Abstract
Triptolide is a biologically active component purified from Chinese herbal plant Tripterygium wilfordii Hook F. It is widely used in East Asia for treatment of systemic lupus erythematosus, rheumatoid arthritis, nephritis, Bechect’s disease, psoriasis, atopic dermatitis, and asthma. However, its immunological mechanisms are poorly understood. IL-12 and IL-23 are closely related heterodimeric cytokines that share the common subunit p40. They are produced by APCs and are key factors in the generation and effector functions of Th1 and Th17 cells, respectively. They have been strongly implicated in the pathogenesis of several autoimmune disorders. In this study, we investigated the molecular mechanism whereby triptolide inhibits the expression of the p40 gene in APCs. We demonstrate that triptolide does so at the transcriptional level in part through targeting CCAAT/enhancer-binding protein-α (C/EBPα), which directly interacts with the p40 promoter and inhibits its transcription in inflammatory macrophages. Triptolide can activate the transcription of C/EBPα, and phosphorylation of Ser21 and Thr222/226 critical for C/EBPα inhibition of p40. Further, activation of C/EBPα by triptolide is dependent on upstream kinases ERK1/2 and Akt-GSK3β. This study provides mechanistic insights into the immunomodulatory capacity of triptolide and has strong implications for its therapeutic applications in autoimmune diseases.
Triptolide, a diterpene triepoxide, is a biologically active component purified from the Chinese herbal plant Tripterygium wilfordii Hook F (TWHF). The therapeutic use of TWHF in China as a natural medicine can be dated back several centuries ago (1). Currently, it is used for treatment of systemic lupus erythematosus, rheumatoid arthritis, nephritis, Bechect’s disease, psoriasis, atopic dermatitis, asthma, and very recently, in the prevention of transplant rejection (2–8). However, until recently, the mechanisms of the antineoplastic and anti-inflammatory effects of triptolide have not been given sufficient attention. The immunosuppressive effect of triptolide on T cells has been somewhat characterized. It inhibits T cell activation and cytokine gene transcription in T cells (9), and suppresses the expression of genes for transcription factors, signal transduction pathway regulators, DNA binding protein, and MAPK in Jurkat cells (10). In addition, triptolide was reported to inhibit lymphocyte activation and T cell expression of IL-2 at the level of transcription by inhibiting NF-κB transcriptional activation (11). However, little is known about the effect of triptolide on accessory cells, particularly the professional APCs, such as dendritic cells (DCs) and macrophages. It has been suggested that DCs are a primary target of the immunosuppressive activity of triptolide. At high concentrations (≥20 ng/ml) triptolide induces apoptosis of DCs through sequential p38 MAP kinase phosphorylation and caspase 3 activation (12). It has also been shown that triptolide inhibits DC-mediated chemoattraction of neutrophils and T cells through inhibiting Stat3 phosphorylation and NF-κB activation (13). Zhu et al. showed that triptolide prevented the differentiation of immature monocyte-derived DC (MoDC) by inhibiting CD1a, CD40, CD80, CD86, and HLA-DR expression, and by reducing the capacity of MoDC to stimulate lymphocyte proliferation in allogeneic MLR. However, expression of surface CD14 and phagocytic capacity of MoDC was enhanced by triptolide (14). Therefore, the suppression of DC differentiation, maturation, and function of immature DCs by triptolide may explain some of its immunosuppressive properties.
IL-12 is a heterodimeric cytokine composed of the p40 and p35 chains. It is a pivotally important cytokine produced by APCs in host defense against intracellular pathogens and malignancies (15). IL-12 and its close relative IL-23, which shares the p40 chain with IL-12 plus its own unique chain p19, have been strongly implicated in the pathogenesis of several types of autoimmune disorders, such as systemic lupus erythematosus, rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, psoriasis, and atopic asthma (10, 16–18). It has been previously demonstrated by several groups that triptolide could inhibit IL-12 expression (8, 19, 20), in DCs (19) and human monocytic cell line (THP-1) (20).
A recent study demonstrated that in (NZB × NZW)F1 mice, which develop spontaneous lupus nephritis (LN) by 28 wk, proteinuria and BUN levels were significantly reduced after treatment with either triptolide or tripdiolide as compared with those treated with vehicle. There was no hypoalbuminemia or apparent evidence of LN in mice treated with either of the two diterpenoids. At 44 wk of age, the survival rate in mice treated with vehicle was markedly lower than that in mice treated with either triptolide or tripdiolide. The mean level of anti-dsDNA Ab in mice treated with tripdiolide was lower than that in the vehicle-treated mice on completion of the treatment course. Production of TNF, IL-6, and MCP-1 by spleen cells was also decreased after diterpenoid therapy (2). These results support the conclusions that the therapeutic value of extracts of TWHF in LN can be accounted for by the triptolide and tripdiolide contents, and that cooperation between components is not required for therapeutic benefit, and that either diterpenoid could be effective therapy for LN.
Given the use of triptolide as an effective alternative therapeutic agent for autoimmune and inflammatory diseases in which APC-derived IL-12 and IL-23 play strong roles, we investigated the molecular mechanism whereby triptolide regulates expression of the IL-12/IL-23p40 gene in inflammatory macrophages and MoDC.
Materials and Methods
Cells
Human PBMCs were obtained from whole blood from disease-free volunteers by density gradient centrifugation with Ficoll (Invitrogen, Carlsbad, CA). CD14+ monocytes were purified from fresh PBMCs with anti-CD14 magnetic beads (Miltenyi Biotec, Auburn, CA), as recommended by the manufacturer. Isolated monocytes were allowed to adhere to plastic by plating 106 cells per/ml in RPMI 1640 medium for 2 h. Adherent monocytes were washed with RPMI 1640 medium and cultured for 6 d at 106 cells/ml in DC culture medium supplemented with 50 ng/ml of rGM-CSF (R&D Systems, Minneapolis, MN) and 1000 U/ml of rIL-4 (R&D Systems). The murine macrophage-like cell line, RAW264.7, was obtained from American Type Culture Collection (Manassas, VA) and maintained in RPMI 1640 containing 10% FCS, 2 mM L-glutamine, and penicillin/streptomycin. Peritoneal macrophages were isolated from C57BL/6J mice. Briefly, mice were injected i.p. with 2 ml 4% thioglycollate. After 3 d, peritoneal exudate cells were isolated by washing the peritoneal cavity with ice-cold HBSS. Cells were incubated for 2 h, and adherent cells were used as peritoneal macrophages.
Mice
CCAAT/enhancer-binding protein-β deficient (C/EBPβ−/−) mice were obtained for this study by breeding heterozygous female mice with a targeted deletion in the gene of C/EBPβ (C/EBPβ+/−) with heterozygous male mice. The generation of the C/EBPβ−/− mice and their genetic background was described previously by Screpanti et al. (21). C/EBPα conditional knockout (KO) mice are homozygous for C/EBPα floxed allele and hemizygous for the Mx1-cre transgene. Mx1-Cre transgene expression can be induced by administration of polyinosinic-polycytidylic acid [poly (I:C)], leading to deletion of the floxed gene. For induction of Cre expression, 4–7-wk-old mice were injected i.p. with 250 μg poly(I:C) (Sigma-Aldrich, St. Louis, MO) every other day for three doses. All mice were housed in microisolator cages, were monitored daily for evidence of disease, and were sacrificed when moribund.
Reagents and plasmids
Recombinant human and murine IFN-γ were purchased from Genzyme (Boston, MA). Recombinant human M-CSF was purchased from PeproTech (Rocky Hill, NJ). LPS from Escherichia coli 0127:B8 was from Sigma-Aldrich. Triptolide (PG490) was from Biomol (Plymouth Meeting, PA). MAPK inhibitors SB 202190, PD 98059, and JNK inhibitor II were from Calbiochem (Madison, WI). Poly(I:C) was from InvivoGen (San Diego, CA). LiCl was from Sigma-Aldrich (Milwaukee, WI). Abs for C/EBPα, C/EBPβ, C/EBPδ, and MKP-1 were from Santa Cruz Biotechnologies (Santa Cruz, CA). Abs for phosphor-C/EBPα (Ser21), phosphor-C/EBPα (Thr222/226), PTEN, PP2A C subunit, phosphor-ERK1/2, ERK1/2, phosphor-p38, p38, phosphor-JNK, JNK, phosphor-MEK1/2, MEK1/2, phosphor-Akt, Akt, phosphor-GSK3β, and GSK3β were from Cell Signaling (Danvers, MA).
All deletions and mutations of human IL-12p40 promoter-luciferase constructs have been described previously (22, 23). The wild-type (WT) C/EBPα expression vector and mutant C/EBPα expression vectors (S21A, T222A/T226A, S192A, and S248A) were generously provided by Dr. MacDougald (University of Michigan Medical School, Ann Arbor, MI). The dominant negative mutant of C/EBPα (3heptad-F) was generously provided by Dr. C. Vinson (National Cancer Institute, National Institutes of Health, Bethesda, MD). The murine C/EBPα promoter construct was generated by PCR (sense primer: 5′-TACGAGCTCCTATCGCTCTGGCCTGGAGAC-3′; antisense primer: 5′-GTCAAGCTTCTCTGGAGGTGACTGCTCATC-3′) from mouse genome. The fragment was inserted into pGL3-basic vector. The construct was confirmed by sequence verified. All plasmids were purified using the Qiagen Endotoxin-free kit (Qiagen, Valencia, CA).
Cytokine assays
Cytokine secretion was measured by ELISA, using appropriately diluted culture supernatants. Human IL-12p40 and p70, and mouse IL-12p40, p70 were measured by the respective ELISA kits from BD Pharmingen (San Diego, CA), mouse IL-23 was measured by an ELISA kit from Biolegend (San Diego, CA), in triplicates. All experiments were performed three times independently.
