SUMMARY
The mammalian PCP pathway regulates diverse developmental processes requiring coordinated cellular movement, including neural tube closure and cochlear stereociliary orientation. Here, we show that epidermal wound repair is regulated by PCP signaling. Mice carrying mutant alleles of PCP genes Vangl2, Celsr1, PTK7, and Scrb1, and the transcription factor Grhl3, interact genetically, exhibiting failed wound healing, neural tube defects and disordered cochlear polarity. Using phylogenetic analysis, ChIP, and gene expression in Grhl3−/− mice, we identified RhoGEF19, a homologue of a RhoA activator involved in PCP signaling in Xenopus, as a direct target of GRHL3. Knockdown of Grhl3 or RhoGEF19 in keratinocytes induced defects in actin polymerisation, cellular polarity and wound healing, and re-expression of RhoGEF19 rescued these defects in Grhl3-kd cells. These results define a role for Grhl3 in PCP signaling, and broadly implicate this pathway in epidermal repair.
Highlights.
Grhl3 is a component of the PCP signaling pathway
Grhl3 acts in this pathway through the RhoA activator, RhoGEF19
PCP signalling regulates mammalian embryonic epidermal wound repair
INTRODUCTION
The PCP pathway governs a diverse range of cellular and developmental events that require coordinated orientation and movement of cells within a plane of epithelium (Axelrod and McNeill, 2002; Seifert and Mlodzik, 2007; Wu and Mlodzik, 2009; Zallen, 2007). In Drosophila, PCP is evident in the organization of cuticular structures, such as wing hairs and body bristles, and in the ommatidial clusters of the eye (Adler, 2002; Klein and Mlodzik, 2005). PCP signaling occurs through the serpentine receptor frizzled (fz), and requires the cytoplasmic factors dishevelled (dsh), and prickle (pk), the transmembrane protein Van Gogh/Strabismus (Vang/Stbm), the cadherin starry night/flamingo (stan/fmi), and the Ankyrin repeat protein Diego (Dgo), known collectively as the core PCP proteins (Adler, 2002; Seifert and Mlodzik, 2007). Downstream of these core factors are effector molecules that act in many distinct contexts. These include the small GTPases of the Rho subfamily (RhoA and Rac1), and the Rho-associated kinase (rok) that provide essential links to the actin cytoskeleton (Fanto et al., 2000; Strutt et al., 1997; Winter et al., 2001).
In vertebrates, the epidermis provides a conspicuous example of PCP, as evidenced by the pattern of scales, feathers and hairs in different organisms. The organization of internal tissues is also dependent on this pathway, as demonstrated by the regimented orientation of stereocilia of the sensory hair cells of the cochlea (Curtin et al., 2003; Dabdoub et al., 2003; Montcouquiol et al., 2003). The process of narrowing and lengthening the embryonic axis in gastrulation and neurulation by convergent extension (CE) has also been linked to the PCP pathway, with all of the vertebrate homologs of Drosophila core PCP genes, and many of the downstream effectors implicated in this process (Keller, 2002; Wallingford et al., 2000). In addition, vertebrate genes that lack Drosophila homologs involved in PCP, such as PTK7 (Lu et al., 2004), have been shown to exhibit defects in CE. In mice, perturbed CE may result in a shortened longitudinal embryonic axis, craniorachischisis, in which the neural tube fails to close from the hindbrain to the most caudal extremity of the embryo (Curtin et al., 2003; Hamblet et al., 2002; Kibar et al., 2001), and diminished elongation of the cochlea, resulting in misorientation of stereocilia (Wang et al., 2005).
Mammalian wound healing is another process requiring coordinated cell movement in the plane of epithelia (Martin and Parkhurst, 2004). Integral to this process is regulation of the actin cytoskeleton mediated through the small Rho GTPases (Brock et al., 1996; Fukata et al., 2003; Van Aelst and Symons, 2002). The small GTPases also play a critical role in PCP signaling in both Drosophila and vertebrates (Habas et al., 2001; Strutt et al., 1997; Yan et al., 2009), leading to the hypothesis that the PCP pathway could be important for epidermal repair. This hypothesis was strengthened with the analysis of mice lacking the Grainy head-like 3 (Grhl3) gene. Grhl3 is a member of a family of developmental transcription factors that includes the Drosophila gene grainy head (grh), which is essential for the fz pathway, and has also been implicated wound healing in the fly (Lee and Adler, 2004; Mace et al., 2005; Wilanowski et al., 2002; Ting et al., 2003a). Mice lacking Grhl3 exhibit severe NTD (Ting et al., 2003b), and a marked disturbance in formation, maintenance and repair of the epidermal barrier (Ting et al., 2005). Based on these findings, we postulated that Grhl3 was a component of the mammalian PCP pathway, and that this pathway may regulate epidermal wound healing in mammals.
RESULTS
Grhl3 and Vangl2 interact genetically in neural tube closure and cochlear hair cell orientation
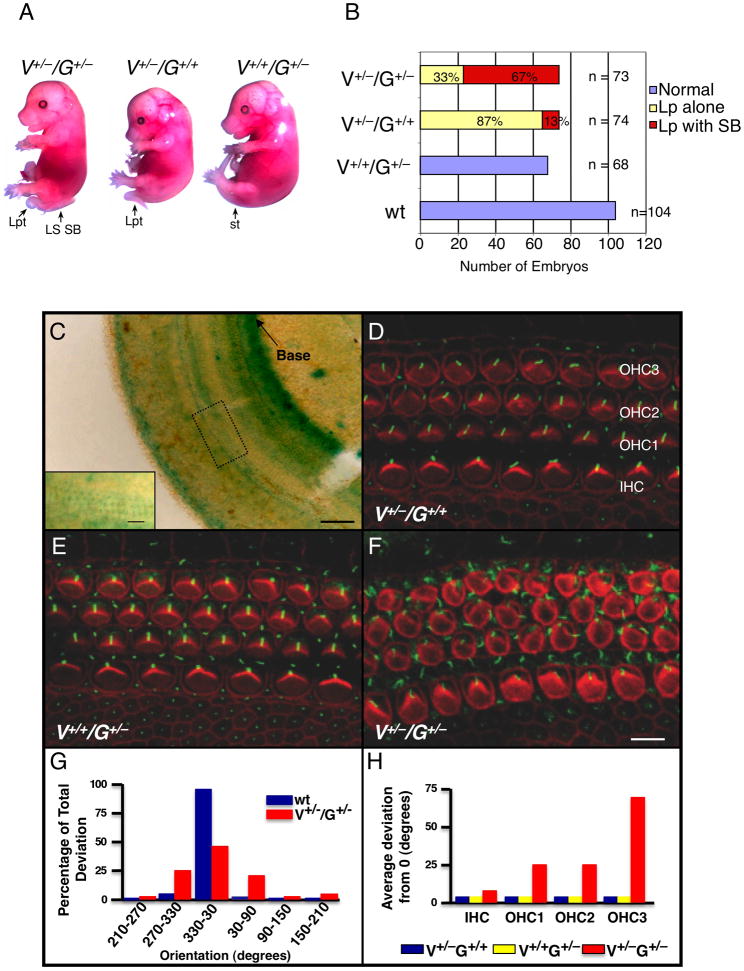
The NTD in mice lacking Grhl3 is accompanied by marked shortening of their longitudinal axis, and a widened midline (see Figures S1A, S1B available online) (Ting et al., 2003b). Similar, but more severe morphological appearances are observed in many of the mouse mutants of PCP genes including, Vangl2 (mutated in the loop-tail (Lp) mouse (Kibar et al., 2001)); Scrb1, (mutated in the circletail (Crc) mouse (Murdoch et al., 2003)); Celsr1, (mutated in the spin cycle (Scy) and crash (Crsh) mice (Curtin et al., 2003)); double null mutants of two of the Dishevelled (Dvl) genes (Dvl1−/−/Dvl2−/−) (Hamblet et al., 2002); and PTK7 (Lu et al., 2004). In view of these phenotypic similarities, and the known role of grh in PCP (Lee and Adler, 2004), we interbred Grhl3+/− mice, which display no neural tube, skin barrier or wound healing defects, with Vangl2+/− mice, which exhibit a loop of the tail and rarely, low sacral spina bifida (Murdoch et al., 2001). We observed that 67% of the Vangl2+/−/Grhl3+/−compound heterozygotes harvested between embryonic day (E) 15.5 and E18.5 exhibited NTD that were lumbo-sacral or high sacral spina bifida (Figure 1A, left embryo). This effect was specific, as no Vangl2+/+/Grhl3+/−embryos exhibited NTD (Figure 1A, right embryo). In 87% of the Vangl2+/− /Grhl3+/+ embryos, we observed a loop tail alone phenotype (Figure 1A, middle embryo), with the remaining 13% exhibiting very low sacral spina bifida (data not shown). The cumulative results of these intercrosses are shown in Figure 1B. This trans-heterozygous interaction suggests that Grhl3 and Vangl2 may function in a common genetic pathway to regulate PCP. This interaction appears less strong than that reported between Vangl2+/− and Scrb1+/− embryos, where a subset of mice display craniorachischisis (Montcouquiol et al., 2003), but is similar to the interaction between Vangl2 and PTK7, which also manifests as incompletely penetrant spina bifida (Lu et al., 2004).
