549 cells
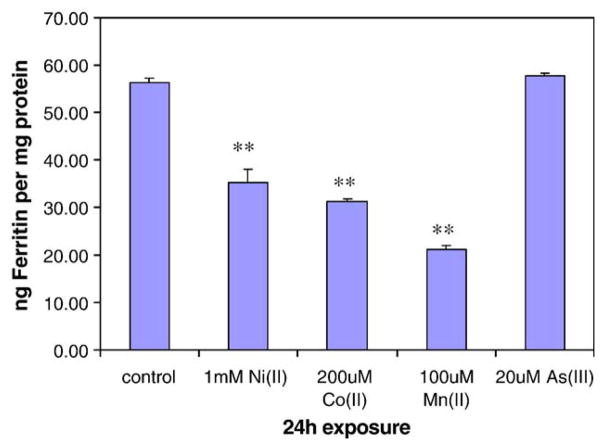
To confirm that IRP-1 and HIF-1α activation by Ni(II), Co(II), and Mn(II) was associated with depletion of intracellular iron, we determined the effects of metal ions on ferritin level in A549 cells after 24 h treatment. It is believed that the binding of active IRP-1 to 5′ IREs blocks the translation of ferritin mRNA. As expected, Fig. 4 showed that 1 mM Ni(II), 0.2 mM Co(II), and 0.1 mM Mn(II) significantly decrease the ferritin protein levels in A549 cells, while 20 μM As(III) did not significantly effect the amount of ferritin.
Fig. 4.
Ni(II), Co(II), and Mn(II) decreased ferritin protein levels in A549 cells. A549 cells were treated with no metal, 1 mM Ni(II), 200 μM Co(II) and 100 μM Mn(II) in 6-well plates. 24 h later, the cells were washed by 1 × PBS, lysed, and analyzed by ELISA. The total proteins were determined by using Coomassie Plus Reagent as described under Materials and methods. Data represent means (n = 3) ± SEM. **Significantly different from control (P < 0.01).
Effects of 12 metal ions on hypoxia-response element (HRE) reporter gene in A549 cells after 20 h treatment
We investigated whether the metals that stabilized HIF could also induce the HRE reporter gene by determining firefly luciferase activity in an HIF-dependent construct following 20 h of exposure. As shown in Fig. 5, only Ni(II), Co(II), and to lesser extents V(V) and Mn(II) activated HRE reporter gene in A549 cells after 20 h treatment. 0.2 mM Co(II) induced the reporter gene approximately 3.7-fold, while 1 mM Ni(II) 2.4-fold, 0.1 mM V(V) 1.9-fold, and 0.1 mM Mn(II) 1.9-fold. Other metal ions failed to induce HRE reporter gene, except Cu(II), which decreased the HRE luciferase activity compared to control. These results indicate that except for Ni, Co, V, and Mn ions, any increase in HIF-1α was inconsequential since it had no effect on its downstream targets in A549 cells.
Fig. 5.
Ni(II), Co(II), V(V), and Mn(II) activated HRE reporter gene in A549 cells. A549 cells transiently transfected with an HRE-containing luciferase reporter gene were treated with 12 metal ions for 20 h at the concentration indicated in the figure. Shown are mean values ± SEM of two independent experiments. Experiment was performed with at least 4 values and shown as mean values ± SEM.
Effects of 12 metal ions on a HIF-1α-regulated Cap43 protein in A549 cells
We next determined whether the metal ions that had consistent effects on HRE reporter gene activity could also induce HIF-1α-regulated Cap43 (NDRG1) protein. As shown in Fig. 6A, the induction of Cap43 protein by Ni(II) and Co(II) was dose-dependent which was consistent with the effects on HRE reporter gene. Fig. 6B shows that only Ni(II), Co(II), V (V), and Mn(II) significantly induced Cap43 protein in A549 cells after 24 h treatment, while the other metals had no observable effects. Again, these results reinforce the findings that only these metal ions stabilized HIF-1α sufficiently to cause the activate downstream effectors.
Fig. 6.