Quantitative real-time PCR
Total RNAs were isolated using the RNeasy mini kit (Qiagen, Hilden, Germany) and reverse-transcribed into cDNA. Real-time PCR was performed with an ABI 7400 System using the SYBR GREEN PCR kit. We select GAPDH as the endogenous control for the real-time PCR relative quantification analysis. The following primers were used for PCR amplification: 1) mouse IL-12p40, upper: 5′-GGAAGCACGGCAGCAGAATAAAT-3′, lower: 5′-AACTTGAGGGAGAAGTAGGAATGG-3′; 2) mouse IL-12p35, upper: 5′-CCCTTGCCCTCCTAAACCAC-3′, lower: 5′-TAGTAGCCAGGCAACTCTCG-3′; 3) immature mouse IL-12p40, upper: 5′-GACACGCCTGAAGAAGATGA-3′, lower: 5′-TTGTGGAGCAGCAGATGTGA-3′; 4) immature mouse IL-12p35, upper: 5′-TAGCCGCTCCTCACTCCTCT-3′, lower: 5′-TGACTGAAGCCTGCGATGAC-3′; 5) mouse C/EBPα, upper: 5′-AGCCAAGAAGTCGGTGGACAAGAA-3′, lower: 5′-GCGGTCATTGTCACTGGTCAACTC-3′; 6) mouse C/EBPβ, upper: 5′-GAAGTGGCCAACTTCTACTACGAG-3′, lower: 5′-AGAGGTCGGAGAGGAAGTCGTGGT-3′; and 7) mouse C/EBPδ, upper: 5′-GCCATGTACGACGACGAGAG-3′, lower: 5′-GT-TGAAGAGGTCGGCGAAGA-3′. PCR cycling conditions were as follows: initial incubation step of 2 min at 50°C, reverse transcription of 60 min at 60°C and 94°C for 2 min, followed by 40 cycles of 15 s at 95°C for denaturation and 2 min at 62°C for annealing and extension. All experiments were performed three times independently.
Transfections
Transient transfections were performed by electroporation as previously described (22). Transfection efficiency was routinely monitored by β-galactosidase assay by cotransfection with 3 μg pCMV–β-galactosidase plasmid. Variability between samples was typically <10%. Lysates were used for both luciferase and β-galactosidase assays. All experiments were performed three times independently.
EMSA
Nuclear extraction, EMSA, and supershifts were performed as described previously (22). Briefly, 5 μg nuclear protein were incubated for 20 min at room temperature with 0.1 pmol IRDye-700 labeled–double-stranded oligonucleotide synthesized by IDT (Coralville, IA) in the presence of 2 μg poly(dI: dC), and complexes were resolved on nondenaturing 4.5% polyacrylamide gels that were scanned by Odyssey-infrared imaging system. Oligo used for EMSA: 5′-TGTTTTCAATGTTGCAACAAGTCAGT-3′. All experiments were performed three times independently.
Chromatin-immunoprecipitation assays
Chromatin-immunoprecipitation (ChIP) assay was performed as previously described (24). The primers for C/EBPα and C/EBPβ in the mouse IL-12p40 promoters: upper primer 5′-GTCTCCAAGCACCTTGGCCATGAT-3′; lower primer 5′-GCTGCTGTTGCTGGTACTGGAACT-3′. All experiments were performed three times independently.
SiRNA
To generate C/EBPα siRNA expression construct siRNA corresponding to the coding sequence of mouse C/EBPα gene (5′-GCAAGAGCCGAGATAAAGC-3′) was cloned into the siRNA expression vector pSUPER.neo (OligoEngine, Seattle, WA) according to the manufacturer’s instructions. Briefly, equimolar amounts of complementary sense and antisense strands were separately mixed, annealed, and slowly cooled to 10°C in a 50 μl reaction buffer (100 mM NaCl and 50 mM HEPES, pH 7.4). The annealed oligonucleotides were inserted into the BglII/HindIII sites of pSUPER.neo vector. RAW264.7 cells stably depleted of C/EBPα by C/EBPα siRNA was established by transfecting the C/EBPα siRNA expression construct into RAW264.7 cells by electroporation. After 24 h, the cells were cultured in 500 μg/ml G418, and expression of C/EBPα was analyzed in the selected cells. All experiments were performed three times independently.
Results
Suppression of p40 gene transcription in macrophages and DCs by triptolide
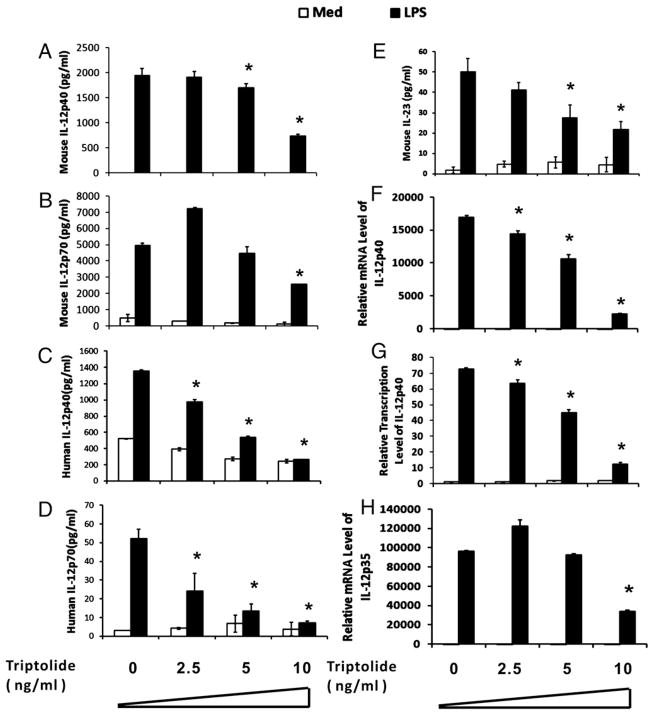
We first investigated whether triptolide could regulate IL-12/IL-23 production in LPS stimulated mouse peritoneal macrophage (MPM) and human MoDCs. Fig. 1A and 1C show that triptolide suppressed IL-12p40 release from both MPM and MoDCs in a dose-dependent manner. No cytotoxic effect of triptolide was observed at these concentrations, measured by crystal violet staining (data not shown). Because the p35 and p40 subunits of IL-12 may be regulated independently, we determined whether triptolide has inhibitory effect on the production of the heterodimeric IL-12 or p70. Whereas p70 levels were markedly reduced by triptolide in LPS-stimulated MoDCs (Fig. 1D), the reduction was less pronounced in MPM (Fig. 1B). Compared with IL-12, IL-23 production by MPM was inhibited significantly by triptolide at all doses (Fig. 1E).
FIGURE 1.
Effects of triptolide on IL-12 gene expression in LPS-treated MPM and MoDC (A–D) MPM (A, B), and MoDCs (C, D) were incubated for 15 h with different concentrations of triptolide and subsequently cultured for an additional 6 h in the presence (filled bars) or absence (open bars) of 1 μg/ml LPS. Mouse IL-12p40 (A), mouse IL-12p70 (B), human IL-12p40 (C), human IL-12p70 (D), and mouse IL-23 (E) levels in the supernatants were measured by ELISA. Experiment shown is representative of three to four independent experiments with mean ± SE. *p < 0.05. F–H, Total RNA was isolated from 1 × 106 MPM incubated with different concentrations of triptolide for 15 h with 1 μg/ml LPS (filled bars) or without (open bar) for an additional 3 h. Real-time PCR was performed to measure p40 (F), p35 (H) mRNA expression, and nascent transcripts for p40 (G). GAPDH mRNA was analyzed as an internal control. The experiment shown is representative of two independent experiments.
To further determine the molecular level at which triptolide exerts its inhibitory effect on IL-12p40 and p70 expression, we analyzed the steady-state mRNA and de novo synthesized transcripts of p40 and p35 in triptolide-treated MPM by real-time quantitative PCR (qPCR). Triptolide dose-dependently suppressed LPS-induced mRNA (Fig. 1F) and new transcripts (Fig. 1G) of the p40 gene, and p35 mRNA levels (Fig. 1H). These data indicate that triptolide suppresses IL-12p40 at the transcriptional level in both MPM and MoDCs. In addition, the inhibitory effect of triptolide is likely independent of IL-10 because no change was observed in IL-10 production in the presence or absence of triptolide (data not shown). We decided also to focus the study on MPM for simplicity.
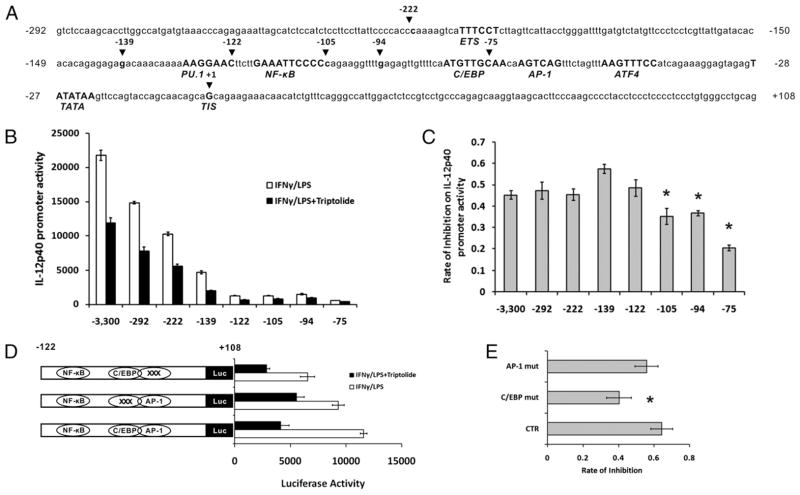
Localization of the triptolide-responsive element in the human p40 promoter
To further understand the transcriptional mechanism of the inhibition of triptolide on p40 expression we took an approach in which we sought to identify the promoter element(s) through which triptolide mediates its inhibitory effects on IL-12p40 transcription, using a series of 5′ deletion mutants of the p40 promoter-luciferase reporter constructs (Fig. 2A) transfected into the mouse macrophage cell line RAW264.7 cells treated or not with triptolide. As shown in Fig. 2B and 2C, 5′ deletion of the 3300-bp p40 promoter to −122 reduced the overall promoter activities but did not affect the triptolide-mediated inhibition of the IFN-γ/LPS-induced transcription. Deletion of the promoter to −105, which eliminated the NF-κB site, resulted in a significant loss of triptolide-induced inhibition. This is consistent with previous finding that NF-κB is a target of triptolide (9). However, further deletion of the promoter to −75 disrupting the functional composite C/EBP/AP-1 site (23) drastically reduced triptolide-mediated inhibition of the IL-12p40 promoter activity, suggesting that this sequence element, in addition to NF-κB, is a critical target in triptolide’s inhibitory activity on p40 expression.