Figure 1.
Genetic interaction between Grhl3 (G) and Vangl2 (V) in neural tube closure and cochlear stereociliary bundle orientation. (A) E15.5 littermates from Vangl2+/−/Grhl3+/−matings. Lp: loop tail; LS SB: lumbo-sacral spina bifida; st: straight tail. (B) Summary of the incidence of spina bifida in the Vangl2/Grhl3 compound heterozygotes. A total of 319 embryos from 46 separate litters of Vangl2 and Grhl3 heterozygote matings were analysed. (C) Section of an entire cochlear duct from a Grhl3+/− embryo at E18.5 stained with β-galactosidase. Inset is high magnification of the boxed region, illustrating the LacZ expressing hair cell layers. (D-F) Luminal surface of the organ of Corti in the middle turn of cochleae from E18.5 embryos with the indicated genotypes. Stereociliary bundles (red) and kinocilia (green) on IHC and OHC are uniformly orientated in (D) and (E). Bundles are non-uniform in (F) with some rotated by greater than 900. The kinocilia are also frequently mispositioned in (F). (G) Distribution histograms of OHC3 bundle orientation in wild type and Vangl2+/− /Grhl3+/− cochleae. Wild type OHCs orientations are largely confined to a 600 segment centered on a line parallel to the neural-abneural axis. In contrast the distributions are noticeably broader in Vangl2+/− /Grhl3+/− mice. (H) Average deviations from a line parallel to the neural-abneural axis for all hair cell layers in the genotypes shown in (D-F). See also Figure S1.
Another key function of the mammalian PCP genes is to co-ordinate the orientation of the actin-based stereociliary bundles of the sensory hair cells in the organ of Corti (Dabdoub et al., 2003). Analysis of LacZ staining in the cochleae of Grhl3+/− mice, which carry a β-galactosidase gene fused in frame to the twelfth codon of exon 2 of the Grhl3 gene as part of the gene targeting strategy (Ting et al., 2003b), revealed expression in the inner and outer hair cell layers (Figure 1C). We therefore tested whether mice deficient for Grhl3 or Vangl2+/− /Grhl3+/− compound heterozygotes exhibited abnormal stereociliary bundle orientation. In wild type mice, every stereociliary bundle is oriented towards the abneural side of the sensory epithelium. Examination of the cochleae from Grhl3-null mice revealed that the regular organization of one row of inner hair cells (IHC) and three rows of outer hair cells (OHCs) separated by pillar cells was preserved (data not shown). Similarly, cochleae from Vangl2+/− /Grhl3+/+ (Figure 1D) and Vangl2+/+/Grhl3+/− (Figure 1E) animals displayed normal orientation of all hair cell layers. In contrast, cochleae from Vangl2+/− /Grhl3+/− animals displayed abnormal bundle orientation throughout the basal, middle and apical turns (Figure 1F). Although the defects were most pronounced in the third row of the OHCs (OHC3), with some bundles rotated 1800 from the wild type position (Figure 1G), the IHC, OHC1 and OHC2 rows also exhibited abnormal orientation (Figure 1H). Overall, the hair bundle phenotype of the Vangl2+/− /Grhl3+/− cochleae was less severe than observed with Vangl2−/− mutants, particularly in the IHC row (Montcouquiol et al., 2003), but more pronounced than either the Scrb1 (Montcouquiol et al., 2003) or PTK7 (Lu et al., 2004) mutants.
Defective wound healing in PCP mutants
In view of the wound healing defects observed in the Grhl3-deficient mice, and the parallels in coordinated cellular movement in PCP and wound repair, we postulated that mice mutant for other PCP genes may also exhibit defective wound healing in vivo. In situ hybridisation studies have previously demonstrated expression of Vangl2 in the embryonic epidermis (Devenport and Fuchs, 2008; Murdoch et al., 2003), and multiple partial Vangl2 cDNA clones have been isolated from adult epidermis. To examine wound healing in Vangl2−/− mutant mice, and Vangl2/Grhl3 compound heterozygotes, we harvested mutant embryos of both genotypes and their littermates with their yolk sac intact at E12.5 (to assess early embryonic wound healing, which involves contraction of a cable of filamentous actin at the wound edge, and is scarless), and E16.5 (to assess late embryonic wound healing, in which keratinocytes crawl inwards to close the wound, as also occurs in adults (Martin, 1997; Redd et al., 2004). After wounding, the embryos were cultured for up to 24 hours in roller bottles and then analysed by scanning electron microscopy (SEM). Under these ex vivo conditions the embryos are viable and continue to develop (McCluskey and Martin, 1995), and time course experiments showed progressive closure occurred across the 24 hour period (Figure S2A). As shown in Figure 2A, at E12.5 wild type, Vangl2+/−, Vangl2−/− , Vangl2+/− /Grhl3+/+, and Vangl2+/+/Grhl3+/− embryos exhibited complete wound closure after 24 hours. In contrast, the Vangl2+/− /Grhl3+/− mutant embryos displayed defective wound repair at 24 hours. The results obtained with all litters examined are presented in Table 1, and the differences between the Vangl2+/−/Grhl3+/− embryos and all other genotypes were statistically significant. In the wound repair assays at E16.5, Vangl2+/− /Grhl3+/− embryos also displayed defective healing (Figure 2B) that was not observed with the other genotypes.
Figure 2.
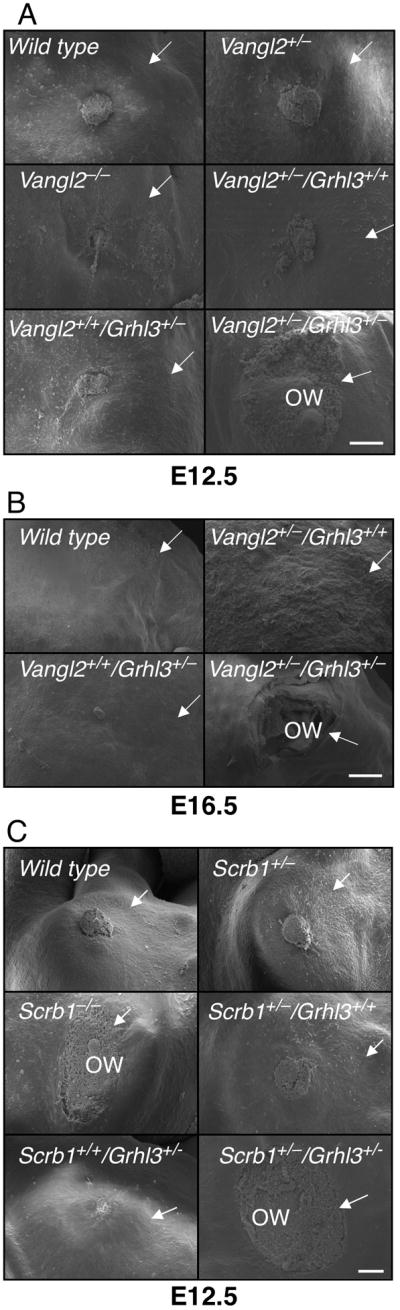
Failed wound healing in PCP mutant mice. SEM of a hind limb amputation wound in embryos with the stated genotypes at (A) E12.5; (B) E16.5; and (C) E12.5. All images are representative of at least six embryos of each genotype. Wounds were classified as open if the residual defect after 24 hours culture was >80% of the original wound diameter. Arrows point to the boundary of the wound. ow - open wound. Scale = 100 μm. See also Figure S2 and Table S1.
