Abstract
The heme oxygenase (HO) reaction consists of three successive oxygenation reactions, i.e. heme to α-hydroxyheme, α-hydroxyheme to verdoheme, and verdoheme to biliverdin-iron chelate. Of these, the least understood step is the conversion of verdoheme to biliverdin-iron chelate. For the cleavage of the oxaporphyrin ring of ferrous verdoheme, involvement of a verdoheme π-neutral radical has been proposed. To probe this hypothetical mechanism in the HO reaction, we performed electrochemical reduction of ferrous verdoheme complexed with rat HO-1 under anaerobic conditions. On the basis of the electrochemical spectral changes, the midpoint potential for the one-electron reduction of the oxaporphyrin ring of ferrous verdoheme was found to be −0.47 ± 0.01 V vs the normal hydrogen electrode (NHE). Because this potential is far lower than those of both flavins of NADPH-cytochrome P450 reductase, and of NADPH, it is concluded that the one-electron reduction of the oxaporphyrin ring of ferrous verdoheme is unlikely to occur and that the formation of the π-neutral radical cannot be the initial step in the degradation of verdoheme by HO. Rather, it appears more reasonable to consider an alternative mechanism in which binding of O2 to the ferrous iron of verdoheme is the first step in the degradation of verdoheme.
Keywords: Heme oxygenase, Verdoheme, Redox potential, Verdoheme π-neutral radical, Oxaporphyrin ring cleavage
INTRODUCTION
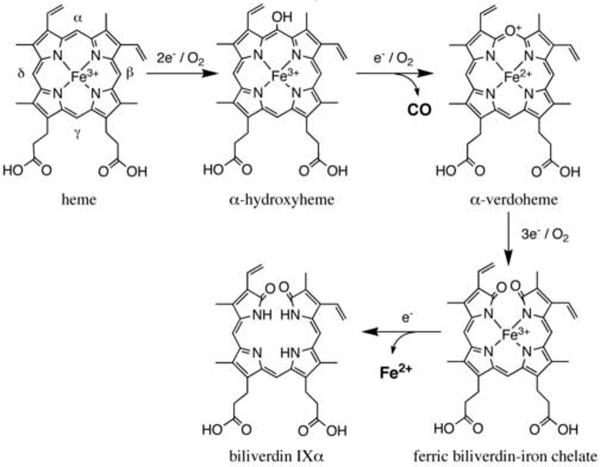
Heme oxygenase (HO1, EC 1.14.99.3) is a microsomal enzyme that catalyzes the O2-dependent degradation of heme to biliverdin IXa, carbon monoxide (CO) and free iron at the expense of molecular oxygen and electrons provided by NADPH-cytochrome P450 reductase (CPR, EC 1.6.2.4) [1–3]. The degradation of heme to biliverdin by HO proceeds through a multi-step mechanism as shown in Fig. 1 [4, 5]. The first step is the oxidation of heme to α-hydroxyheme, requiring O2 and two electrons [6–8]. The second step, also requiring O2 and an electron, is the formation of ferrous α-verdoheme from α-hydroxyheme with the concomitant release of hydroxylated α-meso carbon as CO [9–12]. The third step is the conversion of ferrous α-verdoheme to a ferric biliverdin-iron chelate for which, once again, O2 and three electrons are required [13]. In the last step, the ferric iron of the biliverdin-iron chelate is reduced and finally biliverdin and ferrous ion are released from HO.
Fig. 1.
Degradation of heme catalyzed by HO. HO catalyzes the degradation of heme to biliverdin IXαthrough three distinct intermediates, α-hydroxyheme, α-verdoheme and biliverdin-iron chelate at the expense of O2 and reducing equivalents.
The conversion of verdoheme to the ferric biliverdin-iron chelate along with the release of biliverdin is the rate-limiting step in the HO reaction [14]. The degradation of verdoheme is the least understood of the three successive oxygenation reactions during HO catalysis [13, 15]. It has been well known that hydrolysis of verdoheme to biliverdin can take place in acidic solution [11]. However, the enzymatic degradation of verdoheme to ferric biliverdin-iron chelate by HO requires O2 and reducing equivalents [16–18] and both lactam oxygen atoms present in the biliverdin-iron chelate derive from molecular oxygen [19, 20].