FIGURE 2.
Localization of the triptolide-responsive element in the human IL-12p40 promoter. A, Putative and verified transcription factor binding sites in the −292/+108 p40 promoter region. The core binding sequences are in bold and uppercase, and positions are relative to the transcription initiation site (TIS), which is defined as −1. B, A series of 5′ deletion mutants of the full-length human IL-12p40 promoter-luciferase construct, which spans 3.3 kb upstream and 108-bp downstream of the TIS, were transfected transiently into RAW264.7. Cells were incubated for 15 h with (filled bars) or without 10 ng/ml triptolide (open bars), then stimulated with IFN-γ (15 h), followed by LPS (7 h). Cell lysates were assayed for luciferase activity. Data are summaries of four separate experiments. C, Rate of triptolide-induced inhibition of IL-12p40 promoter activities shown in B, expressed as 1-(triptolide-treated/nontreated). Results represent three independent experiments showing mean ± SE. D, Mutant promoter luciferase constructs of IL-12p40 promoter in the context of −292/+108 were transfected into RAW264.7. Luciferase activity was measured from cell lysates after stimulation of RAW264.7 cells with IFN-γ and LPS in the absence or presence of triptolide (15 h). E, Rate of triptolide-induced inhibition of IL-12p40 promoter activities shown in D. Results represent three independent experiments showing mean ± SE. *p < 0.05.
Because of the reported role of the C/EBP/AP-1 site in the regulation of the mouse IL-12p40 transcription (25), we sought to determine whether it was important for triptolide-mediated inhibition of the human IL-12p40 gene transcription. Base substitutions were introduced into these two elements by site-directed mutagenesis in the context of the −292/−108 IL-12p40-luc construct, which retained full responsiveness to triptolide. Mutation of the C/EBP half of the composite site resulted in a substantial reduction of inhibition of triptolide on the human IL-12p40 promoter activity, whereas the AP-1 site mutation did not (Fig. 2D, 2E). Taken together, we conclude that triptolide inhibitory effects on IL-12p40 promoter activation are most likely mediated through the C/EBP element.
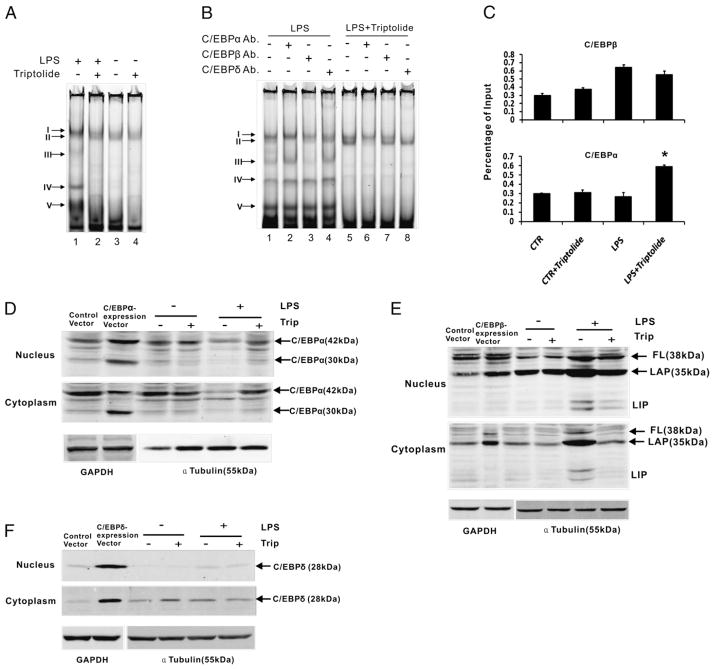
Regulation of C/EBP binding to p40 promoter in vitro and in vivo
Next, we sought to further elucidate the molecular basis of the involvement of the various members of the C/EBP family of transcription factors in the inhibition of IL-12 p40 transcription by triptolide. Six different members of C/EBP family (α, β, γ, δ, ε, and ζ) have been isolated and characterized to date all sharing a strong homology in the C-terminal domain (26). The activity and/or expression level of three C/EBP members (α, β, and δ) are regulated by a number of inflammatory agents, including LPS and a range of cytokines (27–29), which led us to focus mainly on these three members.
We first performed EMSAs to examine physical DNA-protein interactions in MPM using the composite C/EBP/AP-1 element of the p40 promoter as probe. Fig. 3A shows that two discernible binding activities were present constitutively (complexes I and II, lane 3). LPS treatment resulted in a loss of complex II and intensification of complex I plus the appearance of three additional fast-moving complexes (complex III–V, lane 1). Triptolide alone did not impact on the constitutive activities (lane 4). Triptolide treatment in the presence of LPS restored these activities to pre-LPS state (lane 2). Supershift experiment using Abs against the three isoforms of C/EBP (Fig. 3B) revealed that complex II was primarily composed of C/EBPα (lanes 2 and 6), whereas complex III contained C/EBPβ (lane 3), and complex I consisted of C/EBPδ (lane 8). Together, these data indicate that triptolide treatment of LPS-activated MPM likely leads to restoration of the binding activities to the pre-LPS state, that is, by altering the ratios of C/EBPα to C/EBPβ and δ.
FIGURE 3.
Regulation of C/EBPα and β binding to IL-12p40 promoter by triptolide in vitro and in vivo. A, EMSA was performed to examine the DNA-protein interaction at the composite C/EBP/AP-1 site in the IL-12p40 promoter in vitro using nuclear extracts prepared from MPM after incubation with or without triptolide, and stimulation with LPS (3 h). The five discernible complexes are labeled I–V. B, Supershift EMSA was performed to identify the components of the C/EBP complexes. The nuclear extracts used in this procedure were from LPS-stimulated MPM treated with or without triptolide. C, Triptolide regulates C/EBP binding to the p40 promoter in vivo. After incubation with or without triptolide, and stimulation with LPS (3 h), MPM were crosslinked by formaldehyde treatment and lysed. Cell lysates were subjected to immunoprecipitation with control IgG or anti-C/EBPβ (upper) or anti-C/EBPα (lower). Input DNA and DNA recovered from the immunoprecipitation were amplified by real-time PCR with primers spanning the C/EBP/AP-1-binding site in the IL-12p40 promoter. D–F, Western blotting was performed to detect the endogenous C/EBPα (D), C/EBPβ (E), and C/EBPδ (F) expression levels in 3 h LPS-stimulated MPM treated with or without triptolide, in the nucleus and cytoplasm. To aid in the appropriate identification of the various isoforms of endogenous C/EBP recombinant C/EBPs were loaded onto the same blots. Expression of α-tubulin was analyzed as a nuclear loading control whereas GAPDH expression was used as a loading control for whole cell lysate. *p < 0.05.
To demonstrate that triptolide also affects C/EBP binding activities in vivo, we performed ChIP assays. After LPS stimulation of MPM, there was a significant increase of C/EBPβ binding, which was reduced slightly in the presence of triptolide (Fig. 3C, upper), whereas the same treatment resulted in enhanced C/EBPα binding (Fig. 3C, lower). These data further corroborate the results from the in vitro binding studies.
To further establish whether the triptolide-induced change of C/EBP binding activities was caused by changes in C/EBP expression, we analyzed C/EBPα and -β at the protein level in the nucleus and cytoplasm of MPM by Western blot. As revealed in Fig. 3D, the 42 kDa and 30 kDa isoforms of C/EBPα, generated by alternative translational initiation, were present in the nucleus and cytoplasm constitutively. LPS treatment caused a strong reduction of the level of this protein in both compartments, whereas triptolide caused a strong reversal of the reduction, particularly in the nucleus. LPS stimulation induced nuclear levels of the three differentially translated isoforms of C/EBPβ, the 38 kDa full-length protein, the functional 33 kDa LAP, and the dominant negative 20 kDa LIP (30). Triptolide treatment led to an ~50% reduction in the level of the most abundant C/EBPβ isoform, LAP (Fig. 3E), whereas it did not impact on C/EBPδ level (Fig. 3F).
Collectively, these data demonstrate that triptolide causes a shift in the binding activities of C/EBPα and β isoforms in inflammatory macrophages by altering their respective nuclear levels, namely, increasing C/EBPα, whereas decreasing C/EBPβ.