Ni(II), Co(II), V(V), and Mn(II) increased Cap43 protein in A549 cells. A549 cells were treated with 12 metal ions (indicated in panels A and B) for 24 h. After treatment, cells were lysed and the lysates were analyzed by 12.5% SDS-PAGE gel followed by Western blotting with Cap43 antibody and α-tubulin antibody for loading control. In panel A, A549 cells were exposed for 24 h to 0.1, 0.25, 0.5, and 1.0 mM Ni(II) (lanes 1–4), no metal (lane 5), or 0.05, 0.1, 0.2, and 0.3 mM Co(II) (lanes 6–9). Panel B shows the effects of all metal ions on Cap43 protein after 24 h exposure. Fifteen micrograms of total protein for each sample was loaded on the gel. Similar data were obtained in at least 2 independent experiments. Upper panel is a representative blot while the lower panel is the densitometric analysis normalized to α-tubulin. Densitometric data are given as fold-control.
Discussion
We have conducted numerous studies showing that nickel ions interfere with iron uptake and iron-dependent enzymes in A549 cells (Chen et al., 2005), and nickel ions mimic hypoxia to stabilize HIF-1α protein in A549 cells (Salnikow et al., 2003). The purpose of present study was to investigate the effects of other metal ions on both IRP-1 activity and HIF-1α protein, and to study whether the alteration of iron homeostasis as determined by IRP-1 activation and ferritin level by metal ions had downstream effects on the expression of HIF-1α and its regulated genes.
The choice of the exposure concentrations used for the treatment of A549 cells by the metal ions was based on previous cell survival data. Davidson et al. (2003) showed that after exposed to 1 mM NiCl2 and 400 μM CoCl2 for 24 h, A549 cells still had viability as high as (92.78 + 7.9)% and (87.66 + 12.55)%. Other metal ions, including Cr(VI), Cd(II), Cu(II), Fe(II), Zn(II), and Hg(II) were also based on previous studies showing which doses and time interval of exposure utilized were not toxic to A549 cells (Dubrovskaya and Wetterhahn, 1998; Walther et al., 2002; Riley et al., 2005). The remainder of the metal ion doses examined, V(V), Mn (II), As(III), and Al(III), were based upon non-toxic levels studied in other cell types (Oshiro et al., 1998; Zheng and Zhao, 2001; Gao et al., 2002a, 2004). The doses for these 12 metal ions used in the present study were previously shown to be biologically active in additional studies of A549 cells or other cell lines.
IRP-1 is an RNA-binding protein that regulates the expression of several mRNAs in response to the availability of cellular iron (Constable et al., 1992). In this study, we found that 6 of the 12 metals that were studied increased IRP-1 binding activity. There are at least two potential sites for these metals to compete for iron, the membrane iron carrier proteins and iron binding sites in c-aconiotase/IRP-1. In our previous study, we found that Ni(II) decreased the activity of cytosolic aconitase and converted it to an IRP-1 binding form by decreasing cellular iron level. It was shown that Ni(II) depleted cellular iron by competing with it for DMT-1 Fe transporter in A549 cells (Chen et al., 2005). Since DMT-1 can transport a variety of divalent metal ions such as Mn ions. Metals that are not divalent in charge (V and Cr) can also compete with iron for the membrane carrier proteins in human bronchial epithelial cells (Wang et al., 2002), and thus it would be expected that exposure to these metal ions would have similar effects on cellular iron levels as Ni(II). Here, we show that, in addition to Ni(II), only Co(II), Mn(II), V(V), Cu(II), and As(III) activated IRP-1, while all other metal ions were without effect. Our results were consistent with previous reports that 200 μM Mn (II) exposure increased IRP binding activity in PC12 cells (Kwik-Uribe et al., 2003). Martelli and his colleagues reported that the loss of IRP-IRE binding activity could due to the reversible and specific aggregation of the IRP1 apoprotein with Zn(II) and Cd(II), while precipitation did not occur with Co(II) and Mn(II) (Martelli and Moulis, 2004), which may explain why some of these metal ions did not activate IRP-1 and may suggest a new mechanism for the biological toxicity of these metals.