Table 1.
Summary of wound healing data in PCP mutant strains
| Genotype | No. of embryos | Open | Closed | Embryonic stage |
|---|---|---|---|---|
| Wild type | 12 | 2 | 10 | E12.5 |
| Vangl2+/− | 9 | 2 | 7 | E12.5 |
| Vangl2−/− | 6 | 1 | 5 | E12.5 |
| Vangl2+/+/Grhl3+/− | 7 | 0 | 7 | E12.5 |
| Vangl2+/−/Grhl3+/+ | 6 | 1 | 5 | E12.5 |
| Vangl2+/−/Grhl3+/− | 11 | 9* | 2 | E12.5 |
| Wild type | 6 | 0 | 6 | E16.5 |
| Vangl2+/−/Grhl3+/− | 5 | 0 | 5 | E16.5 |
| Vangl2+/−/Grhl3+/− | 4 | 1 | 3 | E16.5 |
| Vangl2+/−/Grhl3+/− | 7 | 6* | 1 | E16.5 |
| Wild type | 8 | 1 | 7 | E12.5 |
| Scrb1+/− | 10 | 2 | 8 | E12.5 |
| Scrb1−/− | 9 | 7* | 2 | E12.5 |
| Scrb1+/+/ Grhl3+/− | 10 | 1 | 9 | E12.5 |
| Scrb1+/−/ Grhl3+/+ | 9 | 1 | 8 | E12.5 |
| Scrb1+/−/ Grhl3+/− | 7 | 6* | 1 | E12.5 |
p<0.05
To determine whether defective wound healing was observed in other PCP mutant strains, we initially assessed the Crc (Scrb1) mutant line. Expression of the defective gene in this line, Scrb1 has been demonstrated in embryonic skin (Murdoch et al., 2003), and multiple partial Scrb1 cDNA clones have been isolated from adult epidermis. We examined wound healing in E12.5 Scrb1−/−embryos, Scrb1/Grhl3 compound heterozygote embryos, and their littermate controls (Figure 2C). In most Scrb1+/− , Scrb1+/+/Grhl3+/− , Scrb1+/− /Grhl3+/+, and wild type embryos complete wound closure was observed after 24 hours. In contrast, the Scrb1−/−and Scrb1+/− /Grhl3+/− embryos displayed a failure of wound closure at 24 hours. Failed wound healing has also been observed in another Scrb1 mutant line, the rumpelstiltzchen (rumz/Line90) mice (Zarbalis et al., 2004). The results obtained with all litters examined are presented in Table 1, and the differences between the Scrb1+/− /Grhl3+/− and Scrb1−/−embryos and all other genotypes were statistically significant. In addition to the wound healing defects, all Scrb1−/−embryos displayed craniorachischisis, as reported previously (Murdoch et al., 2001). Interestingly, the Scrb1+/−/Grhl3+/−embryos did not exhibit any NTD (data not shown). To determine whether genetic interactions between PCP genes other than Grhl3 manifested with impaired wound healing, we intercrossed Vangl2+/− and Scrb1+/− mice (Figure S2B). Healing proceeded normally in wild type, Vangl2+/− /Scrb1+/+, and Vangl2+/+/Scrb1+/− embryos. In contrast, the Vangl2+/− /Scrb1+/− embryos failed to heal. The results obtained with all litters examined are presented in Table S1, and the differences between the Vangl2+/− /Scrb1+/− embryos and all other genotypes were statistically significant. We extended these studies by examining wound healing at E12.5 in PTK7-mutant mice and the Crash (Celsr1 mutant) line (Figure S2C). In both, wild type and heterozygous embryos healed normally, but null embryos exhibited defective wound repair (Table S1). The defect in the Celsr1−/−mice was less penetrant, with 54% of the embryos exhibiting open wounds after 24 hours, and the remaining 46% demonstrating partial or complete closure. These findings confirm that the integrity of the PCP signaling pathway is essential for early and late embryonic wound repair.
In addition to its role in epidermal repair, we have previously demonstrated that Grhl3 is pivotal for the formation of the epidermal barrier in mammals through the regulation of the enzyme involved in cross linking structural proteins and lipids, transglutaminase 1 (Ting et al., 2005). This function appears to be independent of its role in the PCP pathway, as both the Vangl2+/− /Grhl3+/− and Scrb1+/− /Grhl3+/− compound heterozygous mice exhibited normal barrier function (data not shown).
Grhl3 acts through the Rho guanine nucleotide exchange factor, RhoGEF19
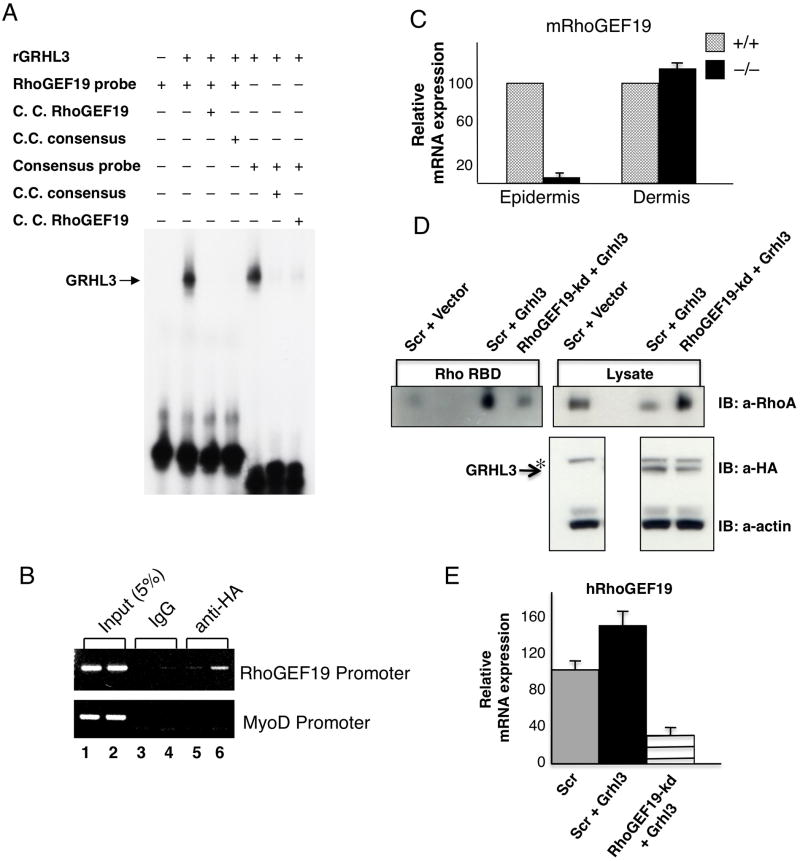
To explore the role of Grhl3 in PCP signaling and wound repair, we initially examined the expression of known PCP genes in our Grhl3-null mice, and the levels of Grhl3 in the PCP mutants (Figure S3). No changes were observed in either setting. To identify potential target genes, we utilised the previously defined GRHL3 DNA consensus binding site (5’-AACCGGTT-3’) (Ting et al., 2005) to interrogate a customised dataset of genomic regions located within 10kb of gene transcriptional start sites that are highly conserved in placental mammals (see Experimental procedures). Consistent with our expression data, no sites were identified in the regulatory regions of Vangl2, Scrb1, PTK7, and Celsr1. However, we did identify a site that was absolutely conserved in all species in the proximal promoter region of the RhoGEF19 gene, situated 109 base pairs (bp) upstream of the murine, and 137 bp upstream of the human transcription start sites (Figure S4A). Mouse RhoGEF19 is a homolog of the Xenopus and human WGEF genes that have been shown to preferentially activate RhoA (Tanegashima et al., 2008; Wang et al., 2004). In addition, XWGEF has recently been shown to be a component of the PCP pathway, and involved in the regulation of CE (Tanegashima et al., 2008). We confirmed specific binding of GRHL3 to the RhoGEF19 site in vitro in electrophoretic mobility shift assays (EMSA) (Figure 3A), and in vivo by chromatin immunoprecipitation (ChIP) (Figure 3B). Consistent with RhoGEF19 being a direct target of GRHL3, expression of the gene was markedly reduced in the epidermis of Grhl3-null mice. RhoGEF19 expression in the dermis, (where Grhl3 is not expressed) was not altered (Figure 3C), and nor was it altered in the other PCP mutant strains (Figure S4B). Q-PCR with epidermal and dermal-specific genes confirmed the integrity of the samples (data not shown). We therefore examined the effects of GRHL3 expression on RhoA activation in the presence, and absence of RhoGEF19 using pull down assays with glutathione-S-transferase (GST) fusion of the Rhotekin Rho-binding domain (RBD) to detect RhoA-GTP. HEK293T cells were co-transfected with a lentivirus carrying an short hairpin RNA (shRNA) targeting RhoGEF19 (RhoGEF19-kd), or a scrambled control shRNA (Scr) and an mammalian expression vector carrying HA-tagged GRHL3, or the control empty vector. Expression of HA-GRHL3 was confirmed by Western blot (Figure 3D, IB:α-HA). A marked increase in active RhoA was detected in the Scr cells expressing GRHL3 compared to Scr cells transfected with vector alone. This increase was completely abrogated in the RhoGEF19-kd cells despite robust expression of HA-GRHL3 in these cells. Consistent with these findings, we showed that expression of RhoGEF19 was increased in the GRHL3-transfected Scr cells by Q-PCR (as no antibody is available for RhoGEF19), but remained low in the RhoGEF19-kd cells transfected with HA-GRHL3 (Figure 3E, graph).