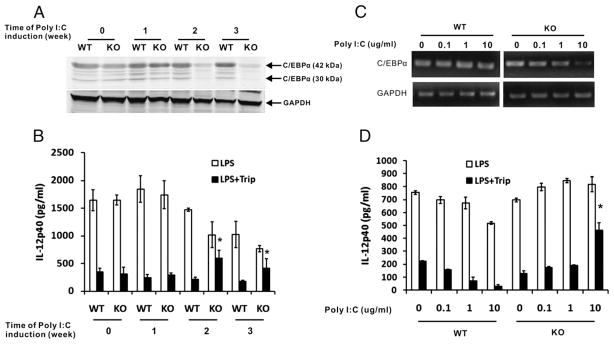
C/EBPα as a major physiological mediator in triptolide’s inhibition of IL-12p40 transcription
To further demonstrate the important role of C/EBPα in the inhibition of IL12p40 transcription by triptolide we analyzed the effect of triptolide on IL-12p40 production in C/EBPα KO mice. C/EBPα conditional KO mice are homozygous for C/EBPα floxed allele and hemizygous for the Mx1-Cre transgene, whose expression can be induced by administration of poly(I:C), leading to deletion of the floxed gene. Mx1-Cre expression was induced by i.p. injection of poly(I:C) for 1, 2, and 3 wk, respectively, and IL-12p40 level in serum was measured by ELISA. Fig. 4A shows that C/EBPα expression was strongly reduced 2 wk after poly(I:C) injection in KO mice. There was an accompanying, significant reversal of the inhibition of p40 production by triptolide (Fig. 4B). Notably, IL-12p40 levels in both WT and KO mice were decreased 2 wk after poly(I:C) injection. This may have been caused by the toxicity of poly(I:C). Studies by others have found that 5 mg/kg poly(I:C) injection for up to 28 d can induce some clinical changes, including anemia, elevations of transaminases, lactic dehydrogenase, and alkaline phosphatase, decreased clotting rate, and increased prothrombin time (31). In addition, we found that loss of C/EBPβ, compared with WT mice, did not influence triptolide-induced inhibition of IL-12p40 production (data not shown).
FIGURE 4.
Effects of triptolide in C/EBPα knockout mice. A–C, Four- to 6-week-old WT and Mx1-cre/C/EBPα KO mice were injected with 250 μg poly(I:C) every other day for three doses in the first week. After 1–3 wk, in the treatment group, a dose of 1 mg/kg (body weight) of triptolide was administrated to mice by i. p. injection overnight, followed by stimulation of LPS (15 mg/kg) for 4 h. Proteins from splenocytes were analyzed for C/EBPα expression by Western blotting (A). Simultaneously, IL-12p40 levels in the serum were measured by ELISA (B). Each group had three mice. Results represent mean ± SE of the three mice. C, MPM from WT and Mx1-cre/C/EBPα KO mice were treated with different concentrations of poly(I:C) for 24 h, incubated with triptolide (filled bars) or without (open bars), followed by stimulation of LPS for 6 h. Total RNA was prepared, and analyzed for mRNA expression of C/EBPα and GAPDH by RT-PCR. Simultaneously, IL-12p40 levels in the supernatants were measured by ELISA (D). Results represent three independent experiments showing mean ± SE. *p < 0.05.
To circumvent the toxicity problems caused by in vivo administration of poly(I:C) we used an exvivo method for the deletion of the C/EBPα gene. MPMs were isolated from WT and C/EBPα conditional KO mice, and treated with different concentrations of poly(I:C) in vitro for 24 h, followed by triptolide and LPS treatment. RT-PCR analysis of C/EBPα mRNA expression confirmed the significant deletion of the gene at this dose of poly(I:C) administration in KO cells (Fig. 4C). As shown in Fig. 4D, the poly(I:C) treatment had no significant effect on IL-12p40 secretion in both WT and KO MPMs. Although inhibition of p40 production by triptolide was not affected by the poly(I:C) treatment in WT cells, it was strongly reversed in C/EBPα KO MPM in a dose-dependent manner, reaching a highly significant level at 10 μg/ml poly(I:C).
Regulation of transcription and phosphorylation of C/EBPα by triptolide
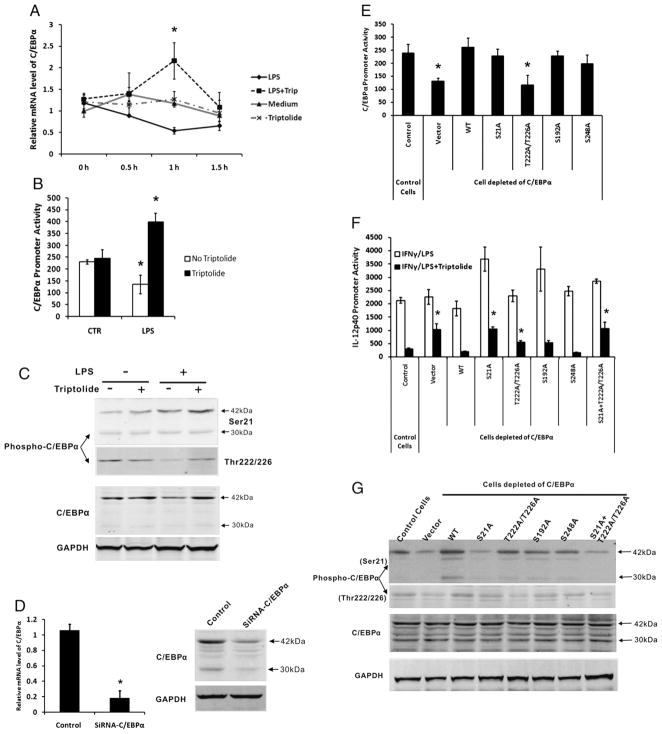
The above data suggested that triptolide may inhibit IL12p40 transcription by increasing the protein level of C/EBPα in the nucleus of MPM. To further understand the mechanism of this effect, we analyzed the mRNA expression and transcription of C/EBPα. Fig. 5A shows that LPS stimulation of MPM inhibited C/EBPα mRNA level, whereas triptolide treatment increased the level by 4-fold 1 h after LPS stimulation.
FIGURE 5.
Regulation of transcription and phosphorylation of C/EBPα by triptolide. A, MPM were incubated for 15 h with (dotted line) or without (solid line) 10 ng/ml triptolide and subsequently cultured for different times in the presence of 1 μg/ml LPS or absence (gray line and gray dotted line). B, A mouse C/EBPα promoter-luciferase construct, which spans 809-bp upstream and 187-bp downstream of the transcription initiation site, was transfected transiently into RAW264.7 cells. Cells were incubated for 15 h with (filled bars) or without 10 ng/ml triptolide (open bars), then stimulated with or without IFN-γ (15 h), followed by LPS (7 h). Cell lysates were assayed for luciferase activity. Data are summaries of four separate experiments. C, Western blotting was performed to detect Ser21- and Thr222/226-phosphorylation of C/EBPα in MPM after incubation with or without triptolide, and stimulation with LPS (1 h). D, Stably expressed siRNA against C/EBPα efficiently inhibited expression of the endogenous C/EBPα at both mRNA (left) and protein (right) levels in RAW264.7 cells. E, Mouse C/EBPα promoter-luciferase construct was cotransfected with WT or various phosphorylation mutants of C/EBPα expression vector into RAW264.7 cells depleted of endogenous C/EBPα by stable siRNA expression. Cell lysates were assayed for luciferase activity 36 h after transfection. F, IL-12p40 luciferase reporter was cotransfected with WT or various phosphorylation mutants of C/EBPα expression vector into RAW264.7 cells depleted of endogenous C/EBPα. Luciferase activity was measured from cell lysates after stimulation of RAW264.7 cells with IFN-γ and LPS in the presence or absence of triptolide (15 h). G, Western blot analysis of recombinant C/EBPα and phosphorylation mutants expressed in transfected RAW264.7 cells that had been depleted of the endogenous C/EBPα via siRNA, then stimulated with IFN-γ/LPS in the presence of triptolide. Expression of GAPDH was analyzed as a loading control. All transfection results represent three independent experiments showing mean ± SE. *p < 0.05.
Next, we sought to determine whether C/EBPα was regulated by triptolide at the level of transcription by using a mouse C/EBPα promoter-luciferase reporter construct, which spans 809-bp upstream and 187-bp downstream of the transcription initiation site. The proximal promoter region of the mouse C/EBPα gene has been characterized and DNA-protein interactions within the first 350-bp are essential for high levels of ubiquitous activity of the promoter (32). Studies have shown that the C/EBPα gene is autoactivated in a species-specific manner. Its promoter can be autoactivated in transfected cells by expression plasmids that specify for C/EBPα or C/EBPβ, which act via a C/EBP recognition sequence (32, 33). C/EBPα promoter contains two potential binding sites for C/EBP: one between nucleotides −252 and −239, the other between nucleotides −203 and −176 (33). We transfected the C/EBPα luciferase construct into RAW264.7, followed by triptolide treatment and IFN-γ/LPS stimulation. As shown in Fig. 5B, in resting RAW264.7 cells, triptolide did not affect C/EBPα promoter activity. However, IFN-γ/LPS stimulation resulted in some inhibition of the promoter activity, which was strongly reversed by triptolide. These data suggest that triptolide could forcefully antagonize the negative signaling of LPS that leads to transcriptional inhibition of C/EBPα. Taken together, these results demonstrate that C/EBPα, not C/EBPβ, plays a major role in triptolide-induced inhibition of IL-12p40 transcription. Triptolide can regulate C/EBPα’s transcription in inflammatory macrophages.
Posttranslational modifications of C/EBPα, such as phosphorylation at different serine/threonine residues, have been found to be an important mechanism of regulating its transcriptional activity (34, 35). We examined the potential effect of triptolide on this type of modification of C/EBPα using Abs specific for phosphorylated C/EBPα at Ser21 and Thr222/Thr226. As shown in Fig. 5C, LPS induced phosphorylation of C/EBPα at Ser21, and triptolide further enhanced this effect. At Thr222/Thr226, LPS alone reduced the phosphorylation, whereas triptolide restored it to the control levels. The phosphorylation patterns of C/EBPα were different from that of its expression (lower panels), suggesting that triptolide regulates both the expression and phosphorylation of C/EBPα, both impacting the latter’s transcriptional activity.
