Abstract
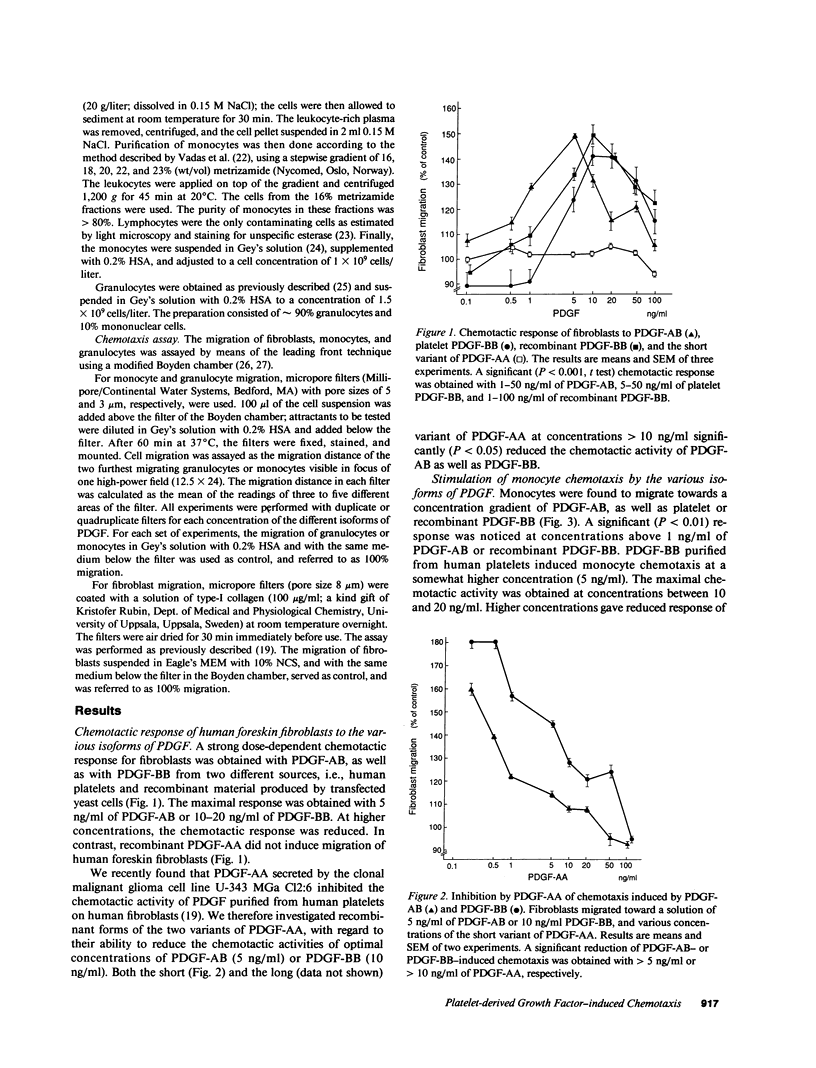
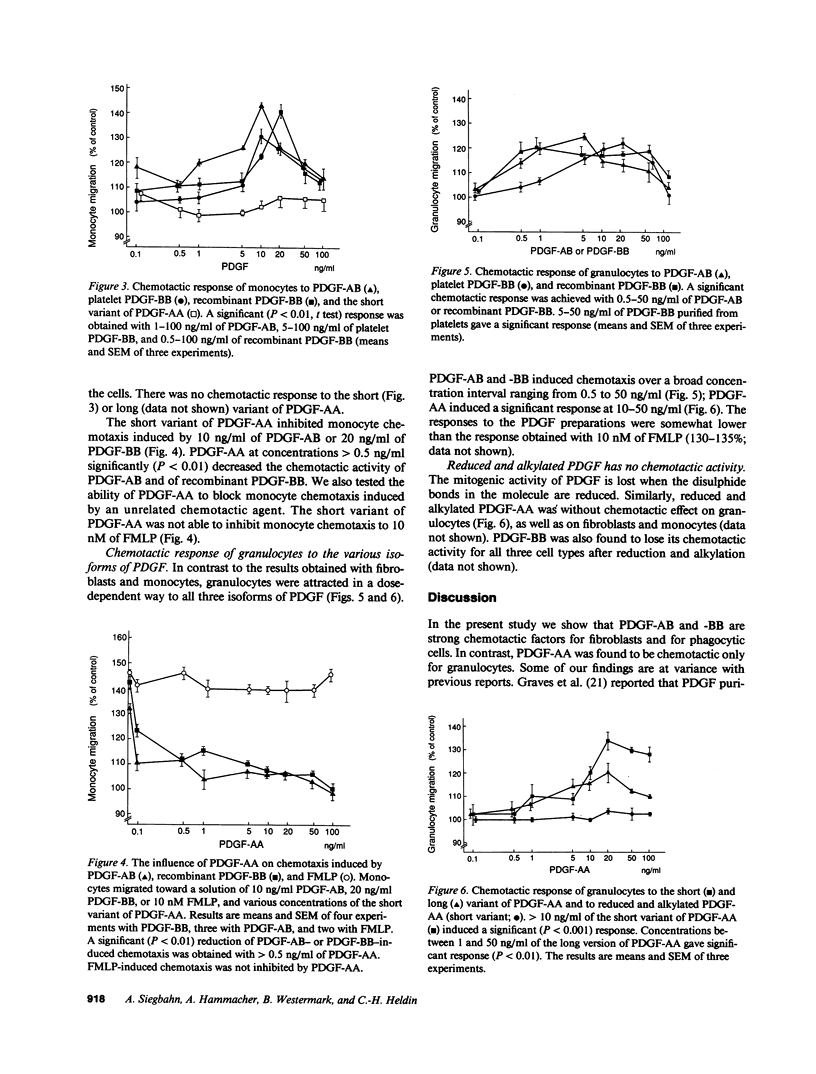
The chemotactic activities of three different isoforms of platelet-derived growth factor (PDGF) on fibroblasts, monocytes, and granulocytes of human origin were investigated. PDGF-AB and PDGF-BB induced strong, dose-dependent responses in both fibroblasts and monocytes, whereas PDGF-AA did not stimulate chemotaxis of these cell types. Instead, PDGF-AA inhibited the chemotactic activity of PDGF-AB and PDGF-BB on fibroblasts and monocytes. However, PDGF-AA was not able to block monocyte chemotaxis induced by FMLP. In contrast, in granulocytes, dose-dependent chemotactic responses were obtained with all three isoforms of PDGF. All isoforms gave maximal responses at concentrations between 5 and 20 ng/ml. At higher concentrations the migration was reduced. Reduction and alkylation of the PDGF molecule, which leads to loss of the mitogenic activity, also caused a loss of the chemotactic activities for all three cell types. The data suggest that the various isoforms of PDGF stimulate and inhibit chemotaxis in an isoform- and cell type-specific manner.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonthron D. T., Morton C. C., Orkin S. H., Collins T. Platelet-derived growth factor A chain: gene structure, chromosomal location, and basis for alternative mRNA splicing. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1492–1496. doi: 10.1073/pnas.85.5.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Hart C. E., Seifert R. A. Sera and conditioned media contain different isoforms of platelet-derived growth factor (PDGF) which bind to different classes of PDGF receptor. J Biol Chem. 1989 Feb 15;264(5):2502–2508. [PubMed] [Google Scholar]
- Deuel T. F., Senior R. M., Huang J. S., Griffin G. L. Chemotaxis of monocytes and neutrophils to platelet-derived growth factor. J Clin Invest. 1982 Apr;69(4):1046–1049. doi: 10.1172/JCI110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devreotes P. N., Zigmond S. H. Chemotaxis in eukaryotic cells: a focus on leukocytes and Dictyostelium. Annu Rev Cell Biol. 1988;4:649–686. doi: 10.1146/annurev.cb.04.110188.003245. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Wright D. G., Schiffmann E. Role of secretory events in modulating human neutrophil chemotaxis. J Clin Invest. 1978 Dec;62(6):1364–1374. doi: 10.1172/JCI109257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauss-Müller V., Kleinman H. K., Martin G. R., Schiffmann E. Role of attachment factors and attractants in fibroblast chemotaxis. J Lab Clin Med. 1980 Dec;96(6):1071–1080. [PubMed] [Google Scholar]
- Graves D. T., Grotendorst G. R., Antoniades H. N., Schwartz C. J., Valente A. J. Platelet-derived growth factor is not chemotactic for human peripheral blood monocytes. Exp Cell Res. 1989 Feb;180(2):497–503. doi: 10.1016/0014-4827(89)90076-1. [DOI] [PubMed] [Google Scholar]