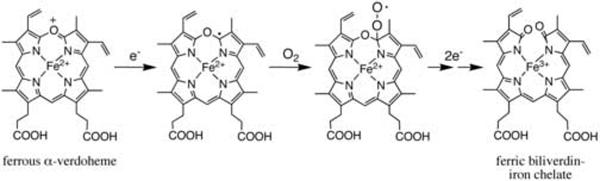
Involvement of a ferrous verdoheme π-neutral radical, i.e. a one-electron reduced form of the oxaporphyrin ring of ferrous verdoheme, to which O2 binds at α-pyrrole carbon, has been proposed as a possible mechanism for the cleavage of the oxaporphyrin ring of ferrous verdoheme [21], as shown in Fig. 2. This proposal is based on the report that the ferrous verdoheme π-neutral radical can be generated in organic solvent by electrochemical reduction of ferrous verdoheme [22]. The redox potential of the bispyridine form of ferrous verdoheme dimethyl ester was −0.903 V vs Ag/Ag+ (−0.269 V vs the normal hydrogen electrode (NHE)) in dimethylformamide/pyridine (6:4) plus 0.1 M tetrα-n-butylammonium perchlorate. The one-electron reduction product was confirmed to be a π-neutral radical species by electron spin resonance. This redox potential value suggests that verdoheme in complex with rat HO-1 (rHO-1) may be reduced by CPR, because the redox potential of FMN (semiquinone/reduced) in rat CPR is −0.270 vs NHE [23]. However, in the degradation of ferrous verdoheme complexed with rHO-1 in the NADPH/CPR system, a spectrum such as that of ferrous verdoheme π-neutral radical has never been observed [8]. On the other hand, the binding of O2 to the ferrous iron of the verdoheme-rHO-1 complex has been hypothesized as an alternative mechanism for the HO reaction [5, 15]. Matsui et al. have recently claimed that they succeeded in detecting the O2- and H2O2-binding forms on the verdoheme iron of ferrous verdoheme-rHO-1 complex [13].
Fig. 2.
Possible reaction mechanism for degradation of ferrous verdoheme to ferric biliverdin-iron chelate.
This situation prompted us to carry out electrochemical titration experiments on ferrous verdoheme-rHO-1 complex as well as heme-rHO-1 complex in order to probe whether or not the one-electron reduction of ferrous verdoheme-rHO-1 complex by CPR is thermodynamically favorable. Here we present electrochemical evidence that the reduction by CPR of the oxaporphyrin ring of ferrous verdoheme complexed with HO is unlikely to occur from the thermodynamic point of view, and conclude that the formation of ferrous verdoheme π-neutral radical cannot be the initial step in the degradation of verdoheme by HO.
EXPERIMENTAL
Materials
Phenazine methosulfate, vitamin K1, anthraquinone-2-sulfonate, benzyl viologen and safranin T were purchased from Nacalai Tesque (Kyoto, Japan), and methyl viologen and 2-hydroxy-1, 4-naphthoquinone from Tokyo Chemical Industry (Tokyo, Japan). Hemin was obtained from Sigma. Ferrous α-verdoheme (hereafter referred to as verdoheme) was synthesized and purified as reported previously [11]. Formation of ferrous verdoheme was confirmed by its optical absorption spectrum (λmax in aqueous pyridine solution: 397, 505, 534 and 679 nm). Concentration of the bispyridine complex of verdoheme was determined spectrophotometrically using ε397 = 53.3 mM−1cm−1 [11]. All spectrophotometric analyses were conducted on a Varian Cary 50 Bio UV-visible spectrophotometer at 25 °C.
Preparation of heme- and ferrous verdoheme-rHO-1 complexes
A soluble form of rHO-1 lacking the 22-amino acid C-terminal hydrophobic segment was expressed in Escherichia coli and purified as described previously [24]. The rHO-1 was reconstituted with 1.2 equiv. of heme and purified by hydroxyapatite (Bio-Rad) column chromatography as previously described [24].