Next, we wanted to investigate the functional effects of the phosphorylation of C/EBPα using different phosphorylation mutants of C/EBPα. To minimize the interference of the endogenous C/EBPα in this cell line, we stably transfected a C/EBPα-specific siRNA expression vector, which resulted in reduction of its target both at the mRNA and protein levels (Fig. 5D). We then tested the ability of the exogenously introduced C/EBPα to regulate the cotransfected C/EBPα promoter, that is, its autoregulation as reported by others (32, 33). Fig. 5E shows that WT C/EBPα increased its own promoter activity ~2-fold, which was consistent with the previously published results. All other phosphorylation mutants, except Thr222A/Thr226A, acted similarly as the WT, suggesting that C/EBPα’s ability to autoregulate is only dependent on Thr222/Thr226. In contrast, for its ability to inhibit IL-12p40, Ser21, and Thr222/Thr226 seemed to be important because their mutations individually and collectively reduced triptolide-induced inhibition of the p40 promoter activity (Fig. 5F). Western blotting analysis of phosphorylation of C/EBPα protein expression at Ser21 and Thr222/Thr226 confirmed the effect of site-mutant constructs of the gene (Fig. 5G).
In conclusion, triptolide induced inhibition of IL-12p40 transcription is mediated by C/EBPα via both elevating the transcription factor’s expression and its serine/threonine phosphorylation.
Regulation of both MAPK and PI3K-Akt-GSK pathways by triptolide
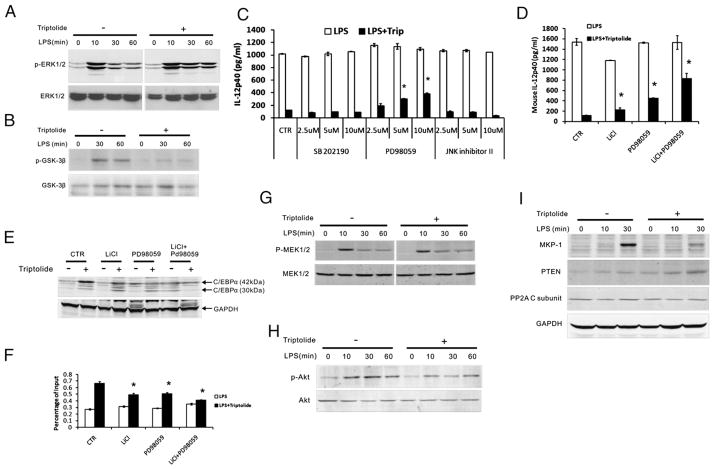
Previous studies by others have shown that in adipocytes and hepatocytes C/EBPα is phosphorylated at Thr222/Thr226 by GSK-3β (36, 37), and in hematopoietic and hepatic cell lines Ser21 is phosphorylated by ERK1/2 (38, 39). To explore the mechanism of triptolide-induced phosphorylation of C/EBPα, we examined the effect of triptolide on these two C/EBPα-modifying kinases by assessing their respective phosphorylation/activation using phosphor-specific Abs. Fig. 6A shows that in MPM, ERK1/2 was activated rapidly in response to LPS, with peak activity attained at ~10 min after the addition of LPS, followed by declines at 30 and 60 min. In contrast, ERk1/2 phosphorylation stimulated by LPS was sustained at higher levels by triptolide during this period. Phosphorylation of GSK-3β on the inactivating residue, Ser9, increased transiently after stimulation of LPS, whereas triptolide strongly suppressed this phosphorylation (Fig. 6B). Because phosphorylation of Ser9 is inhibitory for GSK-3β activity, these results suggest that triptolide may increase the activities of both ERK1/2 and GSK-3β in LPS-activated MPM.
FIGURE 6.
Regulation of MAPK and PI3K-AKT-GSK pathways by triptolide. A and B, Western blot analyses were performed to detect levels of phosphor-ERK1/2 (A) and phosphor-GSK-3β (B) in whole cell lysate prepared from MPM after incubation with or without triptolide, and stimulation with LPS. C, MPM were incubated for 15 h in the presence (filled bars) or absence (open bars) of triptolide, followed by 2 h treatment with p38 inhibitor (SB202190), ERK inhibitor (PD98059), and JNK inhibitor II, and subsequently cultured for an additional 6 h with LPS. Mouse IL-12p40 level in the supernatants was measured by ELISA. D, A similar protocol as that of C was used, except that cells were treated with the GSK-3β inhibitor LiCl or PD98059, or both. E, Same protocol was used as that of D. C/EBPα levels in whole cell lysate were determined by Western blotting. F, Same protocol as D was used. ChIP was performed to examine C/EBPα binding to the IL-12p40 promoter region in vivo. G–I, Western blot analyses were performed to detect phosphor-MEK1/2 (G), phosphor-AKT (H), and expression of MKP-1 and PTEN (I) in whole cell lysate. Results in C, D, and F represent three independent experiments showing mean ± SE. *p < 0.05.
To further demonstrate the role of ERK1/2 in triptolide-induced inhibition of IL-12p40, we treated MPM with small molecule inhibitors of p38(SB202190), ERK1/2(PD98059), and JNK(inhibitor II) 2h prior to LPS stimulation with or without triptolide, and analyzed IL12p40 production. Fig. 6C shows that only PD 98059 rescued to a significant degree the triptolide-induced inhibition of IL-12p40 in a dose-dependent manner without cytotoxicity. These data indicate that ERK1/2, not p38 and JNK, plays a role in mediating the IL-12–inhibiting activity of triptolide. By a similar approach using a selective GSK-3β inhibitor, LiCl (40), we investigated the role of GSK-3β in triptolide’s inhibitory function. As shown in Fig. 6D, LiCl treatment of MPM slightly resisted triptolide’s inhibitory effect. However, when LiCl was combined with PD 98059, it strongly diminished triptolide’s effect on p40 production, by >50%. These data suggest that triptolide inhibits IL-12p40 transcription through ERK1/2- and GSK-3β–dependent pathways separately and interactively.
To further assess the role of ERK1/2 and GSK-3β in mediating the effects of triptolide on C/EBPα’s function, we treated MPM with chemical inhibitors of the kinases, PD 98059 and LiCl, respectively, and examined C/EBPα expression and in vivo binding of C/EBPα to the p40 locus by ChIP analysis (Fig. 6E, 6F). Inhibitors of GSK-3β and ERK1/2 individually resulted in reduction of C/EBPα expression and C/EBPα’s binding activity, whereas the combination of the two inhibitors caused a synergistically greater reduction.
Next, we evaluated the impact of triptolide on the upstream kinases of ERK1/2 and GSK-3β: MEK1/2 and Akt1/2, respectively, by analyzing their phosphorylation. In LPS-stimulated MPM, phosphorylation of MEK1/2 was rapidly induced in 10 min, followed by declines at 30 and 60 min. This pattern was very similar in triptolide-treated cells (Fig. 6G). On the other hand, phosphorylation of Akt was more sustained in LPS-stimulated MPM, but reduced overall by triptolide treatment (Fig. 6H).
The lack of a triptolide-induced effect on the phosphorylation of MEK1/2 prompted us to examine the MAPK phosphatases (MKPs). MKPs are dual-specificity phosphatases that can dephosphorylate both phosphotyrosine and phosphothreonine residues on MAPKs (41). MKP-1, the archetypal member of this family, is widely studied and selectively inactivates all three MAPK families by dephosphorylating them at catalytic tyrosine and threonine residues (42). We examined the effect of triptolide on LPS-induced MKP-1 expression by Western blotting. Fig. 6I (upper) shows that MKP-1 was strongly induced in MPM by LPS at ~1 h, and triptolide treatment strongly decreased the expression. This is consistent with a previous study showing that triptolide blocked MKP-1 induction by LPS in macrophages (43). Triptolide had little effect on the expression of PP2A (lower), which belongs to the same family of dual-specificity phosphatases as MKP-1.
Because triptolide reduced phosphorylation of GSK-3β and Akt, and affected the phosphoinositol 3-kinase (PI3K)-Akt-GSK-3β pathway (Fig. 6B, 6H), we sought to determine whether triptolide has an effect on phosphatase and tensin homolog deleted on chromosome ten (PTEN), one of the enzymes in the protein tyrosine phosphatases (PTPs) superfamily that negatively regulates cell survival mediated by the PI3K-Akt pathway. PTEN acts as a phospholipid phosphatase dephosphorylating the PtdIns(3,4,5)P3 and PtdIns(3,4)P2, resulting in PtdIns(4,5)P2 and PtdIns(4)P, respectively (44). PTEN modulates the PI3K pathway by catalyzing degradation of the PtdIns(3,4,5)P3 generated by PI3K. PTEN inhibits downstream functions mediated by the PI3K pathway, resulting in the activation of Akt/protein kinase B, cell survival, and cell proliferation (45, 46). In this study, we demonstrated that LPS alone did not induce significant PTEN expression in MPM, which was in accord with previous studies (47). On triptolide treatment, however, PTEN expression was elevated at 30 min after stimulation with LPS (Fig. 6I, middle).