Our previous study showed that nickel treatment decreased total cellular iron level in A549 cells (Chen et al., 2005), which likely contributed to the increase of IRP activity. This mechanism is also supported by our ferritin data. Those metal ions, Ni(II), Co(II), and Mn(II), which significantly increased IRP-1 activity, consistently decreased ferritin levels in A549 cells following 24 h exposure. It is of interest that these metal ions are poor inducers of metallothionein and do not induce ferritin since both of these proteins can sequester toxic metal ions and prevent their toxicity, while other metal ions such as Cu, Cd, and Hg are good inducers of metallothionein and either increase or do not decrease ferritin (Joshi and Zimmerman, 1988; Sato and Kondoh, 2002; Araya et al., 2003; Henkel and Krebs, 2004; Beattie et al., 2005; Jaeckel et al., 2005). Cu, Cd, and Hg for example had little effect on hypoxia signaling probably because they are not readily available due to their binding to metallothionein and ferritin.
Another potential iron competing site available to these metals is the [Fe–S] cluster in c-aconitase/IRP-1. It has been reported by Oshiro et al. (2002) that various metal ions can decrease the binding affinity of IRP-1 to IRE probably by forming a non-functional [(1 non-Fe metal + 3Fe) – 4S] IRP-1 complex. However, since they uniformly treated cells with a 150 μM dose, this concentration may be too low for some metals (e.g., Ni ions) to increase the IRP-1 binding activity, but 150 μM may be too high for other more toxic metal ions (e.g., cadmium, copper, and mercury). Because only an apoprotein (no [Fe–S] cluster) form of IRP-1 possesses IRE binding ability, these metals may also destabilize the [Fe–S] cluster in c-aconitase or prevent the formation of its [Fe–S] cluster. However, little is known about the process of [Fe–S] cluster formation in c-aconitase and its proper protein folding in mammalian cells. This possible mechanism must await further investigation. Besides cellular iron level, IRP-1 binding activity can also be modified by cell signaling via reactive oxygen species and protein kinase C.
Hypoxia-inducible factor-1 (HIF-1) is a master regulator of oxygen homeostasis, playing important roles in physiological and pathological processes (Wenger, 2002). Several Fe(II)-dependent enzymes, such as the HIF-prolyl hydroxylase (PHD 1–3) and asparagine hydroxylase (FIH-1), regulate HIF-1α stability and activation. Numerous studies have shown that HIF-1α is not only regulated by oxygen tension, but also by various other stimuli, such as transition metals, which can mimic hypoxia to stabilize HIF-1α protein in cultured cells (Horiguchi et al., 1996; Oshiro et al., 1998; Chun et al., 2000; Hossain et al., 2000; Gao et al., 2002b, 2004; Yuan et al., 2003; Hwang et al., 2004; van Heerden et al., 2004; Zhao et al., 2004). Since Fe(II) is an important cofactor for these hydroxylases and loosely bound to two histidine sites in these enzymes, non-iron metal ions may decrease the activity of these enzymes by either decreasing cellular iron level or directly replacing iron in the enzymes that hydroxylate HIF. Consistent with our finding in EMSA assay, Ni(II), Co(II), Mn (II), and V(V) induced HIF-1α protein and its transcriptional activity, as shown by HRE-driven luciferase reporter activity and the expression of Cap43. Exogenous addition of Fe(II) to cells also exposed to Ni(II) and Co(II) attenuated the induction of HIF-1α, which suggested that the stabilization of HIF-1α by these metal ions was due in part to a competition with iron. Cr(VI) at highly toxic doses induced HIF-1α at a 4 h time point, but when cells were continuously exposed to Cr(VI), HIF-1α became undetectable at later time intervals (24 h). There were no downstream HIF-1α-dependent effects following chromate exposure. Gao et al. (2002b) have shown that Cr(VI) induced HIF-1α through p38 signaling pathway in DU145 human prostate carcinoma cells. However, we found that Fe(II) can attenuate the induction by Cr (VI), suggesting that some Fe depletion may also be involved in HIF-1α induction by Cr(VI) but in either case it is concluded that Cr(VI) is at best a weak inducer of HIF-1α without detectable HIF-1α-dependent downstream effects in A549 cells. It is of interest and importance that metal ions may have different effects in various types of cultured cells since as discussed above Cr (VI) activated HIF-1α in DU145 human prostate cells but was without much effect in A549 cells. However, it is important to study human lung cells since humans are most often exposed to these metal ions by inhalation.