Figure 3.
RhoGEF19 is a direct target gene of GRHL3. (A) EMSA of recombinant (r) GRHL3 binding to the RhoGEF19 promoter. A 100-fold molar excess of unlabelled cold competitor probe was added in the indicated lanes. The migration of the specific GRHL3/DNA complexes is arrowed. (B) ChIP analysis of GRHL3 on the RhoGEF19 promoter. Chromatin from HEK 293T cells transfected with empty vector (lane 5) or HA-tagged Grhl3 (lane 6) was immunoprecipitated using antisera to the HA-tag. As negative controls, we used pre-immune sera (lanes 3 and 4). Lane 1 and 2 shows the input chromatin. The immunoprecipitated chromatin was amplified with RhoGEF19, or control MyoD primers. (C) Q-PCR of RhoGEF19 expression in wild type (hatched boxes) and Grhl3−/− (closed boxes) E18.5 epidermis and dermis. Bars represent standard errors. The HPRT levels served as a reference. (D, E) GRHL3 induced RhoA activation in HEK 293T cells is mediated through increased RhoGEF19 expression. HA-tagged Grhl3 was transfected into HEK 293T cells transduced with an shRNA to RhoGEF19, or a Scrambled control shRNA (Scr), and expression of HA-GRHL3 was confirmed by Western blot (IB:α-HA, α-actin loading control). GTP-Rho was precipitated using GST-RBD and detected by anti-RhoA antibody (IB:αRhoA). Total RhoA was also detected in lysates with anti-RhoA antibody. HA-GRHL3 is arrowed. *non-specific band with HA antibody. Expression of human RhoGEF19 in Scr control cells, Scr cells expressing HA-GRHL3, and RhoGEF19-kd cells expressing HA-GRHL3 was determined by Q-PCR. See also Figure S3.
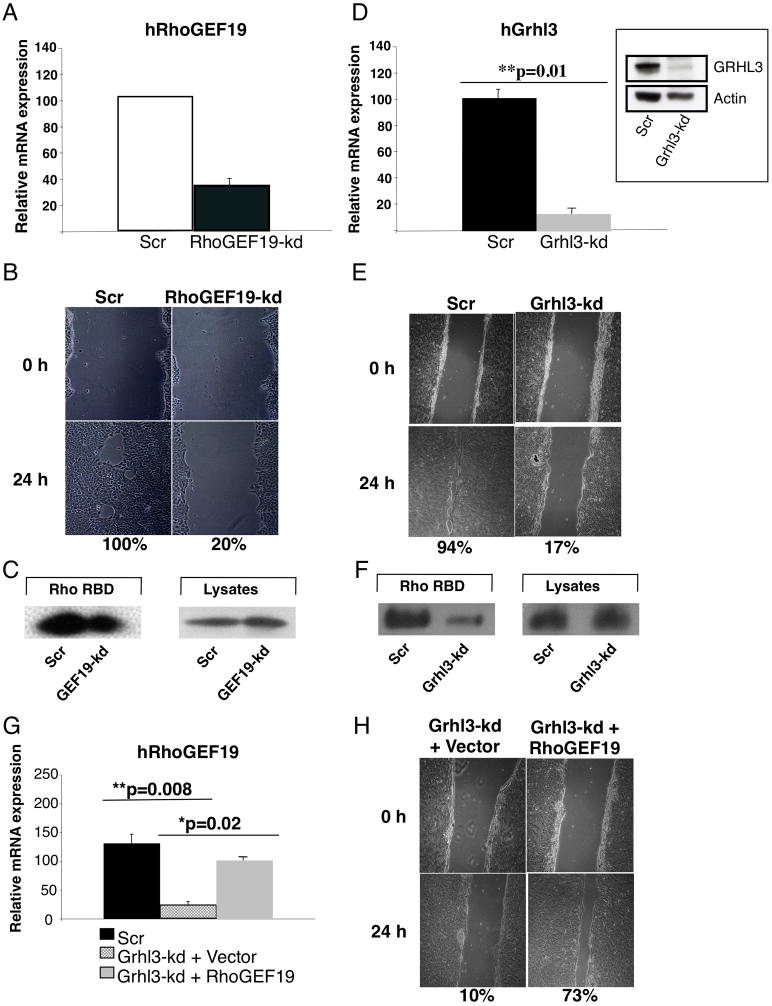
To directly examine the importance of RhoGEF19 in wound repair, we utilized the shRNA lentivirus to target the RhoGEF19 transcript in the human keratinocyte cell line, HaCAT. We confirmed a knockdown of approximately 80% by Q-RT-PCR (Figure 4A) compared to cells transduced with a scrambled control shRNA (Scr). The RhoGEF19-kd HaCAT cells failed to “heal” in scratch assays (20% closure), unlike the Scr cells, which migrated to completely close the scratch within 24 hours (Figure 4B). Activated RhoA was also reduced in these cells (Figure 4C). To confirm that RhoGEF19 was a critical GRHL3 target gene in wound repair, we generated HaCAT cells expressing an shRNA targeting Grhl3 (Grhl3-kd), and a scrambled control line (Scr). Expression of Grhl3 in the Grhl3-kd cells was reduced to less than 20% of the Scr control at both RNA (Figure 4D), and protein level (Figure 4D, inset). RhoGEF19 expression was also reduced to less than 25% in these cells compared to Scr control (Figure 4G). Scratch assays with these cell lines displayed almost complete healing with the Scr control cells (94%), but persistence of the “wound” in the Grhl3-kd cells (17% closure) (Figure 4E). This was not attributable to reduced cell growth, as these cells, and Grhl3−/−keratinocytes, exhibit enhanced proliferation compared to their wild type counterparts (Ting et al., 2005, and not shown). Consistent with our previous data, activated RhoA was reduced in the Grhl3-kd cells (Figure 4F). To determine if re-expression of RhoGEF19 could rescue the wound defect in Grhl3-kd cells, we transfected these cells with a mammalian expression vector containing the RhoGEF19 cDNA (Grhl3-kd + RhoGEF19), or the empty vector as a control (Grhl3-kd + Vector). RhoGEF19 expression levels were restored to near wild type in the Grhl3-kd + RhoGEF19 cells (Figure 4G). Scratch assays with these cells demonstrated that re-expression of RhoGEF19 substantially rescued the wound healing phenotype observed with the Grhl3-kd cells (10% closure versus 73%) (Figure 4H). Taken together, this data identifies RhoGEF19 as a critical direct target of Grhl3 in the context of epidermal migration in wound repair.