Discussion
In this study, we investigated the detailed molecular mechanism, whereby triptolide inhibits the transcription of the p40 gene encoding the shared subunit of IL-12 and IL-23 in APCs. It led to the identification of C/EBPα as the major and direct mediator of triptolide’s inhibitory effect on p40 transcription. C/EBP-related proteins comprise a family of basic region-leucine zipper transcription factors. These proteins dimerize through a leucine zipper and bind to a consensus DNA motif through an adjacent basic region (48). The activity and expression of three C/EBP members (α, β and δ) are regulated by a number of inflammatory signals, including LPS and a range of cytokines (26–29, 49, 50). C/EBPβ-deficient mice display impaired expression of TNF-α, serum amyloid A and P proteins, α1-acid gp, and complement C3 component (28, 51, 52). However, induction of a number of cytokines, such as IL-12, IL-10, IFN-γ, MIP-1α, and GM-CSF, in C/EBPβ−/− mice was comparable to that observed in WT mice, with the exception of G-CSF (51). This may be due to functional compensation by other members that are expressed at normal levels, for example, C/EBPδ (48). These data are consistent with our results (Fig 4) in that IL-12p40 expression was not impaired in C/EBPβ−/− MPM, nor was triptolide-induced inhibition of IL-12p40.
Most of the previously published work on C/EBPα focused on its relationship with cellular proliferation and differentiation. C/EBPα is expressed at high levels in terminally differentiated cells and down-regulated during proliferation (53). C/EBPα is a strong inhibitor of cell proliferation when overexpressed in cultured cells (53, 54). In addition, C/EBPα-deficient mice have defective myeloid cell development, increased hematopoietic stem cell repopulating activity, and a significantly increased myeloblast population in the bone marrow compartment. These phenotypes are probably caused by a specific maturational block of myeloid precursors toward mature granulocyte and macrophage (55). The activity and/or mRNA and protein levels of various C/EBP genes are differentially modulated in response to inflammatory stimuli and to recombinant cytokines. C/EBPα is downregulated under these conditions. The mechanisms on the downregulation and the relationship between C/EBPα and expression of cytokines have not been elucidated. Our study shows that deletion of C/EBPα per se does not impact LPS-induced level of IL-12p40 (Fig. 4B) or those of TNF-α and IL-10 (data not shown); however, it strongly impeded triptolide-induced inhibition of p40 expression, demonstrating the critical role of C/EBPα in this mechanism.
Furthermore, our data indicate that triptolide augments/increases phosphorylation of LPS-induced C/EBPα at Ser21 and Thr222/Thr226 through ERK1/2 and GSK-3β, which potentiates C/EBPα’s transcriptional inhibition of p40 (Figs. 5C, 6E). The MAPK cascade is very important, for many processes in immune responses (56). On TLR4 engagement by LPS, MAPKs are activated by dual phosphorylation at the tripepetide motif Thr-Xaa-Tyr, which leads to the production of inflammatory cytokines, such as IL-12, IL-1, and TNF-α, etc. MAPK inactivation is carried out by a family of MAPK phosphatases (42, 57, 58). MKP-1 selectively inactivates all three MAPK families by dephosphorylating them at catalytic tyrosine and threonine residues (58). Activation of ERK1/2 has been shown to play an important role in Th1/Th2 polarization and in regulating cytokine production from APCs. ERK1-deficient mice display a bias toward Th1 type immune response. Consistent with this observation, DCs from ERK1-deficient mice show enhanced IL-12p70 and reduced IL-10 secretion in response to TLR stimulation (59). This suggests that ERK1/2 is an inhibitor of IL-12. In contrast, mice deficient of the p38-activating kinase, MKK3, exhibit a selective defect in LPS-induced IL-12 production in macrophages (60). We found that triptolide-induced inhibition of p40 transcription was preceded by sustained phosphorylation of ERK1/2 (Fig. 6A), and that blocking the activity of ERK1/2 but not those of p38 and JNK can substantially rescued p40 production inhibited by triptolide (Fig. 6C, 6D).
Numerous studies have implicated the PI3K-AKT pathway in TLR signaling. GSK-3 can act as an intermediary in this pathway. GSK-3 is constitutively active in resting cells but can be inactivated through phosphorylation by AKT or other protein kinases (61, 62). PTEN is a lipid phosphatase and dephosphorylates PIP3 to negatively regulate PI3K signaling (63). However, there are conflicting reports concerning the physiological role of PI3K in the signaling pathway; the inconsistent results are partly due to the specificity of two inhibitors (wortmannin and LY294002) in these investigations. Recently, some research groups used genetic methods to knock out the regulatory subunit (p85) of PI3K, and found that TLR4-mediated induction of IL-12, IFN-γ and TNF-α were increased in DCs (64, 65); and knockdown of GSK-3β by siNA results in increased IL-10 production and suppressed IL-12 synthesis (65). These results are in disagreement with our data. We showed that triptolide strongly inhibited LPS-induced phosphorylation of GSK-3β in MPM (Fig. 6B), and that blocking the activity of GSK-3β by LiCl eased triptolide-induced inhibition of p40 production (Fig. 6E). Further analysis revealed that chemical inhibition of GSK-3β and ERK1/2 resulted also in the inhibition of C/EBPα levels (Fig. 6G). Because we showed that the target of GSK-3β, Thr222/226 in C/EBPα, is critical for the autoregulation of C/EBPα expression (Fig. 5E), it is possible that inhibition of GSK-3β leads to the rescue of triptolide-induced inhibition through reducing the expression or transcriptional potential of C/EBPα, or both. The additive effects of simultaneous chemical inhibition of both ERK1/2 and GSK-3β (Fig. 6F) may be attributed to the independent effects of targeting Ser21 and Thr222/226 of C/EBPα, respectively.
Control over kinase signaling networks is exerted at the level of both phosphorylation and dephosphorylation, catalyzed by protein kinases and phosphatases, respectively (66). The phosphatases include tyrosine-specific phosphatases (PTPs), dual specificity (threonine/tyrosine), MKPs, and protein serine/threonine phosphatases (PSPs). Our study showed that triptolide, in its inhibition of p40 transcription, decreased LPS-induced MKP-1 and increased PTEN (a member of PTPs), whereas having little effect on PP2A (a member of PSPs) (Fig. 6K). The mechanism of the differential regulation of MKP-1 and PTEN expression by triptolide is of great interest for further investigation. One possible mechanism is through induction of reactive oxygen species (ROS) in macrophages. Phagocytic cells, such as macrophages, contain a multiprotein NADPH oxidase complex that produces a burst of ROS as part of the innate immune response to infection in response to a wide variety of physiological stimuli, including LPS (66, 67). There are reports that ROS might be involved in MKP-1 induction in human epithelial and endothelial cells (68–70), and in decreased levels of PTEN in alveolar macrophages and increased activity of AKT (71). Many studies of the relationship between ROS and PTEN focused on inactivation of PTEN by ROS through posttranscriptional regulation (66, 72). Triptolide possesses a 9,11-epoxy-14β-hydroxy system, which may be involved in selective alkylation by nucleophilic groups, such as thiols, present in key target enzymes (73); oxidation and reduction of thiol proteins is thought to be the major mechanism by which reactive oxidants integrate into cellular signal transduction pathway (74).
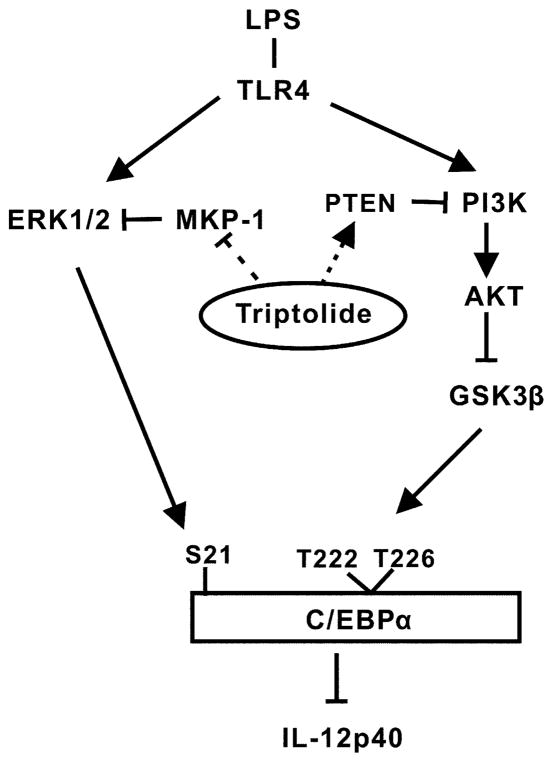
On the basis of these considerations, we propose a new model on how triptolide inhibits IL-12/IL-23p40 in macrophages and DCs (Fig. 7). Our study has uncovered several novel targets of triptolide in MPM: C/EBPα, ERK1/2, GSK-3b, MKP-1, and PTEN, which may prove to have strong implications in guiding the therapeutic applications of triptolide and its derivatives in the treatment of inflammatory auto-immune diseases and cancer.
FIGURE 7.
Model for regulation of IL-12p40 production by triptolide. Triptolide augments TLR4-mediated activation of ERK1/2 and GSK-3β by interfering with the expression of phosphatases MKP-1 and PTEN. ERK1/2 and GSK-3β enhance the phosphorylation of C/EBPα, and its expression because of autoregulation. C/EBPα functions as an inhibitor of p40 transcription via a yet to be elucidated mechanism.