Some metal ions are known to induce oxidative stress, such as Ni(II), Co(II), and Cr(VI). Cr(VI) was reported to induce expression of HIF-1α protein through the production of reactive oxygen species in DU145 cells (Gao et al., 2002b), but our data showed that Cr(VI) did not affect the expression of HIF-1α protein downstream gene Cap43, HRE reporter gene, IRP-1 activity, or ferritin amount in A549 cells. Moreover, Salnikow et al. (2000) reported that ROS are produced during the exposure of cells to metals that mimic hypoxia (e.g., Co ions), but the formation of ROS was not involved in the activation of HIF-1-dependent genes. So, we believe that oxidative stress is not an issue in the activation of IRP-1 and HIF-1α protein by Ni and Co ions in A549 cells.
It should be noted that different agents may induce HIF-1α through different pathway. Ni(II), V(V), and As(III) were found to induce HIF-1α transactivation through PI3K/Akt-dependent pathway (Gao et al., 2002a, 2004; Li et al., 2004), while Cr(VI) was reported to induce expression of HIF-1α protein through p38 signaling pathway (Gao et al., 2002b). However, as shown in Figs. 2D–E, Ni(II) induced much higher HIF-1α protein level than V(V) and As(III) at different time points; moreover, As(III) did not activate HRE reporter gene or induce Cap43 expression, while Ni(II) and V(V) were effective. Additional studies are required to understand the entire mechanisms responsible for activation of hypopxia signaling in various cell types.
Other metal ions, including Cu(II), As(III), Pb(II), and Zn(II), were found to only marginally increase HIF-1α protein, but did not increase HRE-driven luciferase reporter activity and expression of Cap43. However, Martin et al. (2005) reported that Cu(II) dramatically induced HIF-1α in Hep3B human hepatoma cells. A difference in the cell lines utilized in the two studies most likely accounted for the discrepancy in these findings, as shown by the same authors, that reported on the induction of HIF-1α in HeLa cell at considerably higher concentration of Cu(II) compared to the Hep3B cells. In our study, doses higher than 0.1 mM for Cu(II) caused cytotoxicity. Besides the competition with iron, other mechanism may also be involved in HIF-1α stabilization by these metals. For example, it has been reported that As(III) induced HIF-1α through mTOR-dependent pathway in DU145 cells (Skinner et al., 2004). These diverse mechanisms of HIF-1α induction can be operative when several metals were combined, which is a common experience in the real world at superfund sites and in occupational exposure situations (Dayal et al., 1995; Diagomanolin et al., 2004; Roff et al., 2004). As shown in this study, there was synergistic effect on HIF-1α protein level when cells were exposed to the combinations of either [Ni(II) + As(III)] or [Ni(II) + Cr(VI)].
So far, the data presented in this article support that the activation of IRP-1 by Ni(II), Co(II), V(V), and Mn(II) may be caused by cellular iron depletion, by competing with iron-dependent enzymes or DMT-1 iron transporter or both; Ni ions are able to prevent the degradation of HIF-1α protein by von Hippel Lindau (VHL) ubiquitin ligase by their inhibition of prolyl hydroxylases which target HIF-1α for degradation (Ivan et al., 2001; Jaakkola et al., 2001; Costa et al., 2005). Additionally, Ni ions induce HIF-1α protein by permitting its interaction with P300 by inhibiting the asparagines hydroxylase activity of FIH (Zhao et al., 2004).