- Grotendorst G. R., Seppä H. E., Kleinman H. K., Martin G. R. Attachment of smooth muscle cells to collagen and their migration toward platelet-derived growth factor. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3669–3672. doi: 10.1073/pnas.78.6.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammacher A., Hellman U., Johnsson A., Ostman A., Gunnarsson K., Westermark B., Wasteson A., Heldin C. H. A major part of platelet-derived growth factor purified from human platelets is a heterodimer of one A and one B chain. J Biol Chem. 1988 Nov 5;263(31):16493–16498. [PubMed] [Google Scholar]
- Hart C. E., Forstrom J. W., Kelly J. D., Seifert R. A., Smith R. A., Ross R., Murray M. J., Bowen-Pope D. F. Two classes of PDGF receptor recognize different isoforms of PDGF. Science. 1988 Jun 10;240(4858):1529–1531. doi: 10.1126/science.2836952. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Bäckström G., Ostman A., Hammacher A., Rönnstrand L., Rubin K., Nistér M., Westermark B. Binding of different dimeric forms of PDGF to human fibroblasts: evidence for two separate receptor types. EMBO J. 1988 May;7(5):1387–1393. doi: 10.1002/j.1460-2075.1988.tb02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldin C. H., Johnsson A., Wennergren S., Wernstedt C., Betsholtz C., Westermark B. A human osteosarcoma cell line secretes a growth factor structurally related to a homodimer of PDGF A-chains. Nature. 1986 Feb 6;319(6053):511–514. doi: 10.1038/319511a0. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Platelet-derived growth factor: three isoforms and two receptor types. Trends Genet. 1989 Apr;5(4):108–111. doi: 10.1016/0168-9525(89)90040-1. [DOI] [PubMed] [Google Scholar]
- Hoppe J., Weich H. A., Eichner W. Preparation of biologically active platelet-derived growth factor type BB from a fusion protein expressed in Escherichia coli. Biochemistry. 1989 Apr 4;28(7):2956–2960. doi: 10.1021/bi00433a032. [DOI] [PubMed] [Google Scholar]
- Mullink H., Von Blomberg M., Wilders M. M., Drexhage H. A., Alons C. L. A simple cytochemical method for distinguishing EAC rosettes formed by lymphocytes and monocytes. J Immunol Methods. 1979;29(2):133–137. doi: 10.1016/0022-1759(79)90062-0. [DOI] [PubMed] [Google Scholar]
- Nistér M., Hammacher A., Mellström K., Siegbahn A., Rönnstrand L., Westermark B., Heldin C. H. A glioma-derived PDGF A chain homodimer has different functional activities from a PDGF AB heterodimer purified from human platelets. Cell. 1988 Mar 25;52(6):791–799. doi: 10.1016/0092-8674(88)90421-7. [DOI] [PubMed] [Google Scholar]
- Ostman A., Bäckström G., Fong N., Betsholtz C., Wernstedt C., Hellman U., Westermark B., Valenzuela P., Heldin C. H. Expression of three recombinant homodimeric isoforms of PDGF in Saccharomyces cerevisiae: evidence for difference in receptor binding and functional activities. Growth Factors. 1989;1(3):271–281. doi: 10.3109/08977198908998003. [DOI] [PubMed] [Google Scholar]