The reconstitution and purification of verdoheme-rHO-1 complex were performed as reported previously [11]. Unless otherwise stated, the following manipulations were carried out anaerobically in a UNILAB glove box system (MBRAUN, Garching, Germany) filled with N2 gas. Briefly, to rHO-1 solution (30 μM) in 0.1 M potassium phosphate buffer, pH 7.0, was added a slight excess of ferrous verdoheme dissolved in pyridine. The mixture was incubated at 2 °C for 1 h, and then to remove pyridine and unbound verdoheme, ultrafiltration on Microcon YM-10 filter (Millipore) and dilution with 0.1 M potassium phosphate, pH 7.0, were repeated three times. The ferrous verdoheme-rHO-1 complex in 0.1 M potassium phosphate buffer, pH 7.0, exhibited absorption maxima at 400 (ε = 50.2 mM−1 cm−1), 534 and 688 nm [13].
Electrochemical-optical experiments of heme- and verdoheme-rHO-1 complexes
An optically transparent thin-layer electrode cell (6 (height) × 10 (width) × 1 (thickness) mm), in which a mesh working electrode (6 × 7 × 0.2 mm) of platinum for the heme-rHO-1 complex or of gold for the verdoheme-rHO-1 complex, a platinum wire counter electrode and an Ag/AgCl (3 M NaCl) reference electrode were accommodated, was employed for electrochemical-optical experiments (BAS, Tokyo, Japan). The optical path length of the electrochemical cell was 1 mm. The applied potential was controlled by a potentiostat (ALS/CH Instruments electrochemical analyzer-model 600B (BAS, Tokyo, Japan)) and optical absorption spectra were recorded on a Varian Cary 50 Bio UV-visible spectrophotometer.
For electrochemical redox titration of heme-rHO-1 complex, the following electron mediator dyes were included in the sample solution: 15 μM phenazine methosulfate (Em = +0.080 V vs NHE, [25]), 15 μM vitamin K1 (Em = −0.078 V vs NHE, [26]), 15 μM 2-hydroxy-1, 4-naphthoquinone (Em = −0.120 V vs NHE, [27]), 15 μM anthraquinone-2-sulfonate (Em = −0.230 V vs NHE [25]), 15 μM benzyl viologen (Em = −0.350 V vs NHE [25]) and 15 μM methyl viologen (Em = −0.440 V vs NHE, [28]). A heme-rHO-1 complex solution (50 μM) in 0.1 M potassium phosphate buffer, pH 7.0, containing the above mediator dyes was prepared under anaerobic conditions and then a 0.2 ml portion of the sample solution was anaerobically transferred into the electrochemical cell. The electrochemical cell was kept at 25°C in the thermostatic cell holder of the spectrophotometer during electrochemical titration. Absorption spectral changes from 300 to 700 nm were recorded to monitor the oxidation reaction of the heme-rHO-1 complex. The spectra of fully reduced and fully oxidized heme-rHO-1 complex were obtained by applying the potentials of −0.395 V vs NHE and +0.205 V vs NHE, respectively. The data were collected by starting from a fully reduced to a fully oxidized form. It was confirmed that this redox reaction was completely reversible electrochemically. The midpoint potential of the ferric/ferrous heme couple in heme-rHO-1 complex was calculated from the Nernst plot using the least-square method.
For electrochemical redox titration of ferrous verdoheme-rHO-1 complex, the following redox mediator dyes were included in the sample solution: 3 μM safranin T (Em = −0.290 V vs NHE [25]), 10 μM benzyl viologen, and 10 μM methyl viologen. A solution of ferrous verdoheme-rHO-1 complex (0.1 mM) in 0.1 M potassium phosphate buffer, pH 7.0, containing 0.1 M KCl and the mediator dyes listed above was prepared under anaerobic conditions. Then, a 0.2 ml portion of the sample solution was anaerobically transferred into the electrochemical cell. Absorption spectral changes in the region from 300 to 900 nm were recorded at 25°C to monitor the electrochemical reduction reaction of the ferrous verdoheme-rHO-1 complex. The data were fitted to the Nernst equation using the least-square method.