Figure 4.
Re-expression of RhoGEF19 rescues the wound healing defect in Grhl3-deficient keratinocytes. (A) Q-PCR of RhoGEF19 expression in Scr and RhoGEF19-kd HaCAT cells. (B) In vitro scratch wound assays in Scr and RhoGEF19-kd HaCAT cells. Percentages indicate the extent of closure compared to the original defect. (C) RhoA activation in Scr and RhoGEF19-kd HaCAT cells as detailed in Figure 3E. (D) Q-PCR of Grhl3 expression in Scr and Grhl3-kd HaCAT cells. Inset: Western blot of GRHL3 and actin levels in Scr and Grhl3-kd HaCAT cells. (E) In vitro scratch wound assays in Scr and Grhl3-kd HaCAT cells. (F) RhoA activation in Scr and Grhl3-kd HaCAT cells. (G) Q-PCR of RhoGEF19 expression in Scr, Grhl3-kd cells transduced with empty vector, and Grhl3-kd cells transduced with RhoGEF19 expression vector. (H) In vitro scratch wound assays in Grhl3-kd cells transduced with empty vector, and Grhl3-kd cells transduced with RhoGEF19 expression vector. See also Figure S4.
Grhl3 and RhoGEF19 regulate actin polymerisation and cellular polarity during wound repair
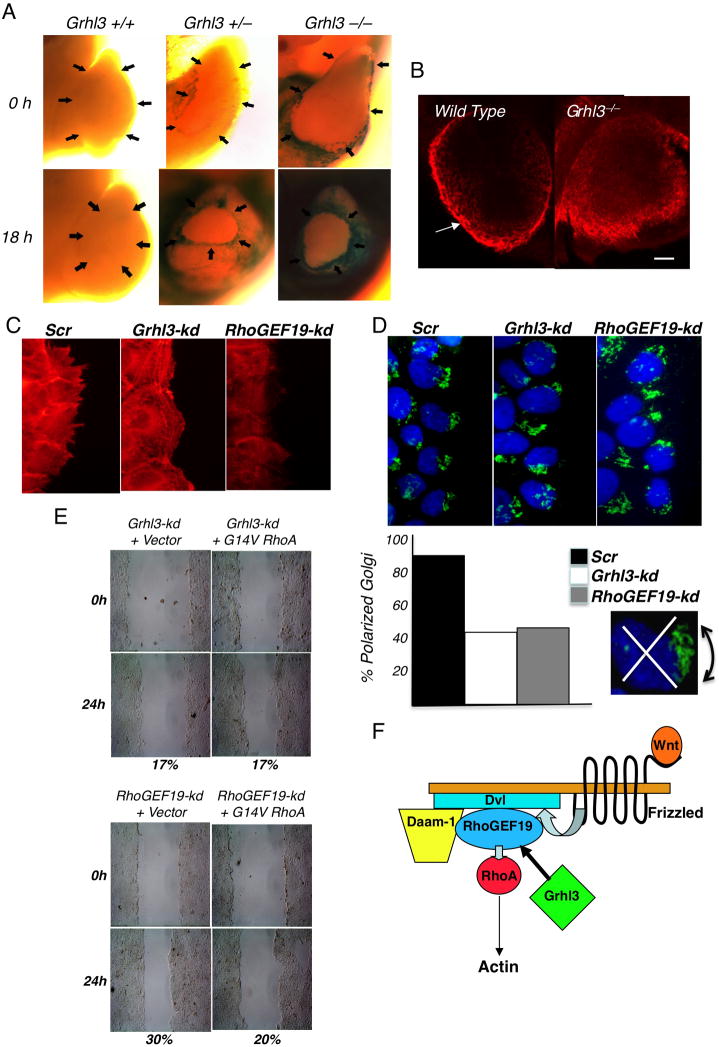
To further explore the mechanism underpinning defective epidermal migration in the absence of Grhl3 or RhoGEF19, we initially examined wound repair in cultured Grhl3-null embryos. A hallmark of wound closure at E12.5 is the formation of a “purse-string” actin cable around the wound margin. Examination of LacZ staining in wounded Grhl3+/− and Grhl3−/−embryos demonstrated a marked induction of circumferential expression between 0 and 18 hours (Figure 5A), suggesting that increased Grhl3 expression in response to wounding would result in enhanced RhoGEF19 expression at the wound margins. Whole mount rhodamine-phalloidin staining of F-actin fibers in wild type and Grhl3−/−embryos revealed marked differences, with clear actin polymerisation and purse string formation in controls, compared with disorganized structure at the wound margins in the mutants (Figure 5B). To examine this further, we compared the effects of loss of Grhl3 and Rho GEF 19 expression on actin polymerisation in human Grhl3-kd, RhoGEF19-kd and Scr control keratinocytes in scratch assays after 18 hours (Figure 5C). The Scr cells, which migrate to completely heal the scratch after 24 hours, displayed extensive actin stress fibers associated with cellular projections aligned in the direction of the migrating cells. In contrast, both the Grhl3-kd, and to an even greater extent, RhoGEF19-kd cells formed only rudimentary stress fibers that showed irregular organization and were not directed towards the scratched area.
Figure 5.
Defective actin polymerization and cellular polarity in keratinocytes lacking Grhl3 or RhoGEF19. (A) LacZ staining of hind-limb amputation wounds in Grhl3+/+, Grhl3+/ −, and Grhl3−/−E12.5 embryos at 0 and 18 hours. The arrows indicate the wound margins. (B) Whole mount phalloidin staining of F-actin cable formation in hind-limb amputation wounds in wild type and Grhl3−/−E12.5 embryos at 18 hours. Scale = 100μm. (C) Phalloidin staining of scratch wound margins at 12 hours in cultured Grhl3-kd, RhoGEF19-kd, or Scr control keratinocytes. (D) Grhl3-kd, RhoGEF19-kd, or Scr keratinocytes were scratch wounded and fixed 6 hours later, prior to labeling with GM130 antibody to stain the Golgi (green) and TOPRO3 to label nuclei (blue). Shown beneath is the quantitation of cells at the leading edge with the Golgi polarized within a 1200 arc (inset) in front of the nucleus. (E) Grhl3-kd or RhoGEF19-kd keratinocytes were transfected with a mammalian expression vector carrying a myc-tagged constitutively active RhoA (G14V RhoA), or the empty vector, prior to scratch wounding and culture for 24 hours. Percentages indicate the extent of closure compared to the original defect. (F) Model of the role of Grhl3 in PCP signaling (adapted from Tanegashima et al. 2008). See also Figure S5.
Cells at the leading edge of a wound polarize the nucleus, actin cytoskeleton, and microtubule organizing centre (MTOC)/Golgi network in the axis of migration (Hall, 2005). We analyzed the polarity of leading edge cells 6 hours following scratch wounding of human Grhl3-kd, RhoGEF19-kd, and Scr control keratinocytes (Figure 5D). In 90% of Scr cells at the wound edge, the Golgi was polarized within a 1200 arc in front of the nucleus. In contrast, both Grhl3-kd, and RhoGEF19-kd cells showed no polarization, and the Golgi remained essentially randomly distributed around the nucleus. This finding is similar to the cellular polarity defects we have previously observed with shRNA-mediated knockdown of Scribble in the context of wounding in MCF10A cells (Dow et al., 2007). To determine the importance of loss of Grhl3 and RhoGEF19 expression on cell polarity at the wound margin, we transfected the human Grhl3-kd, and RhoGEF19-kd keratinocytes with a mammalian expression vector containing a constitutively active form of RhoA, (G14V RhoA) (Ren et al., 1999), tagged with the myc epitope, or the empty vector control, and performed scratch assays (Figure 5E). Both knockdown cell lines transfected with the empty vector failed to heal over 24 hours (17% and 30% closure), and this was not altered with the expression of G14V RhoA, indicating that non-polarized activation of RhoA is insufficient to rescue the wound closure defects observed with loss of Grhl3 or RhoGEF19. Scr control cells transfected with G14V RhoA healed less completely than cells transfected with vector alone (100% versus 75%), and myc expression was equivalent across transfections (Figure S5).