- Ostman A., Rall L., Hammacher A., Wormstead M. A., Coit D., Valenzuela P., Betsholtz C., Westermark B., Heldin C. H. Synthesis and assembly of a functionally active recombinant platelet-derived growth factor AB heterodimer. J Biol Chem. 1988 Nov 5;263(31):16202–16208. [PubMed] [Google Scholar]
- Rorsman F., Bywater M., Knott T. J., Scott J., Betsholtz C. Structural characterization of the human platelet-derived growth factor A-chain cDNA and gene: alternative exon usage predicts two different precursor proteins. Mol Cell Biol. 1988 Feb;8(2):571–577. doi: 10.1128/mcb.8.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R., Raines E. W., Bowen-Pope D. F. The biology of platelet-derived growth factor. Cell. 1986 Jul 18;46(2):155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Griffin G. L., Huang J. S., Walz D. A., Deuel T. F. Chemotactic activity of platelet alpha granule proteins for fibroblasts. J Cell Biol. 1983 Feb;96(2):382–385. doi: 10.1083/jcb.96.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppä H., Grotendorst G., Seppä S., Schiffmann E., Martin G. R. Platelet-derived growth factor in chemotactic for fibroblasts. J Cell Biol. 1982 Feb;92(2):584–588. doi: 10.1083/jcb.92.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegbahn A., Garcia R. C., Kishi K., Nilsson K., Venge P. Specific binding of B-CLL cell-derived chemokinetic inhibitory factor (CIF) to human polymorphonuclear leukocytes. Eur J Haematol. 1987 Aug;39(2):172–179. doi: 10.1111/j.1600-0609.1987.tb00749.x. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Smith C. D., Verghese M. W. Model for leukocyte regulation by chemoattractant receptors: roles of a guanine nucleotide regulatory protein and polyphosphoinositide metabolism. J Leukoc Biol. 1986 Dec;40(6):785–800. doi: 10.1002/jlb.40.6.785. [DOI] [PubMed] [Google Scholar]
- Stroobant P., Waterfield M. D. Purification and properties of porcine platelet-derived growth factor. EMBO J. 1984 Dec 1;3(12):2963–2967. doi: 10.1002/j.1460-2075.1984.tb02241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracio L., Rönnstrand L., Tingström A., Rubin K., Claesson-Welsh L., Funa K., Heldin C. H. Induction of platelet-derived growth factor receptor expression in smooth muscle cells and fibroblasts upon tissue culturing. J Cell Biol. 1988 Nov;107(5):1947–1957. doi: 10.1083/jcb.107.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadas M. A., Nicola N., Lopez A. F., Metcalf D., Johnson G., Pereira A. Mononuclear cell-mediated enhancement of granulocyte function in man. J Immunol. 1984 Jul;133(1):202–207. [PubMed] [Google Scholar]
- Williams L. T., Antoniades H. N., Goetzl E. J. Platelet-derived growth factor stimulates mouse 3T3 cell mitogenesis and leukocyte chemotaxis through different structural determinants. J Clin Invest. 1983 Nov;72(5):1759–1763. doi: 10.1172/JCI111135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]