In conclusions, metals which activate IRP probably by depleting iron in A549 cells, include Ni(II), Co(II), V(V), Mn (II), and with some marginal effects of As(III) and Cu(II). These metal ions can also stabilize HIF-1α, but only Ni(II), Co(II), V(V), and Mn(II) activated HRE reporter gene and induced Cap43 protein. Coexposure of ferrous iron with these metals can reverse the induction of HIF-1α in A549 cells, indicating that Fe depletion is responsible for some of the HIF-1α stabilization. In conclusion, HIF-1α is stabilized and activates transcription of HIF-1α-dependent genes only when A549 cells are exposed to Ni(II), Co(II), V(V), and Mn(II). These cells are human lung cells and people are most often exposed to all of these metal ions by inhalation making it important to understand effects of these metal ions in human lung cells. As discussed above, Ni and Co are known to not only deplete Fe but are also inhibitors of Proline hydroxylases that target HIF-1α for degredation.
Acknowledgments
The authors would like to thank Ping Zhang and Jishen Dai for assistance with the methodology employed in the manuscript and Dr. Salnikow for providing us with the HIF-1α reporter plasmid used in this study. This work was supported by grant numbers ES00260, ES10344, and T32-ES07324 from the National Institute of Environmental Health Sciences, and CA16087 from the National Cancer Institute.
References
- Araya M, Olivares M, Pizarro F, Gonzalez M, Speisky H, Uauy R. Copper exposure and potential biomarkers of copper metabolism. Biometals. 2003;16:199–204. doi: 10.1023/a:1020723117584. [DOI] [PubMed] [Google Scholar]
- Beattie JH, Owen HL, Wallace SM, Arthur JR, Kwun IS, Hawksworth GM, Wallace HM. Metallothionein overexpression and resistance to toxic stress. Toxicol Lett. 2005;157:69–78. doi: 10.1016/j.toxlet.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Chen H, Davidson TL, Singleton S, Garrick MD, Costa M. Nickel decreases cellular iron level and converts cytosolic aconitase to iron-regulatory protein 1 in A549 cells. Toxicol Appl. 2005:275–287. doi: 10.1016/j.taap.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Chun YS, Choi E, Kim GT, Lee MJ, Lee MJ, Lee SE, Kim MS, Park JW. Zinc induces the accumulation of hypoxia-inducible factor (HIF)-1alpha, but inhibits the nuclear translocation of HIF-1beta, causing HIF-1 inactivation. Biochem Biophys Res Commun. 2000;268:652–656. doi: 10.1006/bbrc.2000.2180. [DOI] [PubMed] [Google Scholar]
- Constable A, Quick S, Gray NK, Hentze MW. Modulation of the RNA-binding activity of a regulatory protein by iron in vitro: switching between enzymatic and genetic function? Proc Natl Acad Sci USA. 1992;89:4554–4558. doi: 10.1073/pnas.89.10.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Davidson TL, Chen H, Ke Q, Zhang P, Yan Y, Huang C, Kluz T. Nickel carcinogenesis: epigenetics and hypoxia signaling. Mutat Res. 2005;592:79–88. doi: 10.1016/j.mrfmmm.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Davidson T, Salnikow K, Costa M. Hypoxia inducible factor-1 alpha-independent suppression of aryl hydrocarbon receptor-regulated genes by nickel. Mol Pharmacol. 2003;64:1485–1493. doi: 10.1124/mol.64.6.1485. [DOI] [PubMed] [Google Scholar]
- Dayal H, Gupta S, Trieff N, Maierson D, Reich D. Symptom clusters in a community with chronic exposure to chemicals in two superfund sites. Arch Environ Health. 1995;50:108–111. doi: 10.1080/00039896.1995.9940887. [DOI] [PubMed] [Google Scholar]