RESULTS
Electrochemical titration of heme-rHO-1 complex under anaerobic conditions
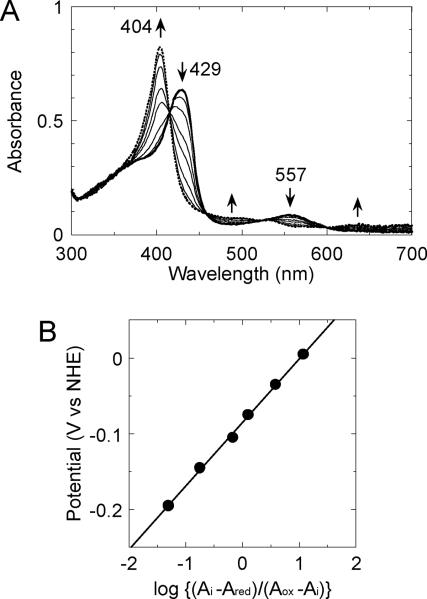
To obtain the redox potential of the heme-rHO-1 complex, we performed the electrochemical oxidation of the ferrous heme complexed with rHO-1 (Fig. 3A). During the electrochemical oxidation of the ferrous heme-rHO-1 complex that had been obtained at an applied potential of −0.395 V vs NHE, the Soret absorption peak at 429 nm and the visible peak at 557 nm decreased and a new Soret peak at 404 nm increased with isosbestic points at 415, 458, 523, and 600 nm. These spectral changes are consistent with oxidation of ferrous heme to ferric heme complexed with rHO-1 [29]. The data were collected by starting from a fully reduced proceeding to the fully oxidized form. It was confirmed that this redox reaction was completely reversible electrochemically (data not shown). From the Nernst plot constructed from the absorbance change at 405 nm of the heme-rHO-1 complex as a function of the applied potentials, the midpoint potential for the ferric/ferrous heme couple in the heme-rHO-1 complex was determined to be −0.087 ± 0.005 V vs NHE (Fig. 3B). This midpoint potential value agreed closely with those reported previously, i.e. −0.076 V vs NHE for heme-rHO-1 complex [30] and −0.065 V vs NHE for heme-human HO-1 complex [31] (Table 1). The slope of the Nernst plot, ~0.084, indicated that this process is a one–electron oxidation of ferrous heme.
Fig. 3.
Electrochemical oxidation of heme-rHO-1 complex under anaerobic conditions. (A) Absorption spectra of the heme-rHO-1 complex (50 μM) during the electrochemical-optical titration at 25 °C were recorded. The experimental conditions and procedures are described in the EXPERIMENTAL section. Applied potentials were: from thick solid line to dotted line, −0.395, −0.195, −0.145, −0.105, −0.075, −0.035, +0.005, and +0.205 V vs NHE. (B) The Nernst plot obtained from the absorbance change at 405 nm of the heme-rHO-1 complex.
Table 1.
Midpoint potentials of heme- and verdoheme-rHO-1 complexes and related compounds at pH 7.0.