In summary, our data defines RhoGEF19 as a direct Grhl3 target gene that is essential for polarized RhoA activation, epidermal migration and wound repair, in the context of the PCP signalling pathway (Figure 5F).
DISCUSSION
Three important findings have emerged from our studies: 1) early and late embryonic epidermal wound healing in mammals is regulated by the PCP signaling pathway; 2) Grhl3 is a component of this pathway, acting through the RhoA activator, RhoGEF19; 3) regulation of expression of RhoGEFs by tissue-restricted transcription factors provides a mechanism for spatio-temporal-specific remodelling of the actin cytoskeleton, and selective cell migration.
Integral to both PCP and wound repair is the need for cells to adopt an axis of polarity, followed by reorganisation of their cytoskeleton leading to directional migration. Consistent with this, regulators of the actin cytoskeleton including RhoA, Rho-associated kinase, and Rac1 have been implicated in both processes (Kodama et al., 2004; Strutt et al., 1997; Tscharntke et al., 2007; Vaezi et al., 2002; Winter et al., 2001). Our data identifies epidermal wound repair defects with five PCP mutant alleles, either in the homozygous state (PTK7, Celsr1, Scrb1, Grhl3), or as compound heterozygotes (Vangl2/Scrb1, Vangl2/Grhl3). The variation in wound healing observed with different mutant alleles is similar to the phenotypic heterogeneity seen with other PCP-regulated processes in various mutant strains, and suggests that the obligate genes required for the diverse processes governed by PCP signaling are context-specific. For wound repair, PTK7, Scrb1 and Grhl3 are all essential, whereas Vangl2 is redundant, and loss of Celsr1 appears to be partially compensated. In cochlear stereociliary organization, Vangl2/Scrb1 compound heterozygotes exhibit marked polarity defects, whereas PTK7/Vangl2 compound heterozygotes have normal cochleas, despite abnormalities in both Vangl2−/−and PTK7-null embryos (Lu et al., 2004; Montcouquiol et al., 2003). In neural tube closure, almost all PTK7/Vangl2 compound heterozygotes display spina bifida (similar to the Grhl3/Vangl2 mice), but only 50% of Vangl2/Scrb1 mutants exhibit craniorachischisis (Murdoch et al., 2001). These findings may be attributable to subtle differences in the PCP pathways regulating the individual processes, or alternatively, variations in the protein complexes involved in wound repair, stereociliary polarity and neurulation.
Several genes implicated in cell motility in response to wounding have also been linked to the PCP pathway. In Drosophila, mutants of Twinstar the homolog of cofilin/ADF (actin depolymerization factor), a key regulator of actin dynamics at the leading edge of motile cells (DesMarais et al., 2005), display polarity defects in the wing, eye and other epithelia (Blair et al., 2006). Although cofilin/ADF has not yet been implicated in mammalian PCP, it is noteworthy that mice lacking cofilin-1, the predominant protein isoform expressed in the embryo, exhibit craniorachischisis, a hallmark of many PCP mutants (Gurniak et al., 2005). In mice, the secreted glycoprotein, Cthrc1 promotes cell migration at wound margins by reducing the deposition of the collagen matrix (Durmus et al., 2006; Pyagay et al., 2005). This factor also functions as a Wnt cofactor, selectively activating the PCP pathway in the context of neural tube closure and cochlear stereociliary organization (Yamamoto et al., 2008). Vangl1 has been linked to epithelial repair in the intestine (Kalabis et al., 2006), and Rho-associated kinase 1 (ROCK-1) is essential for accumulation of filamentous actin in closure of the eyelids and ventral body wall (Shimizu et al., 2005), and has also been implicated in assembly of the stratified epidermis, which may be important for wound repair (Vaezi et al., 2002). Interestingly, several PCP mutants, and the Grhl3-null mice also exhibit defects in eyelid closure at birth (Curtin et al., 2003; Hislop et al., 2008; Lu et al., 2004; Montcouquiol et al., 2003; Yu et al., 2008).
Grhl3 functions as a key transcriptional regulator in the mammalian PCP signaling pathway. It interacts genetically with the core PCP gene Vangl2, in regulating closure of the neural tube, and orientation of the stereociliary bundles of the cochlea, typical features of other recently described PCP genes (Lu et al., 2004; Yamamoto et al., 2008). Unlike many of the other homozygous mutant PCP strains, which exhibit craniorachischisis, and cochlea polarity defects, Grhl3-null animals display thoraco-lumbo-sacral spina bifida (Ting et al., 2003b), and have normal stereociliary bundles. These findings are reminiscent of the phenotypes observed with another PCP gene, Dvl2, one of the three mammalian homologs of the Drosophila dsh gene. In isolation, loss of Dvl2 expression results in thoracic spina bifida (Hamblet et al., 2002), but stereociliary bundle polarity is unaffected (Wang et al., 2005). Only in the context of Dvl1/2 double mutant embryos, or Dvl2−/−/Lp/+ embryos was complete failure of neural tube closure (Hamblet et al., 2002), and stereociliary polarity defects (Wang et al., 2005) observed. The presence of two highly related mammalian homologs of Grhl3 suggests that some functional redundancy also exists in this gene family (Wilanowski et al., 2002).
The identification of Grhl3 as a PCP gene, analogous to its Drosophila homologue grh, further extends the parallels between fly and mammalian PCP signaling. Homologues of all the Drosophila core PCP genes have been identified in vertebrates, and implicated in PCP regulated developmental events (Seifert and Mlodzik, 2007). Mutants of grh display polarity defects of wing hairs and ommatidia, and abnormal localisation of planar polarity proteins in the wing cells. In addition, grh interacts genetically with several of the fz pathway genes, including Vang/Stbm (Lee and Adler, 2004). Reduced levels of Stan protein in both larval wing discs and pupal wings in grh mutants suggests that this factor may also transcriptionally regulate stan/fmi, although putative binding sites for Grh were not evident in the stan/fmi regulatory regions (Lee and Adler, 2004). In mice, Grhl3 interacts genetically with Vangl2, a homologue of Vang/Stbm, but we found no evidence that homologues of stan/fmi (Celsr1-3) were direct target genes of GRHL3. No predicted binding sites for GRHL3 were identified in the Celsr promoters (or any of the other PCP genes examined), and expression of all these genes was not altered in the Grhl3-null mice.
The key GRHL3 target gene that we did identify was the RhoA activator, RhoGEF19. The Xenopus homologue of this gene, WGEF specifically activates RhoA, and co-localizes with Dvl at the plasma membrane in a fz-dependent manner. Upon Wnt signaling and fz activation, Dvl, Daam-1 and Rho are recruited to the cell membrane (Kim and Han, 2007; Park et al., 2006), where they are co-localized and complexed with XWGEF, leading to Rho activation (Tanegashima et al., 2008). Morpholino-induced loss of WGEF expression results in defective CE, with embryos displaying shortened axes, and in severe cases, neural tube defects (Tanegashima et al., 2008). In mouse epidermis, loss of Grhl3 led to a marked reduction in RhoGEF19 expression, with loss of actin polymerisation and defective cellular polarisation and migration at the wound margins. Re-expression of RhoGEF19 in Grhl3-kd cells rescues wound healing indicating that RhoGEF19 is an essential Grhl3 target gene in this process. It is likely that transcriptional regulation of RhoGEF19 will also be important for other Grhl3-dependent morphogenetic events, such as neural tube and eyelid closure.
EXPERIMENTAL PROCEDURES
Experimental Animals
The generation and genotyping of Grhl3 mice has been described previously (Ting et al., 2003b). Lp/+ mice of the LPT/LeJ stock were obtained from the Jackson Laboratory. Crc, Crash and PTK7 mutant mice were obtained from Dr Jennifer Murdoch. Mouse genotyping is detailed in the Supplemental data. All experiments were pre-approved by The University of Melbourne Animal Ethics Committee.
Stereociliary Analysis
Cochleae from E18.5 embryos were dissected and whole mounts from the organ of Corti were prepared. PCP was analyzed by staining for the kinocilium using an anti-acetylated tubulin antibody (Sigma-Aldrich, St.Louis, MO, USA) and filamentous actin was labeled with rhodamine-conjugated phalloidin (Molecular Probes, Eugene, OR, USA). The orientation of individual stereociliary bundles was determined as described previously (Montcouquiol et al., 2003). Data was collected from a minimum of three samples for each genotype.