Abbreviations used in this paper
- C/EBPα
CCAAT/enhancer-binding protein-α
- ChIP
chromatin immunoprecipitation
- DC
dendritic cell
- KO
knockout
- LN
lupus nephritis
- MoDC
monocyte-derived DC
- MPM
mouse peritoneal macrophage
- poly (I:C)
polyinosinic-polycytidylic acid
- PTP
protein tyrosine phosphatase
- qPCR
real-time quantitative PCR
- ROS
reactive oxygen species
- TWHF
Tripterygium wilfordii Hook F
- WT
wild type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Kupchan SM, Court WA, Dailey RG, Jr, Gilmore CJ, Bryan RF. Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J Am Chem Soc. 1972;94:7194–7195. doi: 10.1021/ja00775a078. [DOI] [PubMed] [Google Scholar]
- 2.Tao XL, Sun Y, Dong Y, Xiao YL, Hu DW, Shi YP, Zhu QL, Dai H, Zhang NZ. A prospective, controlled, double-blind, cross-over study of tripterygium wilfodii hook F in treatment of rheumatoid arthritis. Chin Med J (Engl) 1989;102:327–332. [PubMed] [Google Scholar]
- 3.Qin WZ, Zhu GD, Yang SM, Han KY, Wang J. Clinical observations on Tripterygium wilfordii in treatment of 26 cases of discoid lupus erythematosus. J Tradit Chin Med. 1983;3:131–132. [PubMed] [Google Scholar]
- 4.Jiang X. Clinical observations on the use of the Chinese herb Tripterygium wilfordii Hook for the treatment of nephrotic syndrome. Pediatr Nephrol. 1994;8:343–344. doi: 10.1007/BF00866356. [DOI] [PubMed] [Google Scholar]
- 5.Xu WY, Zheng JR, Lu XY. Tripterygium in dermatologic therapy. Int J Dermatol. 1985;24:152–157. doi: 10.1111/j.1365-4362.1985.tb05746.x. [DOI] [PubMed] [Google Scholar]
- 6.Mao H, Chen XR, Q Yi, Li SY, Wang ZL, Li FY. Mycophenolate mofetil and triptolide alleviating airway inflammation in asthmatic model mice partly by inhibiting bone marrow eosinophilopoiesis. Int Immunopharmacol. 2008;8:1039–1048. doi: 10.1016/j.intimp.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Chen T, Xu H, Wang HQ, Zhao Y, Zhu CF, Zhang YH, Ji MJ, Hua YB, Wu WX. Prolongation of rat intestinal allograft survival by administration of triptolide-modified donor bone marrow-derived dendritic cells. Transplant Proc. 2008;40:3711–3713. doi: 10.1016/j.transproceed.2008.06.056. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Chen Y, Liu FQ, Lamb JR, Tam PK. Combined treatment with triptolide and rapamycin prolongs graft survival in a mouse model of cardiac transplantation. Transpl Int. 2008;21:483–494. doi: 10.1111/j.1432-2277.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- 9.Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, Ng JC, Kao PN. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J Biol Chem. 1999;274:13443–13450. doi: 10.1074/jbc.274.19.13443. [DOI] [PubMed] [Google Scholar]
- 10.Numerof RP, Asadullah K. Cytokine and anti-cytokine therapies for psoriasis and atopic dermatitis. BioDrugs. 2006;20:93–103. doi: 10.2165/00063030-200620020-00004. [DOI] [PubMed] [Google Scholar]
- 11.Qiu D, Kao PN. Immunosuppressive and anti-inflammatory mechanisms of triptolide, the principal active diterpenoid from the Chinese medical herb Tripterygium wilfordii Hook F. Drugs R D. 2003;4:1–18. doi: 10.2165/00126839-200304010-00001. [DOI] [PubMed] [Google Scholar]
- 12.Liu Q, Chen T, Chen H, Zhang M, Li N, Lu Z, Ma P, Cao X. Triptolide (PG-490) induces apoptosis of dendritic cells through sequential p38 MAP kinase phosphorylation and caspase 3 activation. Biochem Biophys Res Commun. 2004;319:980–986. doi: 10.1016/j.bbrc.2004.04.201. [DOI] [PubMed] [Google Scholar]
- 13.Liu HK, Perrier S, Lipina C, Finlay D, McLauchlan H, Hastie CJ, Hundal HS, Sutherland C. Functional characterisation of the regulation of CAAT enhancer binding protein alpha by GSK-3 phosphorylation of Threonines 222/226. BMC Mol Biol. 2006;7:14. doi: 10.1186/1471-2199-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu KJ, Shen QY, Cheng H, Mao XH, Lao LM, Hao GL. Triptolide affects the differentiation, maturation and function of human dendritic cells. Int Immunopharmacol. 2005;5:1415–1426. doi: 10.1016/j.intimp.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 16.Tarrant TK, Silver PB, Chan CC, Wiggert B, Caspi RR. Endogenous IL-12 is required for induction and expression of experimental autoimmune uveitis. J Immunol. 1998;161:122–127. [PubMed] [Google Scholar]
- 17.Hue S, Ahern P, Buonocore S, Kullberg MC, Cua DJ, McKenzie BS, Powrie F, Maloy KJ. Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J Exp Med. 2006;203:2473–2483. doi: 10.1084/jem.20061099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyts I, Hellings PW, Hens G, Vanaudenaerde BM, Verbinnen B, Heremans H, Matthys P, Bullens DM, Overbergh L, Mathieu C, et al. IL-12 contributes to allergen-induced airway inflammation in experimental asthma. J Immunol. 2006;177:6460–6470. doi: 10.4049/jimmunol.177.9.6460. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Murakami T, Oppenheim JJ, Howard OM. Triptolide, a constituent of immunosuppressive Chinese herbal medicine, is a potent suppressor of dendritic-cell maturation and trafficking. Blood. 2005;106:2409–2416. doi: 10.1182/blood-2005-03-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Wu QL, Feng YH, Wang YF, Li XY, Zuo JP. Triptolide suppresses CD80 and CD86 expressions and IL-12 production in THP-1 cells. Acta Pharmacol Sin. 2005;26:223–227. doi: 10.1111/j.1745-7254.2005.00035.x. [DOI] [PubMed] [Google Scholar]
- 21.Screpanti I, Musiani P, Bellavia D, Cappelletti M, Aiello FB, Maroder M, Frati L, Modesti A, Gulino A, Poli V. Inactivation of the IL-6 gene prevents development of multicentric Castleman’s disease in C/EBP beta-deficient mice. J Exp Med. 1996;184:1561–1566. doi: 10.1084/jem.184.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma X, Chow JM, Gri G, Carra G, Gerosa F, Wolf SF, Dzialo R, Trinchieri G. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J Exp Med. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao S, Liu J, Chesi M, Bergsagel PL, Ho IC, Donnelly RP, Ma X. Differential regulation of IL-12 and IL-10 gene expression in macrophages by the basic leucine zipper transcription factor c-Maf fibrosarcoma. J Immunol. 2002;169:5715–5725. doi: 10.4049/jimmunol.169.10.5715. [DOI] [PubMed] [Google Scholar]
- 24.Chung EY, Liu J, Homma Y, Zhang Y, Brendolan A, Saggese M, Han J, Silverstein R, Selleri L, Ma X. Interleukin-10 expression in macrophages during phagocytosis of apoptotic cells is mediated by homeodomain proteins Pbx1 and Prep-1. Immunity. 2007;27:952–964. doi: 10.1016/j.immuni.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Plevy SE, Gemberling JH, Hsu S, Dorner AJ, Smale ST. Multiple control elements mediate activation of the murine and human interleukin 12 p40 promoters: evidence of functional synergy between C/EBP and Rel proteins. Mol Cell Biol. 1997;17:4572–4588. doi: 10.1128/mcb.17.8.4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–575. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tengku-Muhammad TS, Hughes TR, Ranki H, Cryer A, Ramji DP. Differential regulation of macrophage CCAAT-enhancer binding protein isoforms by lipopolysaccharide and cytokines. Cytokine. 2000;12:1430–1436. doi: 10.1006/cyto.2000.0711. [DOI] [PubMed] [Google Scholar]
- 28.Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem. 1998;273:29279–29282. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- 29.Ramji DP, Vitelli A, Tronche F, Cortese R, Ciliberto G. The two C/EBP isoforms, IL-6DBP/NF-IL6 and C/EBP delta/NF-IL6 beta, are induced by IL-6 to promote acute phase gene transcription via different mechanisms. Nucleic Acids Res. 1993;21:289–294. doi: 10.1093/nar/21.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Descombes P, Schibler U. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell. 1991;67:569–579. doi: 10.1016/0092-8674(91)90531-3. [DOI] [PubMed] [Google Scholar]
- 31.Homan ER, Zendzian RP, Schott LD, Levy HB, Adamson RH. Studies on poly I:C toxicity in experimental animals. Toxicol Appl Pharmacol. 1972;23:579–588. doi: 10.1016/0041-008x(72)90098-1. [DOI] [PubMed] [Google Scholar]