| Compound | Em(V vs NHE) | Reference |
|---|---|---|
| heme (complexed with rHO-1) | −0.087 ± 0.005 | this work |
| −0.076 | [30] | |
| heme (complexed with human HO-1) | −0.065 | [31] |
| verdoheme (complexed with rHO-1) | −0.47 ± 0.01 | this work |
| verdoheme dimethyl ester (in dimethylformamide /pyridine (6:4)) | −0.269 | [22] |
| FMN (oxidized/semiquinone, rat CPR) | −0.110 | [23] |
| FMN (semiquinone/reduced, rat CPR) | −0.270 | [23] |
| FAD (oxidized/semiquinone, rat CPR) | −0.290 | [23] |
| FAD (semiquinone/reduced, rat CPR) | −0.365 | [23] |
| NADPH | −0.324 | |
| Na2S2O4 | −0.66 | [39] |
Electrochemical reduction of verdoheme-rHO-1 complex under anaerobic conditions
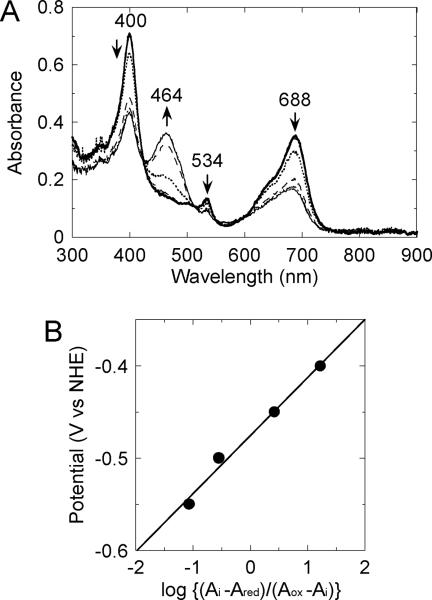
As shown in Fig. 4A, during the electrochemical reduction of the ferrous verdoheme-rHO-1 complex, the 400-, 534- and 688-nm absorption peaks decreased and concomitantly a new absorption peak at 464 nm appeared. The absorption spectrum recorded at the applied potential of −0.60 V vs NHE has never been observed in the normal HO reaction. Rather it bore close resemblance to the absorption spectrum of the π-neutral radical of ferrous verdoheme dimethyl ester in dimethylformamide/pyridine (6:4) reported by Tajima et al. [22]. Thus we judged that a π-neutral radical of ferrous verdoheme in complex with rHO-1 was generated by the electrochemical reduction. This redox reaction was only partially reversible (data not shown), probably due to a reaction leading to nonproductive decomposition of verdoheme, that does not lead to biliverdin [8]. While the immediate product of the reaction has yet to be characterized, a two-electron-reduced anion form of ferrous verdoheme has been indicated as a possible candidate [32]. Indeed, applying potentials below −0.60 V vs NHE caused further spectral changes, and this over-reduction was completely irreversible (data not shown). From the Nernst plot constructed from the absorbance change at 688 nm as a function of the applied potentials, the midpoint potential for the oxaporphyrin ring of verdoheme complexed with rHO-1 was determined to be −0.47 ± 0.01 V vs NHE (Fig. 4B, Table 1). The slope of the Nernst plot, ~0.063, indicated that this process is a one-electron reduction.
Fig. 4.
Electrochemical reduction of ferrous verdoheme-rHO-1 complex under anaerobic conditions. (A) Absorption spectra of the verdoheme-rHO-1 complex (0.1 mM) during the electrochemical-optical titration at 25 °C were recorded. The experimental conditions and procedures are described in the EXPERIMENTAL section. Applied potentials were: +0.22 (rest potential, ), −0.40 ( -.-.-.- ), −0.45 ( …….. ), −0.50 (- - - -), -0.55 ( -…-…- ), and -0.60 V (
), −0.40 ( -.-.-.- ), −0.45 ( …….. ), −0.50 (- - - -), -0.55 ( -…-…- ), and -0.60 V ( ) vs NHE. (B) The Nernst plot obtained from the absorbance change at 688 nm of the ferrous verdoheme-rHO-1 complex.
) vs NHE. (B) The Nernst plot obtained from the absorbance change at 688 nm of the ferrous verdoheme-rHO-1 complex.
DISCUSSION
CPR is an FAD- and FMN-containing protein that functions physiologically as an electron donor for the HO reaction as well as for a variety of other hemoproteins such as cytochrome P450s and cytochrome b5. With the latter hemoproteins, it is established that electrons from NADPH flow first to FAD then to FMN and finally to their heme groups [33–35]. The midpoint reduction potentials of the flavin cofactors in mammalian CPRs have been determined [23, 36]. With rat CPR, they are Em(FADsemiquinone/reduced) = −0.365 V, Em(FADoxidized/semiquinone) = −0.290 V, Em(FMNsemiquinone/reduced) = −0.270 V and Em(FMNoxidized/semiquinone) = −0.110 V vs NHE [23] (see also Table 1). On the other hand, the midpoint potentials of substrate (hexobarbital)-bound rat liver cytochrome P450 and microsomal cytochrome b5 have been reported to be −0.237 V [37] and 0.02 V vs NHE [38], respectively. In the present study, the midpoint potential of the ferric/ferrous couple in the heme-rHO-1 complex was found to be −0.087 V vs NHE. Thus the reduction of the heme-rHO-1 complex, as well as of cytochrome P450s and cytochrome b5, by CPR is a thermodynamically favorable process.