Embryonic wound healing assays
Analysis of wound repair was performed on embryonic day 12.5 and 16.5 embryos as described previously (McCluskey and Martin, 1995). At least 6 embryos of each genotype were analysed for wound closure. Failed wound repair was defined as a residual defect >80% of the original wound diameter. A Fischer’s Exact Test was used to determine statistical difference between wound healing for the different genotypes. P values ≤ 0.05 were considered significant, and results were analysed using the computer software program Prism (GraphPad). The skin permeability assays and histological analysis were performed as described previously (Ting et al., 2005).
Identification of conserved elements in promoter regions
Conserved elements upstream of human genes were identified using annotation data from the UCSC genome browser (http://genome.ucsc.edu/; NCBI Build 36.1). From this, we constructed a new dataset consisting of 10kb regions upstream of the transcription start sites of human RefSeq genes. We then took the intersection of these regions with predicted conserved elements that were identified using a phylo-hidden Markov model, PhastCons (Siepel et al., 2005), on the placental mammal-subset of the 28-way multiple alignment of vertebrate genomes. The resulting 178,611 predicted conserved elements located in upstream regions had a median length of 28nt. The sequence data contained in these elements was then extracted to a fasta file and searched using the GRHL3 DNA binding consensus sequence.
ChIP and EMSA
ChIP was performed as described previously (Wilanowski et al., 2008). EMSAs were performed using recombinant mouse GRHL3 protein, and the Grhl3 DNA consensus binding sequence, as defined previously (Ting et al., 2005). Primer and oligonucleotide sequences for each assay are detailed in the Supplemental data.
RNA preparation and Q-PCR
For expression analysis from skin, the epidermis was separated from dermis using 2 mg/ml Dispase II (Invitrogen, Carlsbad, CA, USA) at 4°C overnight. Both tissues were homogenised in TRIzol (Invitrogen) and RNA extracted according to the manufacturer’s instructions. For expression analysis in the human embryonic kidney cell line (HEK) 293T, cells were co-transfected with the pcDNA3.1 expression vector (Invitrogen) containing hemagglutinin (HA) epitope-tagged murine Grhl3, and an MSCV-based GFP expressing vector using Fugene (Roche, Basel, Switzerland). After 48 hours, GFP+ cells were sorted by FACS and total RNA was extracted using TriZol. Q-PCR carried out as described previously (Ting et al., 2003b) and primer sequences are detailed in the Supplemental data. A student's t-test was used to determine statistical difference in expression levels. P values ≤ 0.05 were considered significant, and results were analysed using the computer software program Prism (GraphPad). The error bars in all expression analyses represent the standard error of the mean.
Activation Assay
Activation assays were performed using Cytoskeleton kits (Cytoskeleton Inc., Denver, CO, USA) in accordance to the manufacturers specifications, as detailed in the Supplemental data. Each experiment in this study was performed in triplicate on at least three separate occasions (n≥9), with parallel control and experimental treatments.
Scratch Assays and Actin Analysis
Scratch assays and phalloidin staining of cultured keratinocyte lines were performed as described previously (Hislop et al., 2008), as detailed in the Supplemental data. Each experiment was performed in triplicate on at least three separate occasions with parallel controls.
RNA interference, lentiviral infection and transduction
The shRNA target oligonucleotides for RhoGEF19 and the scrambled (Scr) control were cloned into the LentiLox3.7 (pLL3.7) lentiviral vector (ATCC, Manassas, VA, USA) using the HpaI and XhoI sites. The shRNA target oligonucleotides for Grhl3 were cloned into the pSUPER.retro.neo+GFP vector using the BglII and HindIII sites. The target sequences and generation of viruses are detailed in the Supplemental data.
Supplementary Material
Acknowledgments
We thank Andrew Copp for Crc mice, Gordon Smyth for statistical analyses, Mary Corbett, Sumitha Vasudevan, Sarah King, and James Pickles for technical assistance. Animal support was provided by staff from the Bio21 and Walter & Eliza Hall Institutes. SMJ is a Principal Research Fellow of the Australian National Health and Medical Research Council (NHMRC). POH is supported by a career development award from the NHMRC. The work was supported by Project Grants from the NHMRC, and The March of Dimes Foundation (SMJ); the Deafness Research Foundation, and the National Institutes of Health (USA) (JZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–535. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- Axelrod JD, McNeill H. Coupling planar cell polarity signaling to morphogenesis. Scientific World Journal. 2002;2:434–454. doi: 10.1100/tsw.2002.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair A, Tomlinson A, Pham H, Gunsalus KC, Goldberg ML, Laski FA. Twinstar, the Drosophila homolog of cofilin/ADF, is required for planar cell polarity patterning. Development. 2006;133:1789–1797. doi: 10.1242/dev.02320. [DOI] [PubMed] [Google Scholar]
- Brock J, Midwinter K, Lewis J, Martin P. Healing of incisional wounds in the embryonic chick wing bud: characterization of the actin purse-string and demonstration of a requirement for Rho activation. J Cell Biol. 1996;135:1097–1107. doi: 10.1083/jcb.135.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Curr Biol. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- Dabdoub A, Donohue MJ, Brennan A, Wolf V, Montcouquiol M, Sassoon DA, Hseih JC, Rubin JS, Salinas PC, Kelley MW. Wnt signaling mediates reorientation of outer hair cell stereociliary bundles in the mammalian cochlea. Development. 2003;130:2375–2384. doi: 10.1242/dev.00448. [DOI] [PubMed] [Google Scholar]
- DesMarais V, Ghosh M, Eddy R, Condeelis J. Cofilin takes the lead. J Cell Sci. 2005;118:19–26. doi: 10.1242/jcs.01631. [DOI] [PubMed] [Google Scholar]
- Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nat Cell Biol. 2008;10:1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow LE, Kauffman JS, Caddy J, Peterson AS, Jane SM, Russell SM, Humbert PO. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene. 2007;26:2272–2282. doi: 10.1038/sj.onc.1210016. [DOI] [PubMed] [Google Scholar]
- Durmus T, LeClair RJ, Park KS, Terzic A, Yoon JK, Lindner V. Expression analysis of the novel gene collagen triple helix repeat containing-1 (Cthrc1) Gene Expr Patterns. 2006;6:935–940. doi: 10.1016/j.modgep.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Fanto M, Weber U, Strutt DI, Mlodzik M. Nuclear signaling by Rac and Rho GTPases is required in the establishment of epithelial planar polarity in the Drosophila eye. Curr Biol. 2000;10:979–988. doi: 10.1016/s0960-9822(00)00645-x. [DOI] [PubMed] [Google Scholar]
- Fukata M, Nakagawa M, Kaibuchi K. Roles of Rho-family GTPases in cell polarisation and directional migration. Curr Opin Cell Biol. 2003;15:590–597. doi: 10.1016/s0955-0674(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Gurniak CB, Perlas E, Witke W. The actin depolymerizing factor n-cofilin is essential for neural tube morphogenesis and neural crest cell migration. Dev Biol. 2005;278:231–241. doi: 10.1016/j.ydbio.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33:891–895. doi: 10.1042/BST20050891. [DOI] [PubMed] [Google Scholar]
- Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boris A. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development. 2002;129:5827–5838. doi: 10.1242/dev.00164. [DOI] [PubMed] [Google Scholar]
- Hislop NR, Caddy J, Ting SB, Auden A, Vasudevan S, King SL, Lindeman GJ, Visvader JE, Cunningham JM, Jane SM. Grhl3 and Lmo4 play coordinate roles in epidermal migration. Dev Biol. 2008;321:263–272. doi: 10.1016/j.ydbio.2008.06.026. [DOI] [PubMed] [Google Scholar]