- Diagomanolin V, Farhang M, Ghazi-Khansari M, Jafarzadeh N. Heavy metals (Ni, Cr, Cu) in the Karoon waterway river, Iran. Toxicol Lett. 2004;151:63–68. doi: 10.1016/j.toxlet.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Dubrovskaya VA, Wetterhahn KE. Effects of Cr(VI) on the expression of the oxidative stress genes in human lung cells. Carcinogenesis. 1998;19:1401–1407. doi: 10.1093/carcin/19.8.1401. [DOI] [PubMed] [Google Scholar]
- Fang R, Aust AE. Induction of ferritin synthesis in human lung epithelial cells treated with crocidolite asbestos. Arch Biochem Biophys. 1997;340:369–375. doi: 10.1006/abbi.1997.9892. [DOI] [PubMed] [Google Scholar]
- Gao N, Ding M, Zheng JZ, Zhang Z, Leonard SS, Liu KJ, Shi X, Jiang BH. Vanadate-induced expression of hypoxia-inducible factor 1 alpha and vascular endothelial growth factor through phosphatidylinositol 3-kinase/Akt pathway and reactive oxygen species. J Biol Chem. 2002a;277:31963–31971. doi: 10.1074/jbc.M200082200. [DOI] [PubMed] [Google Scholar]
- Gao N, Jiang BH, Leonard SS, Corum L, Zhang Z, Roberts JR, Antonini J, Zheng JZ, Flynn DC, Castranova V, Shi X. p38 signaling-mediated hypoxia-inducible factor 1alpha and vascular endothelial growth factor induction by Cr(VI) in DU145 human prostate carcinoma cells. J Biol Chem. 2002b;277:45041–45048. doi: 10.1074/jbc.M202775200. [DOI] [PubMed] [Google Scholar]
- Gao N, Shen L, Zhang Z, Leonard SS, He H, Zhang XG, Shi X, Jiang BH. Arsenite induces HIF-1alpha and VEGF through PI3K, Akt and reactive oxygen species in DU145 human prostate carcinoma cells. Mol Cell Biochem. 2004;255:33–45. doi: 10.1023/b:mcbi.0000007259.65742.16. [DOI] [PubMed] [Google Scholar]
- Henkel G, Krebs B. Metallothioneins: zinc, cadmium, mercury, and copper thiolates and selenolates mimicking protein active site features-structural aspects and biological implications. Chem Rev. 2004;104:801–824. doi: 10.1021/cr020620d. [DOI] [PubMed] [Google Scholar]
- Hirsila M, Koivunen P, Xu L, Seeley T, Kivirikko KI, Myllyharju J. Effect of desferrioxamine and metals on the hydroxylases in the oxygen sensing pathway. FASEB J. 2005;19:1308–1310. doi: 10.1096/fj.04-3399fje. [DOI] [PubMed] [Google Scholar]
- Horiguchi H, Sato M, Konno N, Fukushima M. Long-term cadmium exposure induces anemia in rats through hypoinduction of erythropoietin in the kidneys. Arch Toxicol. 1996;71:11–19. doi: 10.1007/s002040050352. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Bouton CM, Pevsner J, Laterra J. Induction of vascular endothelial growth factor in human astrocytes by lead. Involvement of a protein kinase C/activator protein-1 complex-dependent and hypoxia-inducible factor 1-independent signaling pathway. J Biol Chem. 2000;275:27874–27882. doi: 10.1074/jbc.M002185200. [DOI] [PubMed] [Google Scholar]
- Hwang JT, Lee M, Jung SN, Lee HJ, Kang I, Kim SS, Ha J. AMP-activated protein kinase activity is required for vanadate-induced hypoxia-inducible factor 1alpha expression in DU145 cells. Carcinogenesis. 2004;25:2497–2507. doi: 10.1093/carcin/bgh253. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Jaeckel P, Krauss G, Menge S, Schierhorn A, Rucknagel P, Krauss GJ. Cadmium induces a novel metallothionein and phytochelatin 2 in an aquatic fungus. Biochem Biophys Res Commun. 2005;333:150–155. doi: 10.1016/j.bbrc.2005.05.083. [DOI] [PubMed] [Google Scholar]