One-electron reduction of the bispyridine form of ferrous verdoheme dimethyl ester produces a π-neutral radical of the ferrous verdoheme dimethyl ester in dimethylformamide/pyridine (6:4) plus 0.1 M tetra-n-butylammonium perchlorate, the redox potential of which is −0.903 V vs Ag/Ag+ (−0.269 V vs NHE) [22]. This redox potential value is close to the midpoint potential of FMNsemiquinone/reduced in rat CPR, i.e. −0.270 V vs NHE. Thus we carried out the electrochemical titration of verdoheme in complex with rHO-1 to probe whether or not a π-neutral radical of the ferrous verdoheme could be an intermediate during HO catalysis. From the spectral similarity between the absorption spectrum of ferrous verdoheme in complex with rHO-1 at the applied potential of −0.60 V vs NHE and that of the π-neutral radical of ferrous verdoheme dimethyl ester [22], we concluded that the reduced form of ferrous verdoheme in complex with rHO-1 is a π-neutral radical of the oxaporphyrin ring of verdoheme with a midpoint potential of −0.47 V vs NHE; this is the first report of a redox potential for an intermediate species of the HO reaction. The redox potential of the oxaporphyrin ring of the ferrous verdoheme-rHO-1 complex was far lower than those of both flavins of CPR, and of NADPH (Table 1). It should be noted that a verdoheme π-neutral radical-like spectrum does not appear in the NADPH/CPR-supported degradation of ferrous verdoheme complexed with rHO-1 [8]. Thus, we consider it very unlikely that CPR can catalyze the one-electron reduction of the oxaporphyrin ring of ferrous verdoheme in complex with rHO-1.
Sodium dithionite (Na2S2O4) is a strong reductant with an Em of −0.66 V vs NHE at pH 7.0 and 25 °C [39] (Table 1), and should be capable of reducing the oxaporphyrin ring of the ferrous verdoheme-rHO-1 complex. Indeed, we previously observed a spectrum similar to that shown in Fig. 4A when the verdoheme-rHO-1 complex was reduced with Na2S2O4 under anaerobic conditions and tentatively assigned this spectrum to a π-neutral radical of verdoheme [8].
In summary, it is concluded that the one-electron reduction of the oxaporphyrin ring of ferrous verdoheme cannot be the initial step in the verdoheme degradation by HO. Rather, it appears more reasonable to consider an alternative mechanism in which the binding of O2 to the ferrous iron of verdoheme is the initial step in the degradation of verdoheme [13, 15]. If this is the case, it would be of great interest to determine how electrons are transferred from CPR to the O2-bound ferrous verdoheme complexed with HO. In this connection, we have recently reported, with the aid of FMN-depleted CPR, that the electrons required for verdoheme oxidation can be supplied through a pathway not involving FMN, probably via FAD [40].
ACKNOWLEDGMENTS
This work was supported in part by Grants-in-Aid for Young Scientists 19750150 (to H. Sato) and 18770121 (to Y. H.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, by Grants-in-Aid for Scientific Research 18590278 (to M. N.) and 18550153 (to H. Sakamoto) and for Research Fellow 17-5063 (to M. S.) from the Japan Society for the Promotion of Science, by National Institute of Health Grant GM080575 (to G. P.), and by a grant from the Morikazu Kaibara Medical Science Promotion Foundation (to Y. H.).