- 32.Legraverend C, Antonson P, Flodby P, Xanthopoulos KG. High level activity of the mouse CCAAT/enhancer binding protein (C/EBP alpha) gene promoter involves autoregulation and several ubiquitous transcription factors. Nucleic Acids Res. 1993;21:1735–1742. doi: 10.1093/nar/21.8.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christy RJ, Kaestner KH, Geiman DE, Lane MD. CCAAT/enhancer binding protein gene promoter: binding of nuclear factors during differentiation of 3T3-L1 preadipocytes. Proc Natl Acad Sci USA. 1991;88:2593–2597. doi: 10.1073/pnas.88.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cha HC, Oak NR, Kang S, Tran TA, Kobayashi S, Chiang SH, Tenen DG, MacDougald OA. Phosphorylation of CCAAT/enhancer-binding protein alpha regulates GLUT4 expression and glucose transport in adipocytes. J Biol Chem. 2008;283:18002–18011. doi: 10.1074/jbc.M800419200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Datta J, Majumder S, Kutay H, Motiwala T, Frankel W, Costa R, Cha HC, MacDougald OA, Jacob ST, Ghoshal K. Metallothionein expression is suppressed in primary human hepatocellular carcinomas and is mediated through inactivation of CCAAT/enhancer binding protein alpha by phosphatidylinositol 3-kinase signaling cascade. Cancer Res. 2007;67:2736–2746. doi: 10.1158/0008-5472.CAN-06-4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross SE, Erickson RL, Hemati N, MacDougald OA. Glycogen synthase kinase 3 is an insulin-regulated C/EBPalpha kinase. Mol Cell Biol. 1999;19:8433–8441. doi: 10.1128/mcb.19.12.8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedersen TA, Bereshchenko O, Garcia-Silva S, Ermakova O, Kurz E, Mandrup S, Porse BT, Nerlov C. Distinct C/EBPalpha motifs regulate lipogenic and gluconeogenic gene expression in vivo. EMBO J. 2007;26:1081–1093. doi: 10.1038/sj.emboj.7601563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross SE, Radomska HS, Wu B, Zhang P, Winnay JN, Bajnok L, Wright WS, Schaufele F, Tenen DG, MacDougald OA. Phosphorylation of C/EB-Palpha inhibits granulopoiesis. Mol Cell Biol. 2004;24:675–686. doi: 10.1128/MCB.24.2.675-686.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radomska HS, Bassères DS, Zheng R, Zhang P, Dayaram T, Yamamoto Y, Sternberg DW, Lokker N, Giese NA, Bohlander SK, et al. Block of C/EBP alpha function by phosphorylation in acute myeloid leukemia with FLT3 activating mutations. J Exp Med. 2006;203:371–381. doi: 10.1084/jem.20052242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci USA. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26:3203–3213. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 42.Kuwano Y, Kim HH, Abdelmohsen K, Pullmann R, Jr, Martindale JL, Yang X, Gorospe M. MKP-1 mRNA stabilization and translational control by RNA-binding proteins HuR and NF90. Mol Cell Biol. 2008;28:4562–4575. doi: 10.1128/MCB.00165-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen P, Li J, Barnes J, Kokkonen GC, Lee JC, Liu Y. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J Immunol. 2002;169:6408–6416. doi: 10.4049/jimmunol.169.11.6408. [DOI] [PubMed] [Google Scholar]
- 44.Maehama T, Dixon JE. PTEN: a tumour suppressor that functions as a phospholipid phosphatase. Trends Cell Biol. 1999;9:125–128. doi: 10.1016/s0962-8924(99)01519-6. [DOI] [PubMed] [Google Scholar]
- 45.Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- 46.Tsugawa K, Jones MK, Sugimachi K, Sarfeh IJ, Tarnawski AS. Biological role of phosphatase PTEN in cancer and tissue injury healing. Front Biosci. 2002;7:e245–e251. doi: 10.2741/tsugawa. [DOI] [PubMed] [Google Scholar]
- 47.Okamura H, Yoshida K, Sasaki E, Qiu L, Amorim BR, Morimoto H, Haneji T. Expression of PTEN and Akt phosphorylation in lipopolysaccharide-treated NIH3T3 cells. Cell Biol Int. 2007;31:119–125. doi: 10.1016/j.cellbi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 48.Hu HM, Baer M, Williams SC, Johnson PF, Schwartz RC. Redundancy of C/EBP alpha, -beta, and -delta in supporting the lipopolysaccharide-induced transcription of IL-6 and monocyte chemoattractant protein-1. J Immunol. 1998;160:2334–2342. [PubMed] [Google Scholar]
- 49.Kinoshita S, Akira S, Kishimoto T. A member of the C/EBP family, NF-IL6 beta, forms a heterodimer and transcriptionally synergizes with NF-IL6. Proc Natl Acad Sci USA. 1992;89:1473–1476. doi: 10.1073/pnas.89.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cardinaux JR, Allaman I, Magistretti PJ. Pro-inflammatory cytokines induce the transcription factors C/EBPbeta and C/EBPdelta in astrocytes. Glia. 2000;29:91–97. [PubMed] [Google Scholar]
- 51.Tanaka T, Akira S, Yoshida K, Umemoto M, Yoneda Y, Shirafuji N, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- 52.Screpanti I, Romani L, Musiani P, Modesti A, Fattori E, Lazzaro D, Sellitto C, Scarpa S, Bellavia D, Lattanzio G, et al. Lymphoproliferative disorder and imbalanced T-helper response in C/EBP beta-deficient mice. EMBO J. 1995;14:1932–1941. doi: 10.1002/j.1460-2075.1995.tb07185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lekstrom-Himes J, Xanthopoulos KG. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J Biol Chem. 1998;273:28545–28548. doi: 10.1074/jbc.273.44.28545. [DOI] [PubMed] [Google Scholar]
- 54.Hendricks-Taylor LR, Darlington GJ. Inhibition of cell proliferation by C/EBP alpha occurs in many cell types, does not require the presence of p53 or Rb, and is not affected by large T-antigen. Nucleic Acids Res. 1995;23:4726–4733. doi: 10.1093/nar/23.22.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang DE, Zhang P, Wang ND, Hetherington CJ, Darlington GJ, Tenen DG. Absence of granulocyte colony-stimulating factor signaling and neutrophil development in CCAAT enhancer binding protein alpha-deficient mice. Proc Natl Acad Sci USA. 1997;94:569–574. doi: 10.1073/pnas.94.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dong C, Davis RJ, Flavell RA. MAP kinases in the immune response. Annu Rev Immunol. 2002;20:55–72. doi: 10.1146/annurev.immunol.20.091301.131133. [DOI] [PubMed] [Google Scholar]
- 57.Keyse SM. Protein phosphatases and the regulation of mitogen-activated protein kinase signalling. Curr Opin Cell Biol. 2000;12:186–192. doi: 10.1016/s0955-0674(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 58.Franklin CC, Kraft AS. Conditional expression of the mitogen-activated protein kinase (MAPK) phosphatase MKP-1 preferentially inhibits p38 MAPK and stress-activated protein kinase in U937 cells. J Biol Chem. 1997;272:16917–16923. doi: 10.1074/jbc.272.27.16917. [DOI] [PubMed] [Google Scholar]
- 59.Agrawal A, Dillon S, Denning TL, Pulendran B. ERK1−/− mice exhibit Th1 cell polarization and increased susceptibility to experimental autoimmune encephalomyelitis. J Immunol. 2006;176:5788–5796. doi: 10.4049/jimmunol.176.10.5788. [DOI] [PubMed] [Google Scholar]
- 60.Lu HT, Yang DD, Wysk M, Gatti E, Mellman I, Davis RJ, Flavell RA. Defective IL-12 production in mitogen-activated protein (MAP) kinase kinase 3 (Mkk3)-deficient mice. EMBO J. 1999;18:1845–1857. doi: 10.1093/emboj/18.7.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woodgett JR. Recent advances in the protein kinase B signaling pathway. Curr Opin Cell Biol. 2005;17:150–157. doi: 10.1016/j.ceb.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 62.Hazeki K, Nigorikawa K, Hazeki O. Role of phosphoinositide 3-kinase in innate immunity. Biol Pharm Bull. 2007;30:1617–1623. doi: 10.1248/bpb.30.1617. [DOI] [PubMed] [Google Scholar]
- 63.Deane JA, Fruman DA. Phosphoinositide 3-kinase: diverse roles in immune cell activation. Annu Rev Immunol. 2004;22:563–598. doi: 10.1146/annurev.immunol.22.012703.104721. [DOI] [PubMed] [Google Scholar]
- 64.Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 65.Martin M, Rehani K, Jope RS, Michalek SM. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat Immunol. 2005;6:777–784. doi: 10.1038/ni1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tonks NK. Redox redux: revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 67.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 68.Hannken T, Schroeder R, Stahl RA, Wolf G. Atrial natriuretic peptide attenuates ANG II-induced hypertrophy of renal tubular cells. Am J Physiol Renal Physiol. 2001;281:F81–F90. doi: 10.1152/ajprenal.2001.281.1.F81. [DOI] [PubMed] [Google Scholar]
- 69.Yoneda K, Peck K, Chang MM, Chmiel K, Sher YP, Chen J, Yang PC, Chen Y, Wu R. Development of high-density DNA microarray membrane for profiling smoke- and hydrogen peroxide-induced genes in a human bronchial epithelial cell line. Am J Respir Crit Care Med. 2001;164:S85–S89. doi: 10.1164/ajrccm.164.supplement_2.2106062. [DOI] [PubMed] [Google Scholar]
- 70.Fürst R, Brueckl C, Kuebler WM, Zahler S, Krötz F, Görlach A, Vollmar AM, Kiemer AK. Atrial natriuretic peptide induces mitogen-activated protein kinase phosphatase-1 in human endothelial cells via Rac1 and NAD(P)H oxidase/Nox2-activation. Circ Res. 2005;96:43–53. doi: 10.1161/01.RES.0000151983.01148.06. [DOI] [PubMed] [Google Scholar]
- 71.Flaherty DM, Monick MM, Hinde SL. Human alveolar macrophages are deficient in PTEN. The role of endogenous oxidants. J Biol Chem. 2006;281:5058–5064. doi: 10.1074/jbc.M508997200. [DOI] [PubMed] [Google Scholar]
- 72.Silva A, Yunes JA, Cardoso BA, Martins LR, Jotta PY, Abecasis M, Nowill AE, Leslie NR, Cardoso AA, Barata JT. PTEN posttranslational inactivation and hyperactivation of the PI3K/Akt pathway sustain primary T cell leukemia viability. J Clin Invest. 2008;118:3762–3774. doi: 10.1172/JCI34616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kupchan SM, Schubert RM. Selective alkylation: a biomimetic reaction of the antileukemic triptolides? Science. 1974;185:791–793. doi: 10.1126/science.185.4153.791. [DOI] [PubMed] [Google Scholar]
- 74.Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radic Biol Med. 2008;45:549–561. doi: 10.1016/j.freeradbiomed.2008.05.004. [DOI] [PubMed] [Google Scholar]