- Joshi JG, Zimmerman A. Ferritin: an expanded role in metabolic regulation. Toxicology. 1988;48:21–29. doi: 10.1016/0300-483x(88)90055-8. [DOI] [PubMed] [Google Scholar]
- Kwik-Uribe CL, Reaney S, Zhu Z, Smith D. Alterations in cellular IRP-dependent iron regulation by in vitro manganese exposure in undifferentiated PC12 cells. Brain Res. 2003;973:1–15. doi: 10.1016/s0006-8993(03)02457-0. [DOI] [PubMed] [Google Scholar]
- Li J, Davidson G, Huang Y, Jiang BH, Shi X, Costa M, Huang C. Nickel compounds act through phosphatidylinositol-3-kinase/Akt-dependent, p70(S6k)-independent pathway to induce hypoxia inducible factor transactivation and Cap43 expression in mouse epidermal Cl41 cells. Cancer Res. 2004;64:94–101. doi: 10.1158/0008-5472.can-03-0737. [DOI] [PubMed] [Google Scholar]
- Martelli A, Moulis JM. Zinc and cadmium specifically interfere with RNA-binding activity of human iron regulatory protein 1. J Inorg Biochem. 2004;98:1413–1420. doi: 10.1016/j.jinorgbio.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Martin F, Linden T, Katschinski DM, Oehme F, Flamme I, Mukhopadhyay CK, Eckhardt K, Troger J, Barth S, Camenisch G, Wenger RH. Copper-dependent activation of hypoxia-inducible factor (HIF)-1: implications for ceruloplasmin regulation. Blood. 2005;105:4613–4619. doi: 10.1182/blood-2004-10-3980. [DOI] [PubMed] [Google Scholar]
- Morgan EH, Oates PS. Mechanisms and regulation of intestinal iron absorption. Blood Cells Mol Diseases. 2002;29:384–399. doi: 10.1006/bcmd.2002.0578. [DOI] [PubMed] [Google Scholar]
- Oshiro S, Kawahara M, Mika S, Muramoto K, Kobayashi K, Ishige R, Nozawa K, Hori M, Yung C, Kitajima S, Kuroda Y. Aluminum taken up by transferrin-independent iron uptake affects the iron metabolism in rat cortical cells. J Biochem (Tokyo) 1998;123:42–46. doi: 10.1093/oxfordjournals.jbchem.a021914. [DOI] [PubMed] [Google Scholar]
- Oshiro S, Nozawa K, Hori M, Zhang C, Hashimoto Y, Kitajima S, Kawamura K. Modulation of iron regulatory protein-1 by various metals. Biochem Biophys Res Commun. 2002;290:213–218. doi: 10.1006/bbrc.2001.6182. [DOI] [PubMed] [Google Scholar]
- Piquemal D, Joulia D, Balaguer P, Basset A, Marti J, Commes T. Differential expression of the RTP/Drg1/Ndr1 gene product in proliferating and growth arrested cells. Biochim Biophys Acta. 1999;1450:364–373. doi: 10.1016/s0167-4889(99)00056-7. [DOI] [PubMed] [Google Scholar]
- Riley MR, Boesewetter DE, Turner RA, Kim AM, Collier JM, Hamilton A. Comparison of the sensitivity of three lung derived cell lines to metals from combustion derived particulate matter. Toxicol In Vitro. 2005;19:411–419. doi: 10.1016/j.tiv.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Roff M, Bagon DA, Chambers H, Dilworth EM, Warren N. Dermal exposure to electroplating fluids and metalworking fluids in the UK. Ann Occup Hyg. 2004;48:209–217. doi: 10.1093/annhyg/meh029. [DOI] [PubMed] [Google Scholar]
- Rolfs A, Kvietikova I, Gassmann M, Wenger RH. Oxygen-regulated transferrin expression is mediated by hypoxia-inducible factor-1. J Biol Chem. 1997;272:20055–20062. doi: 10.1074/jbc.272.32.20055. [DOI] [PubMed] [Google Scholar]
- Salnikow K, Su W, Blagosklonny MV, Costa M. Carcinogenic metals induce hypoxia-inducible factor-stimulated transcription by reactive oxygen species-independent mechanism. Cancer Res. 2000;60:3375–3378. [PubMed] [Google Scholar]
- Salnikow K, Davidson T, Zhang Q, Chen LC, Su W, Costa M. The involvement of hypoxia-inducible transcription factor-1-dependent pathway in nickel carcinogenesis. Cancer Res. 2003;63:3524–3530. [PubMed] [Google Scholar]