ABBREVIATIONS
- HO
heme oxygenase
- heme
iron protoporphyrin IX either ferrous or ferric form
- CPR
NADPH-cytochrome P450 reductase
- NHE
the normal hydrogen electrode
- rHO-1
a soluble form of rat HO-1 lacking the 22-amino acid C-terminal hydrophobic segment
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Tenhunen R, Marver HS, Schmid R. J. Biol. Chem. 1969;244:6388–6394. [PubMed] [Google Scholar]
- [2].Schacter BA, Nelson EB, Marver HS, Masters BSS. J. Biol. Chem. 1972;247:3601–3607. [PubMed] [Google Scholar]
- [3].Yoshida T, Noguchi M, Kikuchi G. J. Biol. Chem. 1980;255:4418–4420. [PubMed] [Google Scholar]
- [4].Ortiz de Montellano PR, Auclair K. In: Porphyrin Handbook. Kadish K, Smith K, Guilard R, editors. vol. 12. Academic Press Inc.; New York: 2003. pp. 183–210. [Google Scholar]
- [5].Ortiz de Montellano PR. Curr. Opin. Chem. Biol. 2000;4:221–227. doi: 10.1016/s1367-5931(99)00079-4. [DOI] [PubMed] [Google Scholar]
- [6].Yoshida T, Noguchi M, Kikuchi G, Sano S. J. Biochem. (Tokyo) 1981;90:125–131. doi: 10.1093/oxfordjournals.jbchem.a133441. [DOI] [PubMed] [Google Scholar]
- [7].Sakamoto H, Omata Y, Palmer G, Noguchi M. J. Biol. Chem. 1999;274:18196–18200. doi: 10.1074/jbc.274.26.18196. [DOI] [PubMed] [Google Scholar]
- [8].Sakamoto H, Omata Y, Hayashi S, Harada S, Palmer G, Noguchi M. Eur. J. Biochem. 2002;269:5231–5239. doi: 10.1046/j.1432-1033.2002.03230.x. [DOI] [PubMed] [Google Scholar]
- [9].Yoshida T, Noguchi M, Kikuchi G. J. Biol. Chem. 1982;257:9345–9348. [PubMed] [Google Scholar]
- [10].Mansfield Matera K, Takahashi S, Fujii H, Zohu H, Ishikawa K, Yoshimura T, Rousseau DL, Yoshida T, Ikeda-Saito M. J. Biol. Chem. 1996;271:6618–6624. doi: 10.1074/jbc.271.12.6618. [DOI] [PubMed] [Google Scholar]
- [11].Sakamoto H, Omata Y, Adachi Y, Palmer G, Noguchi M. J. Inorg. Biochem. 2000;82:113–121. doi: 10.1016/s0162-0134(00)00149-5. [DOI] [PubMed] [Google Scholar]
- [12].Sakamoto H, Takahashi K, Higashimoto Y, Harada S, Palmer G, Noguchi M. Biochem. Biophys. Res. Commun. 2005;338:578–583. doi: 10.1016/j.bbrc.2005.08.176. [DOI] [PubMed] [Google Scholar]
- [13].Matsui T, Nakajima A, Fujii H, Mansfield Matera K, Migita CT, Yoshida T, Ikeda-Saito M. J. Biol. Chem. 2005;280:36833–36840. doi: 10.1074/jbc.M503529200. [DOI] [PubMed] [Google Scholar]
- [14].Liu Y, Ortiz de Montellano PR. J. Biol. Chem. 2000;275:5297–5307. doi: 10.1074/jbc.275.8.5297. [DOI] [PubMed] [Google Scholar]
- [15].Lad L, Ortiz de Montellano PR, Poulos TL. J. Inorg. Biochem. 2004;98:1686–1695. doi: 10.1016/j.jinorgbio.2004.07.004. [DOI] [PubMed] [Google Scholar]
- [16].Wilks A, Ortiz de Montellano PR. J. Biol. Chem. 1993;268:22357–22362. [PubMed] [Google Scholar]
- [17].Saito S, Itano HA. Proc. Natl. Acad. Sci. USA. 1982;79:1393–1397. doi: 10.1073/pnas.79.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yoshida T, Noguchi M. J. Biochem. (Tokyo) 1984;96:563–570. doi: 10.1093/oxfordjournals.jbchem.a134868. [DOI] [PubMed] [Google Scholar]