- Kalabis J, Rosenberg I, Podolsky DK. Vangl1 protein acts as a downstream effector of intestinal trefoil factor (ITF)/TFF3 signaling and regulates wound healing of intestinal epithelium. J Biol Chem. 2006;281:6434–6441. doi: 10.1074/jbc.M512905200. [DOI] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Kibar Z, Vogan KJ, Groulx N, Justice MJ, Underhill DA, Gros P. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–255. doi: 10.1038/90081. [DOI] [PubMed] [Google Scholar]
- Kim GH, Han JK. Essential role for beta-arrestin 2 in the regulation of Xenopus convergent extension movements. EMBO J. 2007;26:2513–2526. doi: 10.1038/sj.emboj.7601688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein TJ, Mlodzik M. Planar cell polarization: An emerging model points in the right direction. Annu Rev Cell Dev Biol. 2005;21:155–176. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- Kodama A, Lechler T, Fuchs E. Coordinating cytoskeletal tracks to polarize cellular movements. J Cell Biol. 2004;167:203–207. doi: 10.1083/jcb.200408047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Adler PN. The grainy head transcription factor is essential for the function of the frizzled pathway in the Drosophila wing. Mech Dev. 2004;121:37–49. doi: 10.1016/j.mod.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- Mace KA, Pearson JC, McGinnis W. An epidermal barrier wound repair pathway in Drosophila is mediated by grainy head. Science. 2005;308:381–385. doi: 10.1126/science.1107573. [DOI] [PubMed] [Google Scholar]
- Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Martin P, Parkhurst SM. Parallels between tissue repair and embryo morphogenesis. Development. 2004;131:3021–3034. doi: 10.1242/dev.01253. [DOI] [PubMed] [Google Scholar]
- McCluskey J, Martin P. Analysis of the tissue movements of embryonic wound healing--DiI studies in the limb bud stage mouse embryo. Dev Biol. 1995;170:102–114. doi: 10.1006/dbio.1995.1199. [DOI] [PubMed] [Google Scholar]
- Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- Murdoch JN, Henderson DJ, Doudney K, Gaston-Massuet C, Phillips HM, Paternotte C, Arkell R, Stanier P, Copp AJ. Disruption of scribble (Scrb1) causes severe neural tube defects in the circletail mouse. Hum Mol Genet. 2003;12:87–98. doi: 10.1093/hmg/ddg014. [DOI] [PubMed] [Google Scholar]
- Murdoch JN, Rachel RA, Shah S, Beermann F, Stanier P, Mason CA, Copp AJ. Circletail, a new mouse mutant with severe neural tube defects: chromosomal localization and interaction with the loop-tail mutation. Genomics. 2001;78:55–63. doi: 10.1006/geno.2001.6638. [DOI] [PubMed] [Google Scholar]
- Park E, Kim GH, Choi SC, Han JK. Role of PKA as a negative regulator of PCP signaling pathway during Xenopus gastrulation movements. Dev Biol. 2006;292:344–357. doi: 10.1016/j.ydbio.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Pyagay P, Heroult M, Wang Q, Lehnert W, Belden J, Liaw L, Friesel RE, Lindner V. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ Res. 2005;96:261–268. doi: 10.1161/01.RES.0000154262.07264.12. [DOI] [PubMed] [Google Scholar]
- Redd MJ, Cooper L, Wood W, Stramer B, Martin P. Wound healing and inflammation: embryos reveal the way to perfect repair. Philos Trans R Soc Lond B Biol Sci. 2004;359:777–784. doi: 10.1098/rstb.2004.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XD, Kiosses WB, Schwartz MA. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 1999;18:578–585. doi: 10.1093/emboj/18.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, Noda Y, Matsumura F, Taketo MM, Narumiya S. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol. 2005;168:941–953. doi: 10.1083/jcb.200411179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepel A, Bejerano G, Pedersen JS, Hinrichs AS, Hou M, Rosenbloom K, Clawson H, Spieth J, Hillier LW, Richards S, et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 2005;15:1034–1050. doi: 10.1101/gr.3715005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strutt DI, Weber U, Mlodzik M. The role of RhoA in tissue polarity and Frizzled signalling. Nature. 1997;387:292–295. doi: 10.1038/387292a0. [DOI] [PubMed] [Google Scholar]
- Tanegashima K, Zhao H, Dawid IB. WGEF activates Rho in the Wnt-PCP pathway and controls convergent extension in Xenopus gastrulation. EMBO J. 2008;27:606–617. doi: 10.1038/emboj.2008.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting SB, Caddy J, Hislop N, Wilanowski T, Auden A, Zhao LL, Ellis S, Kaur P, Uchida Y, Holleran WM, et al. A homolog of Drosophila grainy head is essential for epidermal integrity in mice. Science. 2005;308:411–413. doi: 10.1126/science.1107511. [DOI] [PubMed] [Google Scholar]
- Ting SB, Wilanowski T, Cerruti L, Zhao LL, Cunningham JM, Jane SM. The identification and characterization of human Sister-of-Mammalian Grainyhead (SOM) expands the grainyhead-like family of developmental transcription factors. Biochem J. 2003a;370:953–962. doi: 10.1042/BJ20021476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting SB, Wilanowski T, Auden A, Hall M, Voss AK, Thomas T, Parekh V, Cunningham JM, Jane SM. Inositol- and folate-resistant neural tube defects in mice lacking the epithelial-specific factor Grhl-3. Nat Med. 2003b;9:1513–1519. doi: 10.1038/nm961. [DOI] [PubMed] [Google Scholar]
- Tscharntke M, Pofahl R, Chrostek-Grashoff A, Smyth N, Niessen C, Niemann C, Hartwig B, Herzog V, Klein HW, Krieg T, et al. Impaired epidermal wound healing in vivo upon inhibition or deletion of Rac1. J Cell Sci. 2007;120:1480–1490. doi: 10.1242/jcs.03426. [DOI] [PubMed] [Google Scholar]
- Vaezi A, Bauer C, Vasioukhin V, Fuchs E. Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev Cell. 2002;3:367–381. doi: 10.1016/s1534-5807(02)00259-9. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, Symons M. Role of Rho family GTPases in epithelial morphogenesis. Genes Dev. 2002;16:1032–1054. doi: 10.1101/gad.978802. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nat Genet. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Suzuki H, Yokoo T, Tada-Iida K, Kihara R, Miura M, Watanabe K, Sone H, Shimano H, Toyoshima H, et al. WGEF is a novel RhoGEF expressed in intestine, liver, heart, and kidney. Biochem Biophys Res Commun. 2004;324:1053–1058. doi: 10.1016/j.bbrc.2004.09.153. [DOI] [PubMed] [Google Scholar]
- Wilanowski T, Caddy J, Ting SB, Hislop NR, Cerruti L, Auden A, Zhao LL, Asquith S, Ellis S, Sinclair R, et al. Perturbed desmosomal cadherin expression in grainy headlike 1-null mice. EMBO J. 2008;27:886–897. doi: 10.1038/emboj.2008.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilanowski T, Tuckfield A, Cerruti L, O'Connell S, Saint R, Parekh V, Tao J, Cunningham JM, Jane SM. A highly conserved novel family of mammalian developmental transcription factors related to Drosophila grainyhead. Mech Dev. 2002;114:37–50. doi: 10.1016/s0925-4773(02)00046-1. [DOI] [PubMed] [Google Scholar]
- Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, Luo L. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- Wu J, Mlodzik M. A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol. 2009;19:295–305. doi: 10.1016/j.tcb.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Nishimura O, Misaki K, Nishita M, Minami Y, Yonemura S, Tarui H, Sasaki H. Cthrc1 selectively activates the planar cell polarity pathway of Wnt signaling by stabilizing the Wnt-receptor complex. Dev Cell. 2008;15:23–36. doi: 10.1016/j.devcel.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Yan J, Lu Q, Fang X, Adler PN. Rho1 has multiple functions in Drosophila wing planar polarity. Dev Biol. 2009;333:186–199. doi: 10.1016/j.ydbio.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Bhandari A, Mannik J, Pham T, Xu X, Andersen B. Grainyhead-like factor Get1/Grhl3 regulates formation of the epidermal leading edge during eyelid closure. Dev Biol. 2008;319:56–67. doi: 10.1016/j.ydbio.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- Zarbalis K, May SR, Shen Y, Ekker M, Rubenstein JL, Peterson AS. A focused and efficient genetic screening strategy in the mouse: identification of mutations that disrupt cortical development. PLoS Biol. 2004;2:E219. doi: 10.1371/journal.pbio.0020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.