- Sato M, Kondoh M. Recent studies on metallothionein: protection against toxicity of heavy metals and oxygen free radicals. Tohoku J Exp Med. 2002;196:9–22. doi: 10.1620/tjem.196.9. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- Skinner HD, Zhong XS, Gao N, Shi X, Jiang BH. Arsenite induces p70S6K1 activation and HIF-1alpha expression in prostate cancer cells. Mol Cell Biochem. 2004;255:19–23. doi: 10.1023/b:mcbi.0000007257.67733.3b. [DOI] [PubMed] [Google Scholar]
- Templeton DM, Liu Y. Genetic regulation of cell function in response to iron overload or chelation. Biochim Biophys Acta. 2003;1619:113–124. doi: 10.1016/s0304-4165(02)00497-x. [DOI] [PubMed] [Google Scholar]
- van Heerden D, Vosloo A, Nikinmaa M. Effects of short-term copper exposure on gill structure, metallothionein and hypoxia-inducible factor-1alpha (HIF-1alpha) levels in rainbow trout (Oncorhynchus mykiss) Aquat Toxicol. 2004;69:271–280. doi: 10.1016/j.aquatox.2004.06.002. [DOI] [PubMed] [Google Scholar]
- van Vliet AH, Ketley JM, Park SF, Penn CW. The role of iron in Campylobacter gene regulation, metabolism and oxidative stress defense. FEMS Microbiol Rev. 2002;26:173–186. doi: 10.1016/s0168-6445(02)00095-5. [DOI] [PubMed] [Google Scholar]
- Walther UI, Walther SC, Liebl B, Reichl FX, Kehe K, Nilius M, Hickel R. Cytotoxicity of ingredients of various dental materials and related compounds in L2- and A549 cells. J Biomed Mater Res. 2002;63:643–649. doi: 10.1002/jbm.10384. [DOI] [PubMed] [Google Scholar]
- Wang GL, Semenza GL. Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction. Blood. 1993;82:3610–3615. [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix–loop–helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ghio AJ, Yang F, Dolan KG, Garrick MD, Piantadosi CA. Iron uptake and Nramp2/DMT1/DCT1 in human bronchial epithelial cells. Am J Physiol: Lung Cell Mol Physiol. 2002;282:L987–L995. doi: 10.1152/ajplung.00253.2001. [DOI] [PubMed] [Google Scholar]
- Ward RJ, Zhang Y, Crichton RR. Aluminium toxicity and iron homeostasis. J Inorg Biochem. 2001;87:9–14. doi: 10.1016/s0162-0134(01)00308-7. [DOI] [PubMed] [Google Scholar]
- Wenger RH. Cellular adaptation to hypoxia: O2-sensing protein hydroxylases, hypoxia-inducible transcription factors, and O2-regulated gene expression. FASEB J. 2002;16:1151–1162. doi: 10.1096/fj.01-0944rev. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Hilliard G, Ferguson T, Millhorn DE. Cobalt inhibits the interaction between hypoxia-inducible factor-alpha and von Hippel–Lindau protein by direct binding to hypoxia-inducible factor-alpha. J Biol Chem. 2003;278:15911–15916. doi: 10.1074/jbc.M300463200. [DOI] [PubMed] [Google Scholar]
- Zhao J, Yan Y, Salnikow K, Kluz T, Costa M. Nickel-induced down-regulation of serpin by hypoxic signaling. Toxicol Appl Pharmacol. 2004;194:60–68. doi: 10.1016/j.taap.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Zheng W, Zhao Q. Iron overload following manganese exposure in cultured neuronal, but not neuroglial cells. Brain Res. 2001;897:175–179. doi: 10.1016/s0006-8993(01)02049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhao Q, Slavkovich V, Aschner M, Graziano JH. Alteration of iron homeostasis following chronic exposure to manganese in rats. Brain Res. 1999;833:125–132. doi: 10.1016/s0006-8993(99)01558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]