- [19].Docherty JC, Firneisz GD. Arch. Biochem. Biophys. 1984;235:657–664. doi: 10.1016/0003-9861(84)90241-8. [DOI] [PubMed] [Google Scholar]
- [20].Docherty JC, Schacter BA, Firneisz GD, Brown SB. J. Biol. Chem. 1984;259:13066–13069. [PubMed] [Google Scholar]
- [21].Ortiz de Montellano PR. Acc. Chem. Res. 1998;31:543–549. [Google Scholar]
- [22].Tajima K, Shimizu K, Mano H, Mukai K, Azuma N. J. Chem. Soc., Chem. Commun. 1995;1995:601–603. [Google Scholar]
- [23].Vermilion JL, Coon MJ. J. Biol. Chem. 1978;253:2694–2704. [PubMed] [Google Scholar]
- [24].Omata Y, Asada S, Sakamoto H, Fukuyama K, Noguchi M. Acta Crystallogr. D Biol. Crystallogr. 1998;54:1017–1019. doi: 10.1107/s0907444998003448. [DOI] [PubMed] [Google Scholar]
- [25].Zahn JA, Arciero DM, Hooper AB, Dispirito AA. Eur. J. Biochem. 1996;240:684–691. doi: 10.1111/j.1432-1033.1996.0684h.x. [DOI] [PubMed] [Google Scholar]
- [26].Wagner GC, Kassner RJ, Kamen MD. Proc. Natl. Acad. Sci. USA. 1974;71:253–256. doi: 10.1073/pnas.71.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Baymann F, Moss DA, Mäntele W. Anal. Biochem. 1991;199:267–274. doi: 10.1016/0003-2697(91)90100-8. [DOI] [PubMed] [Google Scholar]
- [28].Okura I, Kaji N, Aono S, Kita T, Yamada A. Inorg. Chem. 1985;24:451–453. [Google Scholar]
- [29].Yoshida T, Kikuchi G. J. Biol. Chem. 1979;254:4487–4491. [PubMed] [Google Scholar]
- [30].Omata Y, Noguchi M. In: Oxygen Homeostasis and Its Dynamics. Ishimura Y, Shimada H, Suematsu M, editors. Springer-Verlag; Tokyo: 1998. pp. 322–327. [Google Scholar]
- [31].Liu Y, Moenne-Loccoz P, Hildebrand DP, Wilks A, Loehr TM, Mauk AG, Ortiz de Montellano PR. Biochemistry. 1999;38:3733–3743. doi: 10.1021/bi982707s. [DOI] [PubMed] [Google Scholar]
- [32].Damaso CO, Rubie ND, Moënne-Loccoz P, Rivera M. Inorg. Chem. 2004;43:8470–8478. doi: 10.1021/ic049029k. [DOI] [PubMed] [Google Scholar]
- [33].Strobel HW, Hodgson AV, Shen S. In: Cytochrome P450. Ortiz de Moltellano PR, editor. Plenum Press; New York: 1995. pp. 225–244. [Google Scholar]
- [34].Murataliev MB, Feyereisen R, Walker FA. Biochim. Biophys. Acta. 2004;1698:1–26. doi: 10.1016/j.bbapap.2003.10.003. [DOI] [PubMed] [Google Scholar]
- [35].Iyanagi T. Biochem. Biophys. Res. Commun. 2005;338:520–528. doi: 10.1016/j.bbrc.2005.08.043. [DOI] [PubMed] [Google Scholar]
- [36].Munro AW, Noble MA, Robledo L, Daff SN, Chapman SK. Biochemistry. 2001;40:1956–1963. doi: 10.1021/bi001718u. [DOI] [PubMed] [Google Scholar]
- [37].Sliger SG, Cinti DL, Gibson GG, Schenkman JB. Biochem. Biophys. Res. Commun. 1979;90:925–932. doi: 10.1016/0006-291x(79)91916-8. [DOI] [PubMed] [Google Scholar]
- [38].Velick SF, Strittmatter P. J. Biol. Chem. 1956;221:265–276. [PubMed] [Google Scholar]
- [39].Mayhew SG. Eur. J. Biochem. 1978;85:535–547. doi: 10.1111/j.1432-1033.1978.tb12269.x. [DOI] [PubMed] [Google Scholar]
- [40].Higashimoto Y, Sato H, Sakamoto H, Takahashi K, Palmer G, Noguchi M. J. Biol. Chem. 2006;281:31659–31667. doi: 10.1074/jbc.M606163200. [DOI] [PubMed] [Google Scholar]